Review on Magnesium Hydride and Sodium Borohydride Hydrolysis for Hydrogen Production
Abstract
:1. Introduction
2. Influence of Catalysts on the Hydrogen Production of MgH2
2.1. Why MgH2
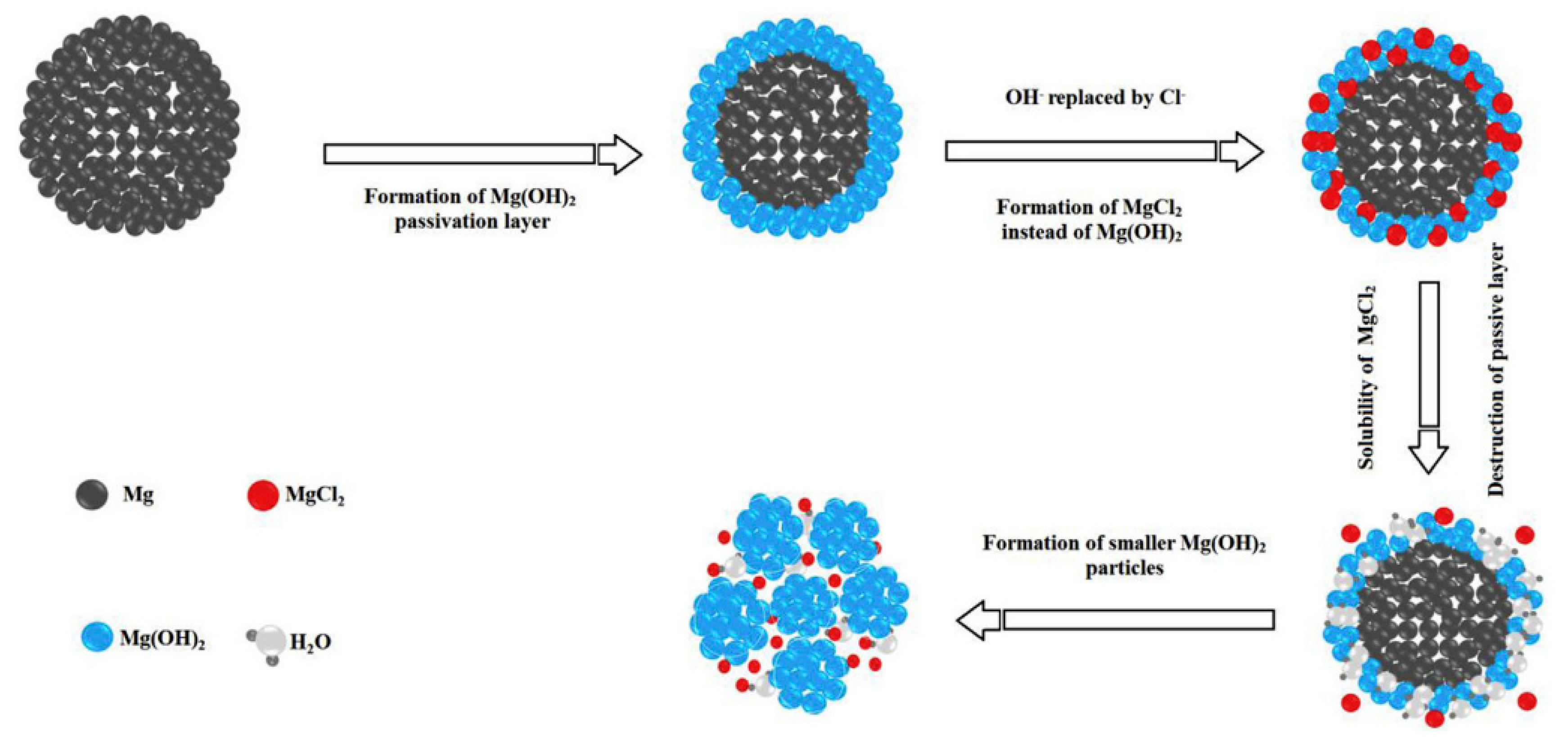
2.2. Chloride-Based Catalysts
2.3. Oxide-Based Catalysts
2.4. Fluoride-Based Catalyst
2.5. Other Catalysts
3. Influence of Catalysts on the Hydrogen Production of NaBH4
3.1. Why NaBH4
3.2. Platinum-Based Catalysts
3.3. Ruthenium-Based Catalysts
3.4. Cobalt-Based Catalysts
3.5. Nickel-Based Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- REN21. Global Status Report, Renewables 2017; REN21: Paris, France, 2017; Volume 72. [Google Scholar]
- BP. BP Statistical Review of World Energy 2022, 71st ed.; BP p.l.c.: London, UK, 2022. [Google Scholar]
- Balbay, A.; Saka, C. The Effect of the Concentration of Hydrochloric Acid and Acetic Acid Aqueous Solution for Fast Hydrogen Production from Methanol Solution of NaBH4. Int. J. Hydrog. Energy 2018, 43, 14265–14272. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Brack, P.; Dann, S.E.; Upul Wijayantha, K.G. Heterogeneous and Homogenous Catalysts for Hydrogen Generation by Hydrolysis of Aqueous Sodium Borohydride (NaBH4) Solutions. Energy Sci. Eng. 2015, 3, 174–188. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen Production from Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, L.Z.; Liu, J.W.; Yao, X.D.; Wang, H.; Liu, Z.W.; Zhu, M. Hydrolysis and Regeneration of Sodium Borohydride (NaBH4)—A Combination of Hydrogen Production and Storage. J. Power Sources 2017, 359, 400–407. [Google Scholar] [CrossRef]
- Zhong, H.; Ouyang, L.; Zeng, M.; Liu, J.; Wang, H.; Shao, H.; Felderhoff, M.; Zhu, M. Realizing Facile Regeneration of Spent NaBH4 with Mg-Al Alloy. J. Mater. Chem. A 2019, 7, 10723–10728. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B. Investigation of the Reaction Mechanism of the Hydrolysis of MgH2 in CoCl2 Solutions under Various Kinetic Conditions. React. Kinet. Mech. Catal. 2021, 132, 93–109. [Google Scholar] [CrossRef]
- Gan, D.; Liu, Y.; Zhang, J.; Zhang, Y.; Cao, C.; Zhu, Y.; Li, L. Kinetic Performance of Hydrogen Generation Enhanced by AlCl3 via Hydrolysis of MgH2 Prepared by Hydriding Combustion Synthesis. Int. J. Hydrog. Energy 2018, 43, 10232–10239. [Google Scholar] [CrossRef]
- Jadhav, A.R.; Bandal, H.A.; Kim, H. NiCo2O4 Hollow Sphere as an Efficient Catalyst for Hydrogen Generation by NaBH4 Hydrolysis. Mater. Lett. 2017, 198, 50–53. [Google Scholar] [CrossRef]
- Tegel, M.; Schöne, S.; Kieback, B.; Röntzsch, L. An Efficient Hydrolysis of MgH2-Based Materials. Int. J. Hydrog. Energy 2017, 42, 2167–2176. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Wang, H.; Liu, J.; Zhu, M. Hydrogen Generation by Hydrolysis of MgH2 and Enhanced Kinetics Performance of Ammonium Chloride Introducing. Int. J. Hydrog. Energy 2015, 40, 6145–6150. [Google Scholar] [CrossRef]
- Shi, L.; Xie, W.; Jian, Z.; Liao, X.; Wang, Y. Graphene Modified Co–B Catalysts for Rapid Hydrogen Production from NaBH4 Hydrolysis. Int. J. Hydrog. Energy 2019, 44, 17954–17962. [Google Scholar] [CrossRef]
- Grosjean, M.H.; Zidoune, M.; Roué, L.; Huot, J.Y. Hydrogen Production via Hydrolysis Reaction from Ball-Milled Mg-Based Materials. Int. J. Hydrog. Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, W.; Wang, Y.; Gao, Y. Fast Hydrogen Generation from NaBH4 Hydrolysis Catalyzed by Nanostructured Co-Ni-B Catalysts. Int. J. Hydrog. Energy 2016, 42, 6072–6079. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen Production by Alkaline Water Electrolysis. Quim. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, H.; Liu, J.W.; Sun, D.L.; Fang, F.; Zhang, Q.A.; Ouyang, L.Z.; Zhu, M. Enhanced Hydrolysis Properties and Energy Efficiency of MgH2-Base Hydrides. J. Alloy. Compd. 2016, 680, 419–426. [Google Scholar] [CrossRef]
- Tessier, J.; Huot, J.; Schulz, R.; Guay, D. Hydrogen Production and Crystal Structure of Ball-Milled MgH2–Ca and MgH2–CaH2 Mixtures. J. Alloy. Compd. 2004, 376, 180–185. [Google Scholar] [CrossRef]
- Ouyang, L.; Ma, M.; Huang, M.; Duan, R.; Wang, H.; Sun, L.; Zhu, M. Enhanced Hydrogen Generation Properties of MgH2-Based Hydrides by Breaking the Magnesium Hydroxide Passivation Layer. Energies 2015, 8, 4237–4252. [Google Scholar] [CrossRef]
- Tayeh, T.; Awad, A.S.; Nakhl, M.; Zakhour, M.; Silvain, J.F.; Bobet, J.L. Production of Hydrogen from Magnesium Hydrides Hydrolysis. Int. J. Hydrog. Energy 2014, 39, 3109–3117. [Google Scholar] [CrossRef]
- Uesugi, H.; Sugiyama, T.; Nii, H.; Ito, T.; Nakatsugawa, I. Industrial Production of MgH2 and Its Application. J. Alloy. Compd. 2011, 509, 650–653. [Google Scholar] [CrossRef]
- Xie, X.B.; Ni, C.; Wang, B.; Zhang, Y.; Zhao, X.; Liu, L.; Wang, B.; Du, W. Recent Advances in Hydrogen Generation Process via Hydrolysis of Mg-Based Materials: A Short Review. J. Alloy. Compd. 2020, 816, 152634. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, Y.; Li, L. Efficient Catalysis by MgCl2 in Hydrogen Generation via Hydrolysis of Mg-Based Hydride Prepared by Hydriding Combustion Synthesis. Chem. Commun. 2012, 48, 5509–5511. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Kimbara, M.; Nakanishi, H.; Matsumoto, S. Hydrogen Generation by Hydrolysis Reaction of Magnesium Hydride. Int. J. Hydrog. Energy 2004, 29, 1213–1217. [Google Scholar] [CrossRef]
- Grosjean, M.H.; Roué, L. Hydrolysis of Mg-Salt and MgH2-Salt Mixtures Prepared by Ball Milling for Hydrogen Production. J. Alloy. Compd. 2006, 416, 296–302. [Google Scholar] [CrossRef]
- Verbovytskyy, Y.V.; Berezovets, V.V.; Kytsya, A.R.; Zavaliy, I.Y.; Yartys, V.A. Hydrogen Generation by the Hydrolysis of MgH2. Mater. Sci. 2020, 56, 9–20. [Google Scholar] [CrossRef]
- Lukashev, R.V.; Yakovleva, N.A.; Klyamkin, S.N.; Tarasov, B.P. Effect of Mechanical Activation on the Reaction of Magnesium Hydride with Water. Russ. J. Inorg. Chem. 2008, 53, 343–349. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Guan, F.; Yu, R.; Zhang, Y.; Qin, H.; Chen, X.; Fu, Q.; Wang, Z. Study on Hydrolysis of Magnesium Hydride by Interface Control. Int. J. Photoenergy 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Nakhl, M.; Castro, F.J.; Bobet, J.L. Effect of Ball Milling Strategy (Milling Device for Scaling-up) on the Hydrolysis Performance of Mg Alloy Waste. Int. J. Hydrog. Energy 2020, 45, 20883–20893. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Castro, F.J.; Nakhl, M.; Bobet, J.L. Hydrogen Generation from Ball Milled Mg Alloy Waste by Hydrolysis Reaction. J. Power Sources 2020, 479, 228711. [Google Scholar] [CrossRef]
- Mao, J.; Zou, J.; Lu, C.; Zeng, X.; Ding, W. Hydrogen Storage and Hydrolysis Properties of Core-Shell Structured Mg-MFx (M=V, Ni, La and Ce) Nano-Composites Prepared by Arc Plasma Method. J. Power Sources 2017, 366, 131–142. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Wu, C.; Chen, Y.; Xu, Y.; Wang, X.; Zhang, Y. Microstructure Characteristics and Hydrolysis Mechanism of Mg-Ca Alloy Hydrides for Hydrogen Generation. Int. J. Hydrog. Energy 2015, 40, 3806–3812. [Google Scholar] [CrossRef]
- Awad, A.S.; El-Asmar, E.; Tayeh, T.; Mauvy, F.; Nakhl, M.; Zakhour, M.; Bobet, J.L. Effect of Carbons (G and CFs), TM (Ni, Fe and Al) and Oxides (Nb2O5 and V2O5) on Hydrogen Generation from Ball Milled Mg-Based Hydrolysis Reaction for Fuel Cell. Energy 2016, 95, 175–186. [Google Scholar] [CrossRef]
- Korablov, D.S.; Bezdorozhev, O.V.; Gierlotka, S.; Yartys, V.A.; Solonin, Y.M. Effect of Various Additives on the Hydrolysis Performance of Nanostructured MgH2 Synthesized by High-Energy Ball Milling in Hydrogen. Powder Metall. Met. Ceram. 2021, 59, 483–490. [Google Scholar] [CrossRef]
- Li, S.; Gan, D.; Zhu, Y.; Liu, Y.; Zhang, G.; Li, L. Influence of Chloride Salts on Hydrogen Generation via Hydrolysis of MgH2 Prepared by Hydriding Combustion Synthesis and Mechanical Milling. Trans. Nonferrous Met. Soc. China 2017, 27, 562–568. [Google Scholar] [CrossRef]
- Berezovets, V.; Kytsya, A.; Zavaliy, I.; Yartys, V.A. Kinetics and Mechanism of MgH2 Hydrolysis in MgCl2 Solutions. Int. J. Hydrog. Energy 2021, 46, 40278–40293. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Zhu, Y.; Liu, Y.; Li, L. Controllable Hydrogen Generation Behavior by Hydrolysis of MgH2-Based Materials. J. Power Sources 2021, 494, 229726. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, H.J.; Song, M.Y. Rate Enhancement of Hydrogen Generation through the Reaction of Magnesium Hydride with Water by MgO Addition and Ball Milling. J. Ind. Eng. Chem. 2012, 18, 405–408. [Google Scholar] [CrossRef]
- Yang, K.; Qin, H.; Lv, J.; Yu, R.; Chen, X.; Zhao, Z.; Li, Y.; Zhang, F.; Xia, X.; Fu, Q.; et al. The Effect of Graphite and Fe2O3 Addition on Hydrolysis Kinetics of Mg-Based Hydrogen Storage Materials. Int. J. Photoenergy 2021, 2021, 6651541. [Google Scholar] [CrossRef]
- Xie, L.; Ding, Y.; Ren, J.; Xie, T.; Qin, Y.; Wang, X.; Chen, F. Improved Hydrogen Generation Performance via Hydrolysis of MgH2 with Nb2O5 and CeO2 Doping. Mater. Trans. 2021, 62, 880–886. [Google Scholar] [CrossRef]
- Naseem, K.; Zhong, H.; Wang, H.; Ouyang, L.; Zhu, M. Promoting Hydrogen Generation via Co-Hydrolysis of Al and MgH2 Catalyzed by Mo and B2O3. J. Alloy. Compd. 2021, 888, 161485. [Google Scholar] [CrossRef]
- Adeniran, J.A.; Akbarzadeh, R.; Lototskyy, M.; Nyamsi, S.N.; Olorundare, O.F.; Akinlabi, E.T.; Jen, T.C. Phase-Structural and Morphological Features, Dehydrogenation/Re-Hydrogenation Performance and Hydrolysis of Nanocomposites Prepared by Ball Milling of MgH2 with Germanium. Int. J. Hydrog. Energy 2019, 44, 23160–23171. [Google Scholar] [CrossRef]
- Ma, M.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Air-Stable Hydrogen Generation Materials and Enhanced Hydrolysis Performance of MgH2-LiNH2 Composites. J. Power Sources 2017, 359, 427–434. [Google Scholar] [CrossRef]
- Hiraki, T.; Hiroi, S.; Akashi, T.; Okinaka, N.; Akiyama, T. Chemical Equilibrium Analysis for Hydrolysis of Magnesium Hydride to Generate Hydrogen. Int. J. Hydrog. Energy 2012, 37, 12114–12119. [Google Scholar] [CrossRef]
- Nunes, H.X.; Silva, D.L.; Rangel, C.M.; Pinto, A.M.F.R. Rehydrogenation of Sodium Borates to Close the NaBH4-H2 Cycle: A Review. Energies 2021, 14, 3567. [Google Scholar] [CrossRef]
- US DOE Go/No-Go Recommendation for Sodium Borohydride for On-Board Vehicular Hydrogen Storage; Office of Energy Efficiency and Renewable Energy: Washington, DC, USA, 2007.
- Zhu, J.; Li, R.; Niu, W.; Wu, Y.; Gou, X. Fast Hydrogen Generation from NaBH4 Hydrolysis Catalyzed by Carbon Aerogels Supported Cobalt Nanoparticles. Int. J. Hydrog. Energy 2013, 38, 10864–10870. [Google Scholar] [CrossRef]
- Lang, C.; Jia, Y.; Liu, J.; Wang, H.; Ouyang, L.; Zhu, M.; Yao, X. NaBH4 Regeneration from NaBO2 by High-Energy Ball Milling and Its Plausible Mechanism. Int. J. Hydrog. Energy 2017, 42, 13127–13135. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, W.; Liu, J.; Felderhoff, M.; Wang, H.; Zhu, M. Enhancing the Regeneration Process of Consumed NaBH4 for Hydrogen Storage. Adv. Energy Mater. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Marrero-Alfonso, E.Y.; Gray, J.R.; Davis, T.A.; Matthews, M.A. Hydrolysis of Sodium Borohydride with Steam. Int. J. Hydrog. Energy 2007, 32, 4717–4722. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Sodium Borohydride versus Ammonia Borane, in Hydrogen Storage and Direct Fuel Cell Applications. Energy Environ. Sci. 2009, 2, 627. [Google Scholar] [CrossRef]
- Demirci, U.B.; Akdim, O.; Miele, P. Ten-Year Efforts and a No-Go Recommendation for Sodium Borohydride for on-Board Automotive Hydrogen Storage. Int. J. Hydrog. Energy 2009, 34, 2638–2645. [Google Scholar] [CrossRef]
- Balbay, A.; Saka, C. Effect of Phosphoric Acid Addition on the Hydrogen Production from Hydrolysis of NaBH4 with Cu Based Catalyst. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 794–804. [Google Scholar] [CrossRef]
- Balbay, A.; Saka, C. Semi-Methanolysis Reaction of Potassium Borohydride with Phosphoric Acid for Effective Hydrogen Production. Int. J. Hydrog. Energy 2018, 43, 21299–21306. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Rodríguez-Pérez, I.A.; Miao, W.; Chen, K.; Wang, W.; Li, Y.; Han, S. Carbon Nanospheres Supported Bimetallic Pt-Co as an Efficient Catalyst for NaBH4 Hydrolysis. Appl. Surf. Sci. 2021, 540, 148296. [Google Scholar] [CrossRef]
- Zabielaitė, A.; Balčiūnaitė, A.; Stalnionienė, I.; Lichušina, S.; Šimkūnaitė, D.; Vaičiūnienė, J.; Šimkūnaitė-Stanynienė, B.; Selskis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Fiber-Shaped Co Modified with Au and Pt Crystallites for Enhanced Hydrogen Generation from Sodium Borohydride. Int. J. Hydrog. Energy 2018, 43, 23310–23318. [Google Scholar] [CrossRef]
- Bozkurt, G.; Özer, A.; Yurtcan, A.B. Development of Effective Catalysts for Hydrogen Generation from Sodium Borohydride: Ru, Pt, Pd Nanoparticles Supported on Co3O4. Energy 2019, 180, 702–713. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Devrim, Y. Hydrogen Generation from Sodium Borohydride Hydrolysis by Multi-Walled Carbon Nanotube Supported Platinum Catalyst: A Kinetic Study. Int. J. Hydrog. Energy 2019, 44, 17586–17594. [Google Scholar] [CrossRef]
- Dai, P.; Zhao, X.; Xu, D.; Wang, C.; Tao, X.; Liu, X.; Gao, J. Preparation, Characterization, and Properties of Pt/Al2O3/Cordierite Monolith Catalyst for Hydrogen Generation from Hydrolysis of Sodium Borohydride in a Flow Reactor. Int. J. Hydrog. Energy 2019, 44, 28463–28470. [Google Scholar] [CrossRef]
- Aygun, A.; Gulbagca, F.; Altuner, E.E.; Bekmezci, M.; Gur, T.; Karimi-Maleh, H.; Karimi, F.; Vasseghian, Y.; Sen, F. Highly Active PdPt Bimetallic Nanoparticles Synthesized by One-Step Bioreduction Method: Characterizations, Anticancer, Antibacterial Activities and Evaluation of Their Catalytic Effect for Hydrogen Generation. Int. J. Hydrog. Energy 2021, in press. [CrossRef]
- Tiri, R.N.E.; Gulbagca, F.; Aygun, A.; Cherif, A.; Sen, F. Biosynthesis of Ag–Pt Bimetallic Nanoparticles Using Propolis Extract: Antibacterial Effects and Catalytic Activity on NaBH4 Hydrolysis. Environ. Res. 2022, 206, 112622. [Google Scholar] [CrossRef]
- Lin, J.; Gulbagca, F.; Aygun, A.; Elhouda Tiri, R.N.; Xia, C.; Van Le, Q.; Gur, T.; Sen, F.; Vasseghian, Y. Phyto-Mediated Synthesis of Nanoparticles and Their Applications on Hydrogen Generation on NaBH4, Biological Activities and Photodegradation on Azo Dyes: Development of Machine Learning Model. Food Chem. Toxicol. 2022, 163, 112972. [Google Scholar] [CrossRef] [PubMed]
- Tuan, D.D.; Lin, K.Y.A. Ruthenium Supported on ZIF-67 as an Enhanced Catalyst for Hydrogen Generation from Hydrolysis of Sodium Borohydride. Chem. Eng. J. 2018, 351, 48–55. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, F.; Yang, L.; He, Z.; Huang, X.; Zhang, D.; Dong, H. Ultrasmall Ru Nanoparticles Supported on Chitin Nanofibers for Hydrogen Production from NaBH4 Hydrolysis. Chin. Chem. Lett. 2020, 31, 2019–2022. [Google Scholar] [CrossRef]
- Avci Hansu, T.; Sahin, O.; Caglar, A.; Kivrak, H. A Remarkable Mo Doped Ru Catalyst for Hydrogen Generation from Sodium Borohydride: The Effect of Mo Addition and Estimation of Kinetic Parameters. React. Kinet. Mech. Catal. 2020, 131, 661–676. [Google Scholar] [CrossRef]
- Dou, S.; Zhang, W.; Yang, Y.; Zhou, S.; Rao, X.; Yan, P.; Isimjan, T.T.; Yang, X. Shaggy-like Ru-Clusters Decorated Core-Shell Metal-Organic Framework-Derived CoOx@NPC as High-Efficiency Catalyst for NaBH4 Hydrolysis. Int. J. Hydrog. Energy 2021, 46, 7772–7781. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Y.; Zhang, W.; Rao, X.; Yan, P.; Isimjan, T.T.; Yang, X. Structure-Regulated Ru Particles Decorated P-Vacancy-Rich CoP as a Highly Active and Durable Catalyst for NaBH4 Hydrolysis. J. Colloid Interface Sci. 2021, 591, 221–228. [Google Scholar] [CrossRef]
- Liu, H.; Ning, H.; Peng, S.; Yu, Y.; Ran, C.; Chen, Y.; Ma, J.; Xie, J. Surface Tailored Ru Catalyst on Magadiite for Efficient Hydrogen Generation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127627. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Yang, L.; Zhang, F.; Li, R.; Dong, H. Ruthenium Nanosheets Decorated Cobalt Foam for Controllable Hydrogen Production from Sodium Borohydride Hydrolysis. Catal. Lett. 2021, 152, 1386–1391. [Google Scholar] [CrossRef]
- Avcı Hansu, T. A Novel and Active Ruthenium Based Supported Multiwalled Carbon Nanotube Tungsten Nanoalloy Catalyst for Sodium Borohydride Hydrolysis. Int. J. Hydrog. Energy 2022, in press. [CrossRef]
- Li, T.; Xiang, C.; Chu, H.; Xu, F.; Sun, L.; Zou, Y.; Zhang, J. Catalytic Effect of Highly Dispersed Ultrafine Ru Nanoparticles on a TiO2-Ti3C2 Support: Hydrolysis of Sodium Borohydride for H2 Generation. J. Alloy. Compd. 2022, 906, 164380. [Google Scholar] [CrossRef]
- Kilinc, D.; Sahin, O. Development of Highly Efficient and Reusable Ruthenium Complex Catalyst for Hydrogen Evolution. Int. J. Hydrog. Energy 2022, 47, 3876–3885. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt-Based Catalysts for the Hydrolysis of NaBH4 and NH3BH3. Phys. Chem. Chem. Phys. 2014, 16, 6872–6885. [Google Scholar] [CrossRef] [PubMed]
- Demirci, U.B.; Akdim, O.; Hannauer, J.; Chamoun, R.; Miele, P. Cobalt, a Reactive Metal in Releasing Hydrogen from Sodium Borohydride by Hydrolysis: A Short Review and a Research Perspective. Sci. China Chem. 2010, 53, 1870–1879. [Google Scholar] [CrossRef]
- Yao, Q.; DIng, Y.; Lu, Z.H. Noble-Metal-Free Nanocatalysts for Hydrogen Generation from Boron- And Nitrogen-Based Hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Akdim, O.; Demirci, U.B.; Muller, D.; Miele, P. Cobalt (II) Salts, Performing Materials for Generating Hydrogen from Sodium Borohydride. Int. J. Hydrog. Energy 2009, 34, 2631–2637. [Google Scholar] [CrossRef]
- Jeong, S.U.; Cho, E.A.; Nam, S.W.; Oh, I.H.; Jung, U.H.; Kim, S.H. Effect of Preparation Method on Co-B Catalytic Activity for Hydrogen Generation from Alkali NaBH4 Solution. Int. J. Hydrog. Energy 2007, 32, 1749–1754. [Google Scholar] [CrossRef]
- Baydaroglu, F.; Özdemir, E.; Hasimoglu, A. An Effective Synthesis Route for Improving the Catalytic Activity of Carbon-Supported Co-B Catalyst for Hydrogen Generation through Hydrolysis of NaBH4. Int. J. Hydrog. Energy 2014, 39, 1516–1522. [Google Scholar] [CrossRef]
- Loghmani, M.H.; Shojaei, A.F. Synthesis and Characterization of Co-La-Zr-B Quaternary Amorphous Nano Alloy: Kinetic Study for Hydrogen Generation from Hydrolysis of Sodium Borohydride. J. Alloy. Compd. 2013, 580, 61–66. [Google Scholar] [CrossRef]
- Manna, J.; Roy, B.; Pareek, D.; Sharma, P. Hydrogen Generation from NaBH4 Hydrolysis Using Co-B/AlPO4 and Co-B/Bentonite Catalysts. Catal. Struct. React. 2017, 3, 157–164. [Google Scholar] [CrossRef]
- Li, T.; Xiang, C.; Zou, Y.; Xu, F.; Sun, L. Synthesis of Highly Stable Cobalt Nanorods Anchored on a Ti4N3Tx MXene Composite for the Hydrolysis of Sodium Borohydride. J. Alloy. Compd. 2021, 885, 160991. [Google Scholar] [CrossRef]
- Bu, Y.; Liu, J.; Chu, H.; Wei, S.; Yin, Q.; Kang, L.; Luo, X.; Sun, L.; Xu, F.; Huang, P.; et al. Catalytic Hydrogen Evolution of NaBH4 Hydrolysis by Cobalt Nanoparticles Supported on Bagasse-Derived Porous Carbon. Nanomaterials 2021, 11, 3259. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Zou, Y.; Xiang, C.; Zhang, H.; Xu, F.; Sun, L.; He, K. Modulating Valence Band to Enhance the Catalytic Activity of Co-Cr-B/NG for Hydrolysis of Sodium Borohydride. J. Alloy. Compd. 2022, 924, 166556. [Google Scholar] [CrossRef]
- Erat, N.; Bozkurt, G.; Özer, A. Co/CuO–NiO–Al2O3 Catalyst for Hydrogen Generation from Hydrolysis of NaBH4. Int. J. Hydrog. Energy 2022, 47, 24255–24267. [Google Scholar] [CrossRef]
- Ozerova, A.M.; Skobelkina, A.A.; Simagina, V.I.; Komova, O.V.; Prosvirin, I.P.; Bulavchenko, O.A.; Lipatnikova, I.L.; Netskina, O.V. Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride. Materials 2022, 15, 10. [Google Scholar] [CrossRef]
- Baydaroglu, F.O.; Özdemir, E.; Gürek, A.G. Polypyrrole Supported Co–W–B Nanoparticles as an Efficient Catalyst for Improved Hydrogen Generation from Hydrolysis of Sodium Borohydride. Int. J. Hydrog. Energy 2022, 47, 9643–9652. [Google Scholar] [CrossRef]
- Guan, S.; An, L.; Chen, Y.; Li, M.; Shi, J.; Liu, X.; Fan, Y.; Li, B.; Liu, B. Stabilized Cobalt-Based Nanofilm Catalyst Prepared Using an Ionic Liquid/Water Interfacial Process for Hydrogen Generation from Sodium Borohydride. J. Colloid Interface Sci. 2022, 608, 3111–3120. [Google Scholar] [CrossRef]
- Sun, L.; Meng, Y.; Kong, X.; Ge, H.; Chen, X.; Ding, C.; Yang, H.; Li, D.; Gao, X.; Dou, J. Novel High Dispersion and High Stability Cobalt-Inlaid Carbon Sphere Catalyst for Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Fuel 2022, 310, 122276. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, J.; Meng, X.; Chen, W.; Guo, J.; Liu, X. Co0.45W0.55 Nanocomposite from ZIF-67: An Efficient and Heterogeneous Catalyst for H2 Generation Upon NaBH4 Hydrolysis. Catal. Lett. 2022, 152, 610–618. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Zhang, J.; Dong, H. Catalytic Hydrolysis of NaBH4 over Titanate Nanotube Supported Co for Hydrogen Production. Int. J. Hydrog. Energy 2022, 47, 5260–5268. [Google Scholar] [CrossRef]
- Netskina, O.V.; Tayban, E.S.; Rogov, V.A.; Ozerova, A.M.; Mukha, S.A.; Simagina, V.I.; Komova, O.V. Solid-State NaBH4 Composites for Hydrogen Generation: Catalytic Activity of Nickel and Cobalt Catalysts. Int. J. Hydrog. Energy 2021, 46, 5459–5471. [Google Scholar] [CrossRef]
- Lee, J.; Shin, H.; Choi, K.S.; Lee, J.; Choi, J.Y.; Yu, H.K. Carbon Layer Supported Nickel Catalyst for Sodium Borohydride (NaBH4) Dehydrogenation. Int. J. Hydrog. Energy 2019, 44, 2943–2950. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Wang, D.; Wu, S.; Cao, Z.; Zhang, K.; Liu, H.; Xin, S. Hydrogen Generation from Hydrolysis of Sodium Borohydride Using Nanostructured Ni-B Catalysts. Int. J. Hydrog. Energy 2016, 41, 16077–16086. [Google Scholar] [CrossRef]
- Lai, Q.; Alligier, D.; Aguey-Zinsou, K.F.; Demirci, U.B. Hydrogen Generation from a Sodium Borohydride-Nickel Core@shell Structure under Hydrolytic Conditions. Nanoscale Adv. 2019, 1, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, Q.; Liu, S.; Liu, X. 3D Porous Ni-Zn Catalyst for Catalytic Hydrolysis of Sodium Borohydride and Ammonia Borane. Funct. Mater. Lett. 2020, 13, 1–4. [Google Scholar] [CrossRef]
- Ma, T.; Qiu, Y.; Zhang, Y.; Ji, X.; Hu, P. Supporting Information Fe-Doped Ni5P4 Ultrathin Nanoporous Nanosheets for Water Splitting and on-Demand Hydrogen Release via NaBH4 Hydrolysis. ACS Appl. Nano Mater. 2019, 2, 3091–3099. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, Z.; Jiang, Y.; Yu, C. Effect of Carriers on Deposition Morphologies and Catalytic Performances of NiCo2O4. Ceram. Int. 2020, 46, 11499–11507. [Google Scholar] [CrossRef]
- Dönmez, F.; Ayas, N. Synthesis of Ni/TiO2 Catalyst by Sol-Gel Method for Hydrogen Production from Sodium Borohydride. Int. J. Hydrog. Energy 2021, 46, 29314–29322. [Google Scholar] [CrossRef]
- Elçiçek, H.; Erol, M.; Özdemir, O.K. Preparation of Highly Efficient NiB Catalyst via Triton-Stabilized for Alkaline NaBH4 Hydrolysis Reaction. Int. J. Energy Res. 2021, 45, 14644–14657. [Google Scholar] [CrossRef]
- Kilinc, D.; Sahin, O. Highly Active and Stable CeO2 Supported Nickel-Complex Catalyst in Hydrogen Generation. Int. J. Hydrog. Energy 2021, 46, 499–507. [Google Scholar] [CrossRef]
- Kiren, B.; Ayas, N. Nickel Modified Dolomite in the Hydrogen Generation from Sodium Borohydride Hydrolysis. Int. J. Hydrog. Energy 2022, 47, 19702–19717. [Google Scholar] [CrossRef]

| Catalysts | H2 Yield Rate | Temperature (°C) | Activation Energy (kJ/mol) | Year | Reference |
|---|---|---|---|---|---|
| PtCofiber/Cu | 3700 mL/min.gcat | 30–70 | 62.60 | 2018 | [58] |
| Pt-Co3O4 | 23,916 mL/min.gcat | 25–55 | 43.52 | 2019 | [59] |
| Pt/MWCNT | 159 mmol/min.gcat | 27–67 | 27.00 | 2019 | [60] |
| Pt/Al2O3/cordierite | 53 mL/min | autothermal | - | 2019 | [61] |
| PdPt | 372.52 mL/min.gcat | 20–35 | 13.93 | 2021 | [62] |
| Ag-Pt | 294.78 mL/min.gcat | 20–35 | 25.61 | 2022 | [63] |
| N@Pt-Ag | 367.01 mL/min.gcat | 20–35 | 16.02 | 2022 | [64] |
| Catalysts | H2 Yield Rate | Temperature (°C) | Activation Energy (kJ/mol) | Year | Reference |
|---|---|---|---|---|---|
| Ru/ZIF | 22,400 mL/min.gcat | 30–60 | 39.00 | 2018 | [65] |
| Ru-Co3O4 | 20,628 mL/min.gcat | 25–55 | 28.26 | 2019 | [59] |
| RuMo/CNT | 82,758.43 mL/min.gcat | 20–50 | 35.11 | 2020 | [67] |
| Ru/CoOx@NPC | 8019.5 mL/min.gcat | 25–45 | 59.30 | 2021 | [68] |
| Ru9.8/r-CoP | 9783.3 mL/min.gcat | 25–45 | 45.30 | 2021 | [69] |
| Ru/NH2-magadiite | 36,515 ± 500 mL/min.gRu | 5–20 | 54.50 | 2021 | [70] |
| Ru/Co | 19.67 mL/min | 30–60 | 30.18 | 2021 | [71] |
| RuW/MWCNT | 198,397.2 mL/min.gcat | 20–50 | 16.32 | 2022 | [72] |
| Ru-TiO2-Ti3C2 | 434,500 mL/min.gRu | 30–60 | 50.96 | 2022 | [73] |
| Ru complex | 299,220 mL/min.gcat | 20–50 | 25.80 | 2022 | [74] |
| Catalysts | H2 Yield Rate | Temperature (°C) | Activation Energy (kJ/mol) | Year | Reference |
|---|---|---|---|---|---|
| Co/Ti4N3Tx | 526 mL/min.gcat | 30–60 | 44.23 | 2021 | [83] |
| Co@150PC | 11,086.4 mL/min.gCo | 15–55 | 31.25 | 2021 | [84] |
| Co-Cr-B/NG | 2231.7 mL/min.gcat | 15–45 | 38.41 | 2022 | [85] |
| Co/CuO–NiO–Al2O3 | 6460 mL/min.gcat | 25–55 | 31.59 | 2022 | [86] |
| Co0 hollow microshells | 1560 mL/min.gcat | 25–40 | 55.5 ± 1.9 | 2022 | [87] |
| Co–W–B/PPy | 13,470 mL/min.gcat | 20–35 | 49.18 | 2022 | [88] |
| Co/CoxOy Nanofilm | 4067 mL/min.gcat | 30–45 | 43.19 | 2022 | [89] |
| Co@C-462-145 | 5392 mL/min.gCo | 25–40 | 32.70 | 2022 | [90] |
| Co0.45W0.55 | 1770 mL/min.gcat | 0–35 | 55.56 | 2022 | [91] |
| Co/HTNT | 1750 mL/min.gCo | 20–50 | 29.68 | 2022 | [92] |
| Catalysts | H2 Yield Rate | Temperature (°C) | Activation Energy (kJ/mol) | Year | Reference |
|---|---|---|---|---|---|
| Ni | 22,500 mL/min.g | 10–60 | 46.60 | 2019 | [96] |
| Ni–Zn | 430 mL/min.g | 25 | - | 2019 | [97] |
| Ni5P4/Fe | 175 mL/min.g | 30 | 53.41 | 2019 | [98] |
| Pine-needle-like NiCo2O4 | 1904.76 mL/min.g | 25–55 | 47.69 | 2020 | [99] |
| Network-like NiCo2O4 | 2251.14 mL/min.g | 25–55 | 52.21 | 2020 | [99] |
| Ball-cactus-like NiCo2O4 | 6219 mL/min.g | 25–55 | 55.79 | 2020 | [99] |
| Dandelion-like NiCo2O4 | 3125 mL/min.g | 25–55 | 63.44 | 2020 | [99] |
| Ni/TiO2 | 110.87 mL/min.gcat | 20–60 | 25.11 | 2021 | [100] |
| NiB25 | 8970 mL/min.gcat | 65 | 48.05 | 2021 | [101] |
| Nickel (II) complex/CeO2 | 43.392 mL/min.gcat | 20–50 | 20.58 | 2021 | [102] |
| Ni/Dolomite | 88.16 mL/min.gcat | 30–60 | 38.33 | 2022 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruslan, N.; Yahya, M.S.; Siddique, M.N.I.; Yengantiwar, A.P.; Ismail, M.; Awal, M.R.; Mohd Yusoff, M.Z.; Abdul Halim Yap, M.F.A.; Mustafa, N.S. Review on Magnesium Hydride and Sodium Borohydride Hydrolysis for Hydrogen Production. Crystals 2022, 12, 1376. https://doi.org/10.3390/cryst12101376
Ruslan N, Yahya MS, Siddique MNI, Yengantiwar AP, Ismail M, Awal MR, Mohd Yusoff MZ, Abdul Halim Yap MFA, Mustafa NS. Review on Magnesium Hydride and Sodium Borohydride Hydrolysis for Hydrogen Production. Crystals. 2022; 12(10):1376. https://doi.org/10.3390/cryst12101376
Chicago/Turabian StyleRuslan, Nuraini, Muhammad Syarifuddin Yahya, Md. Nurul Islam Siddique, Ashish Prabhakar Yengantiwar, Mohammad Ismail, Md. Rabiul Awal, Mohd Zaki Mohd Yusoff, Muhammad Firdaus Asyraf Abdul Halim Yap, and Nurul Shafikah Mustafa. 2022. "Review on Magnesium Hydride and Sodium Borohydride Hydrolysis for Hydrogen Production" Crystals 12, no. 10: 1376. https://doi.org/10.3390/cryst12101376
APA StyleRuslan, N., Yahya, M. S., Siddique, M. N. I., Yengantiwar, A. P., Ismail, M., Awal, M. R., Mohd Yusoff, M. Z., Abdul Halim Yap, M. F. A., & Mustafa, N. S. (2022). Review on Magnesium Hydride and Sodium Borohydride Hydrolysis for Hydrogen Production. Crystals, 12(10), 1376. https://doi.org/10.3390/cryst12101376











