Synthetic Methods towards Energetic Heterocyclic N-Oxides via Several Cyclization Reactions
Abstract
1. Introduction
2. Energetic Heterocyclic N-Oxides via Different Cyclization Reaction
2.1. Azine N-Oxides
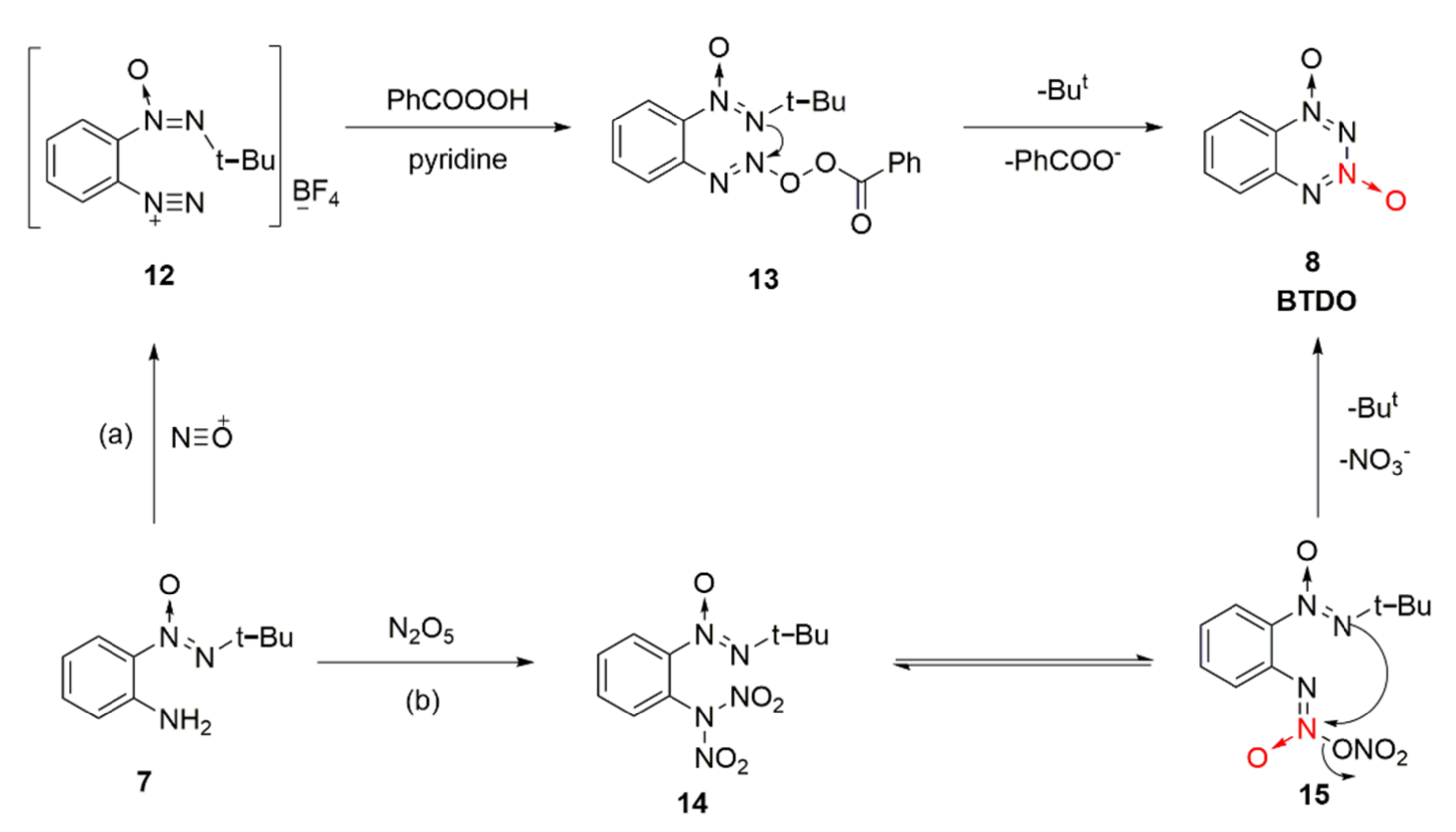
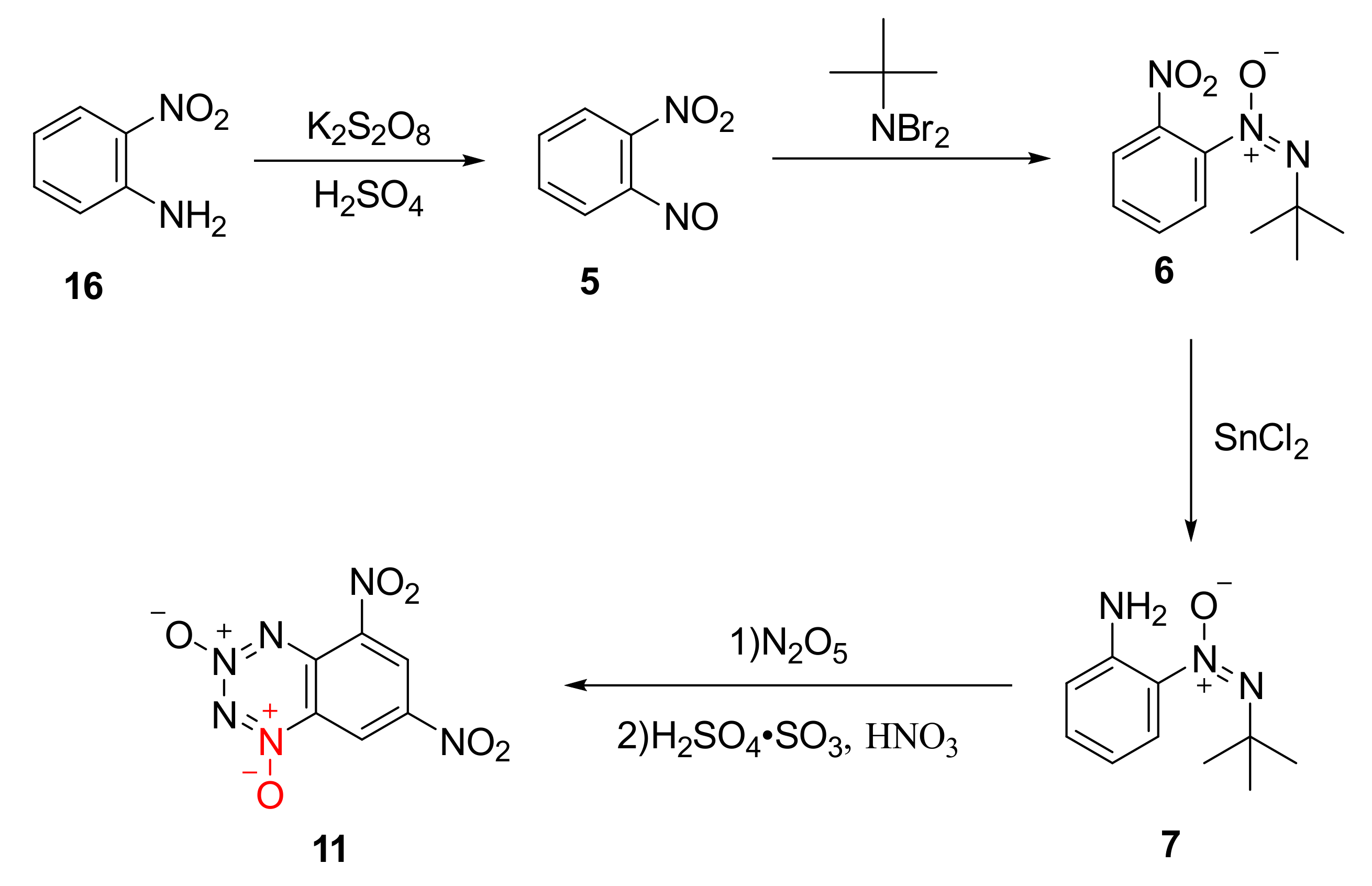
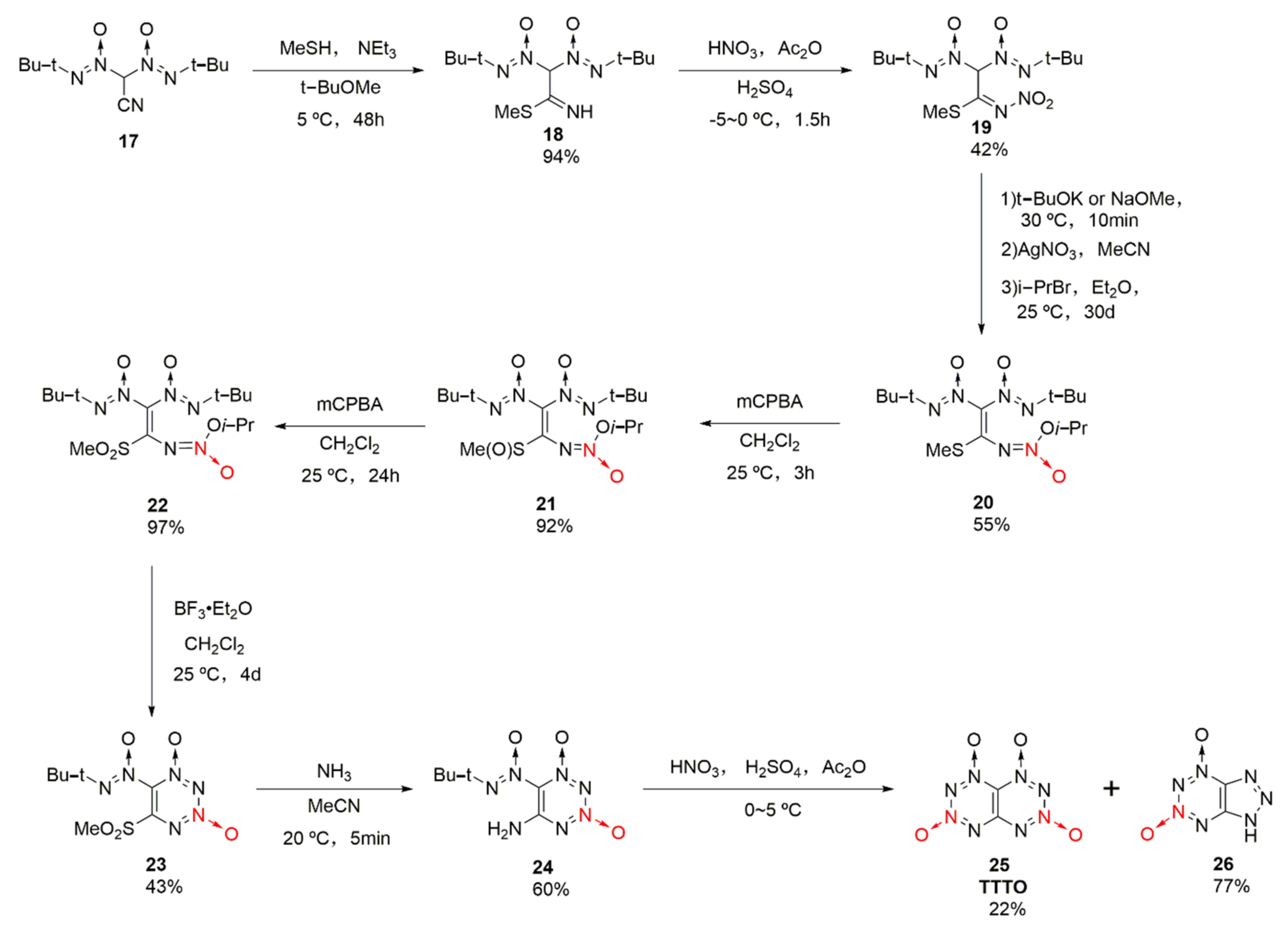
2.1.1. 1,2,3,4-Tetrazine-1,3-Dioxide
2.1.2. 1,2,3,5-Tetrazine-2-Oxides
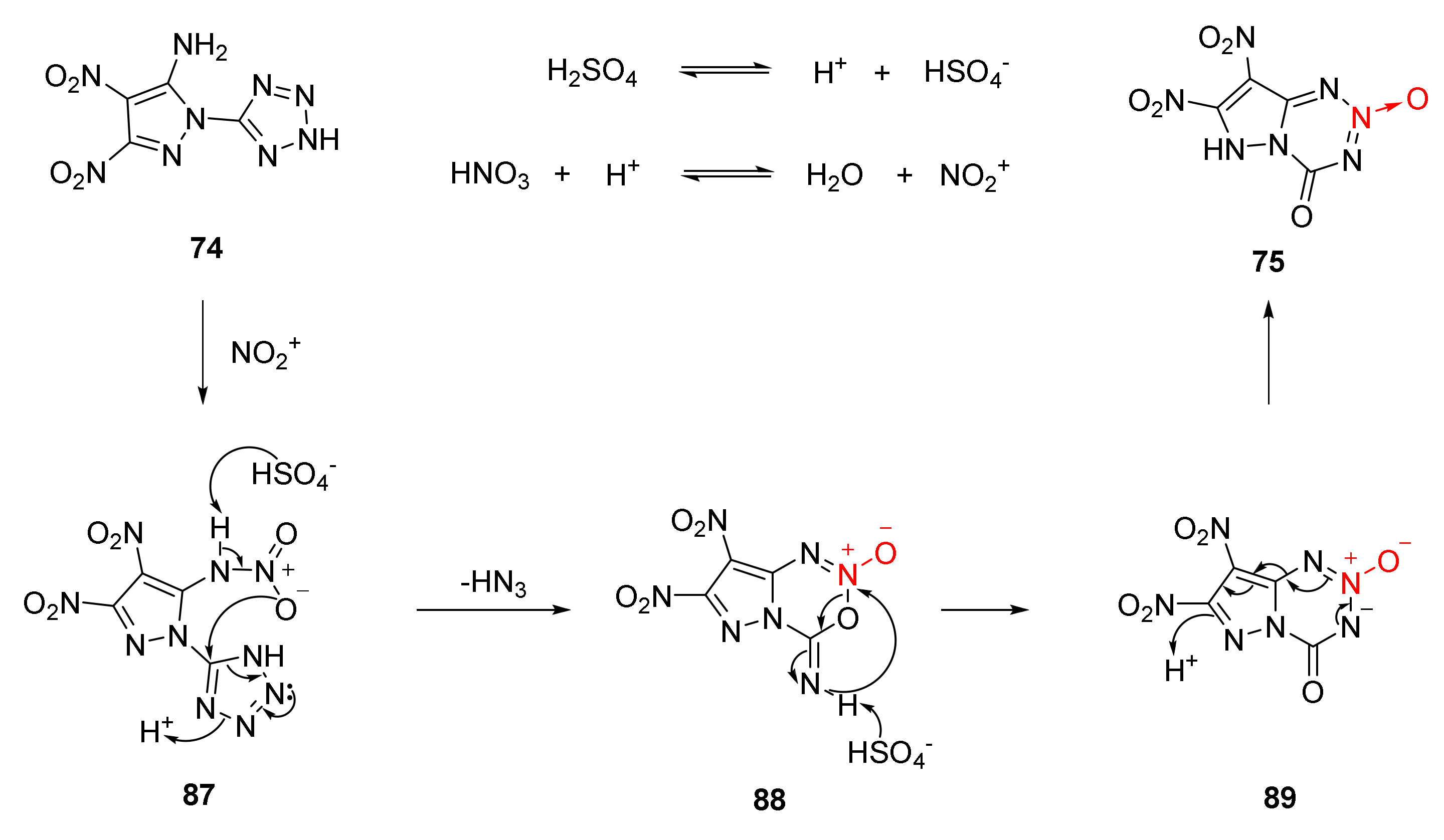
2.1.3. 1,2,3-Triazine-3-Oxides
2.1.4. 1,2,3-Triazine-2-Oxides
2.1.5. Pyridazine-1,2-Dioxide
2.1.6. Pyrazine-1-Oxide
2.2. Oxazole N-Oxide Energetic Compounds
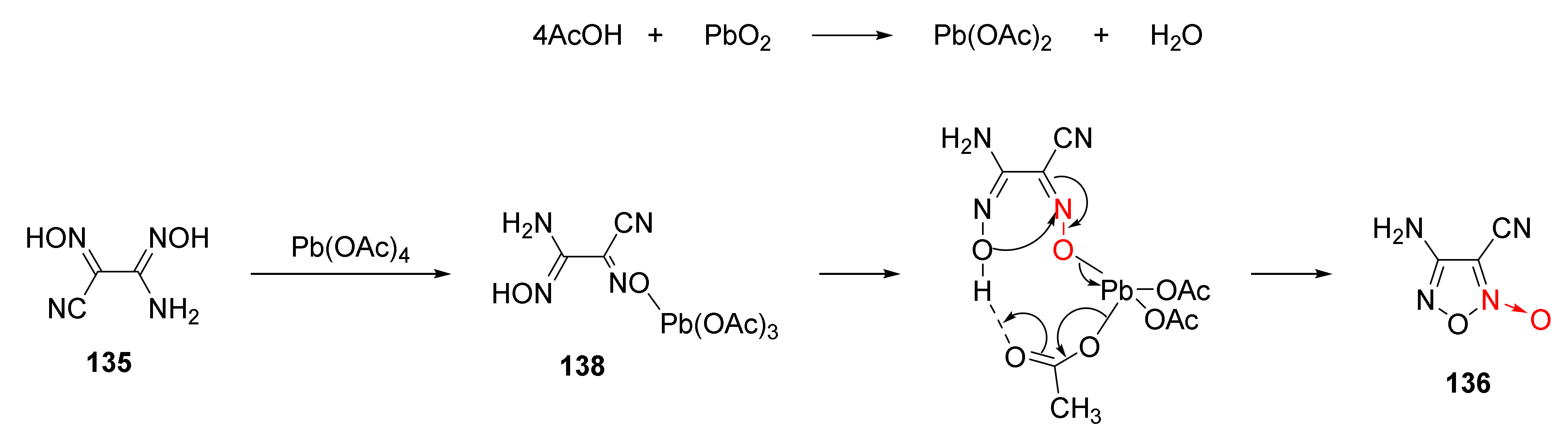
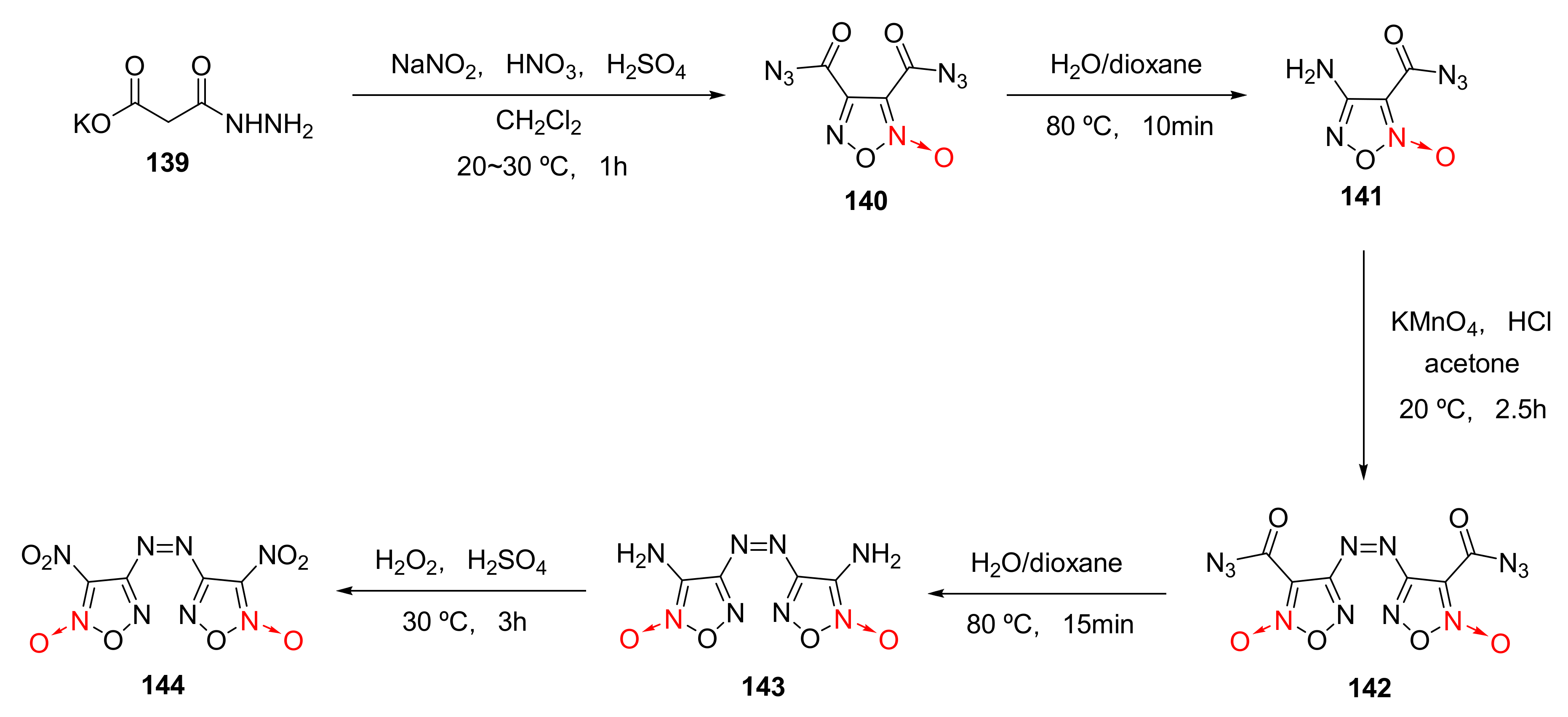
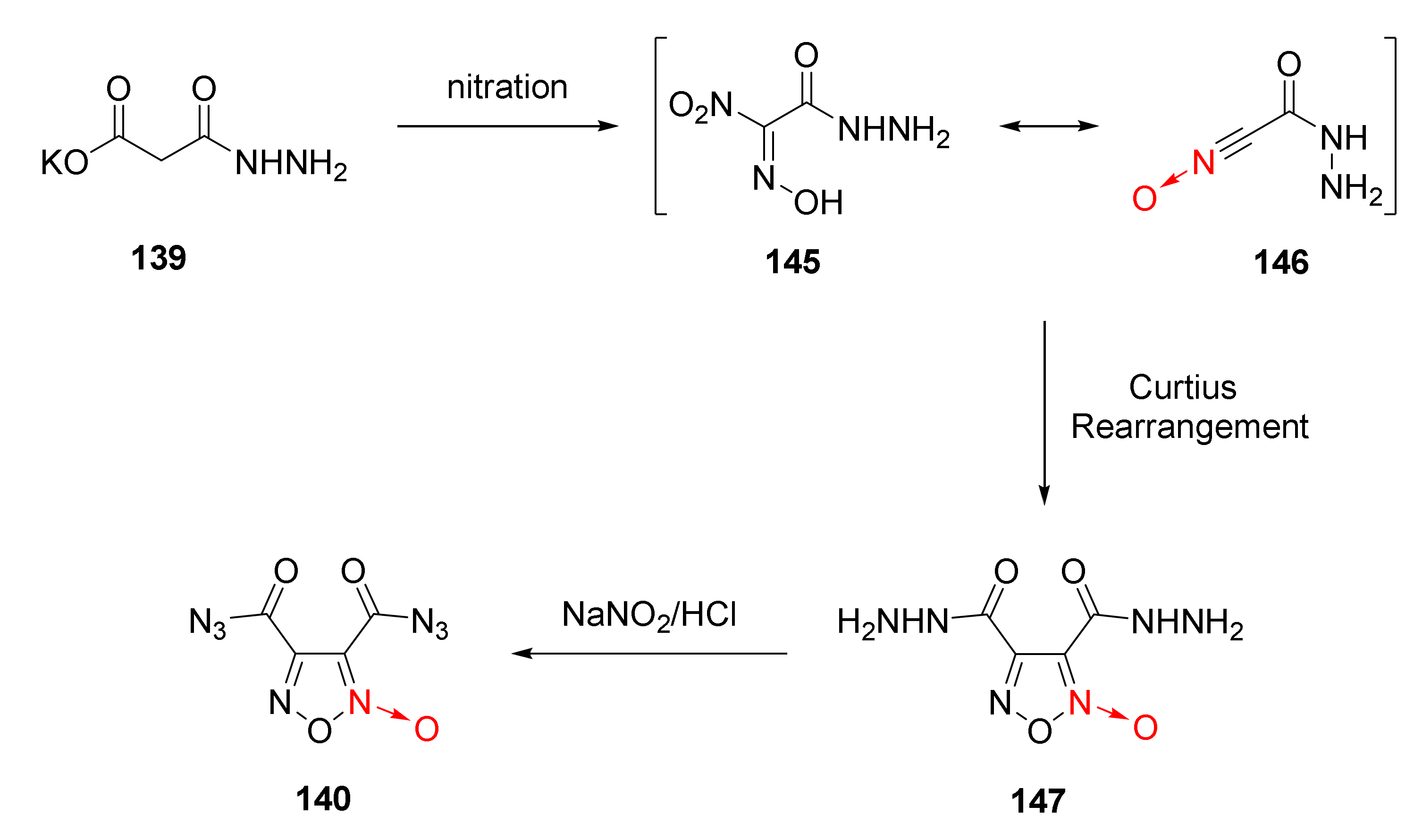
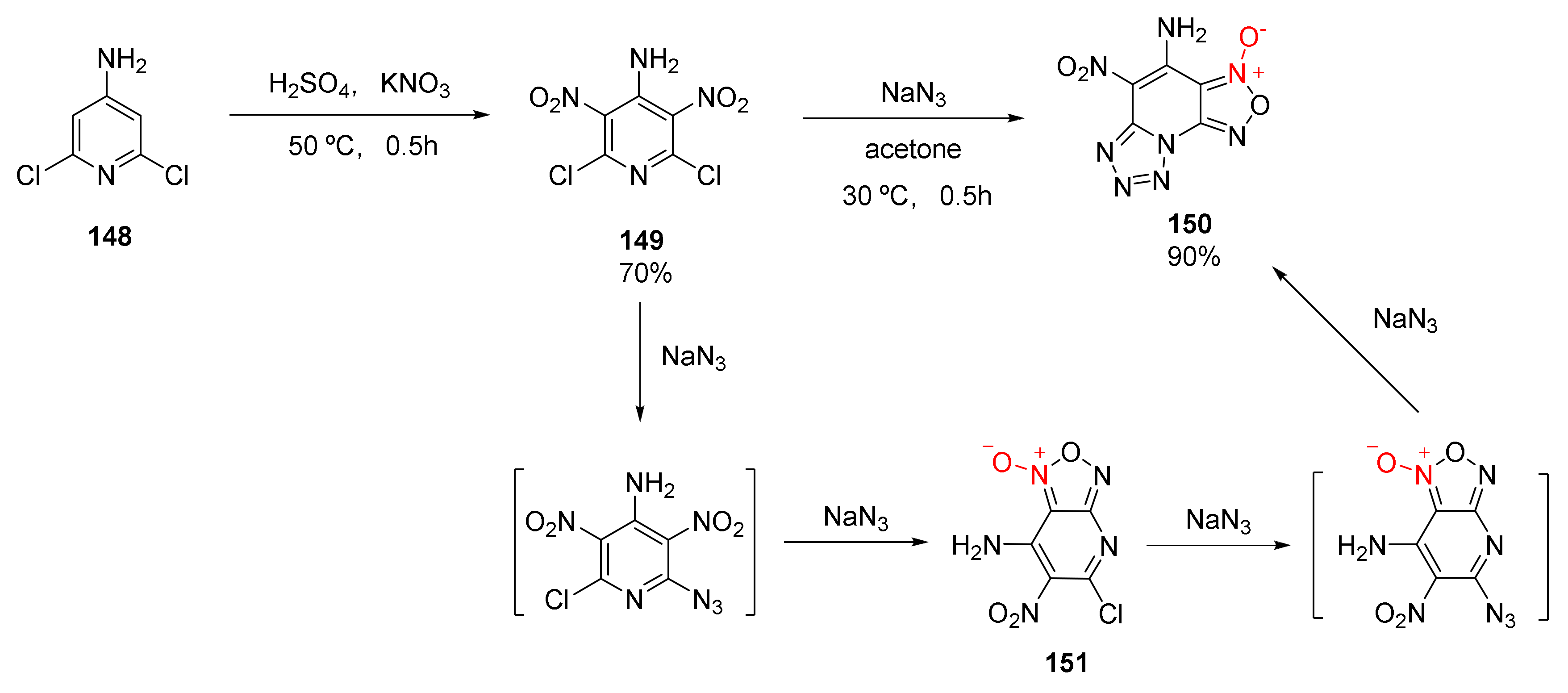
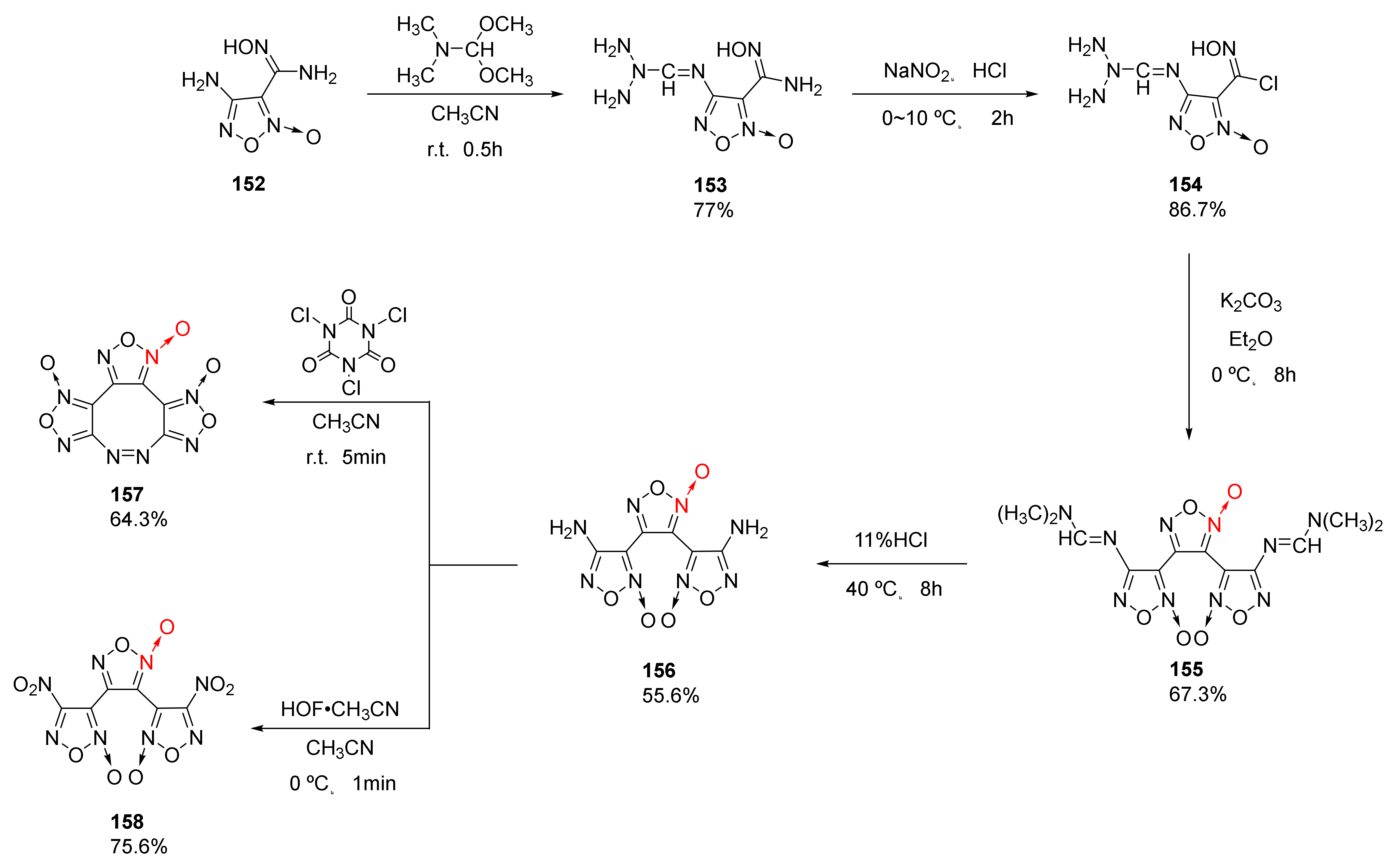
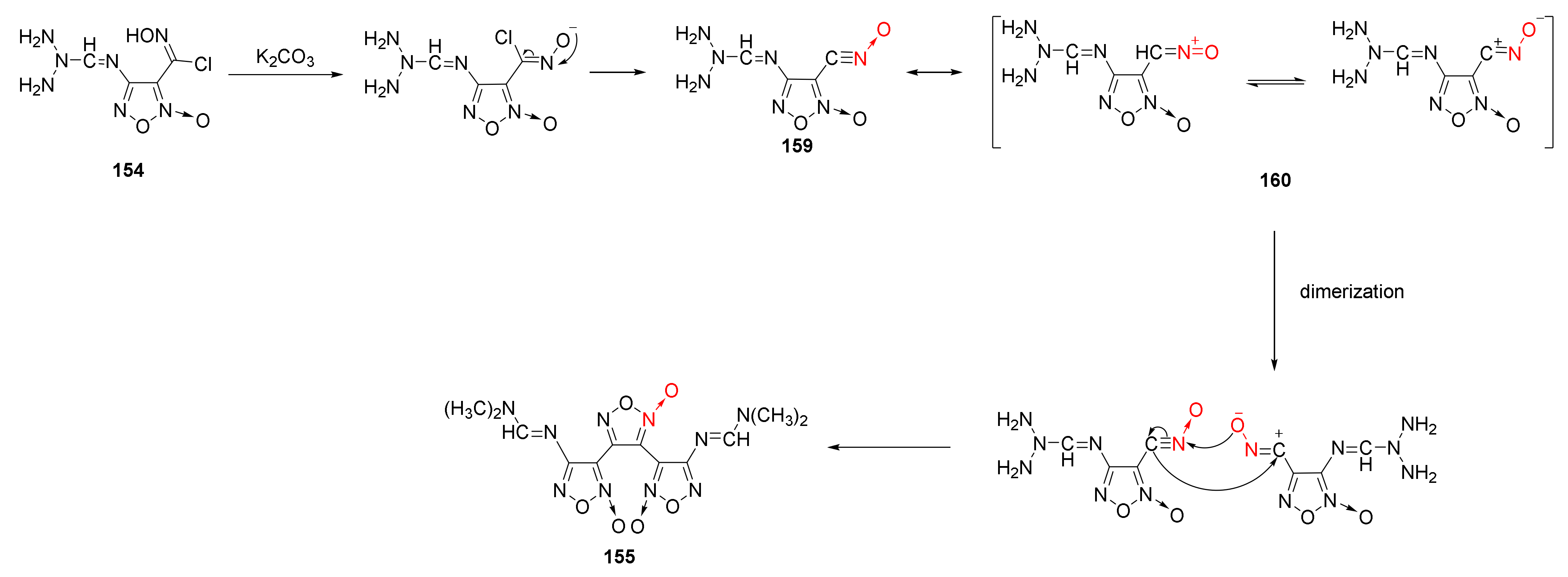
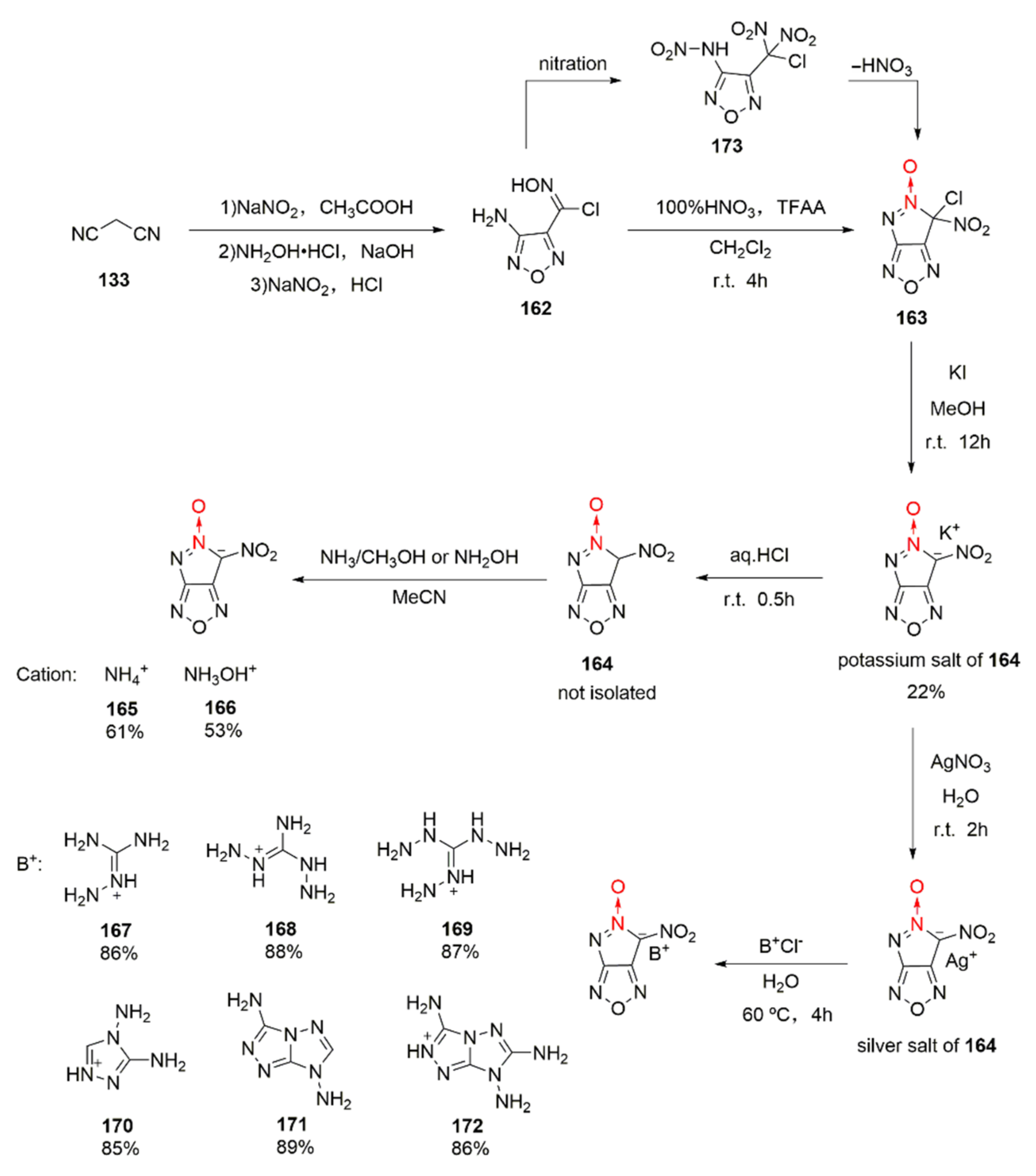
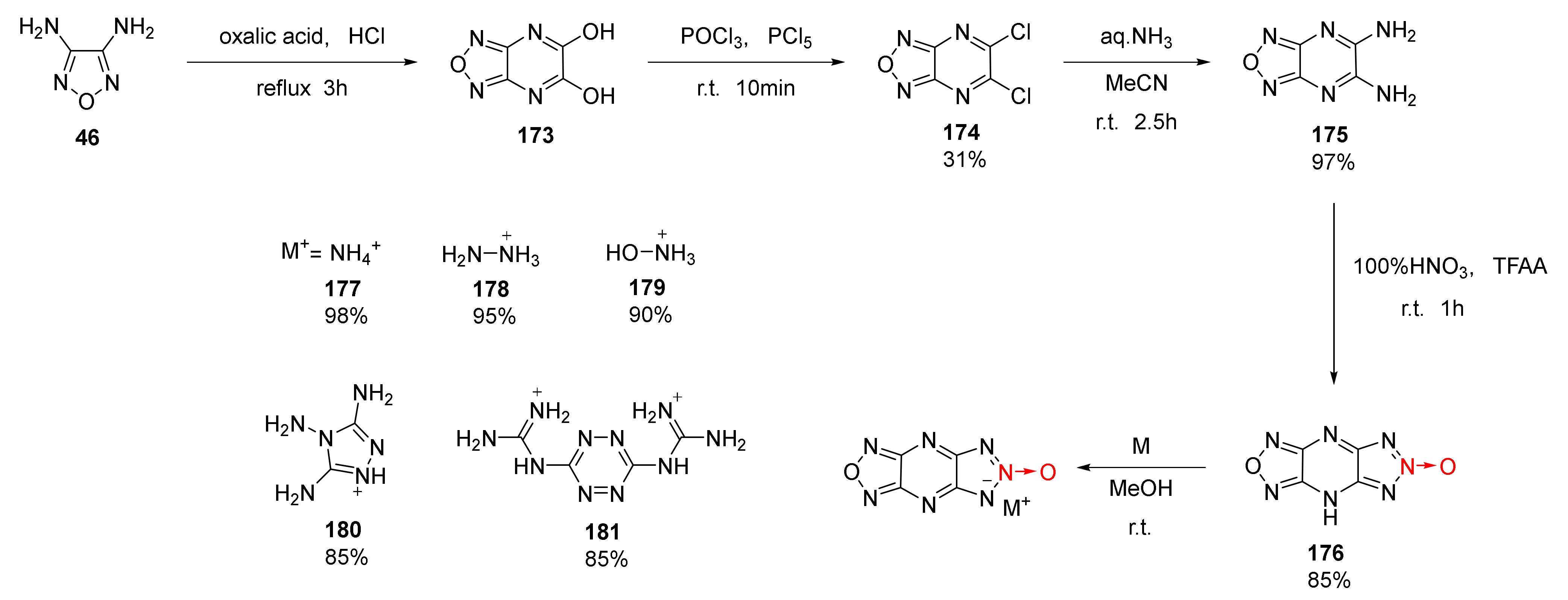
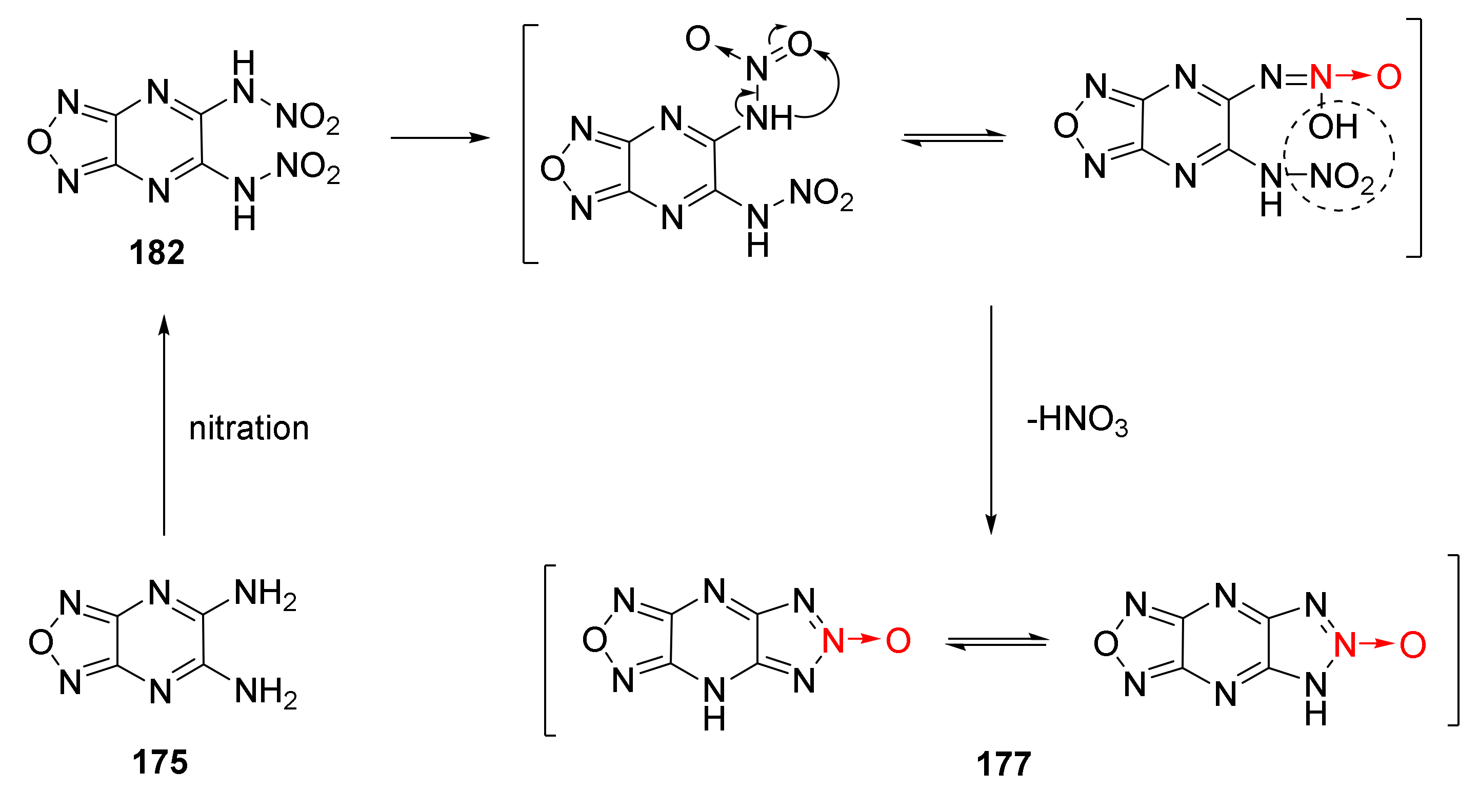
2.2.1. 1,2,5-Oxadiazole-2-Oxides
2.2.2. Pyrazole-1-Oxide
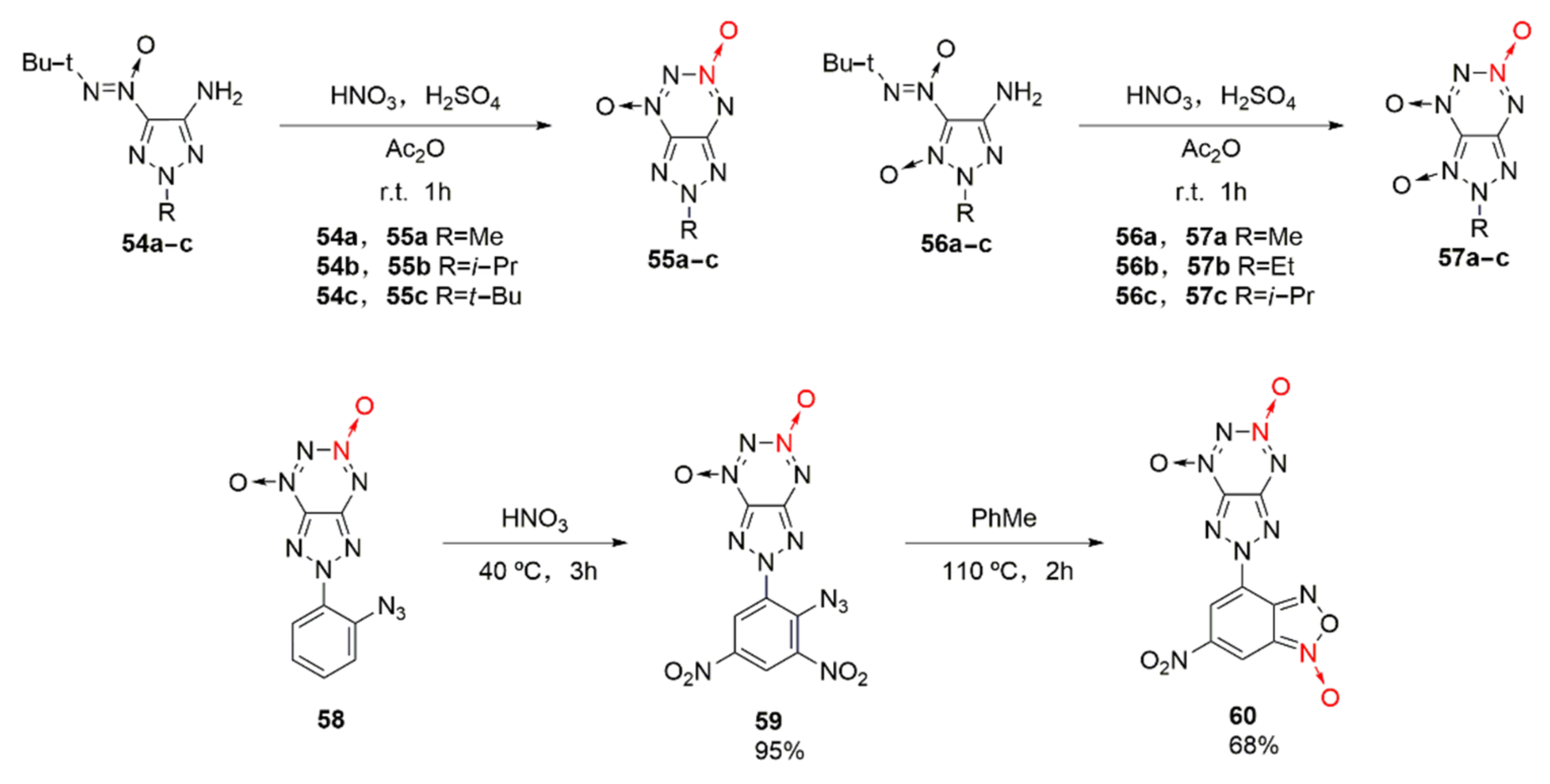
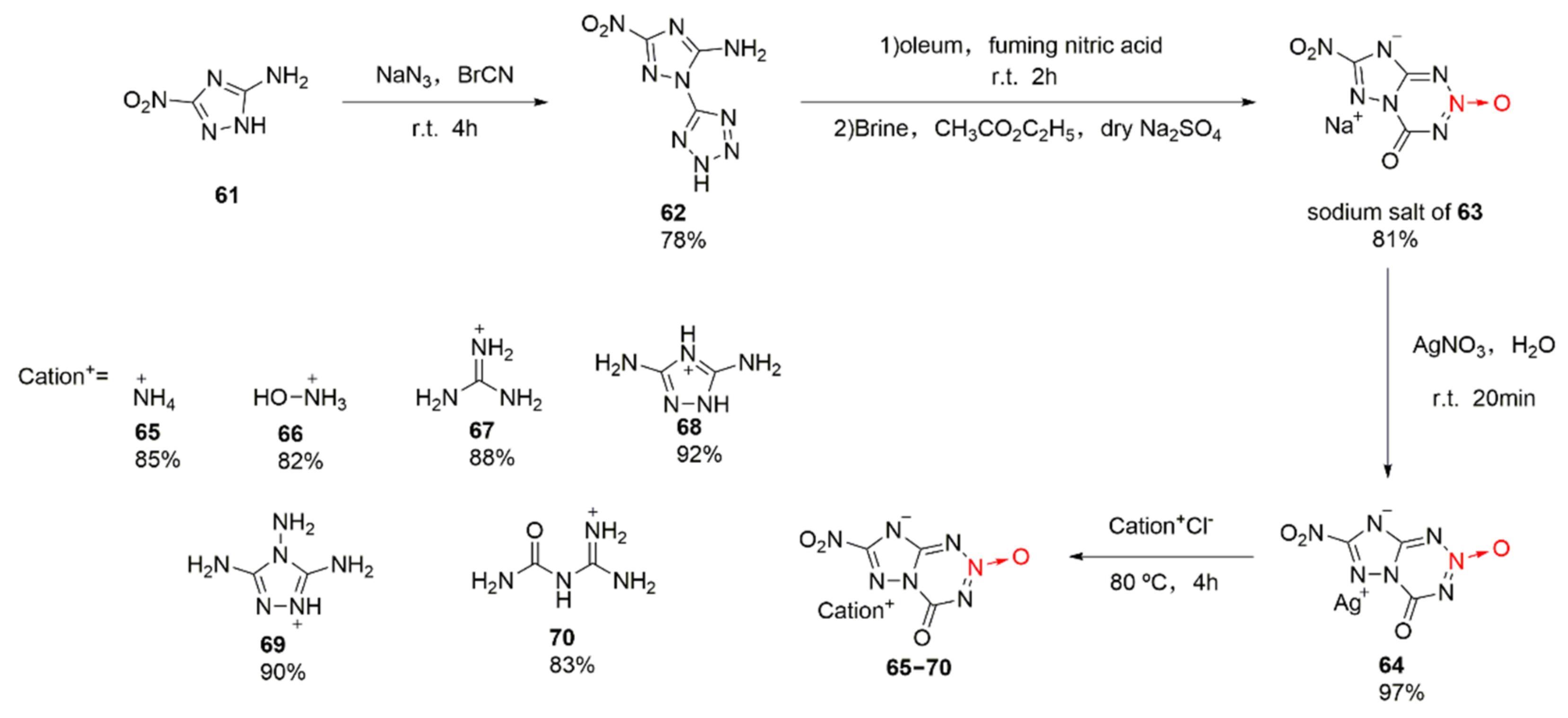
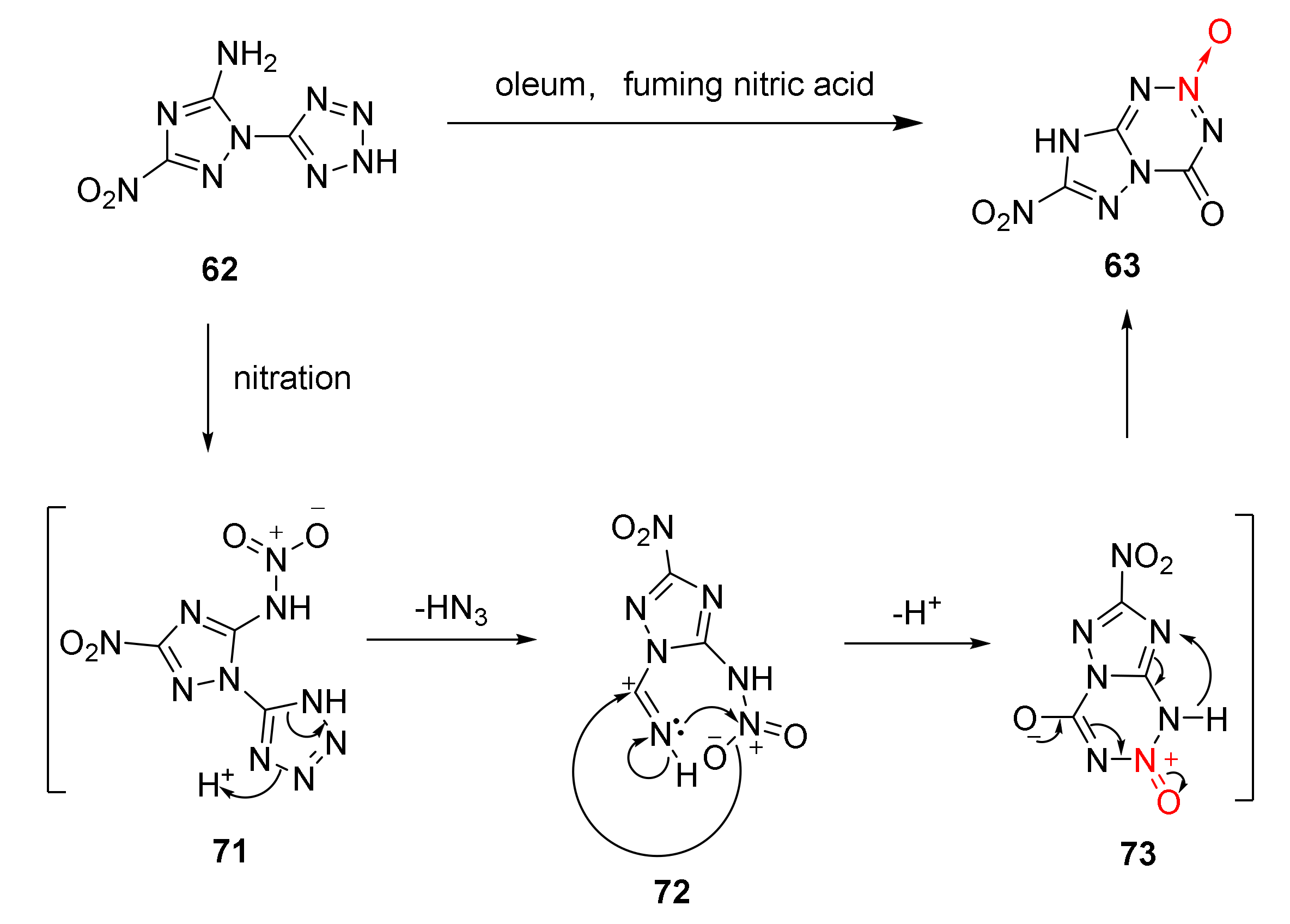
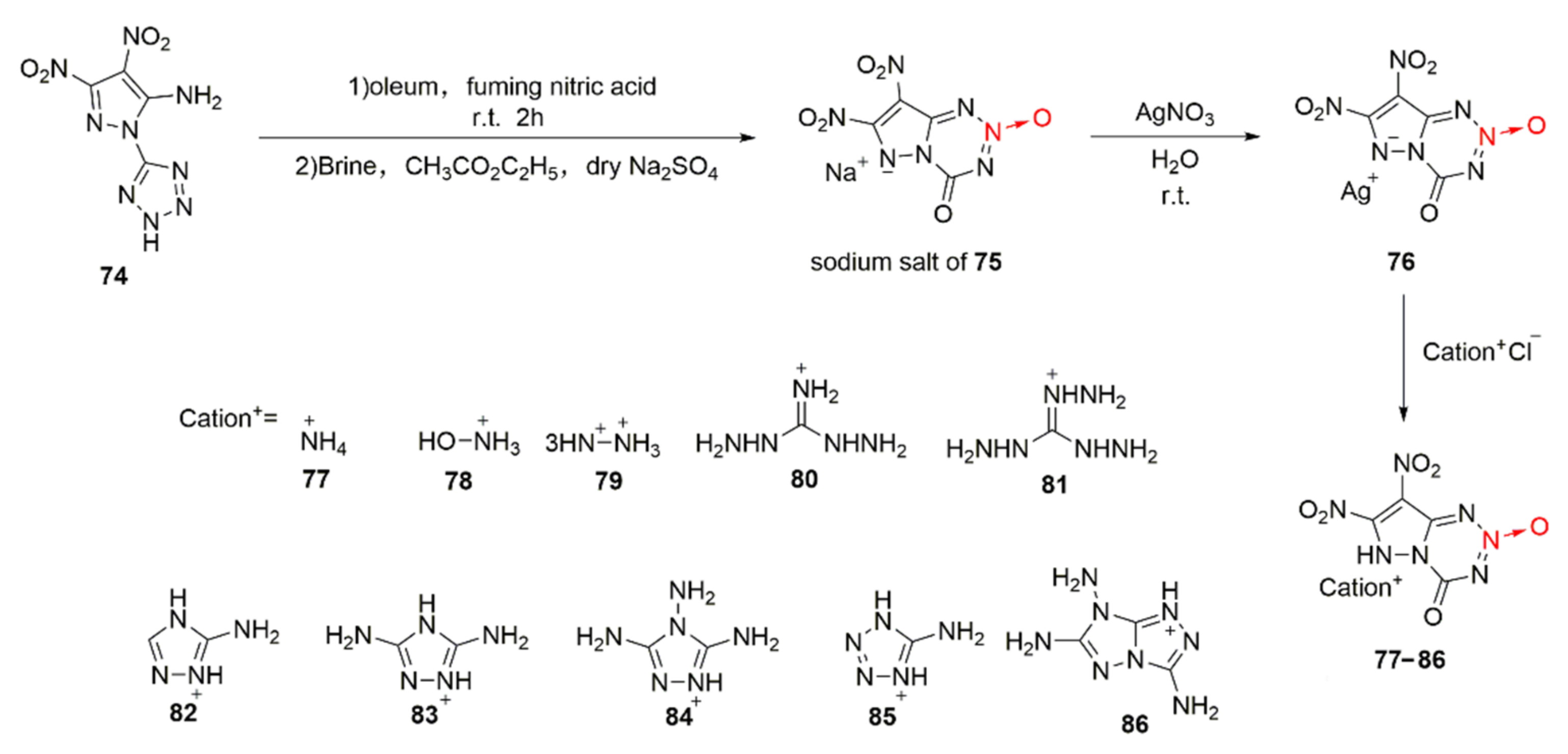
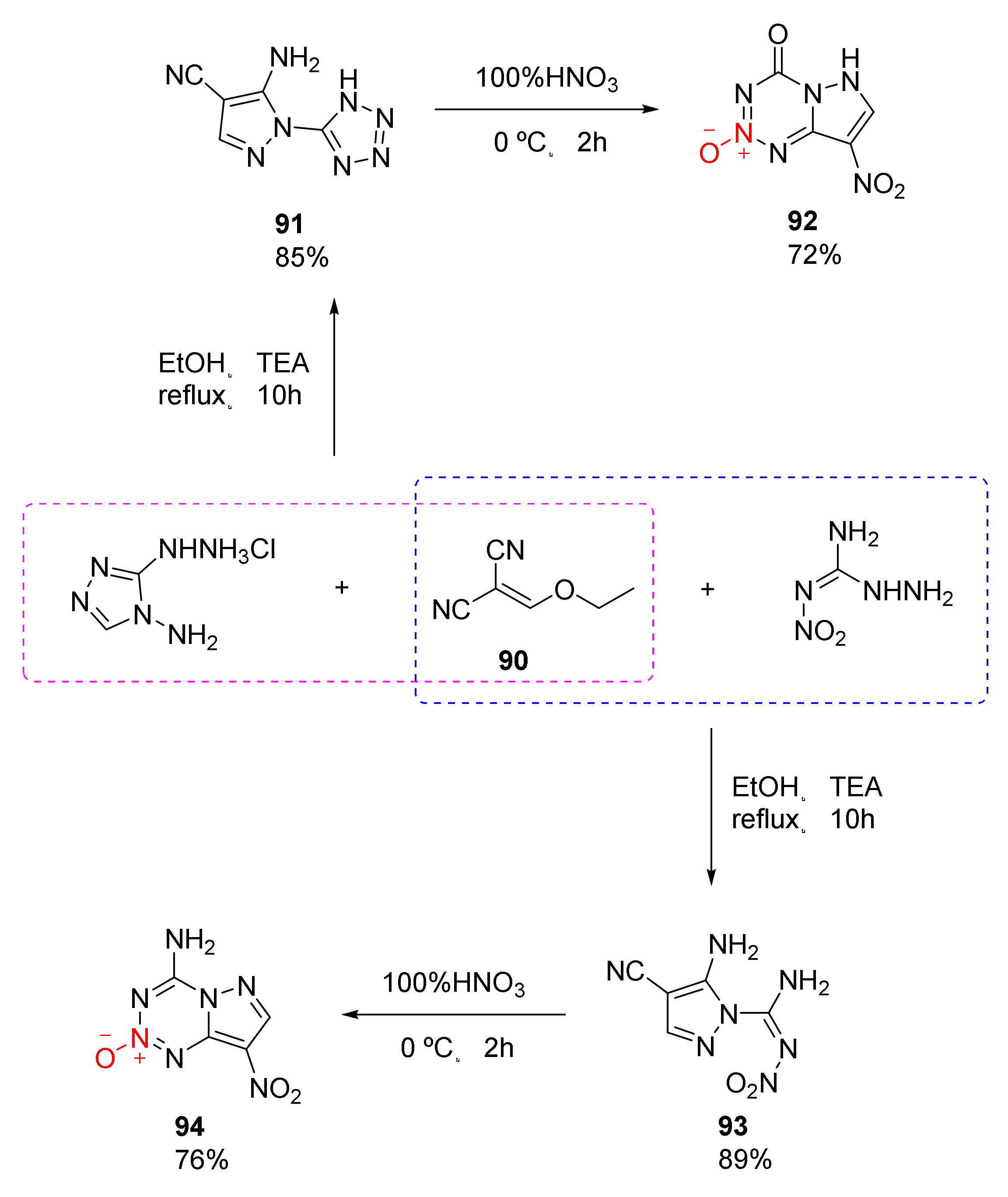
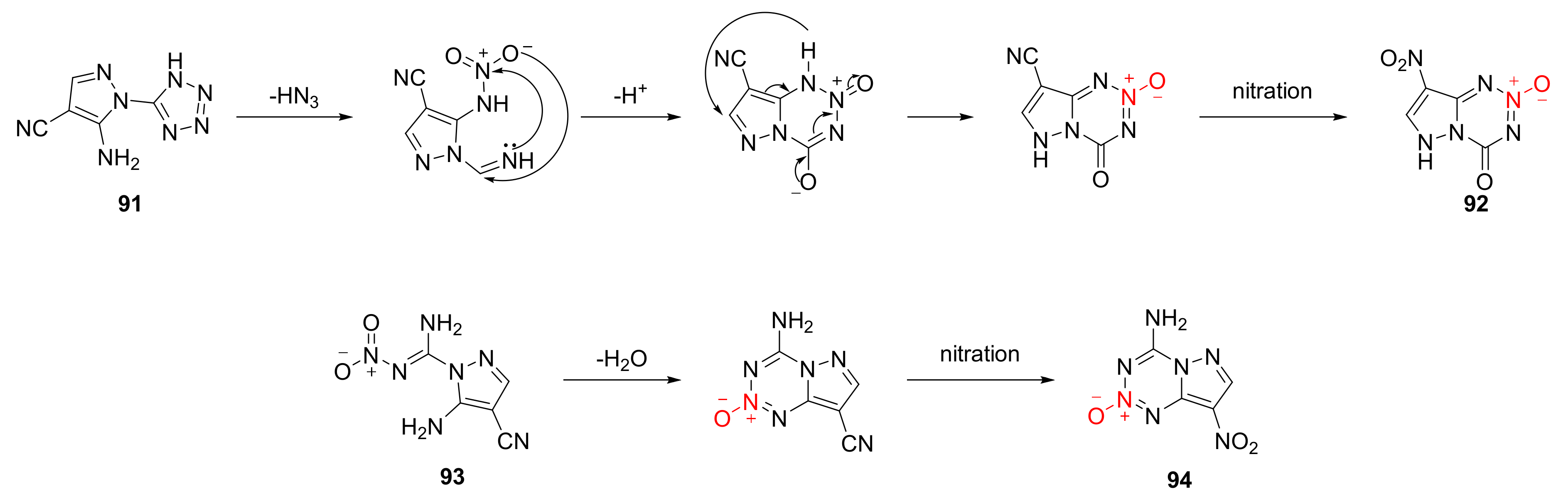
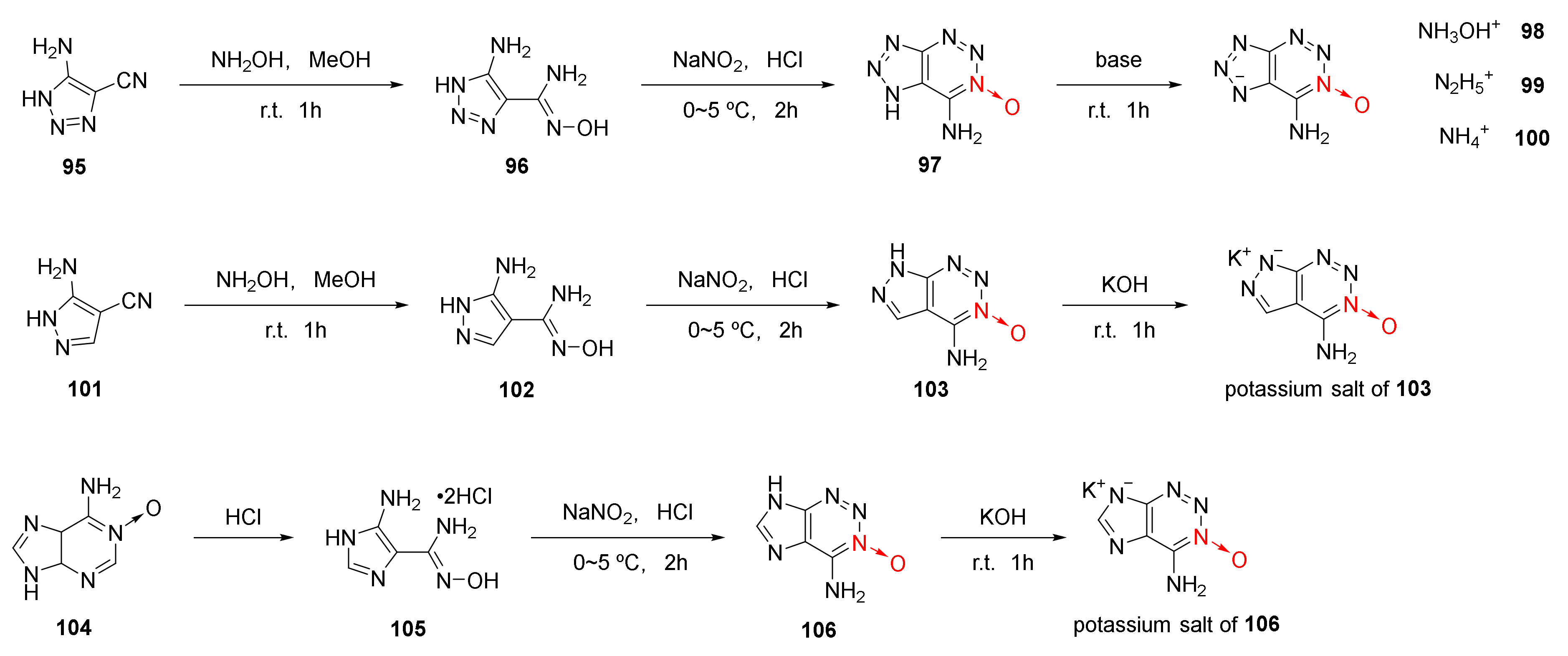
2.2.3. Triazole-1-Oxides
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, H.X.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, D.M.; Talawar, M.B.; Asthana, S.N.; Mahulikar, P.P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Song, S.W.; Yang, Z.J.; Qi, X.J.; Wang, K.C.; Liu, Y.; Zhang, Q.H.; Tian, Y. Accelerating the discovery of insensitive high-energy-density materials by a materials genome approach. Nat. Commun. 2018, 9, 2444. [Google Scholar] [CrossRef]
- Tang, Y.X.; Kumar, D.; Shreeve, J.M. Balancing excellent performance and high thermal stability in a dinitropyrazole fused 1,2,3,4-tetrazine. J. Am. Chem. Soc. 2017, 139, 13684–13687. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.J.; Bi, F.Q.; Huo, H.; Luo, Y.F.; Li, X.Z.; Chen, S.P.; Wang, B.Z. The ingenious synthesis of a nitro-free insensitive high-energy material featuring face-to-face and edge-to-face π-interactions. Front. Chem. 2019, 7, 559. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Zhang, J.L.; Dong, J.; Zhai, L.J.; Guo, T.; Wang, Z.J.; Bi, F.Q.; Wang, B.Z. A promising insensitive energetic material based on a fluorodinitromethyl explosophore group and 1,2,3,4-tetrahydro-1,3,5-triazine: Synthesis, crystal structure and performance. RSC Adv. 2020, 10, 11816–11822. [Google Scholar] [CrossRef]
- Xu, Y.G.; Shen, C.; Lin, Q.H.; Wang, P.C.; Jiang, C.; Lu, M. 1-Nitro-2-trinitromethyl substituted imidazoles: A new family of high performance energetic materials. J. Mater. Chem. A 2020, 4, 17791–17800. [Google Scholar] [CrossRef]
- Barton, L.M.; Edwards, J.T.; Johnson, E.C.; Bukowski, E.J.; Sausa, R.C.; Byrd, E.F.C.; Orlicki, J.A.; Sabatini, J.J.; Baran, P.S. Impact of stereo-and regiochemistry on energetic materials. J. Am. Chem. Soc. 2019, 141, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.X.; Chen, X.; Zhang, C.; Zheng, W.W.; Tian, H.W.; Bai, Y. Reaserch on the S-Tetrazine-based Energetic Compounds. Chin. J. Explos. Propellants 2021, 44, 407–419. (In Chinese) [Google Scholar]
- Han, Y.Z.; Yang, Y.Z.; Du, Z.M.; Zhang, Y.H.; Yao, Q. Progress of Study on Thermal Behaviors of Nitrogen-rich Compounds as Azole, Triazine and Furazan. Chin. J. Explos. Propellants 2016, 39, 1–11. (In Chinese) [Google Scholar]
- Liu, Y.; Gong, L.S.; Yi, X.Y.; He, P.; Zhang, J.G. Tunable 1,2,3-triazole-N-oxides towards high energy density materials: Theoretical insight into structure-property correlations. New J. Chem. 2022, 46, 11741–11750. [Google Scholar] [CrossRef]
- Tang, Y.X.; Zhang, J.H.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Taming of 3, 4-Di(nitramino)furazan. J. Am. Chem. Soc. 2015, 137, 15984–15987. [Google Scholar] [CrossRef] [PubMed]
- Chavez, D.; Klapötke, T.M.; Parrish, D.; Piercey, D.G.; Stierstorfer, J. The Synthesis and Energetic Properties of 3, 4-Bis(2,2,2-trinitroethylamino)furazan (BTNEDAF). Propellants Explos. Pyrotech. 2010, 35, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Ma, Q.; Tang, S.H.; Fan, G.J. Crystal Structure and Thermal Decomposition Properties of N,N’-Bis(2-fluoro-2,2’-dinitroethyl)-3,4’-dinitraminefurazan. Chin. J. Energ. Mater. 2019, 27, 41–46. (In Chinese) [Google Scholar]
- Xiao, X.; Ge, Z.X.; Wang, W.; Liu, Q.; Su, H.P.; Li, T.Q.; Bi, F.Q.; Ji, X.T. Progress of 3-Azido-1,2,4-Triazole and Its Derivatives. Chin. J. Energ. Mater. 2014, 22, 100–107. (In Chinese) [Google Scholar]
- Lai, Y.; Liu, Y.; Huang, W.; Zeng, Z.Z.; Yang, H.W.; Tang, Y.X. Synthesis and Characterization of Pyrazole- and Imidazole- Derived Energetic Compounds Featuring Ortho Azido/nitro Groups. FirePhysChem 2022, 2, 160–164. [Google Scholar] [CrossRef]
- Wu, B.; Yang, L.F.; Zhai, D.D.; Ma, G.M.; Pei, C.H. Facile Synthesis of 4-Amino-3,5-dinitropyrazolated Energetic Derivatives via 4-Bromopyrazole and Their Performances. FirePhysChem 2021, 1, 76–82. [Google Scholar] [CrossRef]
- Vishnevskiy, Y.V.; Tikhonov, D.S.; Schwabedissen, J.; Stammler, H.G.; Moll, R.; Krumm, B.; Klapötke, T.M.; Mitzel, N.W. Tetranitromethane: A nightmare of molecular flexibility in the gaseous and solid States. Angew. Chem. Int. Edit. 2017, 56, 9619–9623. [Google Scholar] [CrossRef]
- Zhai, L.J.; Zhang, J.L.; Zhang, J.R.; Wu, M.J.; Bi, F.Q.; Wang, B.Z. Progress in Synthesis and Properties of High Energy Density Compounds Regulated by N-F Bond. Chin. J. Org. Chem. 2020, 40, 1484–1501. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.L.; Wang, B.Z.; Qiu, L.L.; Xu, R.Q.; Sheremete, A.B. Recent Synthetic Efforts towards High Energy Density Materials: How to Design High-Performance Energetic Structures? FirePhysChem 2022, 2, 83–139. [Google Scholar] [CrossRef]
- Yuan, J.; Long, X.P.; Zhang, C.Y. Influence of N-Oxide Introduction on the Stability of Nitrogen-Rich Heteroaromatic Rings: A Quantum Chemical Study. J. Phys. Chem. A 2016, 120, 9446–9457. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Murray, J.S. Computational analysis of relative stabilities of polyazine N-oxides. Struct. Chem. 2013, 24, 1965–1974. [Google Scholar] [CrossRef]
- Lai, W.P.; Lian, P.; Ge, Z.X.; Liu, Y.Z.; Yu, T.; Lv, J. Theoretical study of the effect of N-oxides on the performances of energetic compounds. J. Mol. Model. 2016, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, W.; Wang, Y.; Zhang, Q.H. Research Progress of Nitrogen⁃rich Fused⁃ring N⁃oxides. Chin. J. Energ. Mater. 2021, 29, 567–578. (In Chinese) [Google Scholar]
- Zhu, J.P.; Ren, J.; Han, X.Y.; Li, Y.X.; Wang, J.L.; Cao, R.L. Study on Structures and Detonation Performance for Polynitropyrazine-N-oxides by Density Functional Theory. Chin. J. Explos. Propllants 2010, 33, 47–51. (In Chinese) [Google Scholar]
- Churakov, A.M.; Ioffe, S.L.; Strelenko, Y.A.; Tartakovskii, V.A. 1,2,3,4-tetrazine 1,3-dioxides-a new class of heterocyclic compounds. Bull. Acad. Sci. USSR Div. Chem. Sci. 1990, 39, 639–640. [Google Scholar] [CrossRef]
- Bi, F.Q.; Wang, B.Z.; Li, X.Z.; Fan, X.Z.; Xu, C.; Ge, Z.X. Progress in the Energetic MateriaIs Based on 1,2,3,4-Tetrazine 1,3-Dioxid. Chin. J. Energ. Mater. 2012, 20, 630–637. (In Chinese) [Google Scholar]
- Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J.; Weyrauther, M. The Synthesis and Energetic Properties of 5,7-Dinitrobenzo-1,2,3,4-tetrazine-1,3-dioxide (DNBTDO). Propellants Explos. Pyrotech. 2012, 37, 527–535. [Google Scholar] [CrossRef]
- Christe, K.O.; Dixon, D.A.; Vasiliu, M.; Wagner, R.I.; Haiges, R.; Boatz, J.A.; Ammon, H.L. Are DTTO and iso-DTTO Worthwhile Targets for Synthesis? Propellants Explos. Pyrotech. 2015, 40, 463–468. [Google Scholar] [CrossRef]
- Khakimov, D.V.; Dzyabchenko, A.V.; Pivina, T.S. Computer simulation of the crystal structure of tetrazino-tetrazine tetraoxide (TTTO) isomers with one and two independent molecules in the unit cell. Russ. Chem. Bull. 2020, 69, 212–217. [Google Scholar] [CrossRef]
- Mendoza-Cortes, J.L.; An, Q.; Goddard, W.A., III; Ye, C.; Zybin, S. Prediction of the crystal packing of di-tetrazine-tetroxide (DTTO) energetic material. J. Comput. Chem. 2016, 37, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Li, J.C.; Hou, H.; Wang, B.S. Extensive theoretical studies of a new energetic material: Tetrazino-tetrazine-tetraoxide (TTTO). J. Comput. Chem. 2009, 30, 1816–1820. [Google Scholar] [CrossRef]
- Klenov, M.S.; Guskov, A.A.; Anikin, O.V.; Churakov, A.M.; Strelenko, Y.A.; Fedyanin, I.V.; Lyssenko, K.A.; Tartakovsky, V.A. Synthesis of Tetrazino-tetrazine 1,3,6,8-Tetraoxide (TTTO). Angew. Chem. Int. Ed. 2016, 55, 11472–11475. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, A.E.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Synthesis of 1,2,3,4-tetrazino[5, 6-g]benzo-1,2,3,4-tetrazine-1,3,7,9-tetraoxides. Russ. Chem. Bull. 2006, 55, 1654–1658. [Google Scholar] [CrossRef]
- Frumkin, A.E.; Churakov, A.M.; Strelenko, Y.A.; Kachala, V.V.; Tartakovsky, V.A. Synthesis of 1,2,3,4-Tetrazino[5, 6-f]benzo-1,2,3,4-tetrazine-1,3,7,9-Tetra-N-oxides. Org. Lett. 1999, 1, 721–724. [Google Scholar] [CrossRef]
- Churakov, A.M.; Loffe, S.L.; Tartakovsky, V.A. Synthesis of [1,2,5]Oxadiazolo [3,4-e][1,2,3,4]tetrazine-4,6-Di-N-oxide. Mendeleev Commun. 1995, 6, 227–228. [Google Scholar] [CrossRef]
- Dong, L.L.; Zhang, G.Q.; Chi, Y.; Fan, G.J.; He, L.; Tao, G.H.; Huang, M. Synthesis of Furazano[3,4-e]-1,2,3,4-tetrazine-1,3-dioxide. Chin. J. Energ. Mater. 2012, 20, 690–692. (In Chinese) [Google Scholar]
- Zelenov, V.P.; Lobanova, A.A.; Sysolyatin, S.V.; Sevodina, N.V. New syntheses of [1,2,5]oxadiazolo[3,4-e][1,2,3,4]tetrazine 4,6-dioxide. Russ. J. Org. Chem. 2013, 49, 455–465. [Google Scholar] [CrossRef]
- Li, X.Z.; Wang, B.Z.; Li, H.; Li, Y.A.; Bi, F.Q.; Huo, H.H.; Fan, X.Z. Novel Synthetic Route and Characterization of [1,2,5]oxadiazolo-[3,4-e][1,2,3,4]tetrazine 4,6-Di-N-oxide (FTDO). Chin. J. Org. Chem. 2012, 32, 1975–1980. [Google Scholar] [CrossRef][Green Version]
- Voronin, A.A.; Zelenov, V.P.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Alkylation of 1-hydroxy-1H-[1,2,3]triazolo[4,5-e][1,2,3,4]tetrazine 5,7-dioxide. Russ. Chem. Bull. 2014, 63, 475–479. [Google Scholar] [CrossRef]
- Voronin, A.A.; Zelenov, V.P.; Churakov, A.M.; Strelenko, Y.A.; Fedyanin, I.V.; Tartakovsky, V.A. Synthesis of 1,2,3,4-tetrazine 1,3-dioxides annulated with 1,2,3-triazoles and 1,2,3-triazole 1-oxides. Tetrahedron 2014, 70, 3018–3022. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zheng, C.M.; Liu, Y.; Gong, X.D.; Xia, M.Z. Theoretical studies of the structure, stability, and detonation properties of vicinal-tetrazine 1,3-dioxide annulated with a five-membered heterocycle. 1. Annulation with a triazole ring. J. Mol. Model. 2015, 21, 1–9. [Google Scholar]
- Shvets, A.O.; Konnov, A.A.; Klenov, M.S.; Churakov, A.M.; Strelenko, Y.A.; Tartakovsky, V.A. Synthesis of 2-(6-nitrobenzofuroxan-4-yl)-2H-[1,2,3]triazolo-[4,5-e][1,2,3,4]tetrazine 4,6-dioxide. Russ. Chem. Bull. 2020, 69, 739–741. [Google Scholar] [CrossRef]
- Bian, C.M.; Dong, X.; Zhang, X.H.; Zhou, Z.M.; Zhang, M.; Li, C. The unique synthesis and energetic properties of a novel fused heterocycle: 7-nitro-4-oxo-4, 8-dihydro-[1,2,4]triazolo[5,1-d][1,2,3,5]tetrazine 2-oxide and its energetic salts. J. Mater. Chem. A 2015, 3, 3594–3601. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Bian, C.M. Energetic ionic salts of 7-nitryl-4-ketone-4,8-dihydro-[1,2,4]triazole[5,1-d][1,2,3,5]tetrazine-2-oxide and preparation method of energetic ionic salt. CN Patent 104447762, 25 March 2015. (In Chinese). [Google Scholar]
- Zhao, B.J.; Wang, P.P.; Fu, W.; Li, C.; Zhou, Z.M. High Density of a New Fused Heterocycle: 7,8-Dinitro-4-oxo-4,6-dihydropyrazolo[5,1-d][1,2,3,5]tetrazine 2-oxide and Its Energetic Salts. ChemistrySelect 2018, 3, 4797–4803. [Google Scholar] [CrossRef]
- Lei, C.J.; Cheng, G.B.; Yi, Z.X.; Zhang, Q.H.; Yang, H.W. A facile strategy for synthesizing promising pyrazole-fused energetic compounds. Chem. Eng. J. 2021, 416, 129190. [Google Scholar] [CrossRef]
- Tang, Y.X.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Energetic and fluorescent azole-fused 4-amino-1,2,3-triazine-3-N-oxides. ACS Appl. Energy Mater. 2019, 2, 8871–8877. [Google Scholar] [CrossRef]
- Deng, M.C.; Feng, Y.G.; Zhang, W.Q.; Qi, X.J.; Zhang, Q.H. A green metal-free fused-ring initiating substance. Nat. Commun. 2019, 10, 1339. [Google Scholar] [CrossRef]
- Deng, M.C.; Feng, Y.A.; Zhang, Q.H. Green environmental protection type primary explosive and preparation method thereof. CN Patent 108752349, 11 June 2018. (In Chinese). [Google Scholar]
- Ogurtsov, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Fakhrutdinov, A.N.; Zlotin, S.G.; Rakitin, O.A. [1,2,5]Oxadiazolo[3,4-d] pyridazine 1,5,6-trioxides: Efficient synthesis via the reaction of 3,4-bis (hydroxyimino) methyl)-1,2,5-oxadiazole 2-oxides with a mixture of concentrated nitric and trifluoroacetic acids and structural characterization. Tetrahedron Lett. 2018, 59, 3143–3146. [Google Scholar] [CrossRef]
- Liu, Y.G.; Huang, Z.; Yu, X.J. Progress of research of new insensitive energetic material LLM-105. Expl. Shock Wave 2004, 24, 465–469. (In Chinese) [Google Scholar]
- Jing, S.M.; Liu, Y.C.; Yuan, J.M. New Synthesis of LLM-105 and Experimental Study of Replacement Process. Initiat. Pyrotech. 2012, 2, 34–36. (In Chinese) [Google Scholar]
- Zhao, X.F.; Liu, Z.L.; Yao, Q.Z.; Chen, J.; Dong, Y. Synthesis of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Chin. J. Explos. Propellants 2012, 35, 15–17. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.M.; Du, Y. Optimization of Synthetic Method of 2,6-Diamino-pyrazine-1-oxide. Chin. J. Explos. Propellants 2017, 40, 25–27. (In Chinese) [Google Scholar]
- Li, Y.H.; Wang, Y.; Liao, L.Y.; Guo, Z.C.; Chen, L.P.; Wu, W.Q. Thermal Risk Analysis Based on Reaction Mechanism: Application to the 2,6-Diaminopyrazine-1-oxide Synthesis Process. Org. Process Res. Dev. 2021, 25, 849–857. [Google Scholar] [CrossRef]
- Luo, Y.F.; Ma, L.; Wang, B.Z.; Zhou, Y.S.; Huo, H.; Jia, S.Y. Synthesis and Characterization of 3,3’-Dicyano-4,4’-azofuroxan. Chin. J. Energ. Mater. 2010, 18, 538–540. (In Chinese) [Google Scholar]
- He, J.X.; Lu, Y.H.; Lei, Q.; Cao, Y.L. Synthesis and Properties of High Energetic Compound 3,3’-Dinitro-4,4’-azofuroxan. Chin. J. Energ. Mater. 2011, 34, 9–12. (In Chinese) [Google Scholar]
- Ma, C.M.; Pan, Y.; Jiang, J.C.; Liu, Z.L.; Yao, Q.Z. Synthesis and thermal behavior of a fused, tricyclic pyridine-based energetic material: 4-amino-5-nitro-[1,2,5]oxadiazolo[3,4-e]tetrazolo[1,5-a]pyridine-3-oxide. New J. Chem. 2018, 42, 11259–11263. [Google Scholar] [CrossRef]
- He, C.L.; Gao, H.X.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Boosting energetic performance by trimerizing furoxan. J. Mater. Chem. A 2018, 6, 9391–9396. [Google Scholar] [CrossRef]
- Sun, K.L.; Wu, N.; Yang, H.; Yang, X.F.; Li, H. Independent Component Analysis Combined with On-line Infrared Spectroscopy for Researching the Synthesis Reaction Mechanism of 3,4’-Bis(4’-aminofurazano-3’)furoxan. Chem. J. Chin. Univ. 2014, 35, 244–249. (In Chinese) [Google Scholar]
- Li, Y.; Huang, H.F.; Shi, Y.M.; Yang, J.; Pan, R.M.; Lin, X.Y. Potassium nitraminofurazan derivatives: Potential green primary explosives with high energy and comparable low friction sensitivities. Chem. Eur. J. 2017, 23, 7353–7360. [Google Scholar] [CrossRef]
- Tang, Y.X.; He, C.L.; Shreeve, J.M. A furazan-fused pyrazole N-oxide via unusual cyclization. J. Mater. Chem. A 2017, 5, 4314–4319. [Google Scholar] [CrossRef]
- Thottempudi, V.; Yin, P.; Zhang, J.H.; Parrish, D.A.; Shreeve, J.M. 1,2,3-Triazolo [4,5,-e]furazano[3,4-b]pyrazine 6-Oxide—A Fused Heterocycle with a Roving Hydrogen Forms a New Class of Insensitive Energetic Materials. Chem. Eur. J. 2014, 20, 542–548. [Google Scholar] [CrossRef]
- Li, Y.N.; Hu, J.J.; Chen, T.; Wang, B.; Chang, P.; Wang, B.Z. Synthesis and Properties of 4-Amino-1,2,3-triazolo[4,5-e]furazano[3,4-b]pyrazine 6-oxide. Chin. J. Energ. Mater. 2020, 28, 664–669. (In Chinese) [Google Scholar]
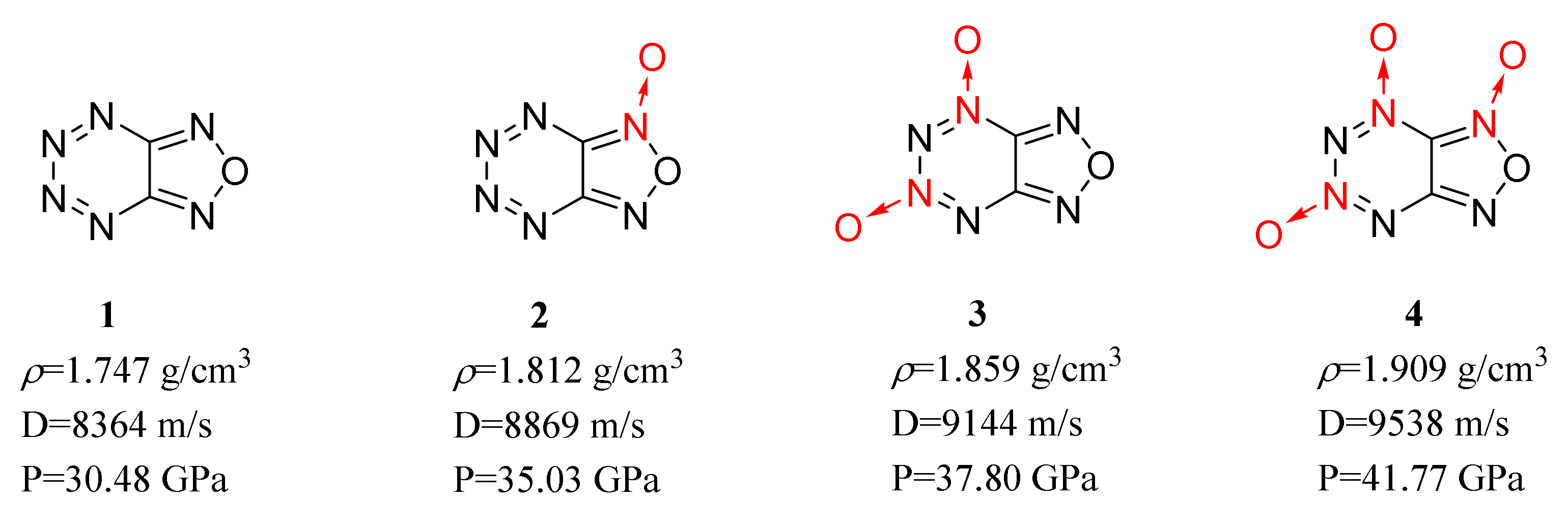

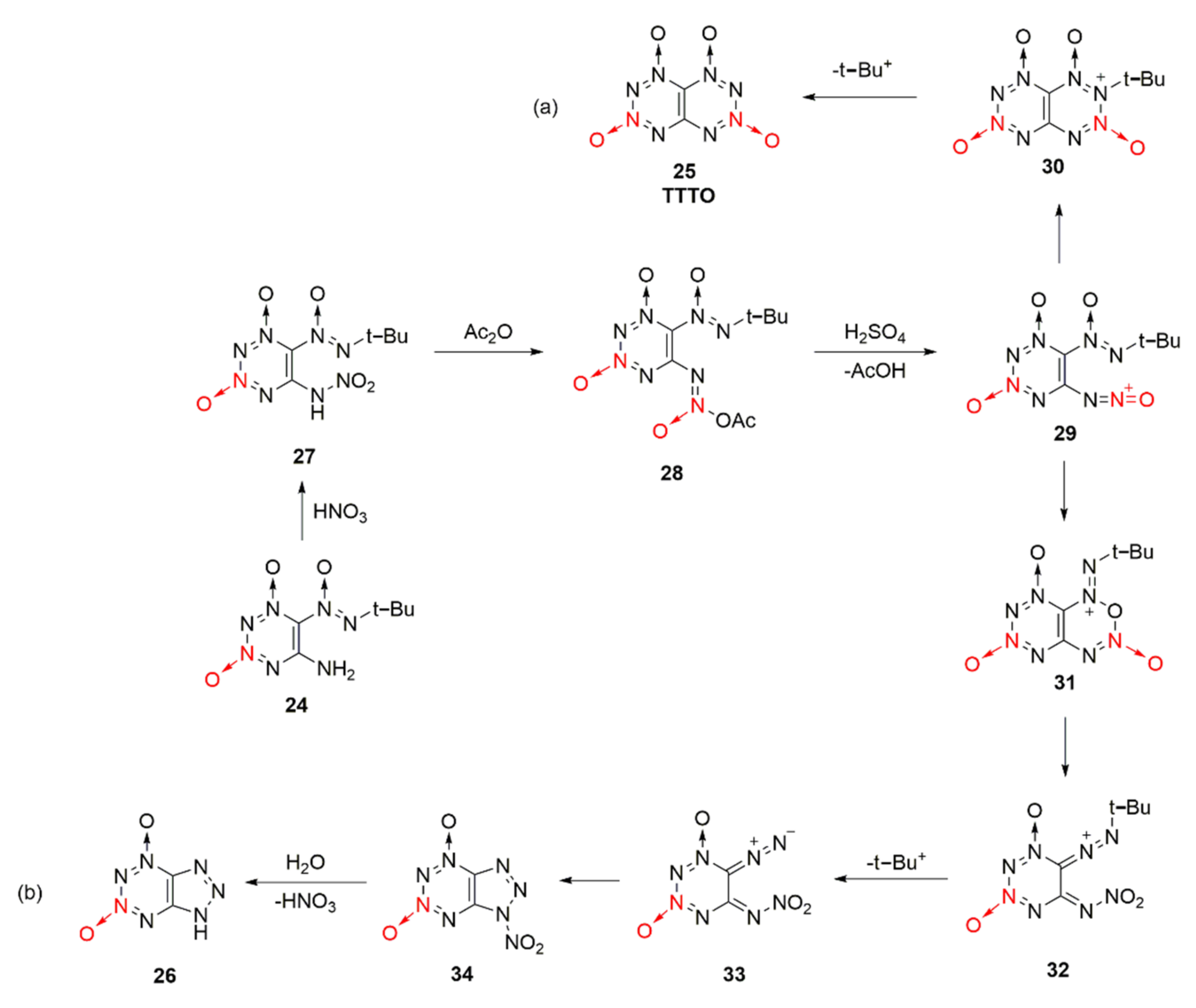
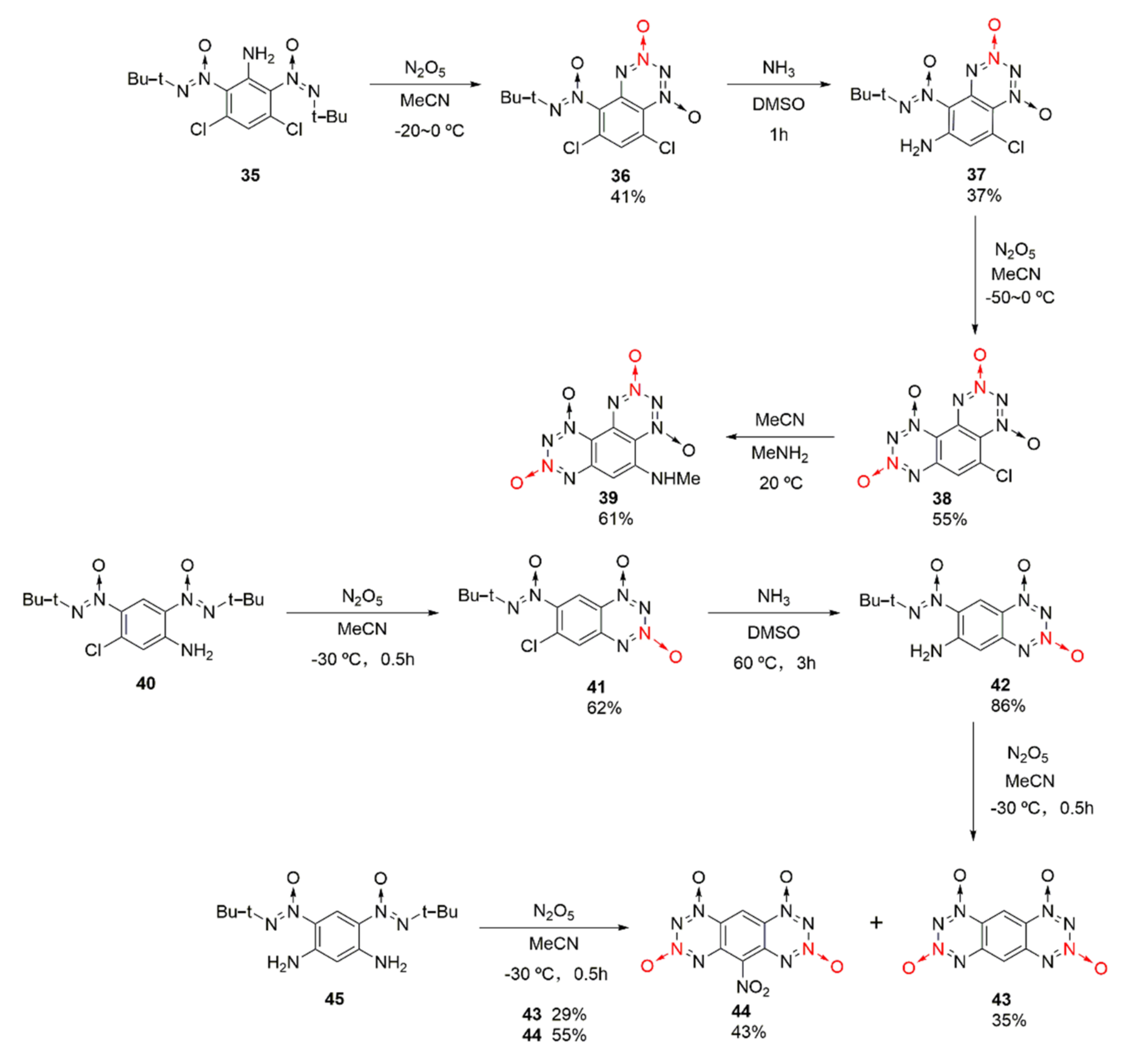
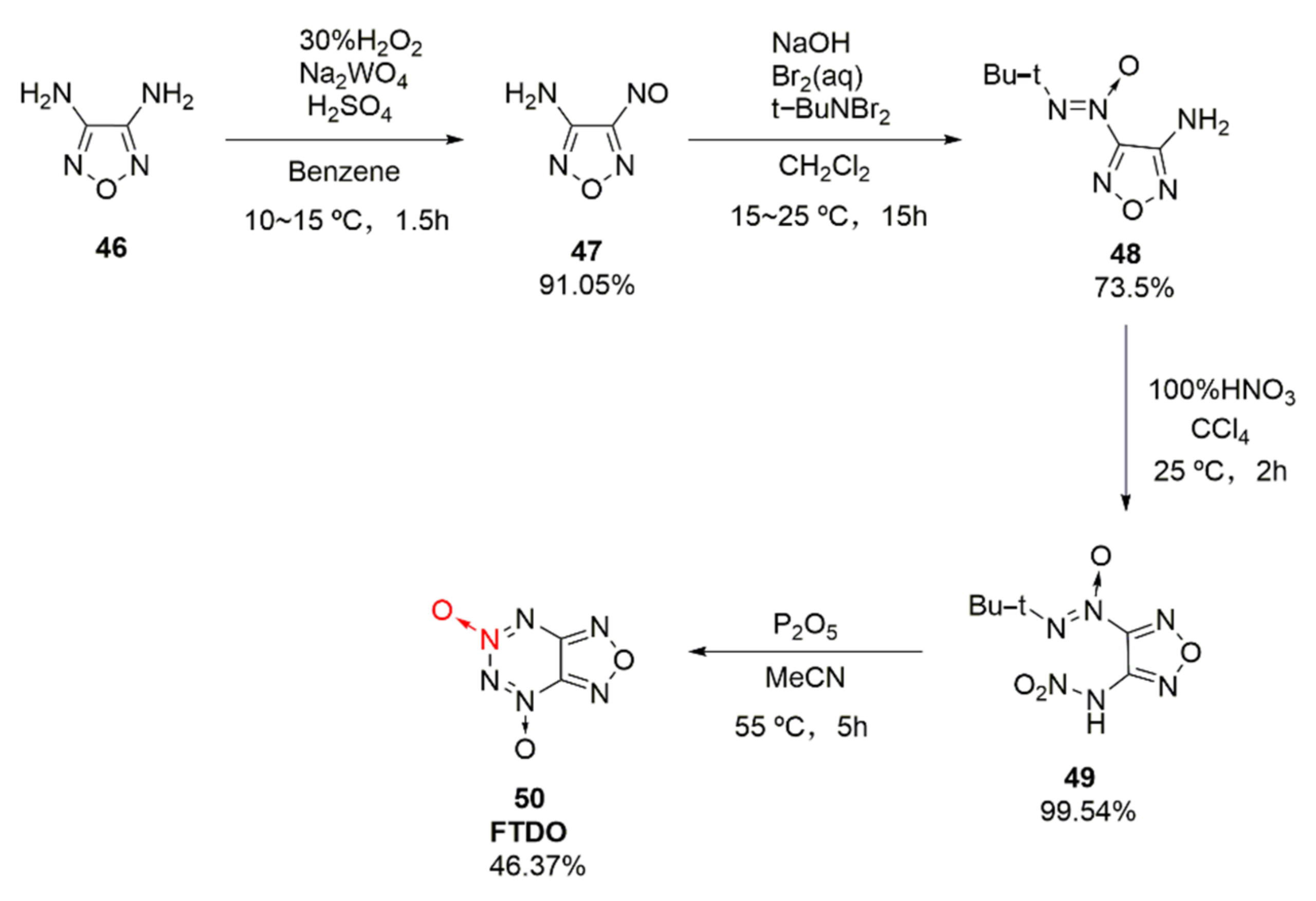
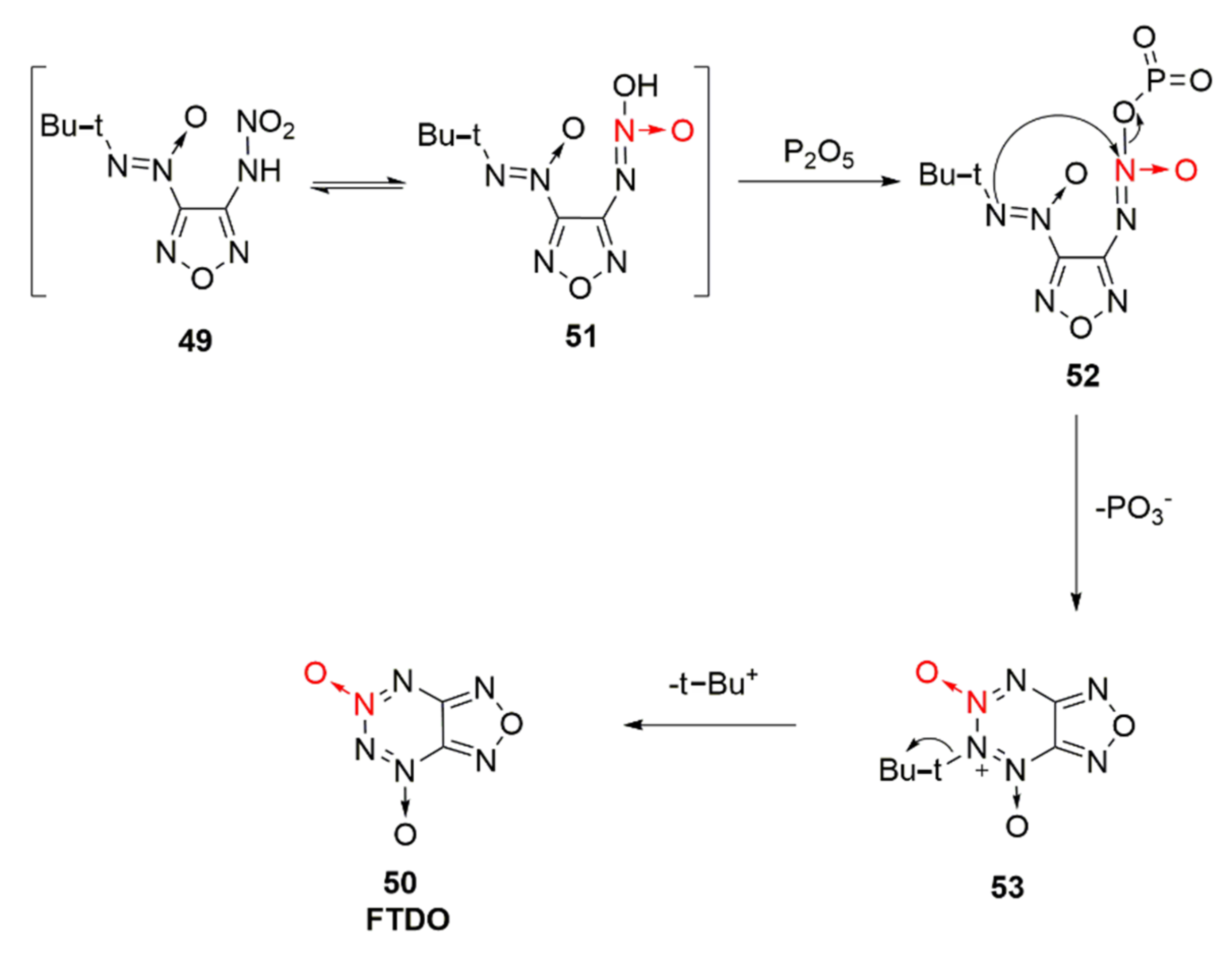
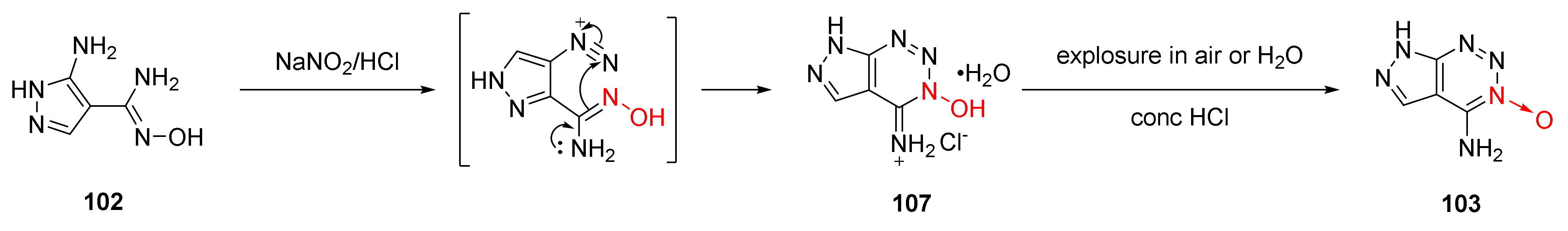
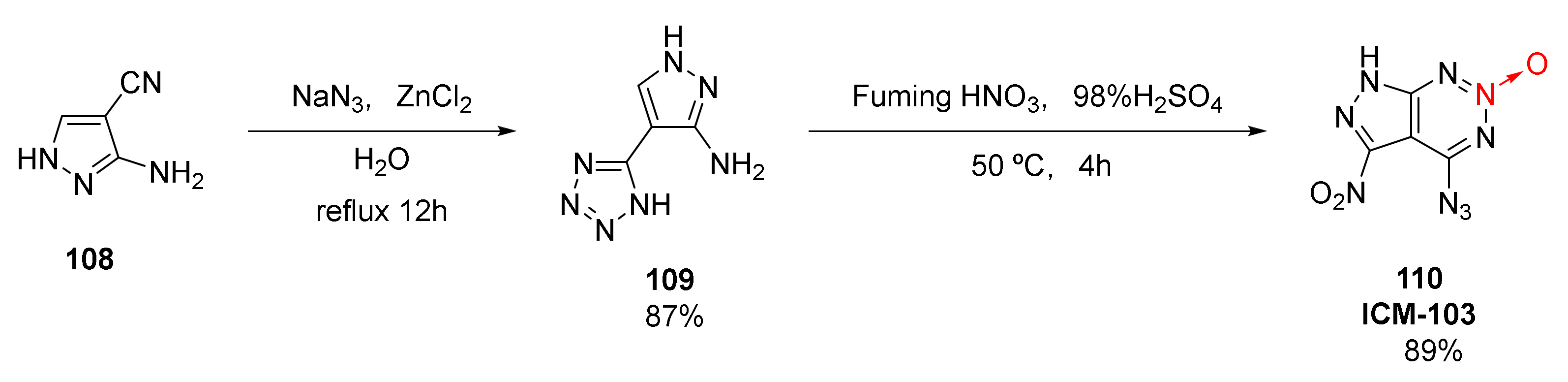
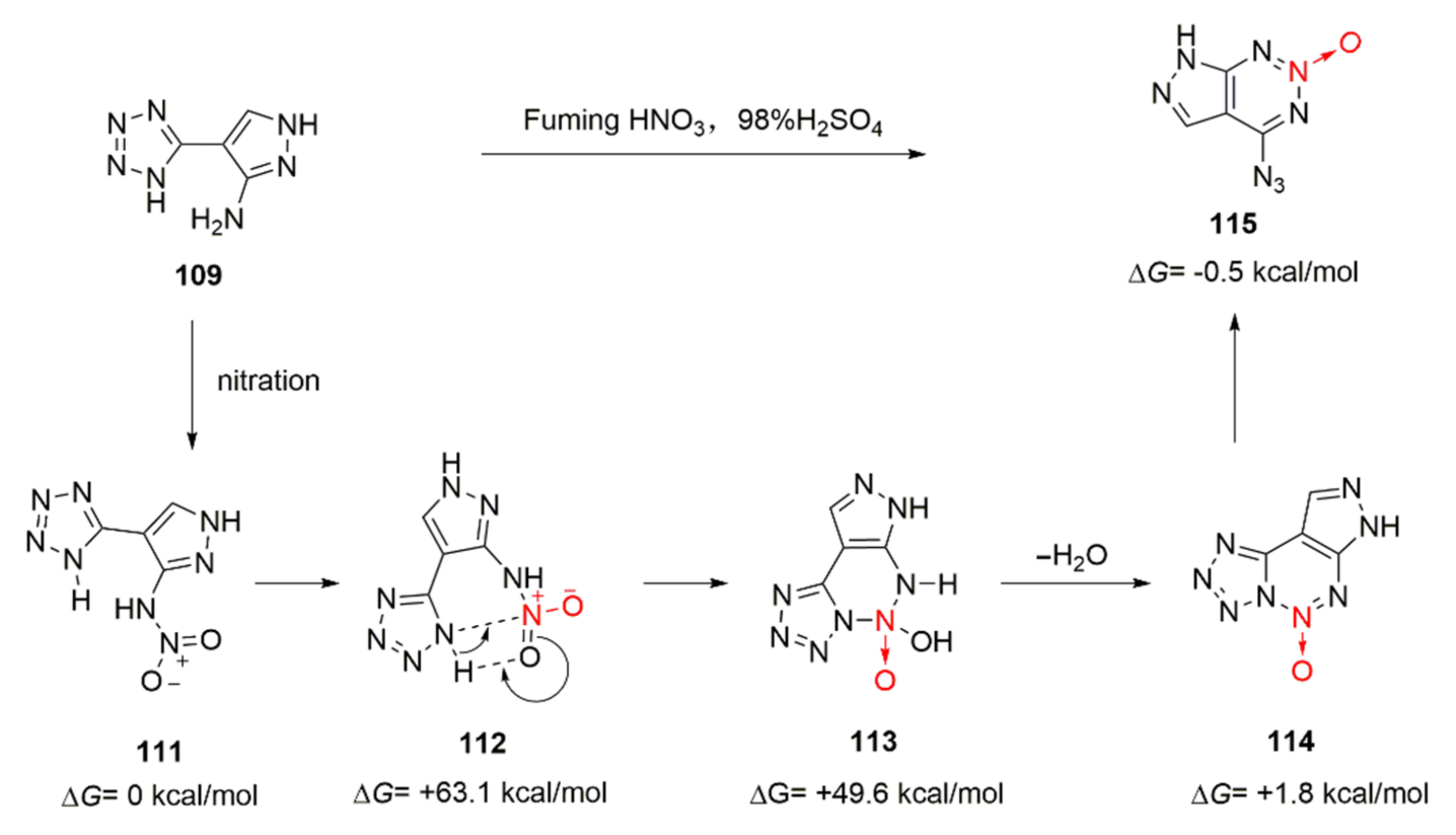
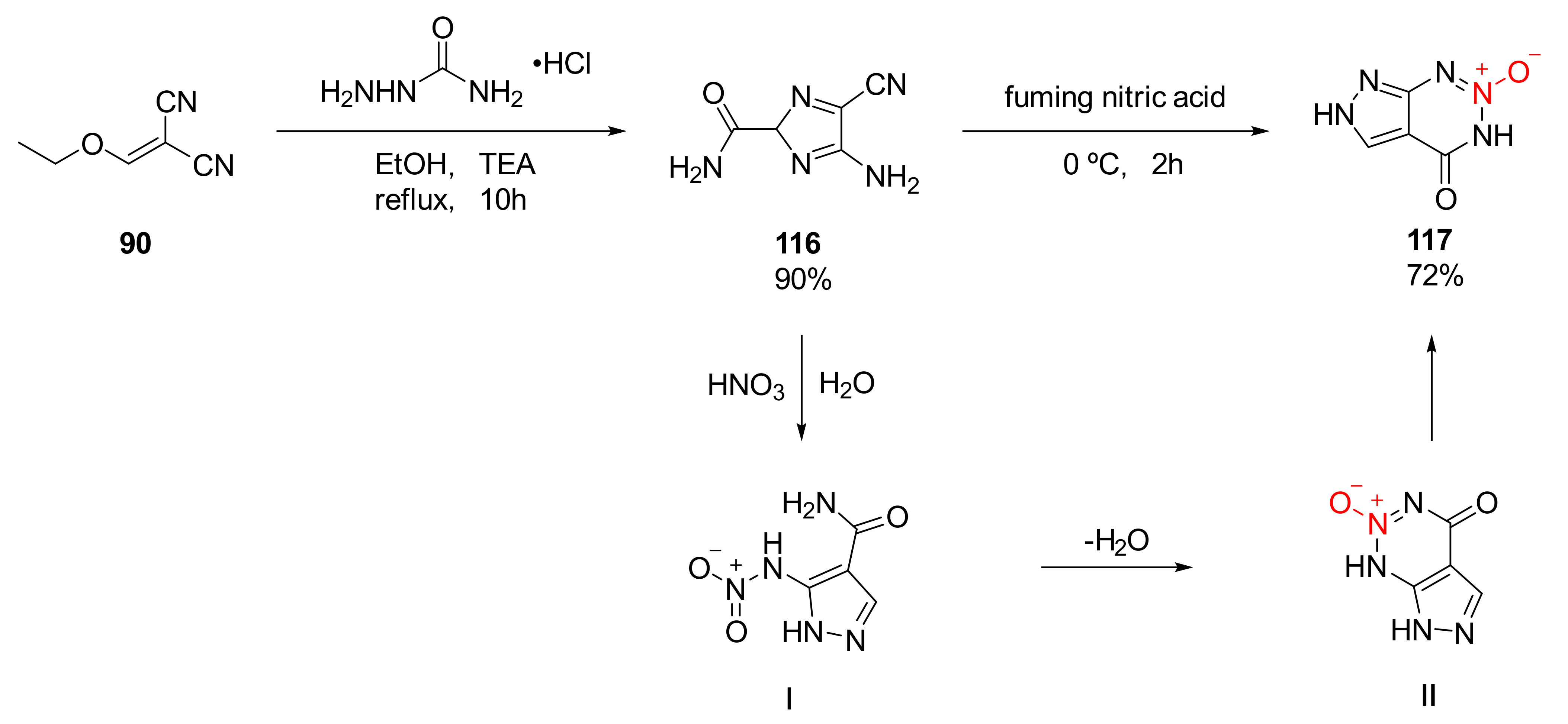
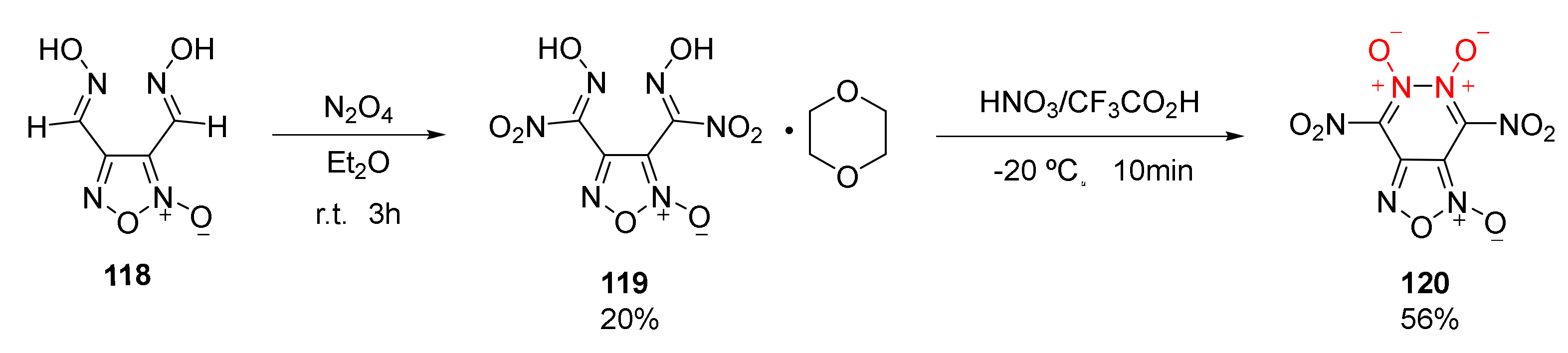
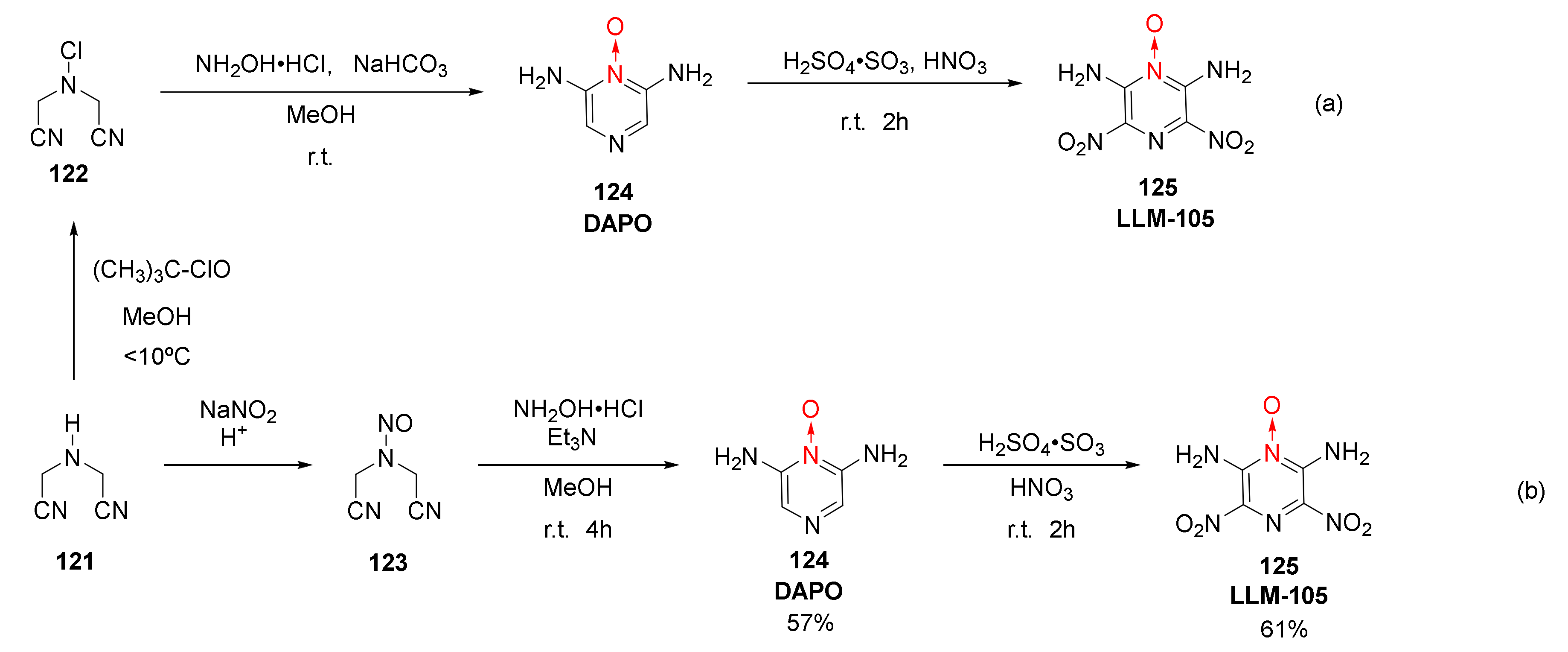
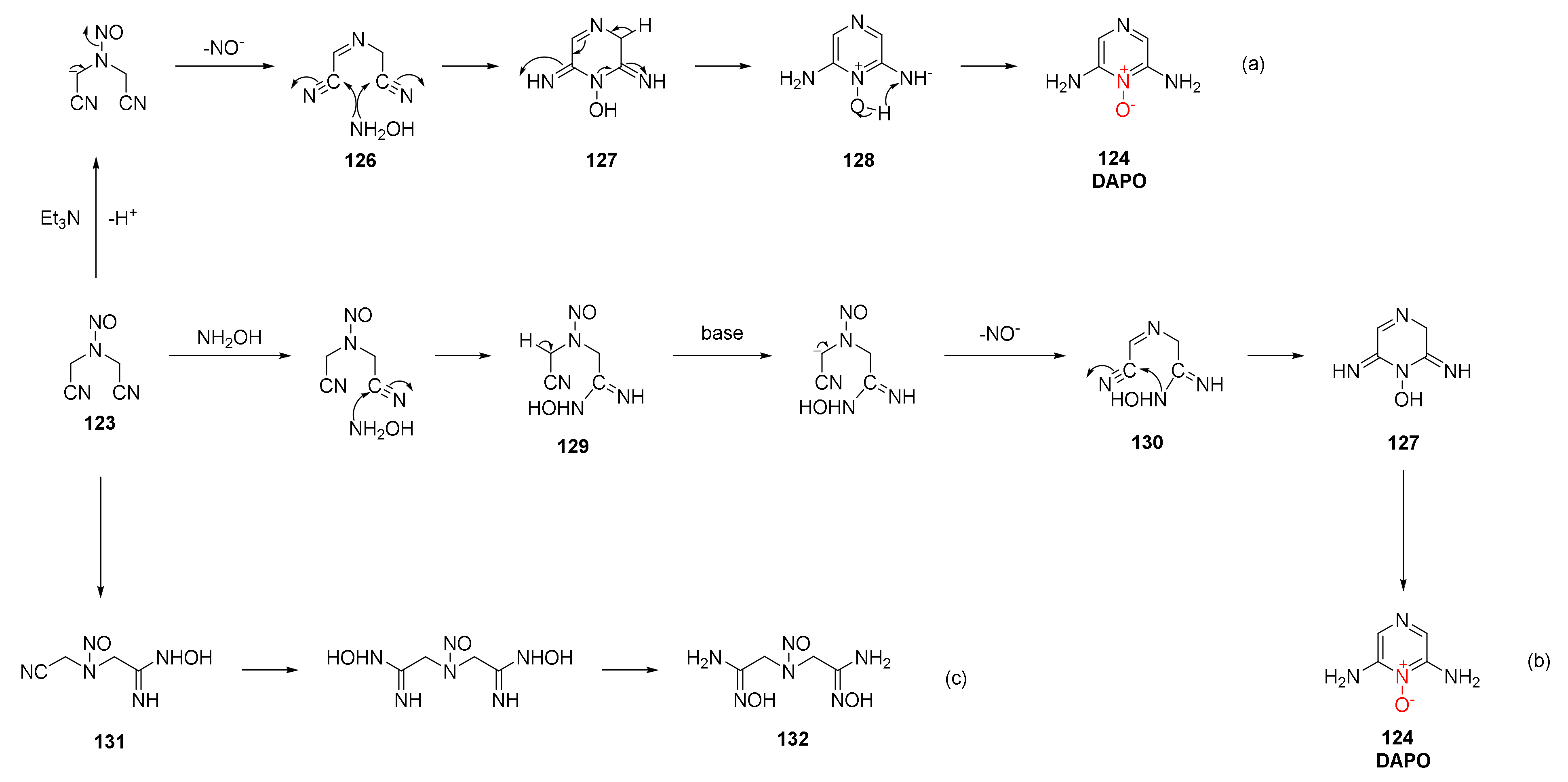
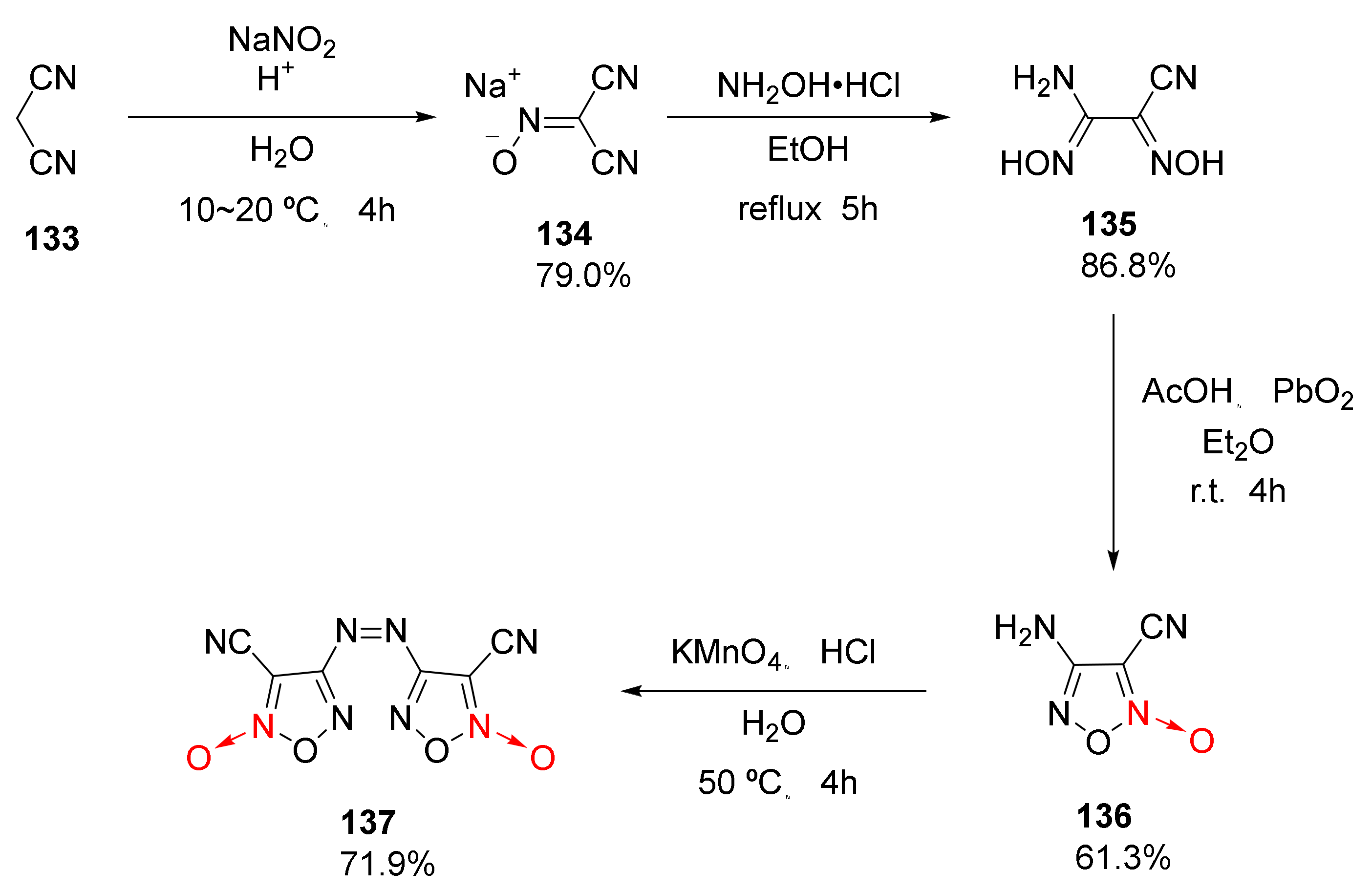
| Compound | ρ/g·cm−3 | D/m·s−1 | P/GPa | IS/J | FS/N | Td/℃ |
|---|---|---|---|---|---|---|
| 65 | 1.77 | 8252 | 29.0 | >40 | 324 | 249 |
| 66 | 1.97 | 9069 | 39.5 | >40 | 324 | 197 |
| 67 | 1.78 | 8113 | 27.1 | >40 | 324 | 269 |
| 68 | 1.89 | 8463 | 30.7 | >40 | 360 | 252 |
| 69 | 1.81 | 8373 | 29.2 | >40 | 324 | 237 |
| 70 | 1.80 | 7856 | 25.2 | >40 | >360 | 241 |
| TATB [43] | 1.93 | 8114 | 31.2 | 50 | >360 | ~360 |
| RDX [43] | 1.82 | 8748 | 34.9 | 7 | 120 | 230 |
| Compound | ρ/g·cm−3 | D/m·s−1 | P/GPa | IS/J | FS/N | Td/℃ |
|---|---|---|---|---|---|---|
| 97 | 1.815 | 8792 | 30.3 | 30 | 360 | 194 |
| 98 | 1.772 | 8670 | 28.0 | 20 | >360 | 137 |
| 99 | 1.800 | 9358 | 33.6 | 25 | >360 | 160 |
| 100 | 1.740 | 8744 | 28.2 | 30 | >360 | 180 |
| 103 | 1.772 | 8271 | 25.6 | >40 | >360 | 221 |
| 106 | 1.743 | 8008 | 23.3 | >40 | >360 | 227 |
| RDX [47] | 1.800 | 8795 | 34.9 | 7.5 | 120 | 204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
She, W.; Xu, Z.; Zhai, L.; Zhang, J.; Huang, J.; Pang, W.; Wang, B. Synthetic Methods towards Energetic Heterocyclic N-Oxides via Several Cyclization Reactions. Crystals 2022, 12, 1354. https://doi.org/10.3390/cryst12101354
She W, Xu Z, Zhai L, Zhang J, Huang J, Pang W, Wang B. Synthetic Methods towards Energetic Heterocyclic N-Oxides via Several Cyclization Reactions. Crystals. 2022; 12(10):1354. https://doi.org/10.3390/cryst12101354
Chicago/Turabian StyleShe, Weiqing, Zhenzhen Xu, Lianjie Zhai, Junlin Zhang, Jie Huang, Weiqiang Pang, and Bozhou Wang. 2022. "Synthetic Methods towards Energetic Heterocyclic N-Oxides via Several Cyclization Reactions" Crystals 12, no. 10: 1354. https://doi.org/10.3390/cryst12101354
APA StyleShe, W., Xu, Z., Zhai, L., Zhang, J., Huang, J., Pang, W., & Wang, B. (2022). Synthetic Methods towards Energetic Heterocyclic N-Oxides via Several Cyclization Reactions. Crystals, 12(10), 1354. https://doi.org/10.3390/cryst12101354







