Effect of Thiourea Containing Composite Additives on Nickel Electrodeposition in Ammoniacal Solution
Abstract
:1. Introduction
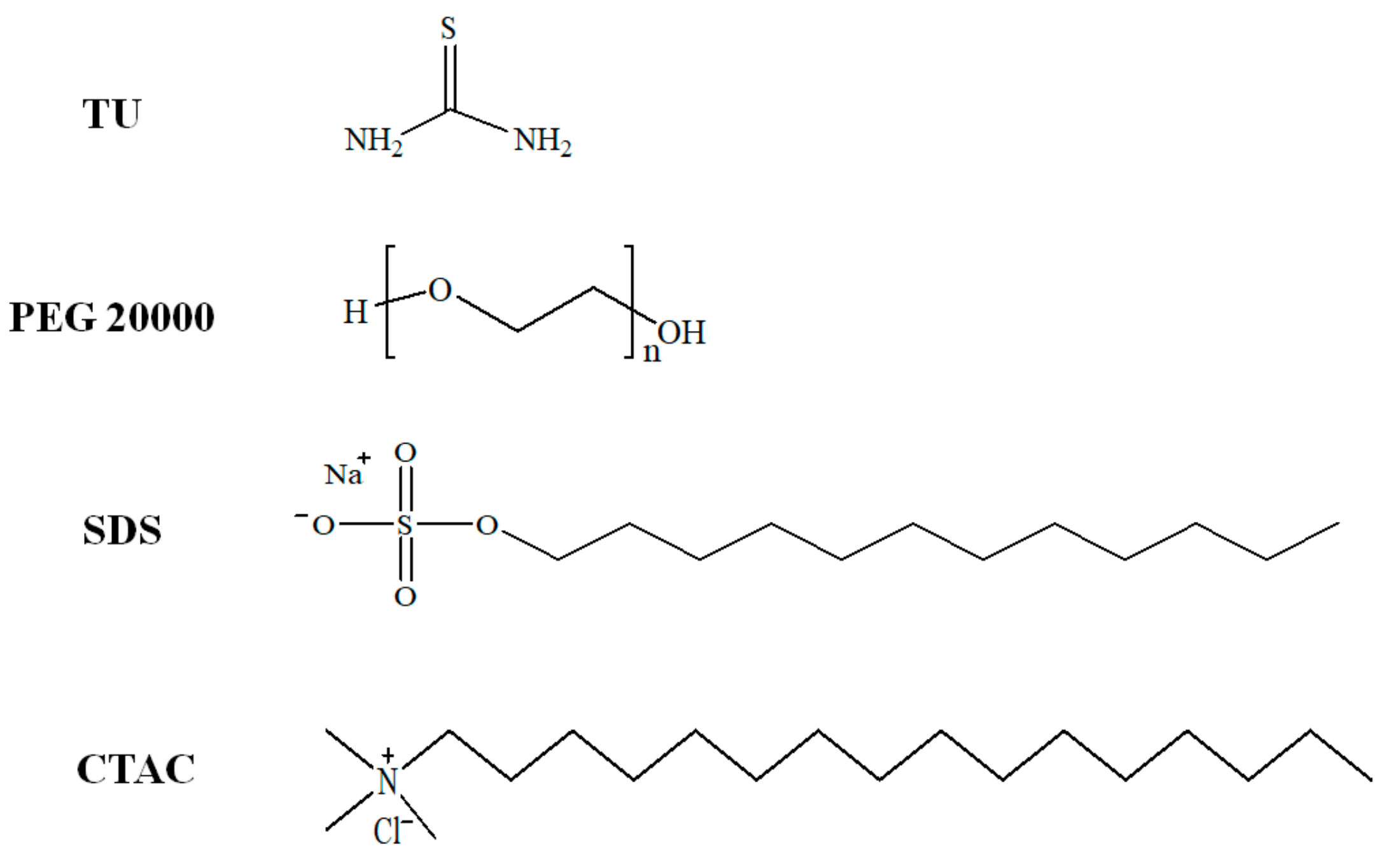
2. Materials and Methods
3. Results and Discussion
3.1. Cyclic Voltammetric Behavior
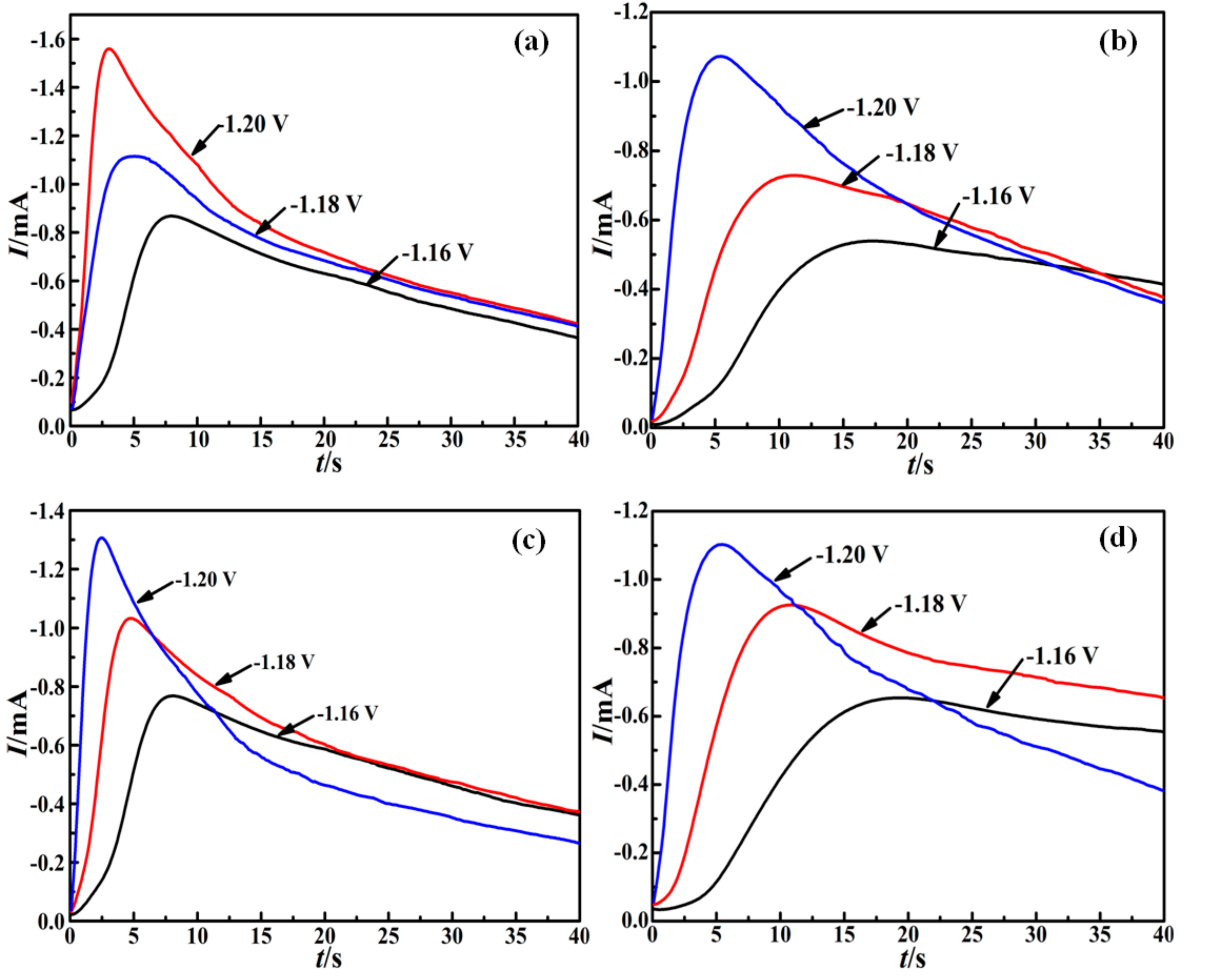
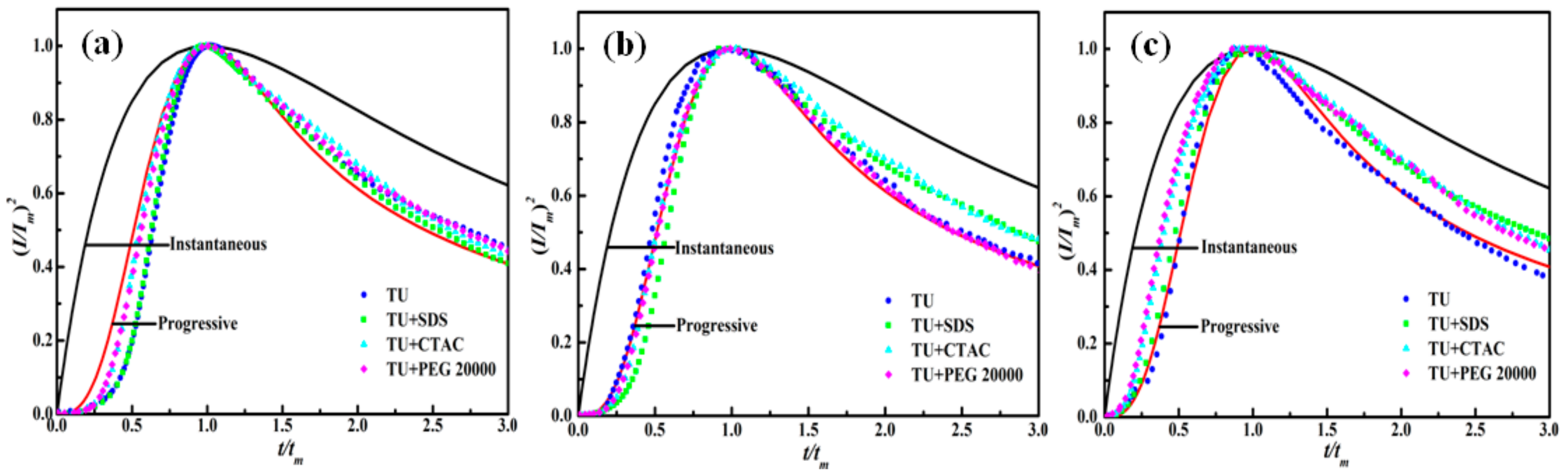
3.2. Nucleation and Growth Mechanism
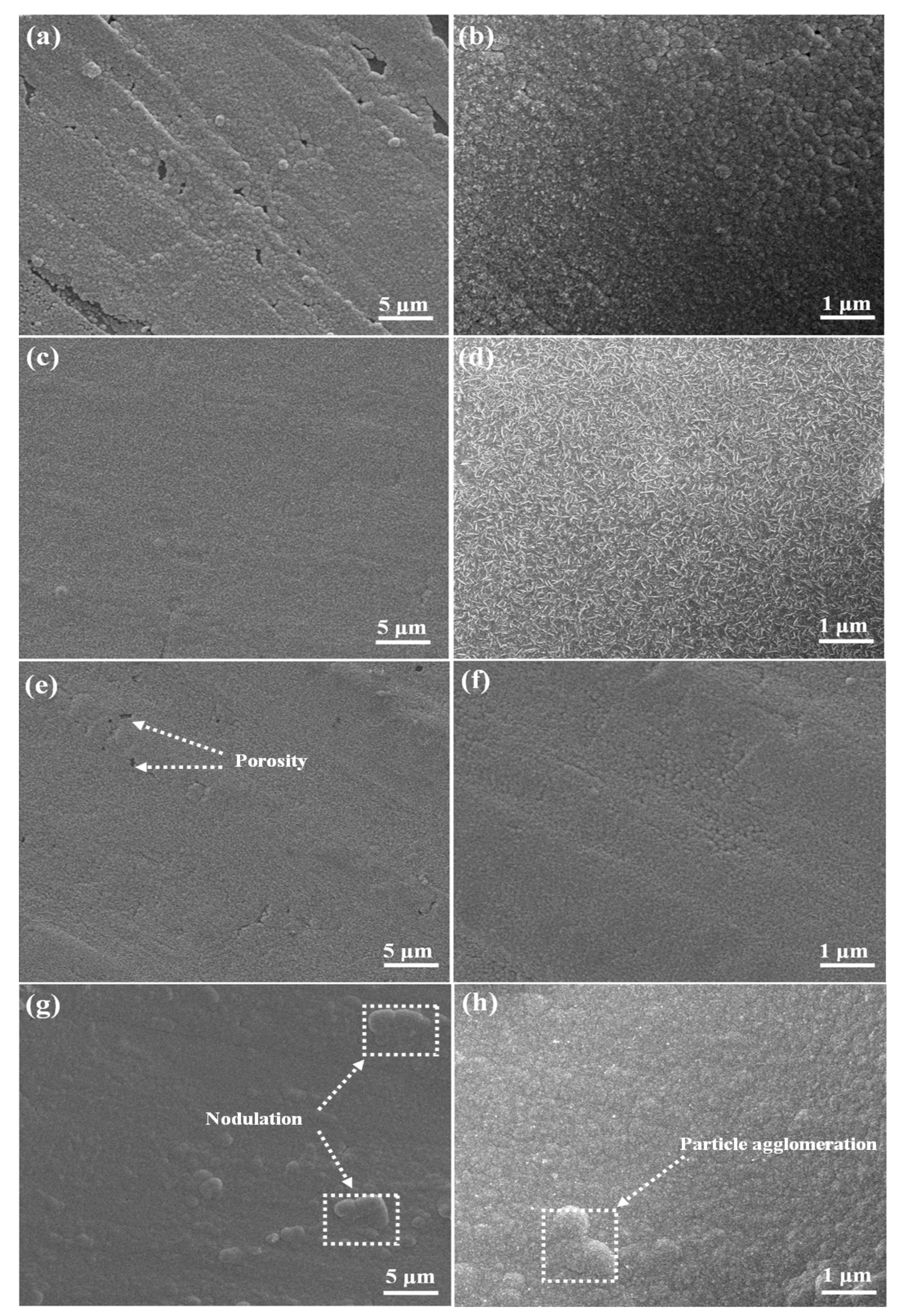
3.3. Deposit Morphology
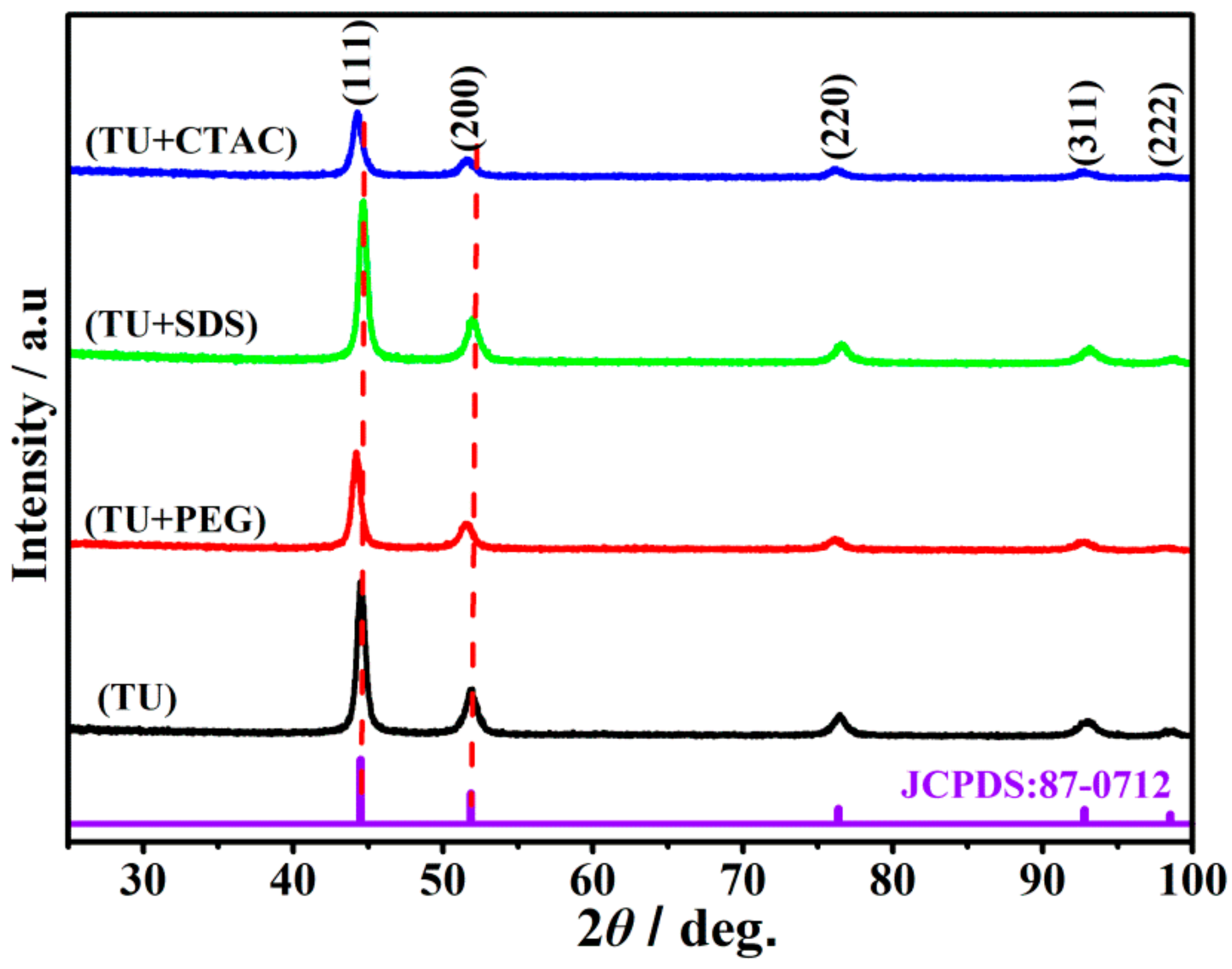
3.4. Crystallographic Orientations
4. Conclusions
- (1)
- The introduction of composite additives resulted in a stronger cathodic polarization and increased the nucleation overpotential significantly.
- (2)
- The reduction in nickel followed the 3D progressive nucleation mechanism in the presence of composite additives at the step potential of −1.16 V and −1.18 V but became ambiguous when thiourea was used in combination with CTAC or PEG20000 at the potential of −1.20 V.
- (3)
- The composite additives had an effect on forming compact and smooth nickel deposits. The introduction of CTAC to the thiourea containing electrolyte resulted in nickel particles changing from needle-like to spherical.
- (4)
- All nickel deposits had the face-centered cubic structure. The diffraction peaks of (111) and (200) crystal planes were slightly shifted toward lower 2θ values when thiourea was used in combination with additive CTAC or PEG20000.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.P.; Cao, H.Z.; Zheng, G.Q.; Hou, G.Y.; Wu, L.K. Electrodeposition of Bright Nickel from Liquid Ammonia Solution Containing Chloride. J. Electrochem. Soc. 2016, 163, D829–D835. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Tripathy, B.C.; Das, S.C.; Singh, P.; Misra, V.N. Effect of sodium lauryl sulphate on nickel electrowinning from acidic sulphate solutions. Hydrometallurgy 2009, 100, 60–64. [Google Scholar] [CrossRef]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. The effects of stabilizers on the bath stability of electroless Ni deposition and the deposit. Appl. Surf. Sci. 2004, 229, 282–300. [Google Scholar] [CrossRef]
- Lin, K.-L.; Hwang, J.-W. Effect of thiourea and lead acetate on the deposition of electroless nickel. Mater. Chem. Phys. 2002, 76, 204–211. [Google Scholar] [CrossRef]
- Tseluikin, V.N.; Vasilenko, E.A. Electrodeposition and properties of composite coatings based on nickel. Russ. J. Appl. Chem. 2011, 84, 2005–2007. [Google Scholar] [CrossRef]
- Gyawali, G.; Joshi, B.; Tripathi, K.; Lee, S.W. Effect of ultrasonic nanocrystal surface modification on properties of electrodeposited Ni and Ni-SiC composite coatings. J. Mater. Eng. Perform. 2017, 26, 4462–4469. [Google Scholar] [CrossRef]
- Lanzutti, A.; Lekka, M.; de Leitenburg, C.; Fedrizzi, L. Effect of pulse current on wear behavior of Ni matrix micro-and nano-SiC composite coatings at room and elevated temperature. Tribol. Int. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Antihovich, I.V. Electrodeposition of nickel and composite nickel-fullerenol coatings from low-temperature sulphate-chloride-isobutyrate electrolyte. Procedia Chem. 2014, 10, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Bhale, J.; Mishra, A.; Malviya, P. Synthesis and X-ray diffraction study of some nickel(II) complexes of urea and thiourea. J. Phys. 2014, 534, 012044. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-L.; Katayama, Y.; Miura, T. Effects of acetone and thiourea on electrodeposition of Ni from a hydrophobic ionic liquid. Electrochimica Acta 2012, 85, 622–627. [Google Scholar] [CrossRef]
- Jing, L.U.; Yang, Q.H.; Zhang, Z. Effects of additives on nickel electrowinning from sulfate system. Trans. Nonferrous Met. Soc. China 2010, 20, 97–101. [Google Scholar]
- Jin, Y.; Hongying, Y.U.; Yang, D.; Sun, D. Effects of complexing agents on acidic electroless nickel deposition. Rare Met. 2010, 29, 401–406. [Google Scholar] [CrossRef]
- Rudnik, E.; Wojnicki, M.; Włoch, G. Effect of gluconate addition on the electrodeposition of nickel from acidic baths. Surf. Coatings Technol. 2012, 207, 375–388. [Google Scholar] [CrossRef]
- Srinivasan, R.; Bapu, G.N.K.R. Effect of additives on electrodeposition of nickel from acetate bath: Cyclic voltammetric study. Trans. Inst. Met. Finish. 2013, 91, 52–56. [Google Scholar] [CrossRef]
- Loto, C.A.; Loto, R.T. Effect of dextrin and thiourea additives on the Zinc electroplated mild steel in acid chloride solution. Int. J. Electrochem. Sci. 2013, 8, 12434–12450. [Google Scholar]
- Cao, H.; Di, Y.; Zhu, S.; Li, D.; Zheng, G. Preparation, characterization, and electrochemical studies of sulfur-bearing nickel in an ammoniacal electrolyte: The influence of thiourea. J. Solid State Electrochem. 2012, 16, 3115–3122. [Google Scholar] [CrossRef]
- Borikar, D.K.; Umare, S.S.; Viswanath, S.G. Electrowinning of nickel from ammonical sulphate bath and effect of aceton on morphology of nickel deposit and its correlation with kinetic parameters. Metalurgija 2006, 45, 3–8. [Google Scholar]
- Jović, V.D.; Jović, B.M.; Pavlović, M.G. Morphology and composition of Ni-Co alloy powders electrodeposited from ammoniacal electrolyte. J. Solid State Electrochem. 2006, 10, 959–966. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Reaction and nucleation mechanisms of copper electrodeposition from ammoniacal solutions on vitreous carbon. Electrochimica Acta 2005, 50, 4426–4443. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrochemical and AFM study of cobalt nucleation mechanisms on glassy carbon from ammonium sulfate solutions. Electrochimica Acta 2004, 49, 4719–4732. [Google Scholar] [CrossRef]
- Burlyaev, D.V.; Tinaeva, A.E.; Tinaeva, K.E. Electrodeposition of Zinc–Nickel Coatings from Glycine-Containing Ammonium-Chloride Electrolyte. Prot. Met. Phys. Chem. Surfaces 2020, 56, 552–559. [Google Scholar] [CrossRef]
- Mech, K.; Żabiński, P.; Kowalik, R.; Tokarski, T.; Fitzner, K. Electrodeposition of Co–Pd alloys from ammonia solutions and their catalytic activity for hydrogen evolution reaction. J. Appl. Electrochem. 2014, 44, 97–103. [Google Scholar] [CrossRef]
- Mech, K.; Żabiński, P.; Kowalik, R.; Fitzner, K. Analysis of Co–Pd alloys deposition from electrolytes based on [Co(NH3)6]3+ and [Pd(NH3)4]2+ complexes. Electrochimica Acta 2013, 104, 468–473. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Bondarenko, Z.V.; Pshenichkina, T.V.; Morozova, N.V.; Kudryavtsev, V.N. Electrodeposition of a cobalt-molybdenum alloy from an ammonia-citrate electrolyte. Russ. J. Electrochem. 2007, 43, 349–354. [Google Scholar] [CrossRef]
- Dergacheva, M.B.; Statsyuk, V.N.; Fogel, L.A. Electrodeposition of CdTe from ammonia-chloride buffer electrolytes. J. Electroanal. Chem. 2005, 579, 43–49. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, J.G.; Ding, Z.Y.; Liu, Z.J. Electrochemical deposition of bright nickel on titanium matrix from ammoniacal solution in the presence of thiourea. Int. J. Electrochem. Sci. 2017, 12, 7312–7325. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, J.G.; Chang, G.; Tang, J.; Ji, X.B.; Liu, Z.J. The Contribution of Heteroatoms in Amide Derivatives with an Identical Structure on Nickel Electrodeposits. J. Electrochem. Soc. 2019, 166, D381–D388. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X. Effects of [HMIM]HSO4 and [OMIM]HSO4 on the electrodeposition of zinc from sulfate electrolytes. J. Appl. Electrochem. 2009, 39, 1185–1192. [Google Scholar] [CrossRef]
- Rooij, D. Electrochemical methods: Fundamentals and applications. J. Chem. Educ. 2003, 50, A25–A26. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochimica Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Scharifker, B.R.; Mostany, J.; Palomar, P.M. On the Theory of the Potentiostatic Current Transient for Diffusion-Controlled Three-Dimensional Electrocrystallization Processes. J. Electrochem. Soc. 1999, 146, 1005–1012. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, Z.-Y.; Liu, S.-J.; He, Y.-N. Cathodic process of nickel electrodeposition from ammonia–ammonium chloride solutions. Rare Met. 2017, 1–6. [Google Scholar] [CrossRef]
- Song, Y.; Tang, J.; Hu, J.; Liu, S.; Fu, Y.; Ji, X. Insights into electrodeposition process of nickel from ammonium chloride media with speciation analysis and in situ synchrotron radiation X-ray imaging. Electrochimica Acta 2016, 210, 812–820. [Google Scholar] [CrossRef]
- Zhao, J.M.; Liu, H.X.; Di, W. The inhibition synergistic effect between imidazoline derivative and thiourea. Electrochemistry 2004, 10, 440–445. [Google Scholar]
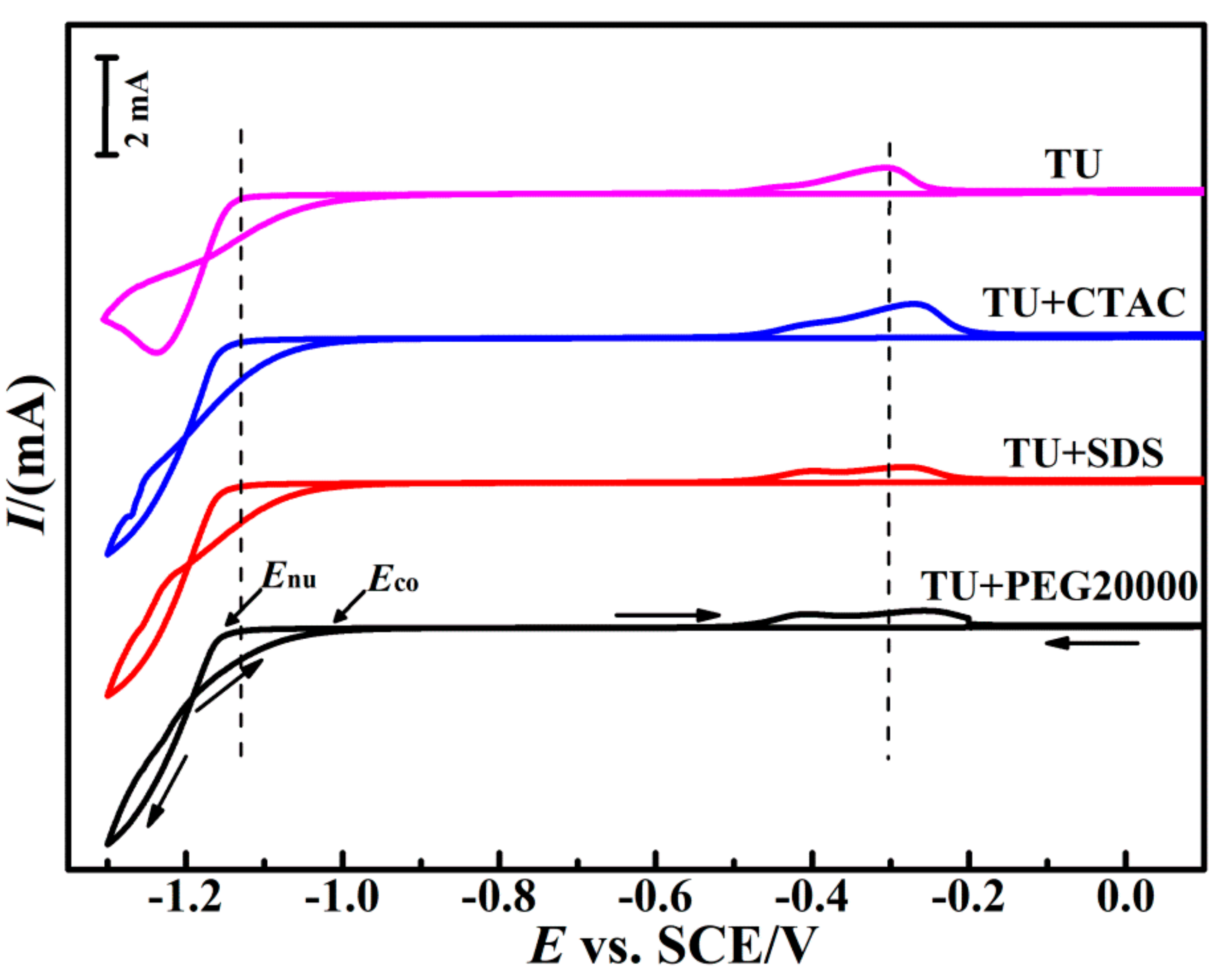
| Additive Composition | −Eco (mV) | −Enu (mV) | NOP (mV) |
|---|---|---|---|
| TU | 995 | 1163 | 168 |
| TU + CTAC | 986 | 1181 | 195 |
| TU + SDS | 1002 | 1186 | 184 |
| TU + PEG20000 | 994 | 1182 | 188 |
| Additive Composition | E V | tm s | Im mA/cm2 | tm mA2/(cm4·s) | 109D cm2/s |
|---|---|---|---|---|---|
| TU | −1.16 | 7.8 | 0.87 | 5.90 | 1.99 |
| −1.18 | 5.1 | 1.11 | 6.28 | 2.12 | |
| −1.20 | 3.2 | 1.56 | 7.79 | 2.63 | |
| TU + CTAC | −1.16 | 17.6 | 0.54 | 5.13 | 1.73 |
| −1.18 | 11.1 | 0.73 | 5.92 | 2.00 | |
| −1.20 | 5.5 | 1.07 | 6.30 | 2.12 | |
| TU + SDS | −1.16 | 8.2 | 0.77 | 4.86 | 1.64 |
| −1.18 | 4.8 | 1.03 | 5.09 | 1.72 | |
| −1.20 | 2.6 | 1.31 | 4.46 | 1.50 | |
| TU + PEG 20000 | −1.16 | 19.3 | 0.65 | 8.15 | 2.75 |
| −1.18 | 10.9 | 0.93 | 9.43 | 3.18 | |
| −1.20 | 5.8 | 1.10 | 7.02 | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Chen, J.; Zhang, J.; Sun, L. Effect of Thiourea Containing Composite Additives on Nickel Electrodeposition in Ammoniacal Solution. Crystals 2022, 12, 43. https://doi.org/10.3390/cryst12010043
Yuan L, Chen J, Zhang J, Sun L. Effect of Thiourea Containing Composite Additives on Nickel Electrodeposition in Ammoniacal Solution. Crystals. 2022; 12(1):43. https://doi.org/10.3390/cryst12010043
Chicago/Turabian StyleYuan, Liang, Jinhong Chen, Jingxiang Zhang, and Lingna Sun. 2022. "Effect of Thiourea Containing Composite Additives on Nickel Electrodeposition in Ammoniacal Solution" Crystals 12, no. 1: 43. https://doi.org/10.3390/cryst12010043
APA StyleYuan, L., Chen, J., Zhang, J., & Sun, L. (2022). Effect of Thiourea Containing Composite Additives on Nickel Electrodeposition in Ammoniacal Solution. Crystals, 12(1), 43. https://doi.org/10.3390/cryst12010043





