Using DFT to Calculate the Parameters of the Crystal Field in Mn2+ Doped Hydroxyapatite Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Calculation of Electron Density
2.2. Calculation of the Parameters of the Electric Crystal Field
2.3. HAp Samples and EPR Technique
3. Results
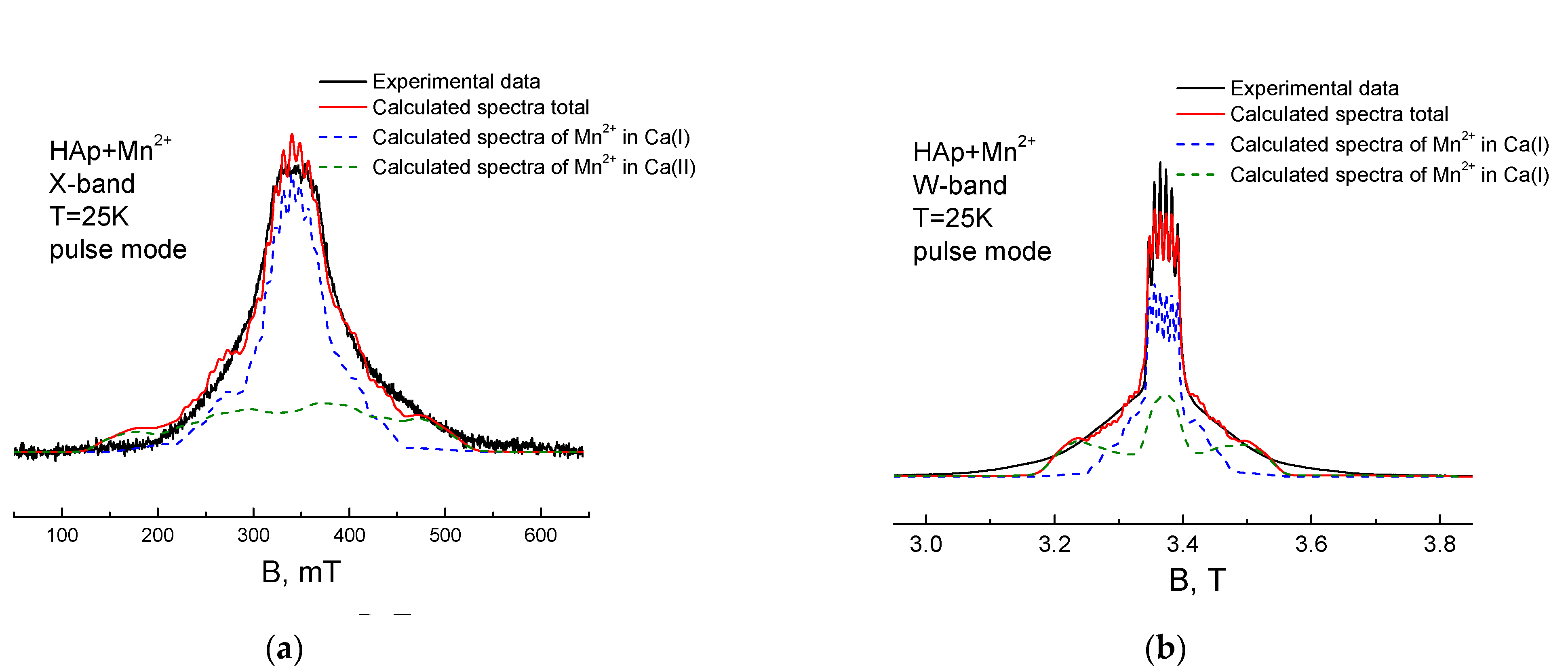
Modeling of “Powder” EPR Spectra of Mn2+
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Silva, L.M.D.; Tavares, D.D.S.; Santos, E.A.D. Isolating the Effects of Mg2+, Mn2+ and Sr2+ Ions on Osteoblast Behavior from those Caused by Hydroxyapatite Transformation. Mater. Res. 2020, 23. [Google Scholar] [CrossRef]
- Matsunaga, K. First-principles study of substitutional magnesium and zinc in hydroxyapatite and octacalcium phosphate. J. Chem. Phys. 2008, 128, 245101. [Google Scholar] [CrossRef] [PubMed]
- Gafurov, M.; Biktagirov, T.; Yavkin, B.; Mamin, G.; Filippov, Y.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Nitrogen-containing species in the structure of the synthesized nano-hydroxyapatite. JETP Lett. 2014, 99, 196–203. [Google Scholar] [CrossRef][Green Version]
- Medvecký, Ľ.; Štulajterová, R.; Parilák, Ľ.; Trpčevská, J.; Ďurišin, J.; Barinov, S. Influence of manganese on stability and particle growth of hydroxyapatite in simulated body fluid. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 281, 221–229. [Google Scholar] [CrossRef]
- Li, Y.; Widodo, J.; Lim, S.; Ooi, C.P. Synthesis and cytocompatibility of manganese (II) and iron (III) substituted hydroxyap-atite nanoparticles. J. Mater. Sci. 2012, 47, 754–763. [Google Scholar] [CrossRef]
- Rau, J.V.; Fadeeva, I.V.; Fomin, A.S.; Barbaro, K.; Galvano, E.; Ryzhov, A.P.; Murzakhanov, F.; Gafurov, M.; Orlinskii, S.; Antoniac, I.V.; et al. Sic Parvis Magna: Manganese-Substituted Tricalcium Phosphate and Its Biophysical Properties. ACS Biomater. Sci. Eng. 2019, 5, 6632–6644. [Google Scholar] [CrossRef]
- Fadeeva, I.; Kalita, V.; Komlev, D.; Radiuk, A.; Fomin, A.; Davidova, G.; Fursova, N.; Murzakhanov, F.; Gafurov, M.; Fosca, M.; et al. In Vitro Properties of Manganese-Substituted Tricalcium Phosphate Coatings for Titanium Biomedical Implants Deposited by Arc Plasma. Materials 2020, 13, 4411. [Google Scholar] [CrossRef]
- Gabbasov, B.; Gafurov, M.; Starshova, A.; Shurtakova, D.; Murzakhanov, F.; Mamin, G.; Orlinskii, S. Conventional, pulsed and high-field electron paramagnetic resonance for studying metal impurities in calcium phosphates of biogenic and synthetic origins. J. Magn. Magn. Mater. 2019, 470, 109–117. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef]
- Liu, H.; Cui, X.; Lu, X.; Liu, X.; Zhang, L.; Chan, T.-S. Mechanism of Mn incorporation into hydroxyapatite: Insights from SR-XRD, Raman, XAS, and DFT calculation. Chem. Geol. 2021, 579, 120354. [Google Scholar] [CrossRef]
- Chelyshev, Y.; Gafurov, M.; Ignatyev, I.; Zanochkin, A.; Mamin, G.; Sorokin, B.; Sorokina, A.; Lyapkalo, N.; Gizatullina, N.; Mukhamedshina, Y.; et al. Paramagnetic Manganese in the Atherosclerotic Plaque of Carotid Arteries. BioMed. Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal Structure of Hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef]
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Klimashina, E.; Putlayev, V.; Kuznetsova, L.; Orlinskii, S. The Interplay of manganese and nitrate in hydroxyapatite nanoparticles as revealed by pulsed EPR and DFT. Phys. Chem. Chem. Phys. 2015, 17, 20331–20337. [Google Scholar] [CrossRef] [PubMed]
- Ignatyev, I.M.; Gafurov, M.R.; Krivosheeva, N.V. Criteria for Carotid Atherosclerotic Plaque Instability. Ann. Vasc. Surg. 2021, 72, 340–349. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Grishin, P.O.; Goldberg, M.A.; Yavkin, B.V.; Mamin, G.V.; Orlinskii, S.B.; Fedotov, A.Y.; Petrakova, N.V.; Antuzevics, A.; Gafurov, M.R.; et al. Radiation-Induced Stable Radicals in Calcium Phosphates: Results of Multifrequency EPR, EDNMR, ESEEM, and ENDOR Studies. Appl. Sci. 2021, 11, 7727. [Google Scholar] [CrossRef]
- Kooser, R.G.; Kirchmann, E.; Matkov, T. Measurements of spin concentration in electron paramagnetic resonance spectros-copy preparation of standard solutions from optical absorption. Concepts Magn. Reson. 1992, 4, 145–152. [Google Scholar] [CrossRef]
- Reviakine, R.; Arbuznikov, A.V.; Tremblay, J.-C.; Remenyi, C.; Malkina, O.L.; Malkin, V.G.; Kaupp, M. Calculation of zero-field splitting parameters: Comparison of a two-component noncolinear spin-density-functional method and a one-component perturbational approach. J. Chem. Phys. 2006, 125, 54110. [Google Scholar] [CrossRef] [PubMed]
- Ganyushin, D.; Neese, F. First-principles calculations of zero-field splitting parameters. J. Chem. Phys. 2006, 125, 024103. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.E.; Terra, J.; Warschkow, O.; Jiang, M.; González, G.B.; Okasinski, J.S.; Bedzyk, M.J.; Rossi, A.M.; Eon, J.-G. A theoretical and experimental study of lead substitution in calcium hydroxyapatite. Phys. Chem. Chem. Phys. 2006, 8, 967–976. [Google Scholar] [CrossRef]
- De Leeuw, N.H. Computer simulations of structures and properties of the biomaterial hydroxyapatite. J. Mater. Chem. 2010, 20, 5376–5389. [Google Scholar] [CrossRef]
- Gelli, R.; Briccolani-Bandini, L.; Pagliai, M.; Cardini, G.; Ridi, F.; Baglioni, P. Exploring the effect of Mg2+ substitution on amorphous calcium phosphate nanoparticles. J. Colloid Interface Sci. 2021, 66, 444–453. [Google Scholar]
- Goldberg, M.; Gafurov, M.; Murzakhanov, F.; Fomin, A.; Antonova, O.; Khairutdinova, D.; Pyataev, A.; Makshakova, O.; Konovalov, A.; Leonov, A.; et al. Mesoporous Iron(III)-Doped Hydroxyapatite Nanopowders Obtained via Iron Oxalate. Nanomaterials 2021, 11, 811. [Google Scholar] [CrossRef]
- Goldberg, M.; Gafurov, M.; Makshakova, O.; Smirnov, A.; Komlev, V.; Barinov, S.; Kudryavtsev, E.; Sergeeva, N.; Achmedova, S.; Mamin, G.; et al. Influence of Al on the Structure and in Vitro Behavior of Hydroxyapatite Nanopowders. J. Phys. Chem. B 2019, 123, 9143–9154. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Akopyan, A.V.; Gafurov, M.R.; Makshakova, O.N.; Donskaya, N.O.; Fomin, A.S.; Polikarpova, P.P.; Anisimov, A.V.; Murzakhanov, F.F.; Leonov, A.V.; et al. Iron-Doped Mesoporous Powders of Hydroxyapatite as Molybdenum-Impregnated Catalysts for Deep Oxidative Desulfurization of Model Fuel: Synthesis and Experimental and Theoretical Studies. J. Phys. Chem. C 2021, 125, 11604–11619. [Google Scholar] [CrossRef]
- Rulis, P.; Ouyang, L.; Ching, W.Y. Electronic structure and bonding in calcium apatite crystals: Hydroxyapatite, fluorapat-ite, chlorapatite, and bromapatite. Phys. Rev. B 2004, 70, 155104. [Google Scholar] [CrossRef]
- Bystrova, A.V.; Dekhtyar, Y.D.; Popov, A.I.; Coutinho, J.; Bystrov, V.S. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modi-fied hydroxyapatite structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Avakyan, L.A.; Paramonova, E.V.; Coutinho, J.; Öberg, S.; Bystrov, V.S.; Bugaev, L.A. Optoelectronics and defect levels in hydroxyapatite by first-principles. J. Chem. Phys. 2018, 148, 154706. [Google Scholar] [CrossRef]
- Biktagirov, T.; Gafurov, M.; Mamin, G.; Klimashina, E.; Putlayev, V.; Orlinskii, S. Combination of EPR Measurements and DFT Calculations To Study Nitrate Impurities in the Carbonated Nanohydroxyapatite. J. Phys. Chem. A 2014, 118, 1519–1526. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Gilinskaya, L.G.; Scherbakova, M.Y. Physics of Apatite; Nauka: NovosiЬirsk, Russia, 1975; Volume C, pp. 7–63. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Sugano, S.; Tanabe, Y.; Kamimura, H. Multiplets of Transition-Metal Ions in Crystals; Academic Press: Cambridge, MA, USA, 1970; pp. 6–16. [Google Scholar]
- Mulak, M.; Mulak, J. The Second and Third Crystal Field Splitting Moments in Verification of the Trigonal and Hexagonal Crystal Field Parametrizations. Phys. Status Solidi 2018, 255, 1800115. [Google Scholar] [CrossRef]
- Kovaleva, E.S.; Shabanov, M.P.; Putlyaev, V.I.; Tretyakov, Y.D.; Ivanov, V.K.; Silkin, N.I. Bioresorbable carbonated hydroxyapatite Ca10-xNax(PO4)6-x(CO3)x(OH)2 powders for bioactive materials preparation. Cent. Eur. J. Chem. 2009, 7, 168. [Google Scholar] [CrossRef]
- Sharma, R.R.; Das, T.P.; Orbach, R. Zero-field splitting of S-state ions. I. Point-multipole model. Phys. Rev. 1966, 149, 257. [Google Scholar] [CrossRef]
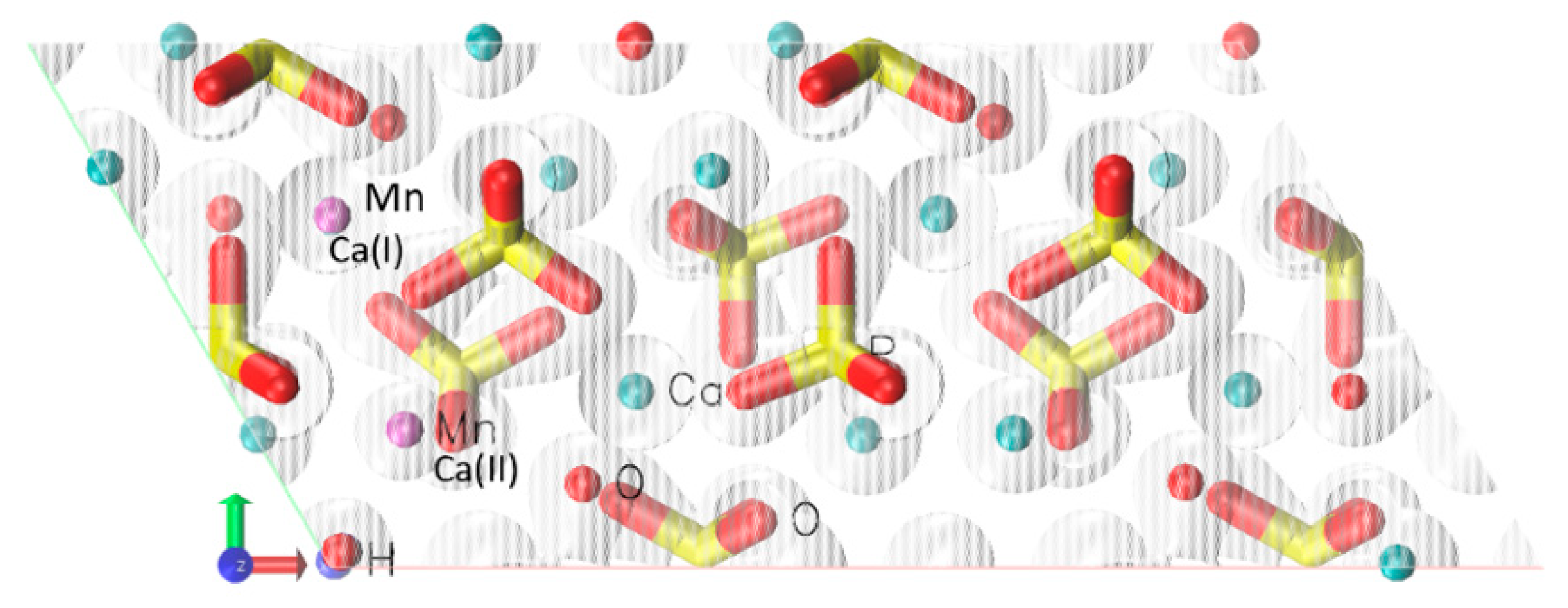
| Ca(I) | Ca(II) | |||
|---|---|---|---|---|
| cm−1 | Electron Density Model | Point-Charge Model | Electron Density Model | Point-Charge Model |
| B20 | 51,443 | 25,831 | 47,183 | 26,702 |
| B22 | 196 | 0 | 1510 | 729 |
| B40 | 3721 | 2688 | 12,229 | 3850 |
| B41 | 52 | 0 | 326 | 44 |
| B42 | 44 | 0 | 272 | 103 |
| B43 | 308 | 39 | 81 | 7 |
| B44 | 8 | 0 | 229 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurtakova, D.V.; Grishin, P.O.; Gafurov, M.R.; Mamin, G.V. Using DFT to Calculate the Parameters of the Crystal Field in Mn2+ Doped Hydroxyapatite Crystals. Crystals 2021, 11, 1050. https://doi.org/10.3390/cryst11091050
Shurtakova DV, Grishin PO, Gafurov MR, Mamin GV. Using DFT to Calculate the Parameters of the Crystal Field in Mn2+ Doped Hydroxyapatite Crystals. Crystals. 2021; 11(9):1050. https://doi.org/10.3390/cryst11091050
Chicago/Turabian StyleShurtakova, Daria Vladimirovna, Peter Olegovich Grishin, Marat Revgerovich Gafurov, and Georgy Vladimirovich Mamin. 2021. "Using DFT to Calculate the Parameters of the Crystal Field in Mn2+ Doped Hydroxyapatite Crystals" Crystals 11, no. 9: 1050. https://doi.org/10.3390/cryst11091050
APA StyleShurtakova, D. V., Grishin, P. O., Gafurov, M. R., & Mamin, G. V. (2021). Using DFT to Calculate the Parameters of the Crystal Field in Mn2+ Doped Hydroxyapatite Crystals. Crystals, 11(9), 1050. https://doi.org/10.3390/cryst11091050







