Abstract
α-Calcium sulfate hemihydrate (α-HH) is an important cementitious material, which can be prepared by hydrothermal method from calcium sulfate dihydrate (DH) in an electrolyte solution. Study of the conversion kinetics of DH to α-HH in NaCl solution is helpful for understanding the control process. In this paper, X-ray diffraction (XRD) patterns were applied to study the effect of temperature on the crystallization kinetics of α-HH to determine the kinetic parameters. The research results show that the sigmoidal shape of the α-HH crystallization curve follows the Avrami-Erofeev model, which describes the crystallization kinetics of α-HH formation. Applying Arrhenius law in experimental data and model calculations, an apparent activation energy of 124 kJ/mol for nucleation and an apparent activation energy of 810 kJ/mol for growth were obtained. By adjusting the temperature of the solution, the number of α-HH nucleation and growth steps increases, which can effectively increase the DH-α-HH conversion rate in the NaCI solution.
1. Introduction
According to the difference in hydration, calcium sulfate can be divided into dihydrate gypsum (CaSO4·2H2O), hemihydrate gypsum (CaSO4·0.5H2O), and anhydrite (CaSO4) [1,2,3]. Phosphogypsum (PG) is mainly composed of calcium sulfate dihydrate (DH), which is a byproduct from the wet process of phosphoric acid production. It is estimated that every ton of phosphoric acid produces about 4.5 to 5.5 tons of PG [4,5] in the world. A large amount of PG is processed through stockpiling, which not only takes up a lot of farmland but also causes serious pollution to the surrounding environment. Therefore, the recycling and comprehensive utilization of PG are very important for environmental protection. In recent years, the high value-added utilization of PG has attracted widespread attention. At present, the most important high value-added utilization technology is the use of PG to produce hemihydrate gypsum (HH) cementing materials, mainly α-type hemihydrate gypsum (α-HH) and β-type hemihydrate gypsum (β-HH). β-HH can be used to produce various gypsum-based building materials such as gypsum blocks and gypsum boards. However, because β-HH crystals are generally irregular fibers or flakes, the structure is loose and crystallinity is poor, resulting in low strength of the hardened body. The α-HH has the advantages of high crystallinity, compact structure, low water consumption for standard consistency, low heat of hydration, and slow hydration rate. In addition, α-HH products have higher mechanical strength and better biocompatibility [6,7,8], which can not only replace β-HH to enhance product quality, but are widely used in precision casting, medical treatment, functional fillers, and decorations. Therefore, the use of PG to prepare α-HH is an important research direction for PG resource utilization. α-HH is a crystal formed by dehydration of DH under normal pressure salt solution conditions [9,10,11,12,13]. By adding an inorganic additive NaCl to the aqueous solution to make the solution supersaturated, DH dehydration yields α-HH. Then the organic additive succinic acid is added to transform it into short cylindrical α-HH with higher strength [14,15,16,17].
The dissolution of DH and the nucleation and growth of α-HH constitute the transformation process under the salt water solution system [18,19]. According to the evolution of solid and liquid components, four main transformation processes are classified, including dissolution control [7,20], growth control, dissolution-nucleation control [21], and nucleation-growth control [22]. Therefore, these steps are very critical for the transformation of DH into α-HH. Understanding and controlling these influencing factors of the crystallization process and directing the synthesis process to produce products with expected properties may have a significant effect on the large-scale preparation of α-HH.
The driving force for the conversion is the solubility difference. According to the solubility balanced formula, the solubility product and water activity are two important factors affecting solubility, and solubility has a great relationship with temperature. For a given temperature, there is a critical water activity at which both DH and α-HH will reach solution equilibrium (the same solubility) [23]. An increase in temperature can enlarge the solubility difference between DH and α-HH, thus accelerating the transformation (crystallinity). However, the potential relationship between DH to α-HH conversion kinetics and temperature is currently unclear.
In the field of crystallization, XRD data is often used to determine the degree of crystallinity. The reaction process can be determined from Raman data [24], thermogravimetric analysis (TGA) [25,26,27], pH value [28], X-ray diffraction (XRD) [29], and atomic absorption spectroscopy (AAS) [28]. Then the empirical data can be used to fit the known dynamic model to understand and predict the appropriate parameters of the control mechanism.
The Avrami-Erofeev model is often used to obtain kinetic data from hydrothermal crystallized samples [29,30,31]. The XRD pattern contributes to determining the induction time, rate constant, frequency factor, and activation energy of the induction and growth period and other kinetic parameters from the Avrami-Erofeev model. The Avrami-Erofeev model was proposed for the first time to study the effect of temperature on the crystallization kinetics of α-HH in hydrothermal reactions. This study used DH to prepare α-HH crystallinity data to determine the effect of temperature on the formation of α-HH.
2. Materials and Methods
2.1. Sample Preparation
The conversion of DH to α-HH was carried out in a 2.47 M NaCl solution at a temperature of 92.0–98.0 °C. The mixed solution was prepared with NaCl (Analytical Grade, Shanghai Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), succinic acid (Analytical Grade, Shanghai Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and deionized water (18.25 MΩ.cm resistances), which was transferred to a 1.0 L three-necked flask equipped with a condenser and a Teflon impeller. The temperature was monitored by a temperature sensor inserted into the three-necked flask, and the temperature was controlled at ±0.5 °C of the set temperature using a heating mantle. When the set temperature was reached, PG was added to a three-neck flask with a solid-liquid ratio of 1:4, and about 20 mL of suspension samples was taken from the middle of the suspension at regular intervals. The suspension was quickly filtered in a G4 molten glass crucible produced by Changchun Glass Instrument Factory, China. The filter cake was quickly washed three times with boiling water, and then washed twice with absolute ethanol, and then dried in an oven at 45 °C for 12 h.
2.2. Material Characterization
The crystal phase of the product was characterized by advanced powder X-ray diffractometer (XRD, X‘Pert Pro MPD/PANalytical, Malvern, UK). The filtered Cu-Ka radiation (I = 0.15 nm) was used to characterize the phase and crystallinity of the sample in the 2θ range of 10°–40°, and the scan speed was 5 °/min. To obtain comparable kinetic parameters, the scan speed and sample weight are controlled unchanged in all XRD tests. The degree of crystallinity of α-HH was calculated by dividing the total area (∑A) of the four main XRD peaks of the prepared sample by the similar total area of the α-HH standard sample (∑As) [29].
In this paper, the high crystallinity of α-HH without an impurity crystal phase was obtained as the standard sample. It had the highest peak region, and no other impurity was detected. At this time, all the DH in the solution was converted to α-HH. Therefore, the sample obtained at 98 °C with a hydrothermal reaction duration of 3 h was selected as the standard sample for comparison. When the time was 0 h, there was no α-HH crystallization in the solution, and the crystallinity was the minimum. In this work, the four main peak areas of the α-HH standard sample with 2θ in the range of 10°–40° were selected to calculate the crystallinity at 14.83°, 25.74°, 29.81°, and 31.92°. At the same time, a scanning electron microscope (SEM, FEI/INSPECT F50) was used to observe the surface morphology of the sample.
3. Results and Discussion
3.1. XRD Analysis
Figure 1a–c depicts the XRD patterns of the synthesized samples under the conditions of 92 °C, 95 °C, and 98 °C in different time ranges.
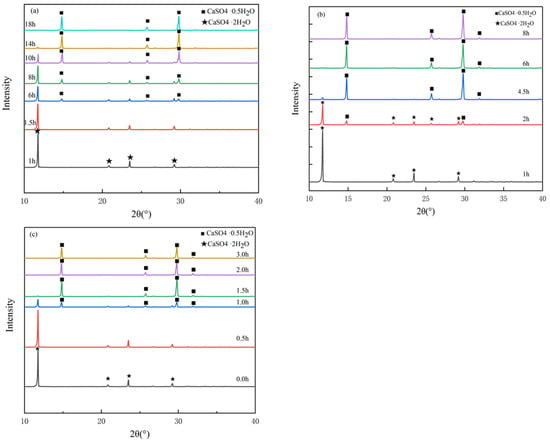
Figure 1.
XRD patterns of samples synthesized at different temperatures (a) 92 °C, (b) 95 °C and (c) 98 °C.
Figure 1a shows that no phase inversion occurred after reacting at 92 °C for 1 h, and all the substances in the solution were DH. However, with increasing reaction time, a α-HH peak appeared at 1.5 h, in this case, a α- HH phase coexisted with a DH phase. The reaction time was further increased to 14 h, an α-HH phase appeared, and the peak area and peak intensity were higher. In addition, there were faint peaks at 2θ of 11.73° and 23.50°. These two peaks matched the diffraction peaks of DH. When the hydrothermal reaction time was further increased to 18 h, only an α-HH phase was found in XRD pattern, and all DH phase was transformed into α-HH phase. It can be concluded from Figure 1a that when the hydrothermal temperature is 92 °C, the prolongation of the reaction time leads to more crystal growth, so the peak area increases. The above results indicate that the hydrothermal reaction of DH at 92 °C for 14 h and 18 h could result in the formation of α-HH with better crystallinity. The diffraction peaks were consistent with the standard α-HH, with only some slight angle changes.
Figure 1b describes the crystal phase of the synthesized sample at 95 °C. It can be seen that after 2 h of reaction time, the main peaks of DH and α-HH were found, and the peak area of α-HH was larger. The results of this analysis indicate that increasing the reaction temperature to 95 °C can promote the formation of α-HH crystals. The hydrothermal reaction time further continued to 4.5 h, and the DH peak basically disappeared. When the hydrothermal reaction time further continued to 6 h and 8 h, all DH in the solution was converted into α-HH.
As shown in Figure 1c, the DH-α-HH reaction is carried out at 98 °C. The increase in temperature can promote the formation of α-HH. The main peak of α-HH was visible even when the reaction duration was 1 h. When the duration was 2 h and 3 h, the α-HH standard sample was formed, and no additional peaks of other substances were observed.
What should be cause for concern is that with the increase of hydrothermal temperature and time, α-HH peak area was bigger. The results show that the crystallinity of α-HH got higher with the increase of time and temperature, and the solution of α-HH quantity increased. In this study, the XRD peak of the α-HH sample (Corresponding to different crystallization temperatures) was very consistent with the XRD peak of the α-HH sample published in previous references [25,32,33]. The effect of the reaction temperature on the crystallinity of the sample is terminated in Table A1 in the Appendix A. The value given in the last column of the table was calculated by Equation (1).
3.2. Crystallization Kinetics Research
Crystallization kinetics are generally studied by analyzing the curve of crystallinity (α) and reaction time (t). In this study, α-HH crystallinity was extracted from the XRD patterns in Figure 1a–c as a function of hydrothermal reaction time. The crystallization curves at 92 °C, 95 °C, and 98 °C are shown in Figure 2.
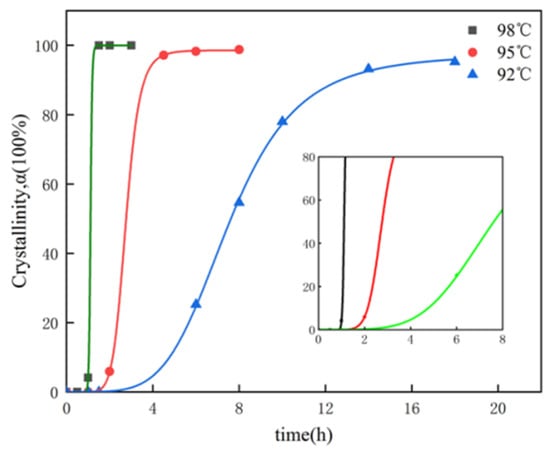
Figure 2.
Crystallization curves for α- HH samples synthesized through the hydrothermal process at various temperatures.
As it can be seen from Figure 2, the degree of crystallinity increased significantly with the reaction temperature and the time increases. Each crystallization curve presents an S shape. To study the crystallization kinetics of α-HH, the conversion process was divided into three stages: (1) the induction period: it is related to temperature; (2) Growth stage: including the interval between the stable stage and the induction stage, the α-HH crystal formed rapidly; (3) Stable period: including deceleration of the growth process [24].
The induction time was defined as the hydrothermal time when the crystallinity of α-HH was about 4%. It is worth noting that the induction time of 4% is determined by considering the degree of crystallinity to realize the transformation of molecular clusters from excited state to relaxation stable state in solution; nucleation and transformation have been included in the induction period [34]. Stabilization time is defined as the time at which the crystallinity of α-HH remains nearly constant. Induction time and stabilization time are listed in Table 1.

Table 1.
Induction and stable times of α- HH samples synthesized with different crystallization temperatures.
Table 1 shows the induction time and stabilization time at different hydrothermal temperatures. The results reveal that with increasing the hydrothermal temperature, the induction time and stabilization time were reduced. At 92 °C, the induction time and stabilization time were 4 h and 13 h, respectively. At 98 °C, they were reduced to 1h and 1.5 h, respectively. The reason can be attributed to the increase in the solubility of DH with increasing temperature, resulting in more nucleation centers at higher temperatures [27].
The nucleation rate can generally be calculated by the reciprocal of the induction time [35], where the suddenly gradual and rapid upward increase of the crystallization curve indicates that the induction period was over and the α-HH crystal was rapidly generated. The slope of the steepest part of the crystallization curve is considered to be the rate of transformation. Figure 2 demonstrates that both nucleation and growth rates increase with the increase of hydrothermal temperature.
3.2.1. Activation Energy and Frequency Factor of the Induction Period
In order to extract the accurate activation energy data of the α-HH conversion process from the crystallization curve, it was first assumed that the hemihydrate gypsum nucleation process was an energy activation step. It is important that the nucleation process of α-HH is assumed to control the rate during the induction period. Secondly, the volume and temperature remained unchanged at this time, because all components in the container were evenly mixed at this time, and the growth of the α-HH crystal was largely unaffected by the crystal size. The most important thing is that at the early stage of the reaction, the solution concentration was at least constant [29,34,35,36].
According to the Equation (2) [37], we determined the values of activation energy (En) and frequency factor (lnAn) in the induction period by the change of temperature due to nucleation rate (1/t0).
where is the nucleation rate, which is calculated by the reciprocal of induction time, An is the frequency factor, En is the apparent activation energy of nucleation, T is the absolute temperature, and R is the universal gas constant.
Figure 3 shows the application of the data of three different temperatures on the Arrhenius Equation (2) for fitting the linear graph. The values of activation energy En and frequency factor lnAn were calculated from the slope and intercept of the linear curve, as shown in Table 2 below.
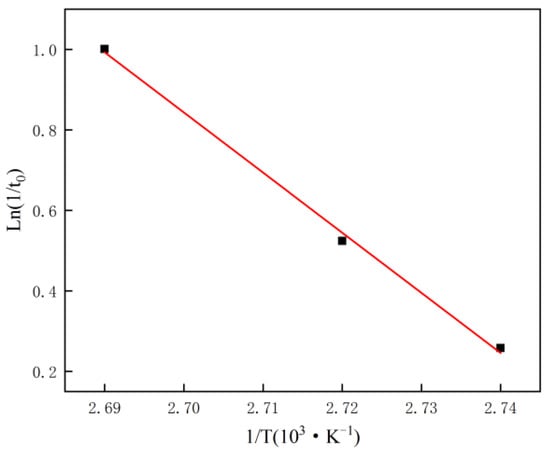
Figure 3.
Arrhenius diagram during crystal induction period of hemihydrate gypsum prepared by hydrothermal method.

Table 2.
Activation energies (Ei), frequency factors (ln Ai), and fit coefficient (R2) for the nucleation and growth stages of the α-HH crystallization.
3.2.2. Activation Energy and Frequency Factor of Growth Period
Crystallization of crystals is often referred to as a nucleation based process, and the crystallization rate model is described by the Avrami-Erofeev model, which is a general form of the nucleation process rate law. In the published literature, there have been several reports on crystallization kinetics in hydrothermal synthesis processes based on the application of the Avrami equation. On the other hand, the crystallization curve shows an S shape. The crystal growth rate follows the Avrami-Erofeev expression, also known as the KJMA (Kolmogorov-Johnson-Mehl-Avrami) equation [38,39]:
It can be written as:
The reaction index n represents the number of steps in the nucleation process and the dimension of the growth process. The crystallization mechanism is expressed by the sum of two constants, and k is the rate constant. If the function image of ln(−ln(1 − α)) and lnt within the specified range shows a linear or semi-linear law, the formation rate of α-HH follows the Avrami kinetic model of slow conversion at the beginning and end of the reaction. The maximum rate is reached in the intermediate stage. In the current study, α uses the range from 0.2 to 0.7 to eliminate the influence of the early and late parts of crystallization [31].The values of K, N, and R2 for hemihydrate gypsum are calculated from the fitted line at each temperature(see Table 3 and Figure 4). Table 3 shows the kinetic parameters of α-HH transformation at different hydrothermal temperatures, where the approximate value of n = 3 at 92 °C is characteristic of the three-dimensional growth process, while the high values of n = 6.4 and 22.4 at 95 °C and 98 °C indicate three-dimensional growth and multiple nucleation

Table 3.
Avrami-Erofeev kinetics parameters of α-HH samples synthesized at different hydrothermal temperatures.
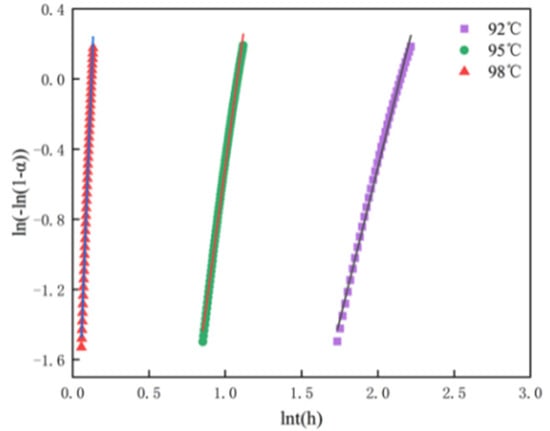
Figure 4.
The plot of ln(−ln(1 − α)) vs. ln(t) for α-HH sample synthesized via hydrothermal method.
The analysis of the reaction index n value corresponding to each temperature in the hydrothermal process shows that the growth of the sample prepared at 92 °C was a three-dimensional growth. At 95 °C and 98 °C, the three-dimensional growth of α-HH crystals had multiple nucleation effects. Obviously, increasing the temperature may change the crystallization mechanism when preparing α-HH, which may be due to the formation of more crystal nuclei, which also proves the above conjecture.
Using the above data, the kinetic equation of crystal formation in the hydrothermal process at temperatures of 92 °C (365.15 K), 95 °C (367.15 K), and 98 °C (369.15 K) can be written as:
The apparent activation energy Eg and frequency factor lnAg of the growth cycle can be calculated from the Arrhenius equation in Equation (8) [35].
The values of Eg and lnAg were determined by drawing lnk and 1/T and matching the best line (as shown in Figure 5).
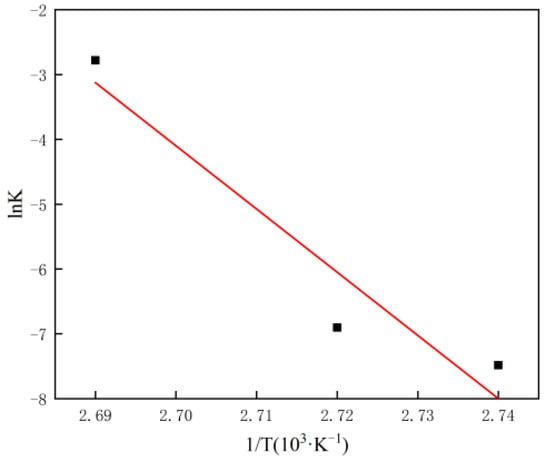
Figure 5.
Arrhenius plots of the growth process for the α-HH samples synthesized by the hydrothermal method.
As shown in Table 2, En and Eg are 124 kJ/mol and 810 kJ/mol, respectively. Therefore, it can be concluded that in the transformation process of DH to α-HH, the growth phase consumed more energy than the nucleation phase, and the energy consumption in the growth phase was about 7 times that of the nucleation phase.
3.3. Morphology Analysis
Under different hydrothermal times at 98 °C, the solid forms in the evolution of the process are DH -α-HH, as shown in Figure 6; most DH crystals showed an irregular shape tabular form, but some of the crystals showed a slender cylindrical shape, as shown in Figure 6a. At 1.5 h, more α-HH crystals were clustered together, and short column α-HH crystals were observed with relatively smooth sides and rough end faces, and the transformation of α-HH to DH was not observed in Figure 6b. When the conversion was complete, the solid was mainly composed of short columnar α-HH crystals (Figure 6c). Notably, a large number of short columnar α-HH crystals were linked to each other, so that the crystal faces of some α-HH crystals were underdeveloped. As can be seen from Figure 6, with the extension of hydrothermal time, the number of α-HH gradually increased, and the crystal development of α-HH became more and more complete.
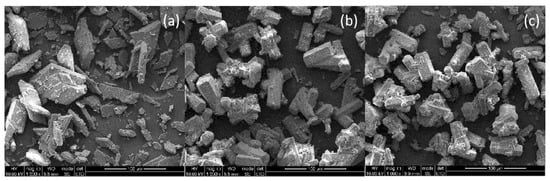
Figure 6.
SEM images of the solids withdrawn at 0 h (a), 1.5 h (b), 3.0 h (c) during the DH to α-HH transformation in a 2.47 M NaCl solution at 98.0 °C.
4. Conclusions
The crystallization kinetics of DH-α-HH transformation was studied by XRD data. The kinetic parameters were determined, including induction time, frequency factor, and activation energy during the induction and growth stages. The transformation of DH-α-HH in salt solution was a process of nucleation and growth, and the dynamic data of the growth period can be well simulated by the Avrami-Erofeev model. The crystallinity of α-HH increased with the increase of hydrothermal time and temperature, and the nucleation and growth processes became multiple nucleation and three-dimensional growth processes. The apparent activation energies of the nucleation and growth phases were 124 kJ/mol and 810 kJ/mol, respectively. The apparent activation energy of nucleation stage was related to the initial induction stage, while the apparent activation energy of the growth stage included the stable period of rapid crystal growth and deceleration of the growth process. In this paper, α-HH samples with high crystallinity were synthesized in a short time at higher hydrothermal temperature. The results show that increasing the temperature can effectively promote the conversion of DH−α-HH, which provides important theoretical guidance for the preparation of α-HH in salt water solution.
Author Contributions
Writing—original draft preparation, Z.W.; writing—review and editing, X.L.; Q.Z.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Graduate Research Foundation of Guizhou Province, China (grant YJSCXJH [2020] 060).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Hydrothermal synthesis conditions and crystallinity of the α-HH samples.
Table A1.
Hydrothermal synthesis conditions and crystallinity of the α-HH samples.
| Sample Name | Temperature (°C) | Time (h) | Phase | Crystallinity (%) |
|---|---|---|---|---|
| S1 | 92 | 1 | DH | 0 |
| S2 | 92 | 1.5 | DH | 0 |
| S3 | 92 | 6 | DH + α-HH | 25 |
| S4 | 92 | 8 | DH + α-HH | 54 |
| S5 | 92 | 10 | DH + α-HH | 77 |
| S6 | 92 | 14 | DH + α-HH | 93 |
| S6 | 92 | 18 | α-HH | 95 |
| S7 | 95 | 1 | DH | 0 |
| S8 | 95 | 2 | DH + α-HH | 5.8 |
| S9 | 95 | 4.5 | DH + α-HH | 97 |
| S10 | 95 | 6 | α-HH | 98 |
| S11 | 95 | 8 | α-HH | 98 |
| S12 | 98 | 0 | DH | 0 |
| S13 | 98 | 0.5 | DH | 0 |
| S14 | 98 | 1 | DH + α-HH | 4.1 |
| S15 | 98 | 1.5 | DH + α-HH | 97 |
| S16 | 98 | 2 | α-HH | 99 |
| S17 | 98 | 3 | α-HH | 100 |
References
- Nasreddine, M.; Bertaut, E.F.; Roubin, M.; Paris, J. Crystallographic Study on Low-Temperature Cr(1 − X)V(X)N. Acta Crystallogr. Sect. B Struct. Sci. 1977, 33, 3010–3013. [Google Scholar]
- Freyer, D.; Voigt, W. Crystallization and Phase Stability of CaSO4 and CaSO4—Based Salts. Mon. Chem. Chem. Mon. 2003, 134, 693–719. [Google Scholar] [CrossRef]
- Reiss, A.G.; Gavrieli, I.; Rosenberg, Y.O.; Reznik, I.J.; Luttge, A.; Emmanuel, S.; Ganor, J. Gypsum Precipitation under Saline Conditions: Thermodynamics, Kinetics, Morphology, and Size Distribution. Minerals 2021, 11, 141. [Google Scholar] [CrossRef]
- Abd El Aleem, S. Changes in Mobility of Toxic Elements during the Production of phosp.pdf[Z]. 2014. Available online: https://www.researchgate.net/publication/262723310_Changes_in_mobility_of_toxic_elements_during_the_production_of_phosppdf (accessed on 20 July 2021).
- Mohammed, F.; Biswas, W.K.; Yao, H.; Tadé, M. Sustainability assessment of symbiotic processes for the reuse of phosphogypsum. J. Clean. Prod. 2018, 188, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Wang, H.; Chen, Q.; Zhang, X.; Wu, Z.; Guan, B. Preparation of alpha-calcium sulfate hemihydrate from FGD gypsum in chloride-free Ca(NO3)2 solution under mild conditions. Fuel 2016, 174, 235–241. [Google Scholar] [CrossRef]
- Garg, M.; Jain, N.; Singh, M. Development of alpha plaster from phosphogypsum for cementitious binders. Constr. Build. Mater. 2009, 23, 3138–3143. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q. Hydration Mechanism and Hardening Property of α-Hemihydrate Phosphogypsum. Minerals 2019, 9, 733. [Google Scholar] [CrossRef] [Green Version]
- Dvořák, K.; Fridrichová, M.; Gazdič, D. Possibilities of Alpha Gypsum Preparation in Chloride Salt Solutions. Adv. Mater. Res. 2012, 598, 310–313. [Google Scholar] [CrossRef]
- Marinkovic, S.; Kostic-Pulek, A.; Tomanec, R. Influence of Some Process Parameters on the Quantity of Hemihydrate Produced in Aqueous Salt Solutions[M]. 1990. Available online: https://www.researchgate.net/publication/264943562_INFLUENCE_OF_SOME_PROCESS_PARAMETERS_ON_THE_QUANTITY_OF_HEMIHYDRATE_PRODUCED_IN_AQUEOUS_SALT_SOLUTIONS (accessed on 20 July 2021).
- Li, X.; Zhang, Q.; Ke, B.; Wang, X.; Li, L.; Li, X.; Mao, S. Insight into the effect of maleic acid on the preparation of α-hemihydrate gypsum from phosphogypsum in Na2SO4 solution. J. Cryst. Growth 2018, 493, 34–40. [Google Scholar] [CrossRef]
- Xlab, C.; Yhab, C. Preparation of α-hemihydrate gypsum from phosphogypsum in glycerol and Na2SO4 mixed solutions under atmospheric pressure. J. Cryst. Growth 2021, 568, 126184. [Google Scholar]
- Li, X.; Zhang, Q. Effect of Molecular Structure of Organic Acids on the Crystal Habit of α-CaSO4•0.5H2O from Phosphogypsum. Crystals 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Guan, B.; Yang, L.; Fu, H.; Kong, B.; Li, T.; Yang, L. α-calcium sulfate hemihydrate preparation from FGD gypsum in recycling mixed salt solutions. Chem. Eng. J. Lausanne 2011, 174, 296–303. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Shen, Z.; Li, L.; Li, X.; Mao, S. L-aspartic acid: A crystal modifier for preparation of hemihydrate from phosphogypsum in CaCl2 solution. J. Cryst. Growth 2019, 511, 48–55. [Google Scholar] [CrossRef]
- Mi, Y.; Chen, D.; Wang, S. Utilization of phosphogypsum for the preparation of α-calcium sulfate hemihydrate in chloride-free solution under atmospheric pressure. J. Chem. Technol. Biotechnol. 2018, 93, 2371–2379. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Yang, G.; Pan, Z.; Ni, X.; Xu, H.; Huang, Q. Effect of pH and succinic acid on the morphology of α-calcium sulfate hemihydrate synthesized by a salt solution method. J. Cryst. Growth 2013, 374, 31–36. [Google Scholar] [CrossRef]
- Guan, B.; Yang, L.; Wu, Z.; Shen, Z.; Ma, X.; Ye, Q. Preparation of α-calcium sulfate hemihydrate from FGD gypsum in K, Mg-containing concentrated CaCl2 solution under mild conditions. Fuel 2009, 88, 1286–1293. [Google Scholar] [CrossRef]
- Li, Z.; Demopoulos, G.P. Model-based construction of calcium sulfate phase-transition diagrams in the HCl-CaCl2-H2O system between 0 and 100 °C. Ind. Eng. Chem. Res. 2006, 45, 4517–4524. [Google Scholar] [CrossRef]
- Chung, S.; Kim, J.; Ban, E.; Yun, J.; Park, B.; Kim, A. Solution-Mediated Phase Transformation of Aripiprazole: Negating the Effect of Crystalline Forms on Dissolution and Oral Pharmacokinetics. J. Pharm. Sci. 2020, 109, 3668–3677. [Google Scholar] [CrossRef]
- Ouyang, J.; Chen, J.; Rosbottom, I.; Chen, W.; Guo, M.; Heng, J.Y. Supersaturation and solvent dependent nucleation of carbamazepine polymorphs during rapid cooling crystallization. CrystEngComm 2021, 23, 813–823. [Google Scholar] [CrossRef]
- Fang, C.; Tang, W.; Wu, S.; Wang, J.; Gao, Z.; Gong, J. Ultrasound-assisted intensified crystallization of L-glutamic acid: Crystal nucleation and polymorph transformation. Ultrason. Sonochem. 2020, 68, 105227. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Jiang, G.; Fu, H.; Yang, L.; Wu, Z. Thermodynamic Preparation Window of Alpha Calcium Sulfate Hemihydrate from Calcium Sulfate Dihydrate in Non-Electrolyte Glycerol-Water Solution under Mild Conditions. Ind. Eng. Chem. Res. 2011, 50, 13561–13567. [Google Scholar] [CrossRef]
- Özen, M.; Mertens, M.; Snijkers, F.; Cool, P. Hydrothermal synthesis and formation mechanism of tetragonal barium titanate in a highly concentrated alkaline solution. Ceram. Int. 2016, 42, 10967–10975. [Google Scholar] [CrossRef]
- Ma, B.; Lu, W.; Su, Y.; Li, Y.; Gao, C.; He, X. Synthesis of α-hemihydrate gypsum from cleaner phosphogypsum. J. Clean. Prod. 2018, 195, 396–405. [Google Scholar] [CrossRef]
- Lu, W.; Ma, B.; Su, Y.; He, X.; Jin, Z.; Qi, H. Preparation of α-hemihydrate gypsum from phosphogypsum in recycling CaCl2 solution. Constr. Build. Mater. 2019, 214, 399–412. [Google Scholar] [CrossRef]
- Fu, H.; Jiang, G.; Wang, H.; Wu, Z.; Guan, B. Solution-Mediated Transformation Kinetics of Calcium Sulfate Dihydrate to α-Calcium Sulfate Hemihydrate in CaCl2 Solutions at Elevated Temperature. Ind. Eng. Chem. Res. 2013, 52, 17134–17139. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hosseini, M.H.R.; Masoudi, A.A. Avrami behavior of magnetite nanoparticles formation in co-precipitation process. J. Min. Metall. 2011, 47, 211–218. [Google Scholar] [CrossRef]
- Marzpour Shalmani, F.; Halladj, R.; Askari, S. An investigation of the crystallization kinetics of zeotype SAPO-34 crystals synthesized by hydrothermal and sonochemical methods. Ultrason. Sonochem. 2016, 29, 354–362. [Google Scholar] [CrossRef]
- Rossetti, G.A.; Watson, D.J.; Newnham, R.E.; Adair, J.H. Kinetics of the hydrothermal crystallization of the perovskite lead titanate. J. Cryst. Growth 1992, 116, 251–259. [Google Scholar] [CrossRef]
- Hassanzadeh, N.; Sadrnezhaad, S.K.; Ghorbanzadeh, M. An investigation of crystallization kinetics of the Na3MnCO3PO4 cathode material, synthesized by the hydrothermal method. Mater. Chem. Phys. 2018, 214, 73–79. [Google Scholar] [CrossRef]
- Guan, Q.; Hu, Y.; Tang, H.; Sun, W.; Gao, Z. Preparation of α-CaSO4•½H2O with tunable morphology from flue gas desulphurization gypsum using malic acid as modifier: A theoretical and experimental study. J. Colloid Interface Sci. 2018, 530, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Sun, W.; Hu, Y.; Yin, Z.; Guan, C. A facile method of transforming FGD gypsum to α-CaSO4•0.5H2O whiskers with cetyltrimethylammonium bromide (CTAB) and KCl in glycerol-water solution. Sci. Rep. 2017, 7, 7085. [Google Scholar] [CrossRef] [PubMed]
- Niphadkar, P.S.; Tangale, N.P.; Joshi, P.N.; Awate, S.V. Crystallization kinetics of Sn-MFI molecular sieve formation by dry gel conversion method. Microporous Mesoporous Mater. 2013, 182, 73–80. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Wang, Y.; Chen, S.; Wang, T.; Yan, Y. Synthesis and crystallization kinetics of ZSM-5 without organic template from coal-series kaolinite. Microporous Mesoporous Mater. 2014, 184, 134–140. [Google Scholar] [CrossRef]
- Gualtieri, A.; Norby, P.; Artioli, G.; Hanson, J. Kinetics of formation of zeolite Na-A [LTA] from natural kaolinites. Phys. Chem. Miner. 1997, 24, 191–199. [Google Scholar] [CrossRef]
- Turta, N.A.; Veltri, M.; Vuono, D.; De Luca, P.; Bilba, N.; Nastro, A. Effect of crystallization temperature on the synthesis of ETS-4 and ETS-10 titanosilicates. J. Porous Mater. 2009, 16, 527. [Google Scholar] [CrossRef]
- Uzcátegui, D.; González, G. Study of the kinetics of crystallization of zeolite MEL. Catal. Today 2005, 107–108, 901–905. [Google Scholar] [CrossRef]
- House, J.E. Principles of Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).