Synthesis of Unexpected Dimethyl 2-(4-Chlorophenyl)-2,3-dihydropyrrolo[2,1-a]isoquinoline-1,3-dicarboxylate via Hydrolysis/Cycloaddition/Elimination Cascades: Single Crystal X-ray and Chemical Structure Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Dimethyl 2-(4-Chlorophenyl)-2,3-dihydropyrrolo[2,1-a]isoquinoline-1,3-dicarboxylate 3
2.2. X-ray Single Crystal Measurements of 3
2.3. Computational Study
3. Results and Discussion
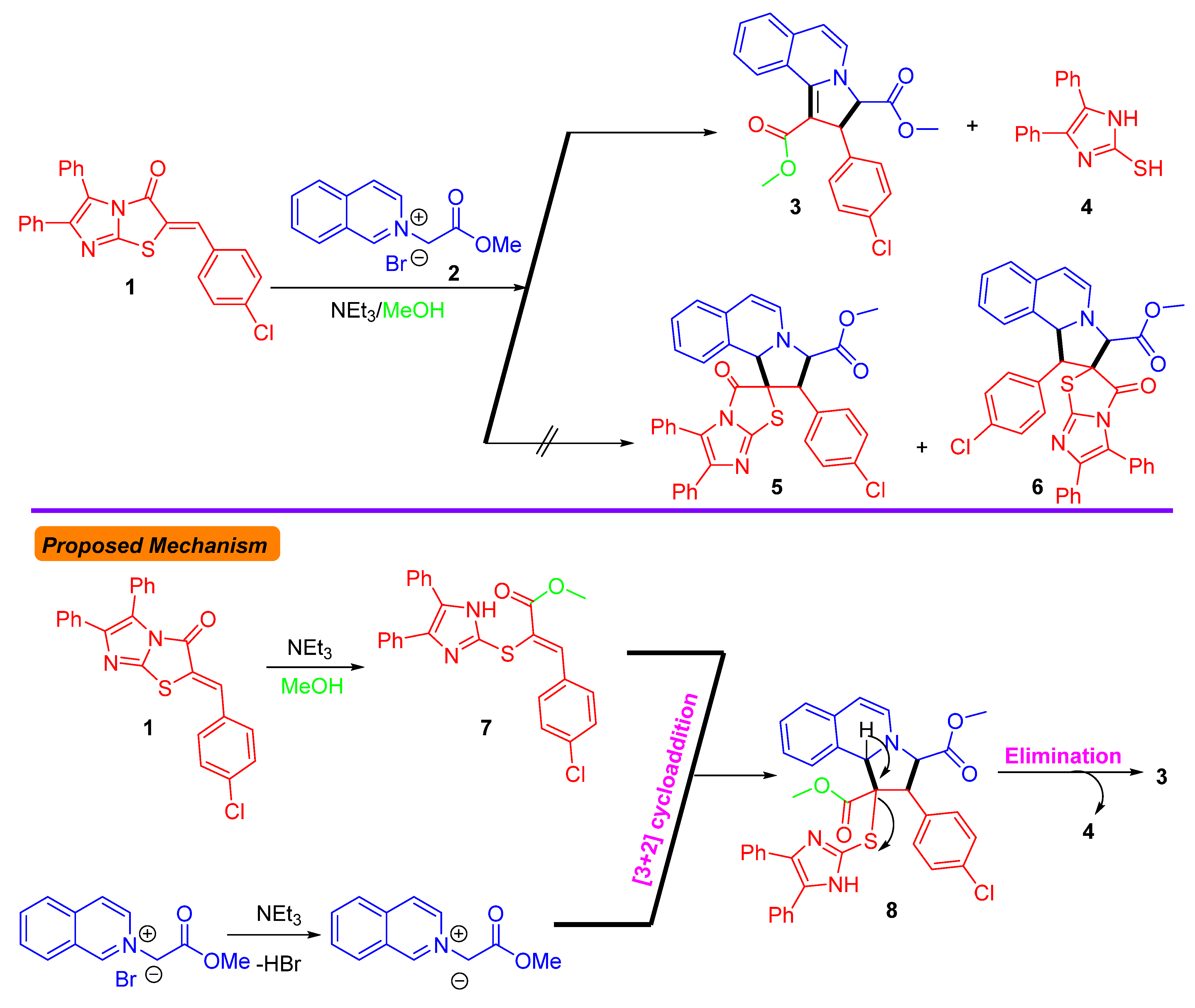
3.1. Chemistry
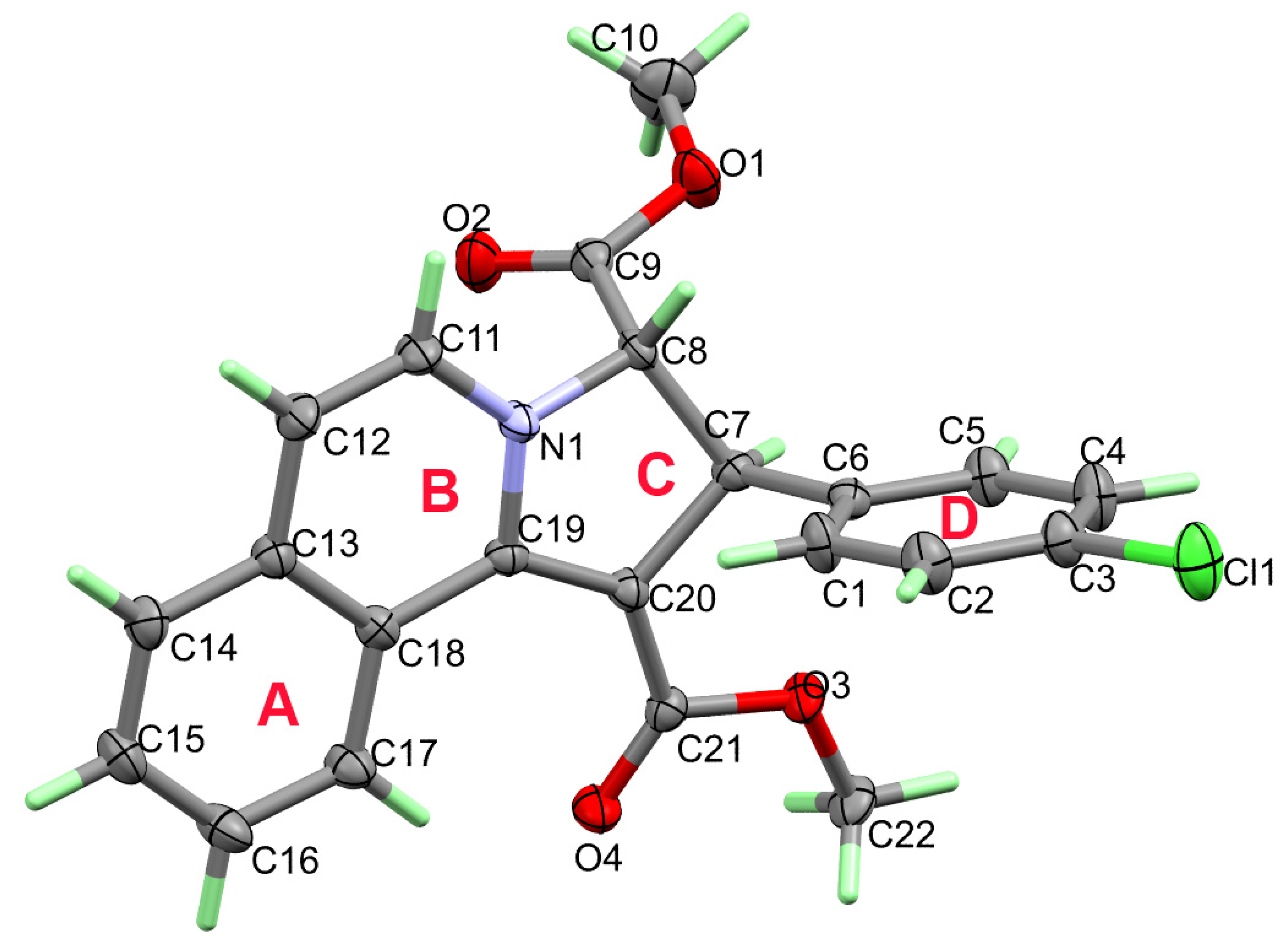
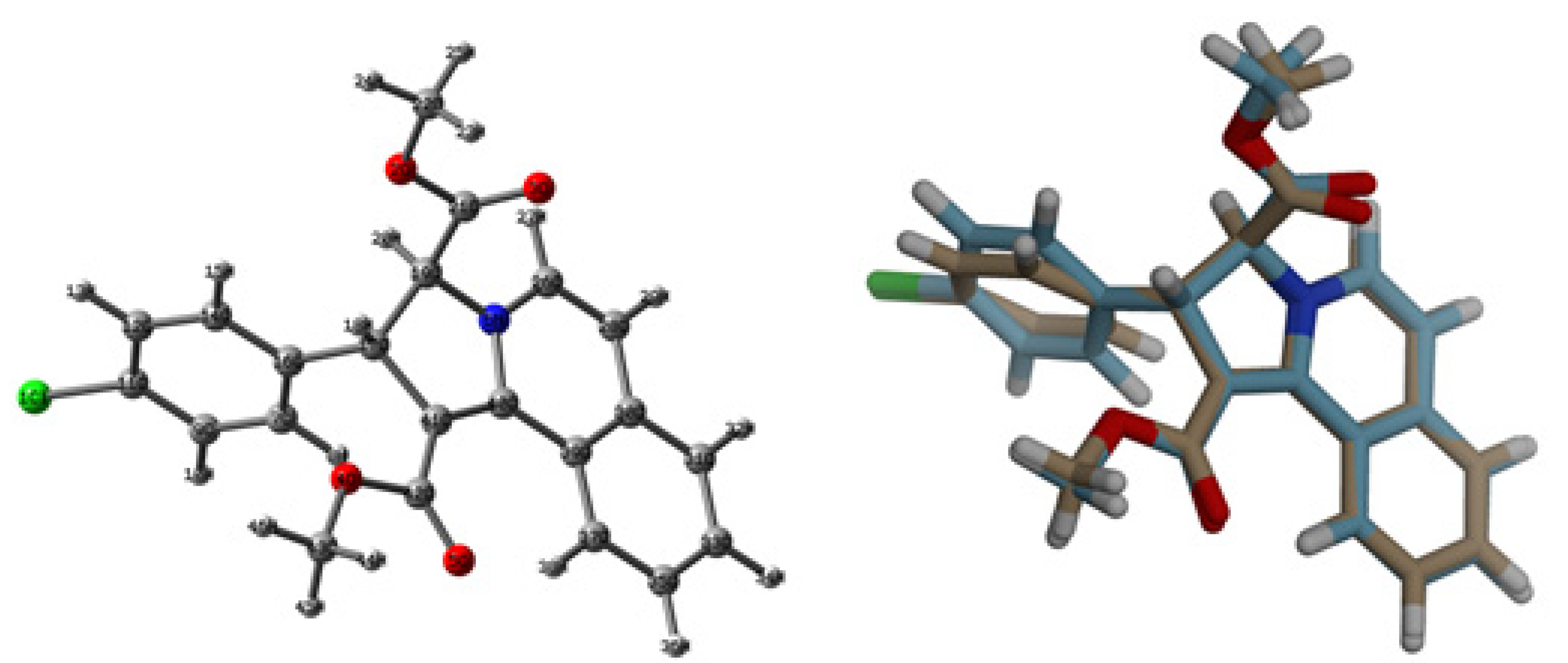
3.2. Crystal Structure Description of 3
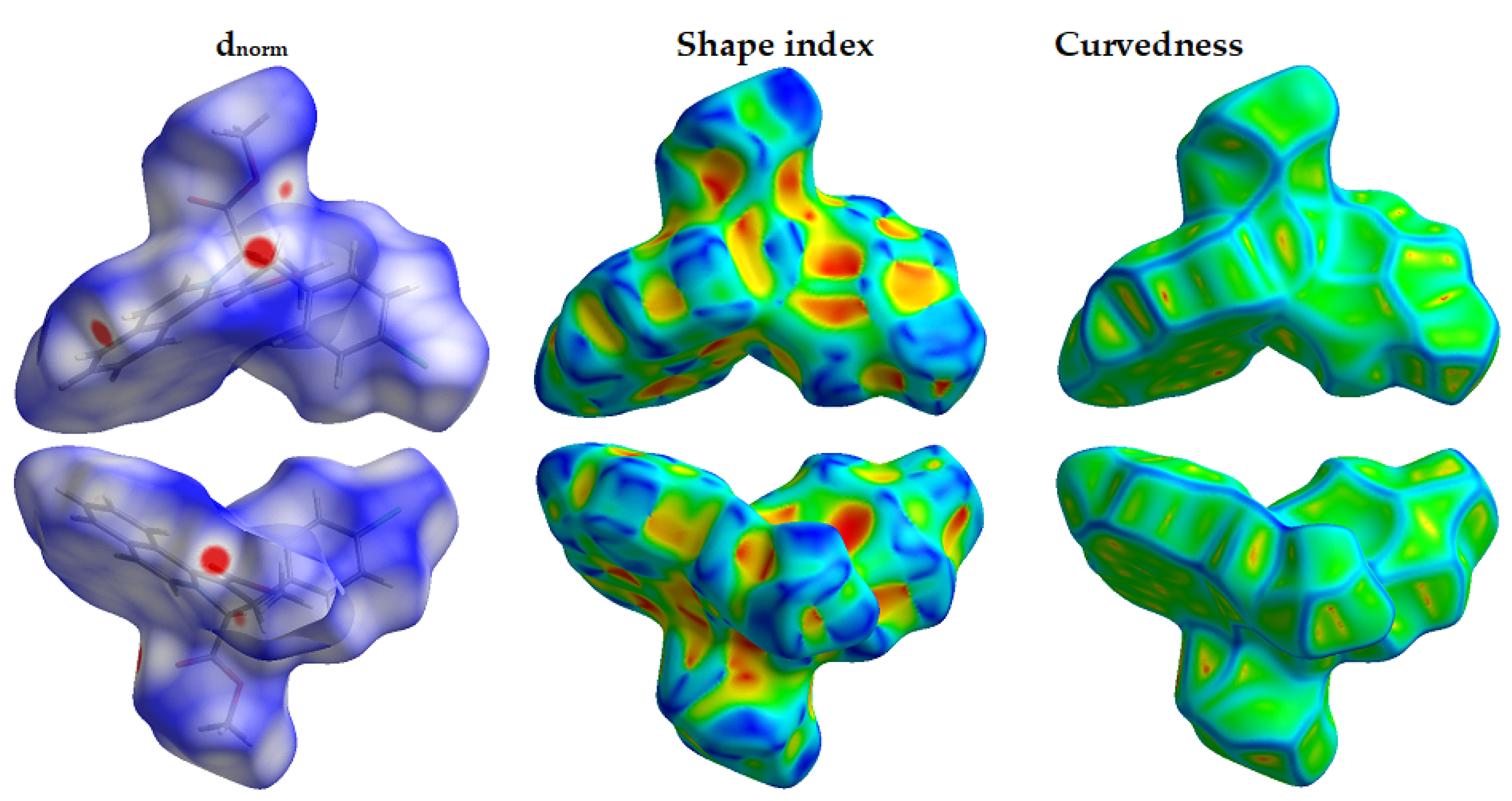
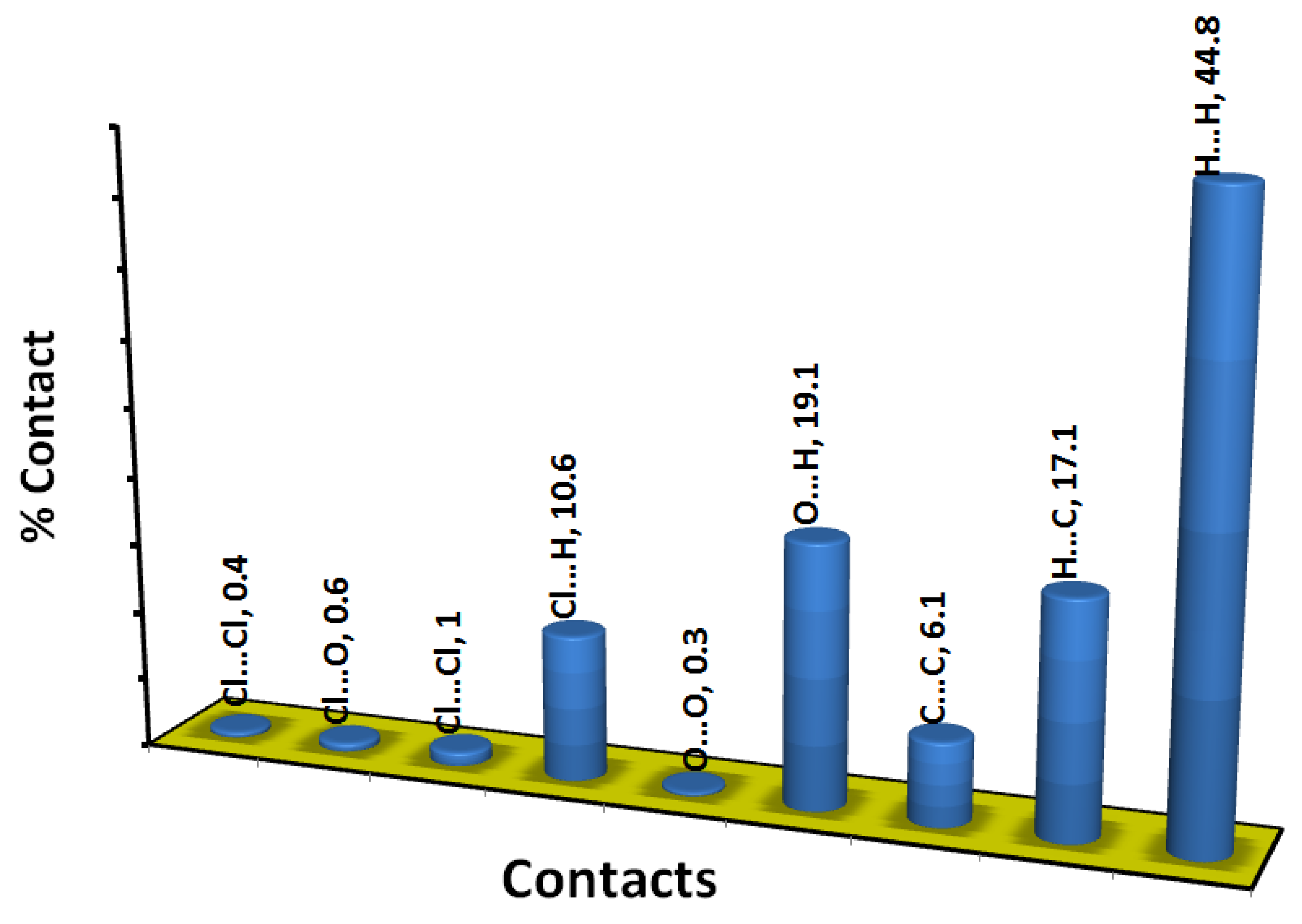
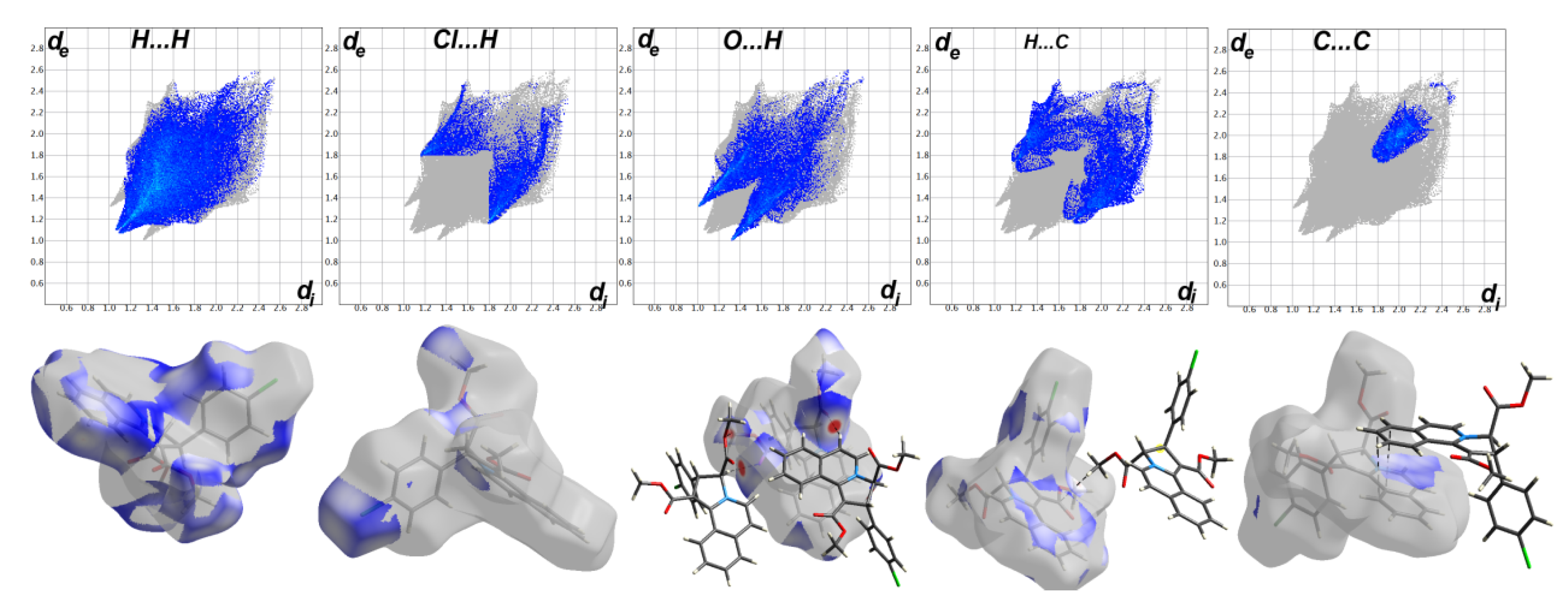
3.3. Analysis of Molecular Packing
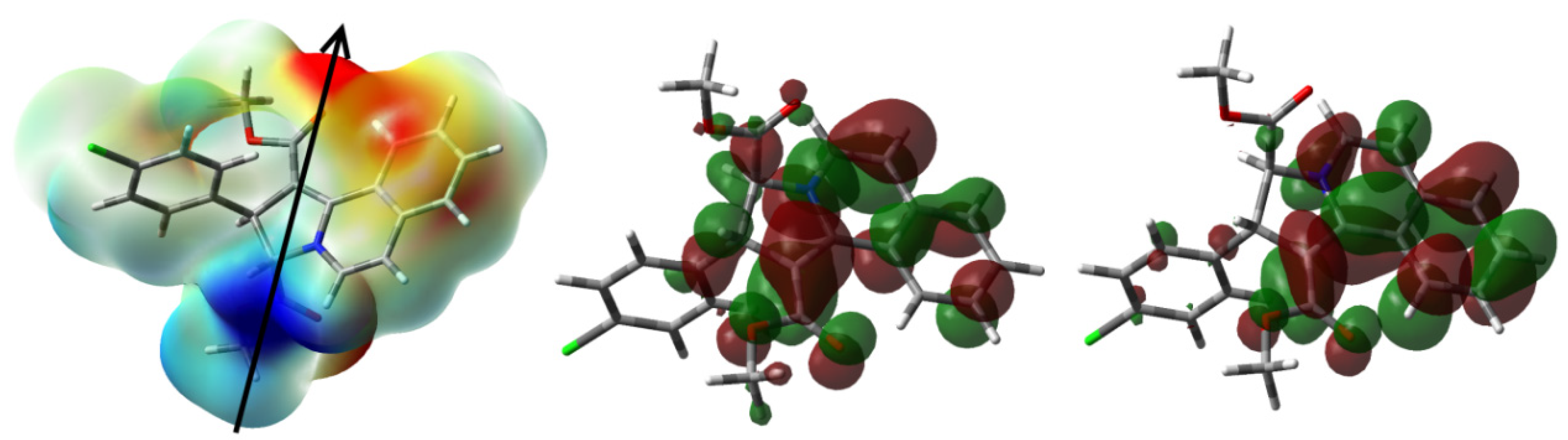
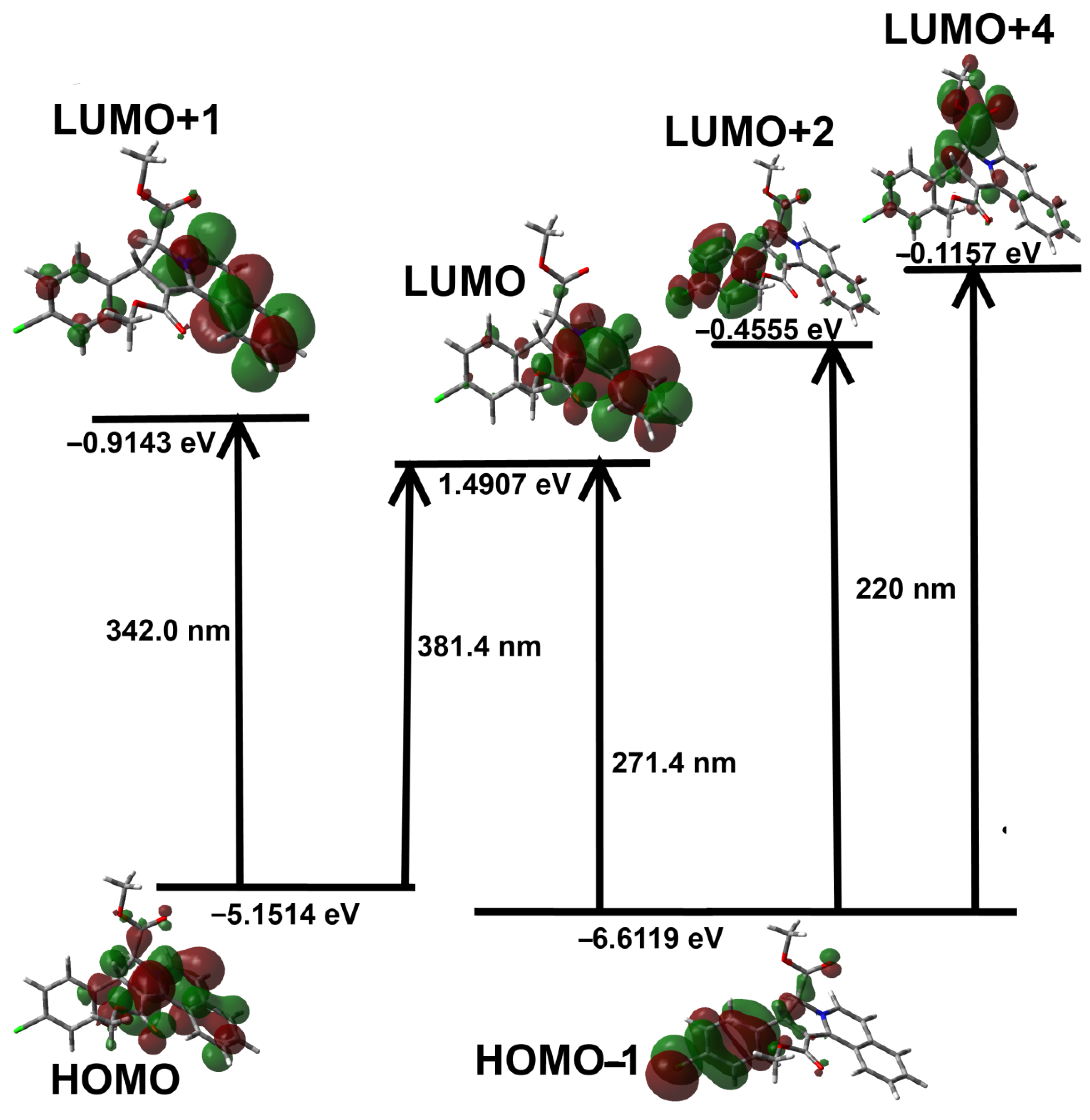
3.4. DFT Studies
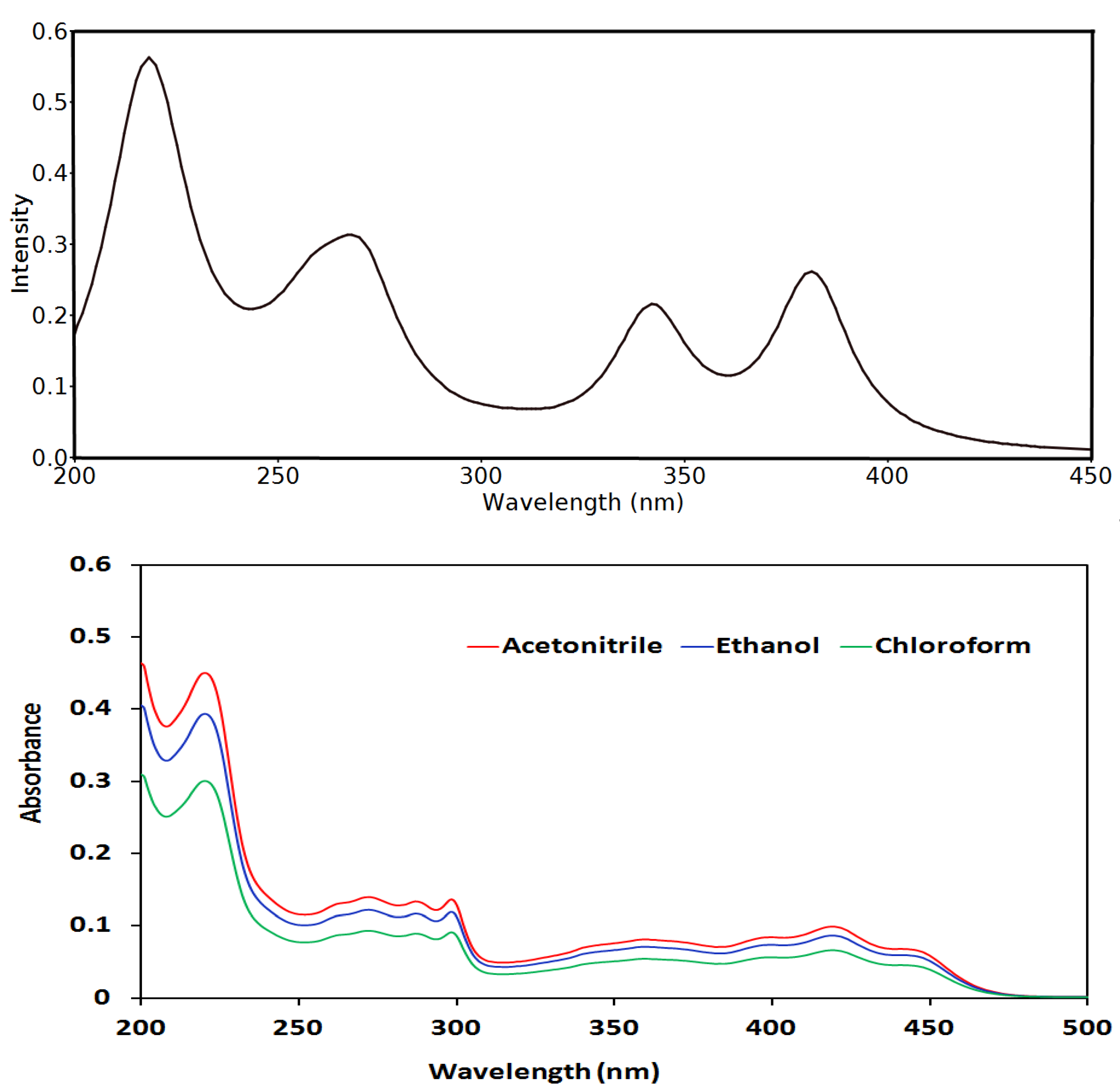
3.5. UV-Vis and NMR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roesch, E.S. Isoquinolines. In Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Brase, S., Ed.; RSC: Cambridge, UK, 2015; pp. 147–213. [Google Scholar]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- García-Castro, M.; Zimmermann, S.; Sankar, M.G.; Kumar, K. Scaffold diversity synthesis and its application in probe and drug discovery. Angew. Chem. Int. Ed. 2016, 55, 7586–7605. [Google Scholar] [CrossRef] [PubMed]
- Sau, P.; Santra, S.K.; Rakshit, A.; Patel, B.K. tert-Butyl nitrite-mediated domino synthesis of isoxazolines and isoxazoles from terminal aryl alkenes and alkynes. J. Org. Chem. 2017, 82, 6358–6365. [Google Scholar] [CrossRef]
- Gang, M.Y.; Liu, J.Q.; Wang, X.S. CuI-catalyzed Sonogashira reaction for the efficient synthesis of 1H-imidazo[2,1-a]isoquinoline derivatives. Tetrahedron 2017, 73, 4698–4705. [Google Scholar] [CrossRef]
- Shi, R.G.; Sun, J.; Yan, C.G. Tandem double [3 + 2] cycloaddition reactions at both C-1 and C-3 atoms of N-cyanomethylisoquinolinium ylide. ACS Omega 2017, 2, 7820–7830. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Villacampa, M.; Menéndez, J.C. Mild and general synthesis of pyrrolo[2,1-a]isoquinolines and related polyheterocyclic frameworks from pyrrole precursors derived from a mechanochemical multicomponent reaction. J. Org. Chem. 2017, 82, 2570–2578. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Apostu, M.O.; Mangalagiu, I.I.; Danac, R. Reactions of ethylcyanoformate with cycloimmonium salts: A direct pathway to fused or substituted azaheterocycles. Tetrahedron 2016, 72, 4230–4238. [Google Scholar] [CrossRef]
- Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. Molecular determinants of topoisomerase I poisoning by Lamellarins: Comparison with Camptothecin and structure−activity relationships. J. Med. Chem. 2005, 48, 3796–3807. [Google Scholar] [CrossRef]
- Reddy, M.V.R.; Rao, M.R.; Rhodes, D.; Hansen, M.S.T.; Rubins, K.; Bushman, F.D.; Venkateswarlu, Y.; Faulkner, D. Lamellarin α 20-sulfate, an inhibitor of HIV-1 integrase active against HIV-1 virus in cell culture. J. Med. Chem. 1999, 42, 1901–1907. [Google Scholar] [CrossRef]
- Aubry, A.; Pan, X.-S.; Fisher, L.M.; Jarlier, V.; Cambau, E. Mycobacterium tuberculosis DNA gyrase: Interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob. Agents Chemother. 2004, 48, 1281–1288. [Google Scholar] [CrossRef]
- Ploypradith, P.; Petchmanee, T.; Sahakitpichan, P.; Litvinas, N.D.; Ruchirawat, S. Total synthesis of natural and unnatural Lamellarins with saturated and unsaturated D-rings. J. Org. Chem. 2006, 71, 9440–9448. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Porco, J.A., Jr. Synthesis of pyrrolo-isoquinolines related to the lamellarins using silver-catalyzed cycloisomerization/dipolar cycloaddition. J. Am. Chem. Soc. 2007, 129, 7744–7745. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Y.; Zhang, S.; Li, H.; Wang, W. Cu (II) catalyzed oxidation-[3+ 2] cycloaddition-aromatization cascade: Efficient synthesis of pyrrolo [2,1-a] isoquinolines. Chem. Commun. 2011, 47, 1036–1038. [Google Scholar] [CrossRef]
- Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Visible-light-induced oxidation/[3 + 2] cycloaddition/oxidative aromatization sequence: A photocatalytic strategy to construct pyrrolo [2,1-a] isoquinolines. Angew. Chem. Int. Ed. 2011, 50, 7171–7175. [Google Scholar] [CrossRef]
- Dondas, H.A.; Fishwick, C.W.; Gai, X.; Grigg, R.; Kilner, C.; Dumrongchai, N.; Kongkathip, B.; Kongkathip, N.; Polysuk, C.; Sridharan, V. Stereoselective palladium-catalyzed four-component cascade synthesis of pyrrolidinyl-, pyrazolidinyl-, and isoxazolidinyl isoquinolines. Angew. Chem. Int. Ed. 2005, 44, 7570–7574. [Google Scholar] [CrossRef]
- Choi, A.; Morley, R.M.; Coldham, I. Synthesis of pyrrolo [1, 2-a] quinolines by formal 1, 3-dipolar cycloaddition reactions of quinolinium salts. Beilstein J. Org. Chem. 2019, 15, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, T.; Liu, Q.; Xu, Y.; Xie, G.; Lv, X.; Ding, S.; Wang, X.; Li, C. External oxidant-free oxidation/[3+ 2] cycloaddition/aromatization cascade: Electrochemical synthesis of polycyclic N-heterocycles. Chem. Commun. 2019, 55, 8398–8401. [Google Scholar] [CrossRef]
- Andreani, A.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Synthesis and antitubercular activity of imidazo [2, 1-b] thiazoles. Eur. J. Med. Chem. 2001, 36, 743–746. [Google Scholar] [CrossRef]
- Maglich, J.M.; Parks, D.J.; Moore, L.B.; Collins, J.L.; Goodwin, B.; Billin, A.N.; Stoltz, C.A.; Kliewer, S.A.; Lambert, M.H.; Willson, T.M.; et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 2003, 278, 17277–17283. [Google Scholar] [CrossRef]
- Hozien, Z.A.; Abd El-Wareth, A.S.; El-Sherief, H.A.; Mahmoud, A.M. An efficient route for synthesis of 5, 6-diphenylimidazo-[2,1-b] thiazoles as antibacterial agents. J. Heterocycl. Chem. 2000, 37, 943–949. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2–p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Biol. Sci. 2020, 27, 1208–1216. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H. Spiroindolone analogues as potential hypoglycemic with dual inhibitory activity on α-amylase and α-glucosidase. Molecules 2019, 24, 2342. [Google Scholar] [CrossRef]
- Islam, M.S.; Ghawas, H.M.; El-Senduny, F.F.; Al-Majid, A.M.; Elshaier, Y.A.; Badria, F.A.; Barakat, A. Synthesis of new thiazolo-pyrrolidine-(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorg. Chem. 2019, 82, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Al Majid, A.M.; Ghawas, H.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335. [Google Scholar] [CrossRef]
- Lotfy, G.; Aziz, Y.M.A.; Said, M.M.; El Sayed, H.; El Sayed, H.; Abu-Serie, M.M.; Teleb, M.; Dömling, A.; Barakat, A. Molecular hybridization design and synthesis of novel spirooxindole-based MDM2 inhibitors endowed with BCL2 signaling attenuation; a step towards the next generation p53 activators. Bioorg. Chem. 2021, 117, 105427. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Soliman, S.M.; Al-majid, A.M.; Ali, M.; Islam, M.S.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Regioselective synthesis of novel spiro-oxindole constructed with pyrrolidine/thioxothiazolidin-4-one derivatives: X-ray crystal structures, Hirshfeld surface analysis, DFT, docking and antimicrobial studies. J. Mol. Struc. 2018, 1152, 101–114. [Google Scholar] [CrossRef]
- Aziz, Y.M.A.; Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Soliman, S.M.; Abu-Serie, M.M.; Teleb, M.; Yousuf, S.; Dömling, A.; et al. Design, synthesis, chemical and biochemical insights into novel hybrid spirooxindole-based p53-MDM2 inhibitors with potential Bcl2 signaling attenuation. Front. Chem. 2021, 9, 735236. [Google Scholar] [CrossRef]
- Al-Majid, A.M.; Soliman, S.M.; Haukka, M.; Ali, M.; Islam, M.S.; Shaik, M.R.; Barakat, A. Design, construction, and characterization of a new regioisomer and diastereomer material based on the spirooxindole scaffold incorporating a sulphone function. Symmetry 2020, 12, 1337. [Google Scholar] [CrossRef]
- Mustafa, A.; Ali, M.I.; Abou-State, M.A.; Hammam, A.E.G. Reactions with 4, 5-disubstituted 2-mercaptoimidazoles and their derivatives. J. Für Prakt. Chem. 1972, 314, 785–792. [Google Scholar] [CrossRef]
- Gherasim, C.; Airinei, A.; Tigoianu, R.; Craciun, A.M.; Danac, R.; Nicolescu, A.; Isac, D.L.; Mangalagiu, I.I. Synthesis and photophysical insights of new fused N-heterocyclic derivatives with isoquinoline skeleton. J. Mol. Liq. 2020, 310, 113196. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Yarnton, Oxfordshire, UK, 2018. [Google Scholar]
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17. 2017, University of Western Australia: Perth, WA, Australia. Available online: http://hirshfeldsurface.net (accessed on 21 July 2017).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- GaussView; Version 4.1; Dennington, R., II; Keith, T.; Millam, J. (Eds.) Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New model for calculation of solvation free energies: Correction of self-consistent reaction field continuum dielectric theory for short-range hydrogen-bonding effects. J. Phys. Chem. 1996, 100, 11775–11788. [Google Scholar] [CrossRef]
- Tannor, D.J.; Marten, B.; Murphy, R.; Friesner, R.A.; Sitkoff, D.; Nicholls, A.; Ringnalda, M.; Goddard, W.A.; Honig, B. Accurate first principles calculation of molecular charge distributions and solvation energies from ab initio quantum mechanics and continuum dielectric theory. J. Am. Chem. Soc. 1994, 116, 11875–11882. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, Æ. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]

| Contact | 3 |
|---|---|
| CCDC | 2075387 |
| empirical formula | C22H18ClNO4 |
| fw | 395.82 |
| temp (K) | 120(2) K |
| λ (Å) | 0.71073 Å |
| cryst syst | Monoclinic |
| space group | P21/c |
| a (Å) | 7.9337(3) |
| b (Å) | 32.6514(8) |
| c (Å) | 7.7034(3) |
| β (deg) | 111.687(4) |
| V (Å3) | 1854.29(12) Å3 |
| Z | 4 |
| ρcalc (mg/m3) | 1.418 mg/m3 |
| μ (Mo Kα) (mm−1) | 0.236 mm−1 |
| No. reflns. | 16,269 |
| Unique reflns. | 4791 |
| GOOF (F2) | 1.027 |
| Rint | 0.0434 |
| R1 a (I ≥ 2σ) | 0.0477 |
| wR2 b (I ≥ 2σ) | 0.1017 |
| Bond | Length/Å | Bond | Length/Å |
|---|---|---|---|
| Cl1-C3 | 1.7425(19) | C6-C7 | 1.521(2) |
| O1-C9 | 1.338(2) | C7-C20 | 1.532(2) |
| O1-C10 | 1.455(2) | C7-C8 | 1.548(2) |
| O2-C9 | 1.194(2) | C8-C9 | 1.522(3) |
| O3-C21 | 1.370(2) | C11-C12 | 1.336(2) |
| O3-C22 | 1.436(2) | C12-C13 | 1.436(2) |
| O4-C21 | 1.223(2) | C13-C14 | 1.408(2) |
| N1-C11 | 1.376(2) | C13-C18 | 1.419(2) |
| N1-C19 | 1.382(2) | C14-C15 | 1.373(2) |
| N1-C8 | 1.457(2) | C15-C16 | 1.398(3) |
| C1-C2 | 1.388(3) | C16-C17 | 1.377(2) |
| C1-C6 | 1.389(2) | C17-C18 | 1.408(2) |
| C2-C3 | 1.385(3) | C18-C19 | 1.459(2) |
| C3-C4 | 1.379(3) | C19-C20 | 1.393(2) |
| C4-C5 | 1.392(3) | C20-C21 | 1.440(2) |
| Bonds | Angle/∲∲ | Bonds | Angle/∲∲ |
| C9-O1-C10 | 114.88(15) | O1-C9-C8 | 110.34(14) |
| C21-O3-C22 | 115.83(13) | C12-C11-N1 | 120.44(15) |
| C11-N1-C19 | 125.37(14) | C11-C12-C13 | 119.38(16) |
| C11-N1-C8 | 122.19(14) | C14-C13-C18 | 119.63(15) |
| C19-N1-C8 | 111.59(13) | C14-C13-C12 | 119.95(15) |
| C2-C1-C6 | 121.37(16) | C18-C13-C12 | 120.42(15) |
| C3-C2-C1 | 118.70(18) | C15-C14-C13 | 120.59(16) |
| C4-C3-C2 | 121.40(18) | C14-C15-C16 | 119.95(16) |
| C4-C3-Cl1 | 118.83(14) | C17-C16-C15 | 120.69(16) |
| C2-C3-Cl1 | 119.77(15) | C16-C17-C18 | 120.64(17) |
| C3-C4-C5 | 118.86 (17) | C17-C18-C13 | 118.48(15) |
| C6-C5-C4 | 121.23(17) | C17-C18-C19 | 122.55(15) |
| C5-C6-C1 | 118.43(16) | C13-C18-C19 | 118.97(14) |
| C5-C6-C7 | 119.92(16) | N1-C19-C20 | 109.02(14) |
| C1-C6-C7 | 121.62(15) | N1-C19-C18 | 115.39(14) |
| C6-C7-C20 | 114.86(14) | C20-C19-C18 | 135.58(15) |
| C6-C7-C8 | 111.81(13) | C19-C20-C21 | 131.92(15) |
| C20-C7-C8 | 101.56(13) | C19-C20-C7 | 109.13(13) |
| N1-C8-C9 | 109.27(14) | C21-C20-C7 | 118.95(14) |
| N1-C8-C7 | 103.02(13) | O4-C21-O3 | 120.31(14) |
| C9-C8-C7 | 111.47(14) | O4-C21-C20 | 130.41(16) |
| O2-C9-O1 | 124.11(18) | O3-C21-C20 | 109.25(14) |
| O2-C9-C8 | 125.55(16) |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| C8-H8…O4 1 | 1.00 | 2.40 | 3.245(2) | 142 |
| C12-H12…O2 2 | 0.95 | 2.46 | 3.346(2) | 155 |
| C17-H17…O4 | 0.95 | 2.14 | 2.980(2) | 147 |
| C22-H22A…O1 3 | 0.98 | 2.59 | 3.520(2) | 158 |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| O2…H12 1 | 2.343 | C17…C15 4a | 3.644 |
| O4…H8 2 | 2.332 | C18…C14 4a | 3.559 |
| O1…H22A 3 | 2.499 | C21…H10C 5a | 2.879 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Soliman, S.M.; Haukka, M.; Al-Shaalan, N.H.; Alkharboush, A.A.; Barakat, A. Synthesis of Unexpected Dimethyl 2-(4-Chlorophenyl)-2,3-dihydropyrrolo[2,1-a]isoquinoline-1,3-dicarboxylate via Hydrolysis/Cycloaddition/Elimination Cascades: Single Crystal X-ray and Chemical Structure Insights. Crystals 2022, 12, 6. https://doi.org/10.3390/cryst12010006
Altowyan MS, Soliman SM, Haukka M, Al-Shaalan NH, Alkharboush AA, Barakat A. Synthesis of Unexpected Dimethyl 2-(4-Chlorophenyl)-2,3-dihydropyrrolo[2,1-a]isoquinoline-1,3-dicarboxylate via Hydrolysis/Cycloaddition/Elimination Cascades: Single Crystal X-ray and Chemical Structure Insights. Crystals. 2022; 12(1):6. https://doi.org/10.3390/cryst12010006
Chicago/Turabian StyleAltowyan, Mezna Saleh, Saied M. Soliman, Matti Haukka, Nora H. Al-Shaalan, Aminah A. Alkharboush, and Assem Barakat. 2022. "Synthesis of Unexpected Dimethyl 2-(4-Chlorophenyl)-2,3-dihydropyrrolo[2,1-a]isoquinoline-1,3-dicarboxylate via Hydrolysis/Cycloaddition/Elimination Cascades: Single Crystal X-ray and Chemical Structure Insights" Crystals 12, no. 1: 6. https://doi.org/10.3390/cryst12010006
APA StyleAltowyan, M. S., Soliman, S. M., Haukka, M., Al-Shaalan, N. H., Alkharboush, A. A., & Barakat, A. (2022). Synthesis of Unexpected Dimethyl 2-(4-Chlorophenyl)-2,3-dihydropyrrolo[2,1-a]isoquinoline-1,3-dicarboxylate via Hydrolysis/Cycloaddition/Elimination Cascades: Single Crystal X-ray and Chemical Structure Insights. Crystals, 12(1), 6. https://doi.org/10.3390/cryst12010006










