Abstract
In the quality control of crystalline particles, the uniformity of the distribution of each characteristic, such as size and shape, is important. In particular, the problem in reaction crystallization is that the comprehensive uniformity of characteristic distributions is frequently reduced by the agglomeration phenomena. In this study, we designed an operation method to improve the comprehensive uniformity in a liquid–liquid reaction crystallization by evaluating the dynamic variation in the uniformity of particle size and crystal shape using homogeneity. The homogeneity of final particles increased when the supersaturation was lowered by intermittent operation with inner seed production. Since the ratios of the uniformities of particle sizes and crystal shapes constituting homogeneity varied dynamically, the intermittent operation was designed by focusing on individual uniformities. The uniformity of particle size for the final particles was increased via modulation operation using reverse addition for the dissolution of the microparticles. In the growth stage after the reverse addition, the uniformity of the shape of the final particles was increased by raising the number of times of adding solution for decreasing the supersaturation. In addition, we proposed suitable addition methods to improve comprehensive uniformity by controlling uniformity constituting homogeneity at each stage of intermittent operation.
1. Introduction
There are many crystalline products present in daily life, such as batteries, foods, medicines, cosmetics, and detergents. The qualities of these products are greatly affected by the qualities of their crystalline particles. Therefore, quality control in the crystallization process is particularly important.
The characteristics of crystalline particles, such as size and shape, must meet the requirements of the purposes of crystalline products [1,2]. Many studies have investigated the operation methods that control certain characteristics and achieve the target average values of the characteristics. Malwade et al. controlled polymorphism and size using the seed-crystal method and modulation operation with a dissolution process [3]. Yamaguchi et al. used a new crystallizer, namely, the Taylor vortex continuous crystallizer, to control morphology [4]. Along with various quality controls being performed on crystalline particles, problems specific to crystalline particles were also focused on. For example, Malwade et al. evaluated particle size distribution, although it was not the objective of the study. Yamaguchi et al. and Yeung et al. evaluated morphology distribution, although they were not the direct objectives of the studies [5]. In other words, in many previous studies, not only the target average value but also the distribution of the characteristic was investigated, because characteristic distribution significantly complicates quality control. Fridolin et al. and Boyang et al. also indicated that particle size distribution, which is one of the characteristic distributions, significantly affected the performance of crystalline products and filtration [6,7]. Therefore, improvement in characteristic distribution is significant.
The operation methods for controlling the particle size distribution have been indicated in many papers. For example, Ramisetty et al. proposed the seed-crystal method [8,9], and Takiyama et al. proposed the modulation operation with a dissolution process [10]. However, the distributions of not only the particle size but also other characteristics are noted. Funakoshi et al. observed agglomerates and showed the distributions of the shape and purity of the agglomerates and the number of single crystals constituting an agglomerate [11,12,13,14]. In such a case, a new index is needed for controlling the characteristic distributions.
Therefore, in a previous study [15], homogeneity was proposed as a novel index for evaluating the comprehensive uniformity of characteristic distributions of crystalline particles. The novel index, i.e., homogeneity, was applied to crystalline particles obtained by reaction crystallization [16,17,18]. The results showed that homogeneity could evaluate the comprehensive uniformity of the distributions of not only the particle size but also other characteristics, such as crystal shape.
Liquid–liquid reaction crystallization is one of the industrial crystallization processes for producing pharmaceuticals and electronic materials. In this industrial field, there is no theoretical method for improvement in the quality of the crystalline particles. Some empirical methods such as the seed-crystal method [19,20] and solution addition method [21,22] (e.g., intermittent operation [23]), exist for obtaining crystalline particles. These methods are controlled by changing the quality of the initial particles and the solution conditions (especially supersaturation [24]) to vary the crystallization phenomena consisting of crystal nucleation and growth. These studies showed that changes in solution conditions strongly affected crystal qualities. Therefore, crystal qualities should be analyzed from the viewpoint of dynamic variation in characteristic distributions to improve the operation method of reaction crystallization.
In this study, we designed an operation method to enable careful quality control of liquid–liquid reaction crystallization by evaluating the dynamic variation in the uniformity of distributions of particle size and crystal shape using homogeneity as a novel index. In particular, the effects of the different addition methods of feed solutions, such as the number of times of adding solution or the order of feed solutions, on individual uniformities consisting of the distributions of particle size and crystal shape were investigated. In addition, we propose suitable addition methods to improve comprehensive uniformity.
2. Materials and Methods
In this study, L-aspartate sodium (L-AspNa, 99.7%, Wako Pure Chemical Industries, Ltd., Osaka, Japan) and hydrochloric acid (HCl, 6 M, Wako Pure Chemical Industries, Ltd., Osaka, Japan) were used for a reaction system. L-aspartic acid was used as an objective crystalline material. We used 250-mL 0.30-mol/L L-AspNa aq. and 250-mL 0.25-mol/L HCl aq. as feed solutions. HCl aq. was added to L-AspNa aq. in a crystallizer. A semi-batch crystallizer (500 mL) was used, and the mixed solution temperature was kept constant at 303 K throughout the experiment. Four marine blades were used and agitated at 400 rpm. In addition, a pH meter (HM-30R and ELP-037, TOA DKK Co. Shinjuku, Japan) was used to measure the pH of the mixed solution in real time. Sampling was performed as appropriate, and the obtained slurry was separated into filtrate and crystals using suction filtration. The filtrate was subjected to high-performance liquid chromatography (HPLC, SPD-10A, LC-10AD, DGU-12A, SIL-20A, and CTO-10AS, SHIMADZU Co., Kyoto, Japan) to determine the concentration. Supersaturation, which is the driving force for crystallization, was calculated using lnS = ln (C/C*). where C is the L-Asp concentration of the mixed solution measured by HPLC and C* is the equilibrium L-Asp concentration corresponding to the solution pH. Supersaturation was calculated from the measured pH and concentration of the mixed solution. The obtained crystals were observed with scanning electron microscopy (SEM, JEOL JSM model 6510). The particle sizes and crystal shapes of 300 of individual particles were measured. Each uniformity of distribution of particle size (PL) and crystal shape (PS) and homogeneity (H) was calculated by the following equation proposed in a previous study [15]. PS is calculated from each probability of four different crystal shape (PSA, PSB, PSC, PSD) classified in each experiment. Since homogeneity is calculated based on the index for distributions of particle size and crystal shape in previous studies, the accuracy and reproducibility of homogeneity depend on the accuracy and reproducibility of those distributions.
The homogeneity is constituted of the uniformity of distributions of particle size and crystal shape: the higher the uniformity of the characteristic, the lower the characteristic distribution. A higher homogeneity implies a more preferable crystalline quality.
In Run 1, 250-mL HCl aq. was continuously added for 3 h and the mixed solution was held for 1 h. In Runs 2 and 3, HCl aq. was intermittently added twice to L-AspNa aq. in the crystallizer, and the first addition volume of HCl aq. was changed to vary the quality of the crystals precipitated initially. The addition rate was 10 mL/min. The detailed addition methods in each operation method are shown in Table 1. The adjustment in HCl aq. volume ratio Vr against the crystallization time in each operation method is shown in Figure 1. Vr is defined by the following equation.

Table 1.
The detailed addition methods in each operation method.
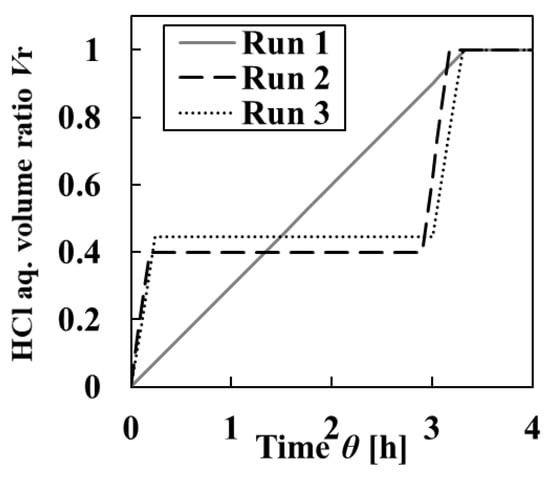
Figure 1.
Changes in HCl aq. volume ratio Vr against crystallization time. Run 1 contains continuous addition of HCl aq., whereas Runs 2 and 3 contain continuous intermittent addition of HCl aq.
3. Results and Discussion
The operation method for feed solutions has a significant impact on crystal quality. As shown in Figure 2, the homogeneity of the final particles obtained in Run 1 was 26.0%. The entire bar charts in Figure 2b show the homogeneity, and the black and white bars show each ratio of the uniformities of particle size and crystal shape constituting homogeneity of crystalline particles, respectively. The homogeneity of crystalline particles decreased monotonically. Furthermore, Figure 2b shows that the ratios of uniformities of particle size and crystal shape constituting homogeneity of crystalline particles varied dynamically. Therefore, the operation for increasing homogeneity should be designed by focusing on individual uniformities. The measured mixed solution conditions are shown in Figure 3. The supersaturation lnS was extremely high (Figure 3c). The homogeneity of crystalline particles was low because a high supersaturation condition caused rapid nucleation and agglomeration [25]. From this result, the continuous operation in which high supersaturation occurred was inappropriate as an operation method for controlling comprehensive uniformity. Therefore, the intermittent operation with inner seed production was performed to increase homogeneity.
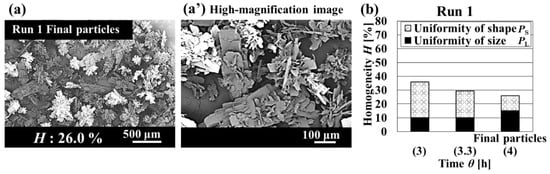
Figure 2.
Quality of crystalline particles obtained in Run 1. (a) SEM photomicrograph of the final particles. (a’) High-magnification image of the final particles. (b) Dynamic variation in homogeneity and uniformity of distributions of particle size and crystal shape with the crystallization time.
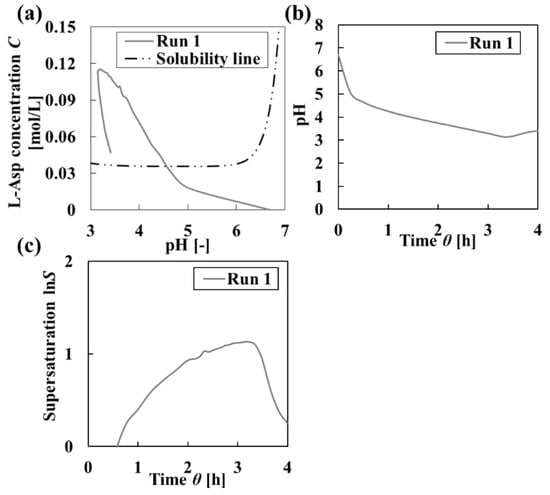
Figure 3.
Changing in the solution condition during crystallization under Run 1. (a) L-Asp concentration to pH. (b) Solution pH. (c) Supersaturation calculated from (a).
The homogeneity of all crystalline particles obtained in Runs 2 and 3 was high because the supersaturation was low compared with Run 1, as can be seen in Figure 4 and Figure 5. In the intermittent operation, the crystallization stage can be considered separately. In this paper, the crystallization stage from which the initial particles were produced was defined as the initial stage and the stage after that as the growth stage. Figure 5 shows that the change in homogeneity of from initial particles to final particles was nonexistent or increased. From focusing on each uniformity of particle size and crystal shape, two excellent features were observed. The uniformity of the crystal shape of initial particles obtained in Run 2 was high, and the uniformity of particle size of final particles obtained in Run 3 was high. It was thought that these two excellent features should be combined to increase homogeneity. Therefore, in Run 2, the addition method for increasing uniformity of particle size of final crystals was considered.
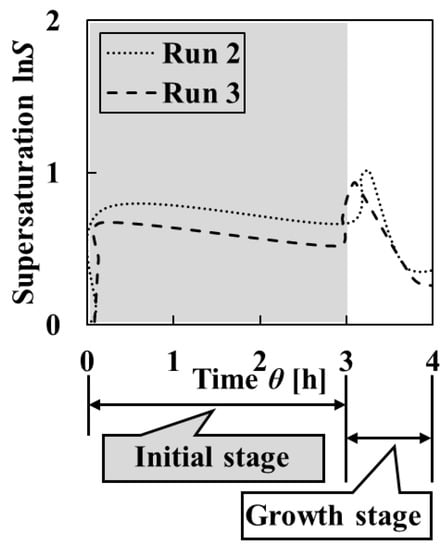
Figure 4.
Changes in the supersaturations calculated from the measured pH and L-Asp concentration of the solutions in Runs 2 and 3. The crystallization stage when the initial particles were produced was defined as the initial stage, and the stage after that as the growth stage.
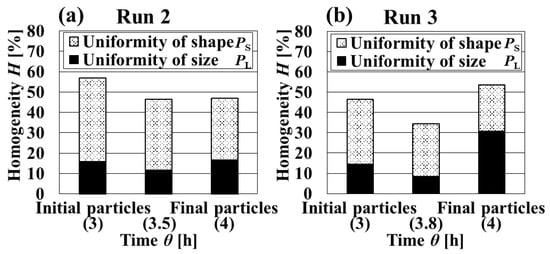
Figure 5.
Dynamic variation in the homogeneity and uniformity of the distributions of particle size and crystal shape with the crystallization time. (a) Run2. (b) Run3.
One of the operation methods for controlling the uniformity of distribution of particle size is a modulation operation with a dissolution process. The uniformity of the distribution of particle size is increased by the dissolution of the microparticles. For example, in cooling crystallization, the modulation operation with a dissolution process is performed by operating temperature because the supersaturation condition depends on the temperature [26]. In reaction crystallization, the supersaturation condition depends on the order of the two feed solutions. In this study, the supersaturation is increased by adding HCl aq. to L-AspNa aq. in the crystallizer. It is considered that the reverse addition of L-AspNa aq. to the mixed solution in the crystallizer creates a dissolution process. Therefore, a modulation operation with the reverse addition of L-AspNa aq. was performed to initiate the dissolution process after the initial high supersaturation.
3.1. Proposed Operation Method in the Initial Stage
In Run 4, for the initial stage, 100-mL HCl aq. was added to 225-mL L-AspNa aq. in the crystallizer as the first addition, and the mixed solution was held for 1 h and 30 min. Then, 25-mL L-AspNa aq. was added to the crystallizer as the second addition, and the mixed solution was held for 1 h and 30 min. Subsequently, in the growth stage, 150-mL HCl aq. was added to the crystallizer as the third addition, and the mixed solution was held for 1 h to obtain the final particles. This detailed addition method of Run 4 is shown in Table 2 and Figure 6.

Table 2.
The detailed addition methods in Run 4.
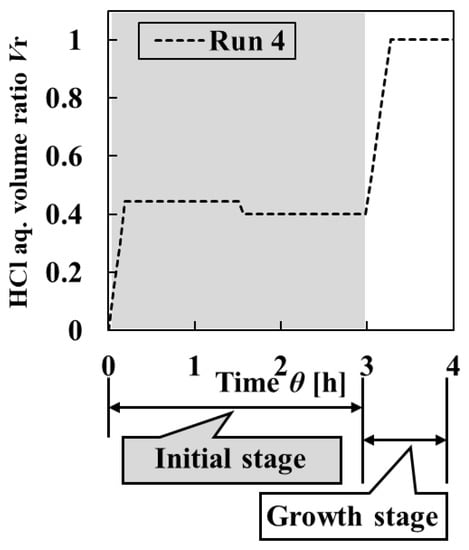
Figure 6.
Change in HCl aq. volume ratio Vr against the crystallization time in Run 4 with intermittent operation of HCl aq. with reverse addition of L-Asp aq.
As designed, in the initial stage, the initial supersaturation was set high at the first addition, and it was decreased after nucleation by the modulation operation with the reverse addition of L-AspNa aq. at the second addition (Figure 7). As shown in Figure 8 and Figure 9, the homogeneity of the final particles obtained in Run 4 was higher than in Run 3. Initial particles with high uniformity of crystal shape were obtained. Furthermore, the uniformity of particle size greatly increased over time. On the other hand, the comparison of the initial and final particles revealed that the uniformity of crystal shape decreased because of the agglomeration of final particles. Since the ratios of the uniformities of particle size and crystal shape constituting the homogeneity of crystalline particles varied dynamically, it is possible to consider increasing the uniformity of crystal shape. When the agglomeration in the growth stage is prevented, it is expected that the uniformity of the crystal shape of the final particles is increased. It is necessary to control supersaturation by increasing the number of times of adding solution in the growth stage for preventing agglomeration.
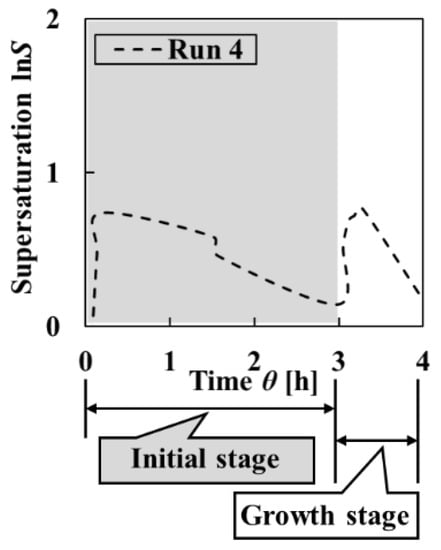
Figure 7.
Change in the supersaturation calculated from the measured pH and L-Asp concentration of the solution in Run 4.

Figure 8.
SEM photomicrograph of crystalline particles obtained in Run 4. (a) Initial particles. (b) Final particles.
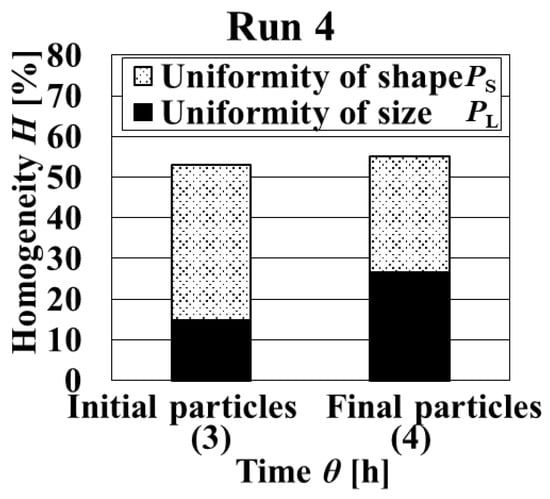
Figure 9.
Dynamic variation in homogeneity and uniformity of the distributions of particle size and crystal shape with crystallization time in Run 4.
3.2. Proposed Operation Method in the Growth Stage
In Run 5, in the initial stage, 104-mL HCl aq. was added to 225-mL L-AspNa aq. in the crystallizer as the first addition, and the mixed solution was held for 1 h and 30 min. Then, 25-mL L-AspNa aq. was added to the crystallizer as the second addition, and the mixed solution was held for 1 h. Subsequently, in the growth stage, 40-mL HCl was added to the crystallizer as the third addition, and the mixed solution was held for 20 min. Then, 40-mL HCl was added to the crystallizer as the fourth addition, and the mixed solution was held for 20 min, and 66-mL HCl was added to the crystallizer as the fifth addition, and the mixed solution was held for 50 min to obtain the final particles. This detailed addition method of Run 5 is shown in Table 3 and Figure 10.

Table 3.
The detailed addition methods in Run 5.
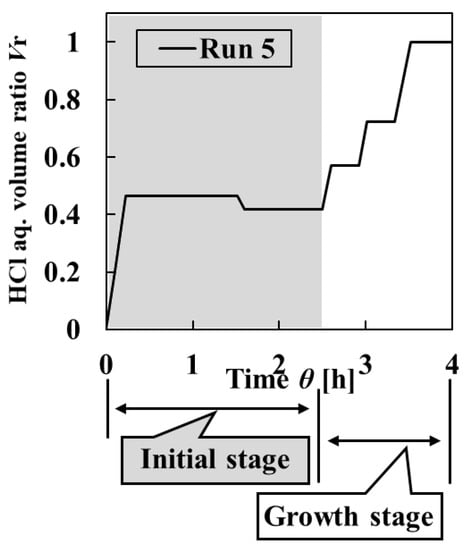
Figure 10.
Change in HCl aq. volume ratio Vr against the crystallization time in Run 5.
Increasing the number of times of adding solution in the growth stage by three times clearly decreased the supersaturation (Figure 11). As indicated in Figure 12 and Figure 13, final particles with the highest homogeneity were obtained. Initial particles with increased uniformity of size and shape were obtained. In addition, the decreasing uniformity of crystal shape of the final particles was controlled by preventing the agglomeration phenomena in the growth stage. From these results, it is possible to improve comprehensive uniformity by proposing each addition method in the initial stage and growth stage.
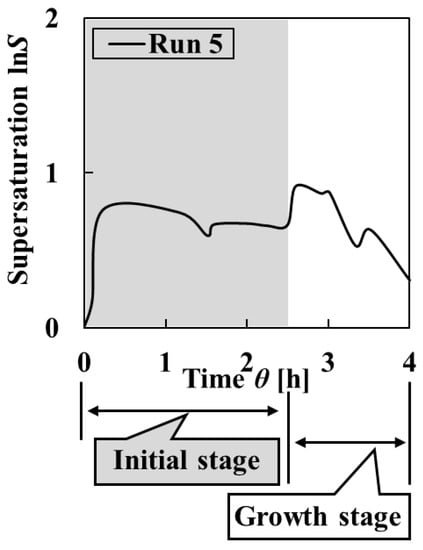
Figure 11.
Change in the supersaturation calculated from the measured pH and L-Asp concentration of the solution in Run 5.

Figure 12.
SEM photomicrograph of crystalline particles obtained in Run 5. (a) Initial particles. (b) Final particles. (b’) High-magnification image of the final particles.
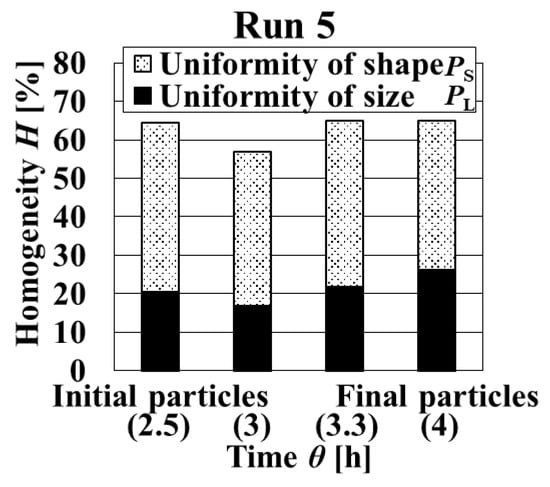
Figure 13.
Dynamic variation in homogeneity and uniformity of distributions of particle size and crystal shape with the crystallization time in Run 5.
4. Conclusions
In this study, we designed an operation method to improve the comprehensive uniformity of crystalline particles by evaluating the dynamic variation in the uniformity of distributions of particle size and crystal shape using homogeneity as a novel index term. Since the ratios of the uniformities of particle sizes and crystal shapes constituting the homogeneity of crystalline particles varied dynamically, it showed the possibility of proposing the operation method to improve uniformity.
The experiment results showed that there were differences in the variation of homogeneity between continuous and intermittent operation. In addition, the differences could be estimated by the effect of supersaturation (lnS). Comparing the results of the intermittent operations with different changes in supersaturation over time, it was found that a significant difference in the ratios of the uniformities constituting the homogeneity at each crystallization stage in the intermittent operation was observable.
In addition, it was revealed that the supersaturation condition in the intermittent operation should combine the features of each stage for improving the homogeneity of the final particles. In particular, in the initial stage of intermittent operation, modulation operation with a dissolution process was necessary to increase the uniformity of particle size, while in the growth stage, raising the number of times of adding solution was necessary to increase the uniformity of crystal shape. Consequently, we found that the concept of the homogeneity could be used to increase the uniformity of both crystal shape and particle size in the reaction crystallization.
Therefore, we expect that using homogeneity is a favorable approach to design operation methods for careful quality control of liquid–liquid reaction crystallization processes.
Author Contributions
M.O.: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft preparation, visualization, S.A.: conceptualization, writing—reviewing and editing, H.T.: conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data have been included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, Y.N.; Wang, H.Y.; Du, S.C.; Wang, Y.; Huang, C.; Qin, Y.J.; Gong, J.B. The liquid-liquid phase separation and crystallization of vanillin in 1-propanol/water solution. Fluid Phase Equilibria 2016, 409, 84–91. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.M.; Xie, M.; He, Y.H.; Huang, G.Y.; Yang, Y.C. Growth mechanisms for spherical mixed hydroxide agglomerates prepared by co-precipitation method: A case of Ni1/3Co1/3Mn1/3(OH)(2). J. Alloys Compd. 2015, 619, 846–853. [Google Scholar] [CrossRef]
- Malwade, C.R.; Qu, H. Antisolvent Crystallization of Indomethacin from a Ternary Solvent System with High Productivity, Better Polymorphism, and Particle Size Control. Org. Proc. Res. Dev. 2019, 23, 968–976. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kim, W.-S.; Takiyama, H. Crystal Quality Control by Using the Millimeter-Size Tube Type Taylor Vortex Continuous Crystallizer. J. Chem. Eng. Jpn. 2019, 52, 308–310. [Google Scholar] [CrossRef]
- Yeung, K.L.; Wolf, E.E. Stm Studies of the Effect of Pretreatment on the Morphology and Aspect Ratio Distribution of Crystallites on Pt/Hopg. Catal. Lett. 1992, 12, 213–226. [Google Scholar] [CrossRef]
- Röder, F.; Sonntag, S.; Schröder, D.; Krewer, U. Simulating the Impact of Particle Size Distribution on the Performance of Graphite Electrodes in Lithium-Ion Batteries. Energy Technol. 2016, 4, 1588–1597. [Google Scholar] [CrossRef]
- Li, B.; Dobosz, K.M.; Zhang, H.; Schiffman, J.D.; Saranteas, K.; Henson, M.A. Predicting the performance of pressure filtration processes by coupling computational fluid dynamics and discrete element methods. Chem. Eng. Sci. 2019, 208. [Google Scholar] [CrossRef] [PubMed]
- Ramisetty, K.A.; Kumar, K.V.; Rasmuson, Å.C. Advanced Size Distribution Control in Batch Cooling Crystallization Using Ultrasound. Org. Proc. Res. Dev. 2019, 23, 935–944. [Google Scholar] [CrossRef]
- Ramisetty, K.A.; Rasmuson, Å.C. Controlling the Product Crystal Size Distribution by Strategic Application of Ultrasonication. Cryst. Growth Des. 2018, 18, 1697–1709. [Google Scholar] [CrossRef]
- Takiyama, H.; Shindo, K.; Matsuoka, M. Effects of undersaturation on crystal size distribution in cooling type batch crystallization. J. Chem. Eng. Jpn. 2002, 35, 1072–1077. [Google Scholar] [CrossRef]
- Taborga, P.; Brito, I.; Graber, T.A. Effect of additives on size and shape of lithium carbonate crystals. J. Cryst. Growth 2017, 460, 5–12. [Google Scholar] [CrossRef]
- Terdenge, L.-M.; Kossuch, J.A.; Schembecker, G.; Wohlgemuth, K. Potential of gassing crystallization to control the agglomeration degree of crystalline products. Powder Technol. 2017, 320, 386–396. [Google Scholar] [CrossRef]
- Heisel, S.; Ernst, J.; Emshoff, A.; Schembecker, G.; Wohlgemuth, K. Shape-independent particle classification for discrimination of single crystals and agglomerates. Powder Technol. 2019, 345, 425–437. [Google Scholar] [CrossRef]
- Funakoshi, K.; Takiyama, H.; Matsuoka, M. Influences of seed crystals on agglomeration phenomena and product purity of m-chloronitrobenzene crystals in batch crystallization. Chem. Eng. J. 2001, 81, 307–312. [Google Scholar] [CrossRef]
- Ohyama, M.; Kudo, S.; Amari, S.; Takiyama, H. Production of crystalline particles with high homogeneity in reaction crystallization by using pH-solubility-profile. J. Ind. Eng. Chem. 2019, 75, 38–43. [Google Scholar] [CrossRef]
- Bramley, A.S.; Hounslow, M.J.; Newman, R.; Paterson, W.R.; Pogessi, C. The Role of Solution Composition on Aggregation During Precipitation. Chem. Eng. Res. Des. 1997, 75, 119–124. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, W.-S.; Kim, K.-S.; Kim, J.-S.; Jung, T.; Ward, M.D. Dynamic Behavior of Particle Agglomeration of Europium Oxalate during Reaction Crystallization in Semi-Batch Reactor. Chem. Eng. Commun. 2006, 193, 370–385. [Google Scholar] [CrossRef]
- Thai, D.K.; Mayra, Q.-P.; Kim, W.-S. Agglomeration of Ni-rich hydroxide crystals in Taylor vortex flow. Powder Technol. 2015, 274, 5–13. [Google Scholar] [CrossRef]
- Ali, M.I.; Schneider, P.A. A fed-batch design approach of struvite system in controlled supersaturation. Chem. Eng. Sci. 2006, 61, 3951–3961. [Google Scholar] [CrossRef]
- Hatakka, H.; Alatalo, H.; Louhi-Kultanen, M.; Lassila, I.; Haeggström, E. Closed-Loop Control of Reactive Crystallization PART II: Polymorphism Control of L-Glutamic Acid by Sonocrystallization and Seeding. Chem. Eng. Technol. 2010, 33, 751–756. [Google Scholar] [CrossRef]
- Torbacke, M.; Rasmuson, k.C. Mesomixing in semi-batch reaction crystallization and influence of reactor size. AIChE J. 2004, 50, 3107–3119. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Tan, R.B.H.; Chow, P.S. Effects of operating conditions on agglomeration and habit of paracetamol crystals in anti-solvent crystallization. J. Cryst. Growth 2005, 279, 477–488. [Google Scholar] [CrossRef]
- Sato, E.; Seki, Y.; Takiyama, H. Control of Reaction Crystallization of Organic Compounds Using the Supersaturation Profile. J. Chem. Eng. Jpn. 2019, 52, 599–604. [Google Scholar] [CrossRef]
- Alatalo, H.M.; Hatakka, H.; Louhi-Kultanen, M.; Kohonen, J.; Reinikainen, S.P. Closed-Loop Control of Reactive Crystallization. Part I: Supersaturation-Controlled Crystallization of L-Glutamic Acid. Chem. Eng. Technol. 2010, 33, 743–750. [Google Scholar] [CrossRef]
- Trampuž, M.; Teslić, D.; Likozar, B. Crystallization of fesoterodine fumarate active pharmaceutical ingredient: Modelling of thermodynamic equilibrium, nucleation, growth, agglomeration and dissolution kinetics and temperature cycling. Chem. Eng. Sci. 2019, 201, 97–111. [Google Scholar] [CrossRef]
- Shoji, M.; Eto, T.; Takiyama, H. A Kinetic Study of the Influence of Modulated Undersaturation Operation on Crystal Size Distribution in Cooling-Type Batch Crystallization. J. Chem. Eng. Jpn. 2011, 44, 191–196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).