Pressure-Induced Structural Phase Transition and Metallization in Ga2Se3 Up to 40.2 GPa under Non-Hydrostatic and Hydrostatic Environments
Abstract
:1. Introduction
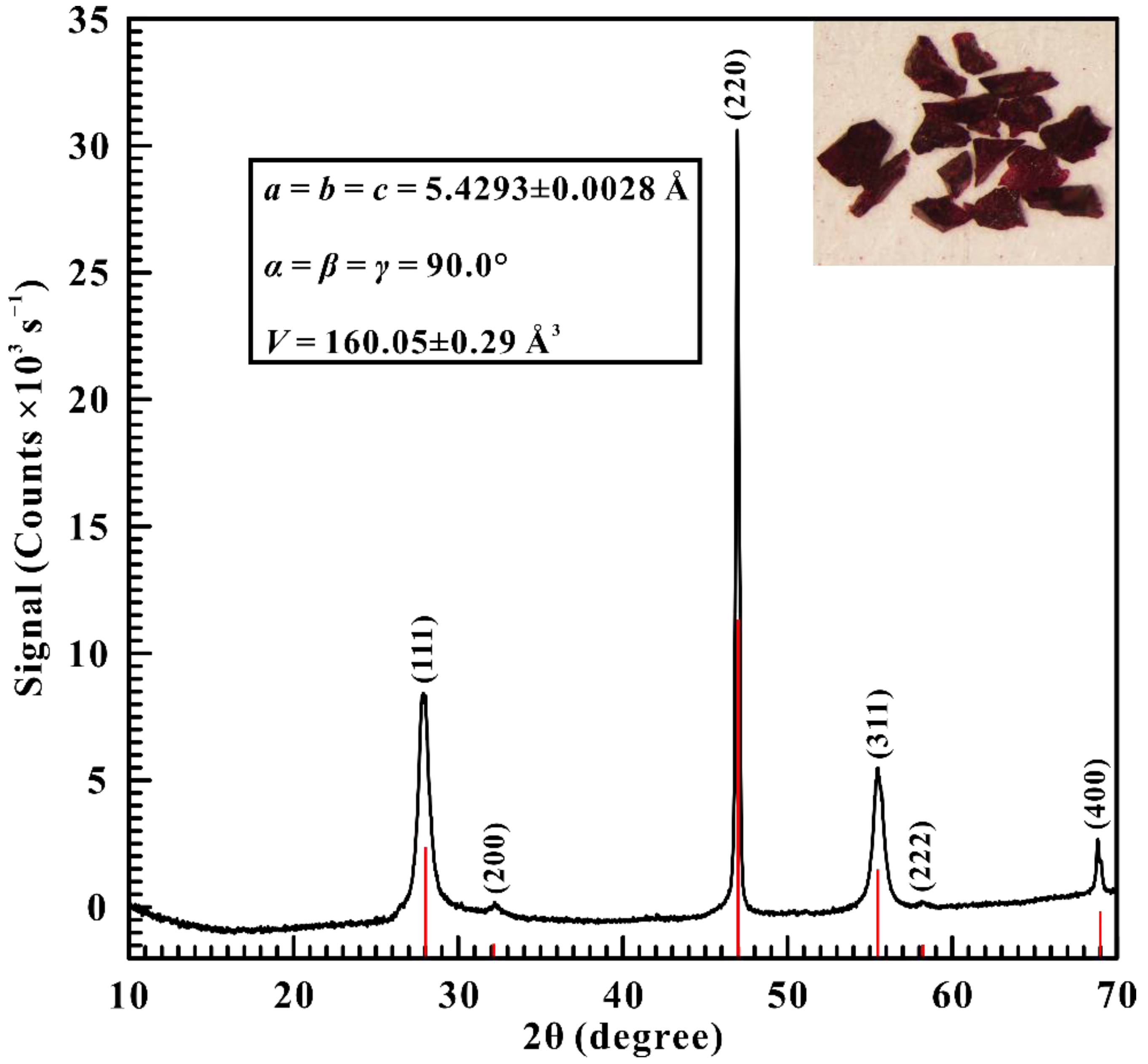
2. Materials and Methods
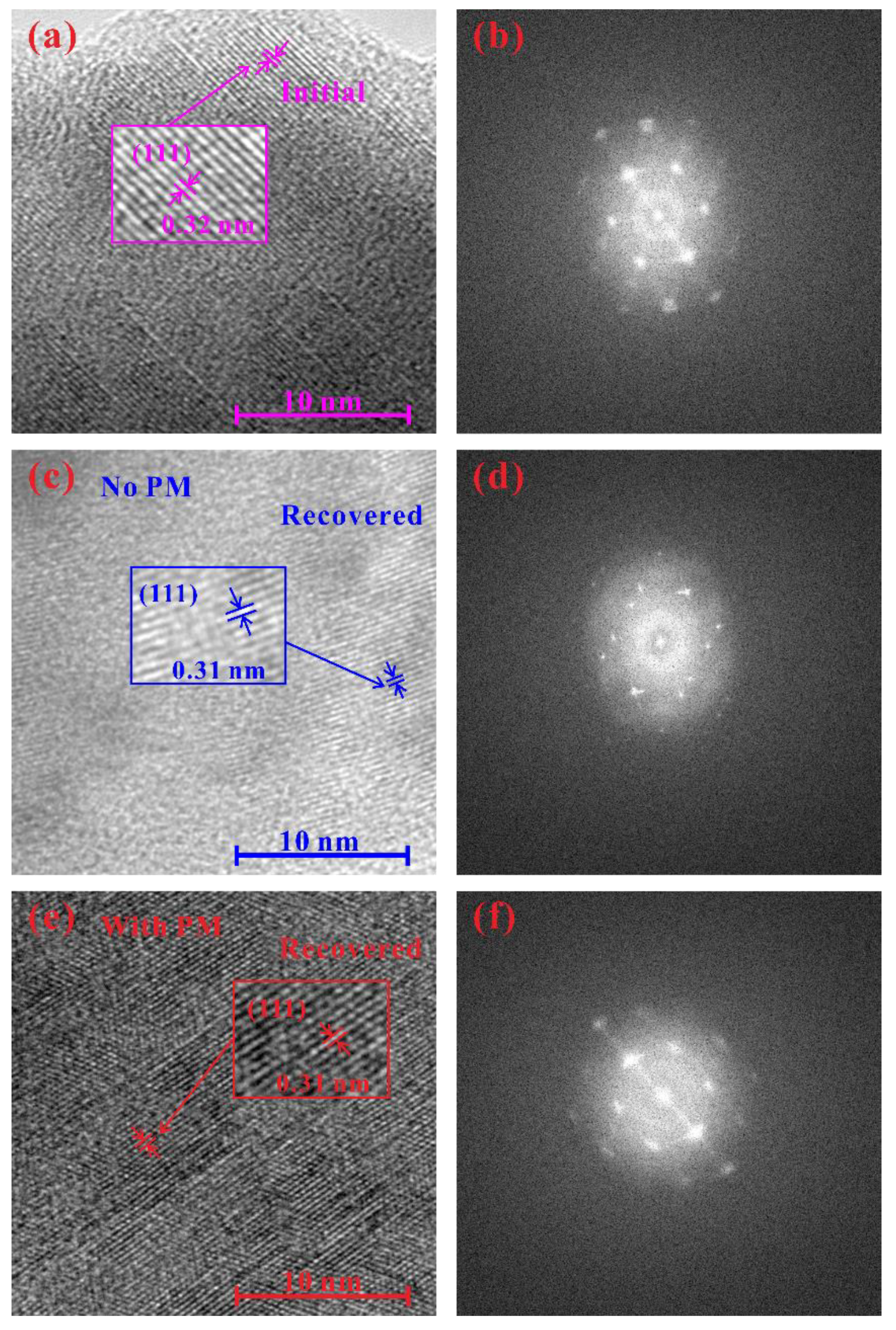
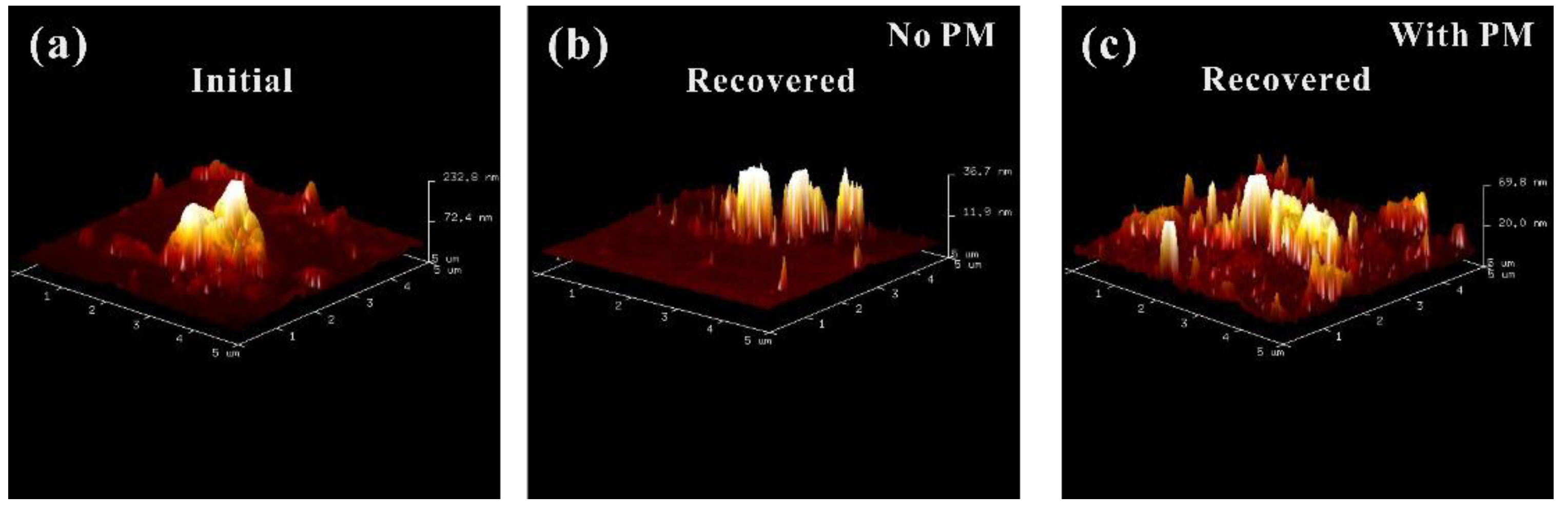
3. Results and Discussion
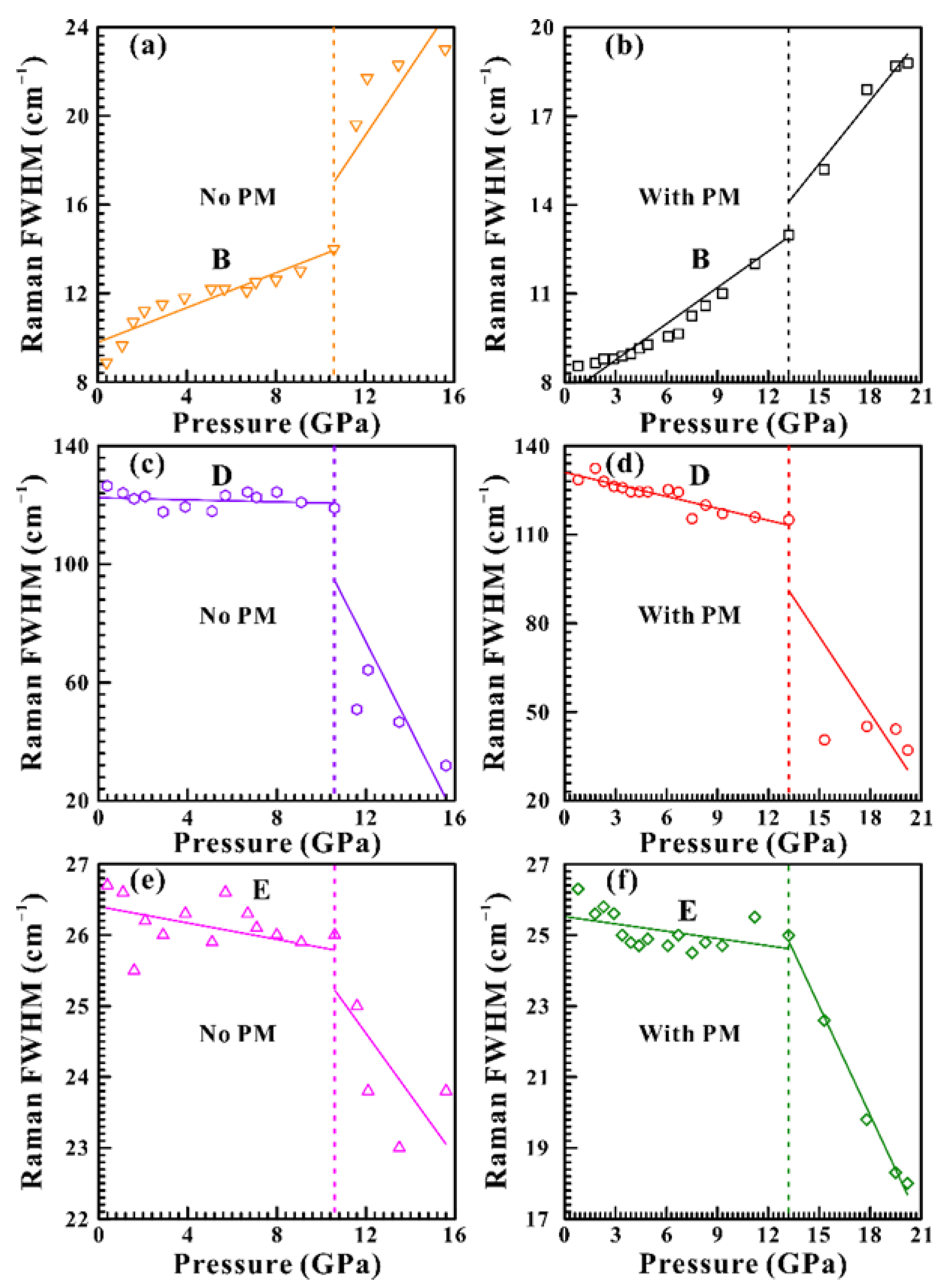
3.1. High-Pressure Raman Spectra Results
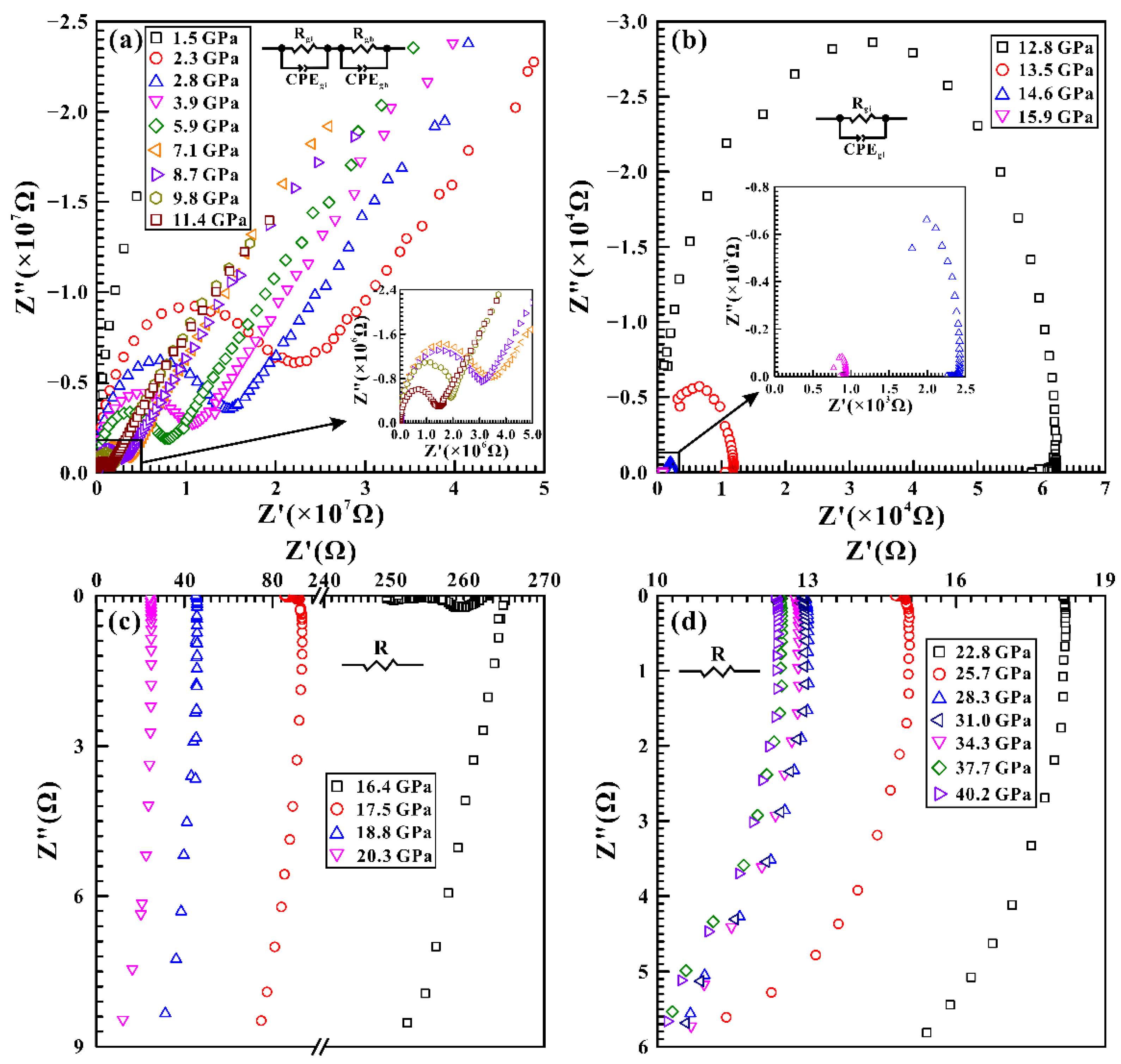
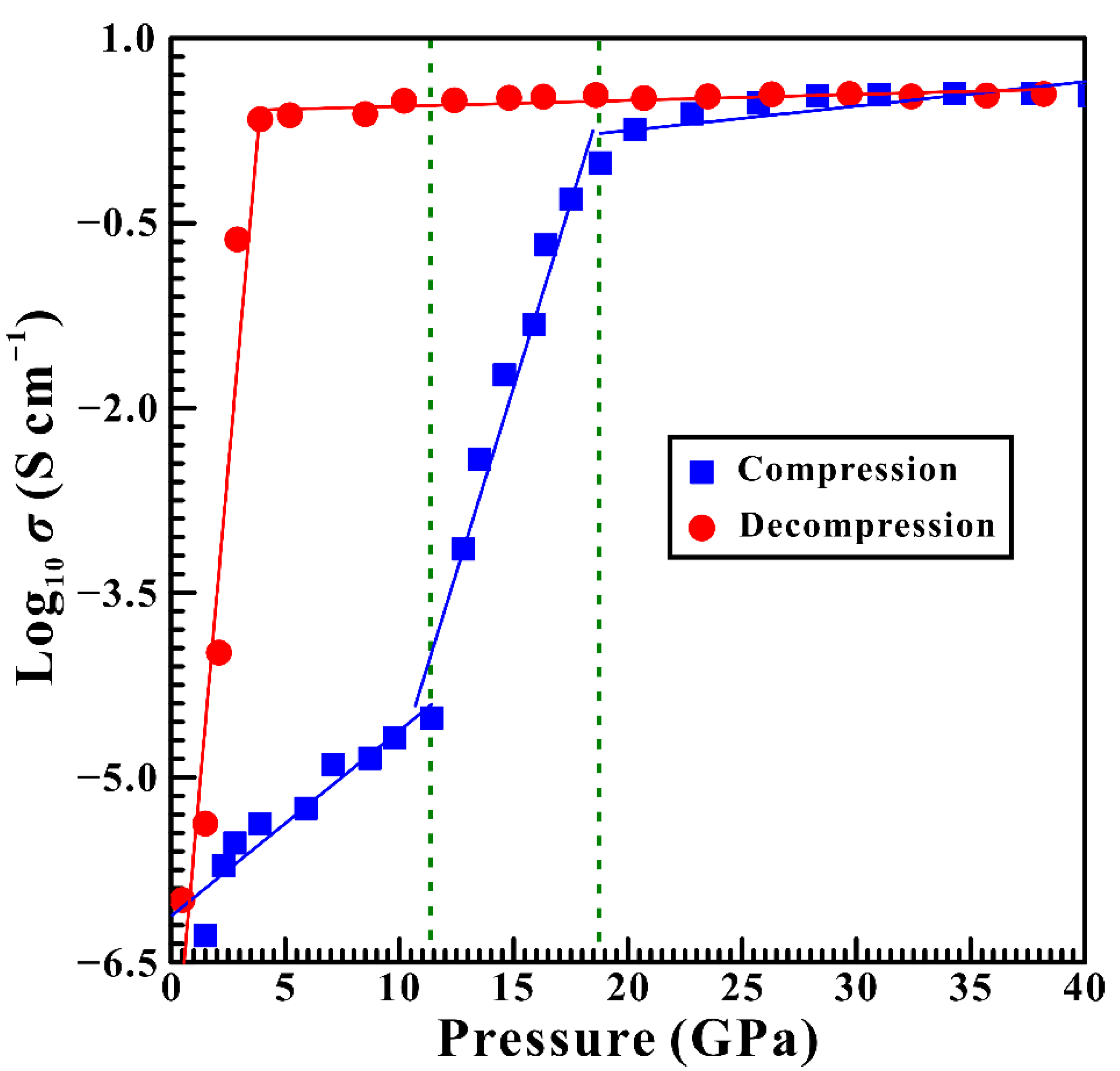
3.2. High-Pressure Electrical Conductivity Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurevich, Y.G.; Koshkin, V.M.; Volovichev, I.N. The heterocontact of two intrinsic semiconductors and radiation-stable electronics. Solid-State Electron. 1995, 38, 235–242. [Google Scholar] [CrossRef]
- Guler, I.; Isik, M.; Gasanly, N.M.; Gasanova, L.G.; Babayeva, R.F. Structural and optical properties of Ga2Se3 crystals by spectroscopic ellipsometry. J. Electron. Mater. 2019, 48, 2418–2422. [Google Scholar] [CrossRef]
- Ho, C.H. Ga2Se3 defect semiconductors: The study of direct band edge and optical properties. ACS Omega 2020, 5, 18527–18534. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.P.; Cheng, X.Y.; Sun, Z.D.; Chi, Y.; Liu, B.W.; Jiang, X.M.; Li, S.F.; Xue, H.G.; Deng, S.Q.; Duppel, V.; et al. Large SHG effect and high LIDT observed coexisting in gallium selenide: A simple but perfect case. Angew. Chem. Int. Ed. 2019, 58, 8087–8091. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, K.V. X-ray-induced conductivity in gallium sesqui-selenide. Tech. Phys. Lett. 2008, 34, 964–966. [Google Scholar] [CrossRef]
- Yitamben, E.N.; Lovejoy, T.C.; Pakhomov, A.B.; Heald, S.M.; Negusse, E.; Arena, D.; Ohuchi, F.S.; Olmstead, M.A. Correlation between morphology, chemical environment, and ferromagnetism in the intrinsic-vacancy dilute magnetic semiconductor Cr-doped Ga2Se3/Si (001). Phys. Rev. B 2011, 83, 045203. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.J.; Zhou, Y.N.; Cui, Y.H.; Fu, Z.W. Ga2Se3 thin film as a negative electrode material for lithium-ion batteries. ECS Electrochem. Lett. 2012, 1, A7–A9. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, H.J.; Li, J.W.; Wang, T.; Wan, L.H.; Guo, H.; Wei, Y.D. Data-driven systematic search of promising photocatalysts for water splitting under visible light. J. Phys. Chem. Lett. 2019, 10, 5211–5218. [Google Scholar] [CrossRef]
- Dai, L.D.; Zhuang, Y.K.; Li, H.P.; Wu, L.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Pu, C. Pressure-induced irreversible amorphization and metallization with a structural phase transition in arsenic telluride. J. Mater. Chem. C 2017, 5, 12157–12162. [Google Scholar] [CrossRef]
- Dai, L.D.; Liu, K.X.; Li, H.P.; Wu, L.; Hu, H.Y.; Zhuang, Y.K.; Yang, L.F.; Pu, C.; Liu, P.F. Pressure-induced irreversible metallization accompanying the phase transitions in Sb2S3. Phys. Rev. B 2018, 97, 024103. [Google Scholar] [CrossRef]
- Liu, K.X.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Yang, L.F.; Pu, C.; Hong, M.L.; Liu, P.F. Phase transition and metallization of orpiment by Raman spectroscopy, electrical conductivity and theoretical calculation under high pressure. Materials 2019, 12, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.X.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Yang, L.F.; Pu, C.; Hong, M.L. Evidences for phase transition and metallization in β-In2S3 at high pressure. Chem. Phys. 2019, 524, 63–69. [Google Scholar] [CrossRef]
- Yang, L.F.; Jiang, J.J.; Dai, L.D.; Hu, H.Y.; Hong, M.L.; Zhang, X.Y.; Li, H.P.; Liu, P.F. High-pressure structural phase transition and metallization in Ga2S3 under non-hydrostatic and hydrostatic conditions up to 36.4 GPa. J. Mater. Chem. C 2021, 9, 2912–2918. [Google Scholar] [CrossRef]
- Savchenko, K.V.; Shchennikov, V.V. A phase transition in Ga2Se3 under high pressure. Can. J. Phys. 1994, 72, 681–682. [Google Scholar] [CrossRef]
- Takumi, M.; Hirata, A.; Ueda, T.; Koshio, Y.; Nishimura, H.; Nagata, K. Structural phase transitions of Ga2Se3 and GaSe under high pressure. Phys. Status Solidi (B) 2001, 223, 423–426. [Google Scholar] [CrossRef]
- Zhao, J.G.; Yang, L.X. Structure evolutions and metallic transitions in In2Se3 under high pressure. J. Phys. Chem. C 2014, 118, 5445–5452. [Google Scholar] [CrossRef]
- Shchennikov, V.V.; Savchenko, K.V.; Popova, S.V. Electrical properties of the high-pressure phases of gallium and indium tellurides. Phys. Solid State 2000, 42, 1036–1040. [Google Scholar] [CrossRef]
- Dai, L.D.; Pu, C.; Li, H.P.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Hong, M.L. Characterization of metallization and amorphization for GaP under different hydrostatic environments in diamond anvil cell up to 40.0 GPa. Rev. Sci. Instrum. 2019, 90, 066103. [Google Scholar] [CrossRef]
- Pu, C.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Hong, M.L. Pressure-induced phase transitions of ZnSe under different pressure environments. AIP Adv. 2019, 9, 025004. [Google Scholar] [CrossRef] [Green Version]
- Finkman, E.; Tauc, J.; Kershaw, R.; Wold, A. Lattice dynamics of tetrahedrally bonded semiconductors containing ordered vacant sites. Phys. Rev. B 1975, 11, 3785–3794. [Google Scholar] [CrossRef]
- Yamada, A.; Kojima, N.; Takahashi, K.; Okamoto, T.; Konagai, M. Raman study of epitaxial Ga2Se3 films grown by molecular beam epitaxy. Jpn. J. Appl. Phys. 1992, 31, L186–L188. [Google Scholar] [CrossRef]
- Ho, C.H.; Lai, X.R.; Chuang, C.A.; Kuo, W.L.; Tiong, K.K. The study of optical properties of III2–VI3 defect semiconductor group compounds Ga2S3, Ga2Se3, In2S3, and In2Se3. Adv. Photonics Res. 2021, 2, 2000110. [Google Scholar] [CrossRef]
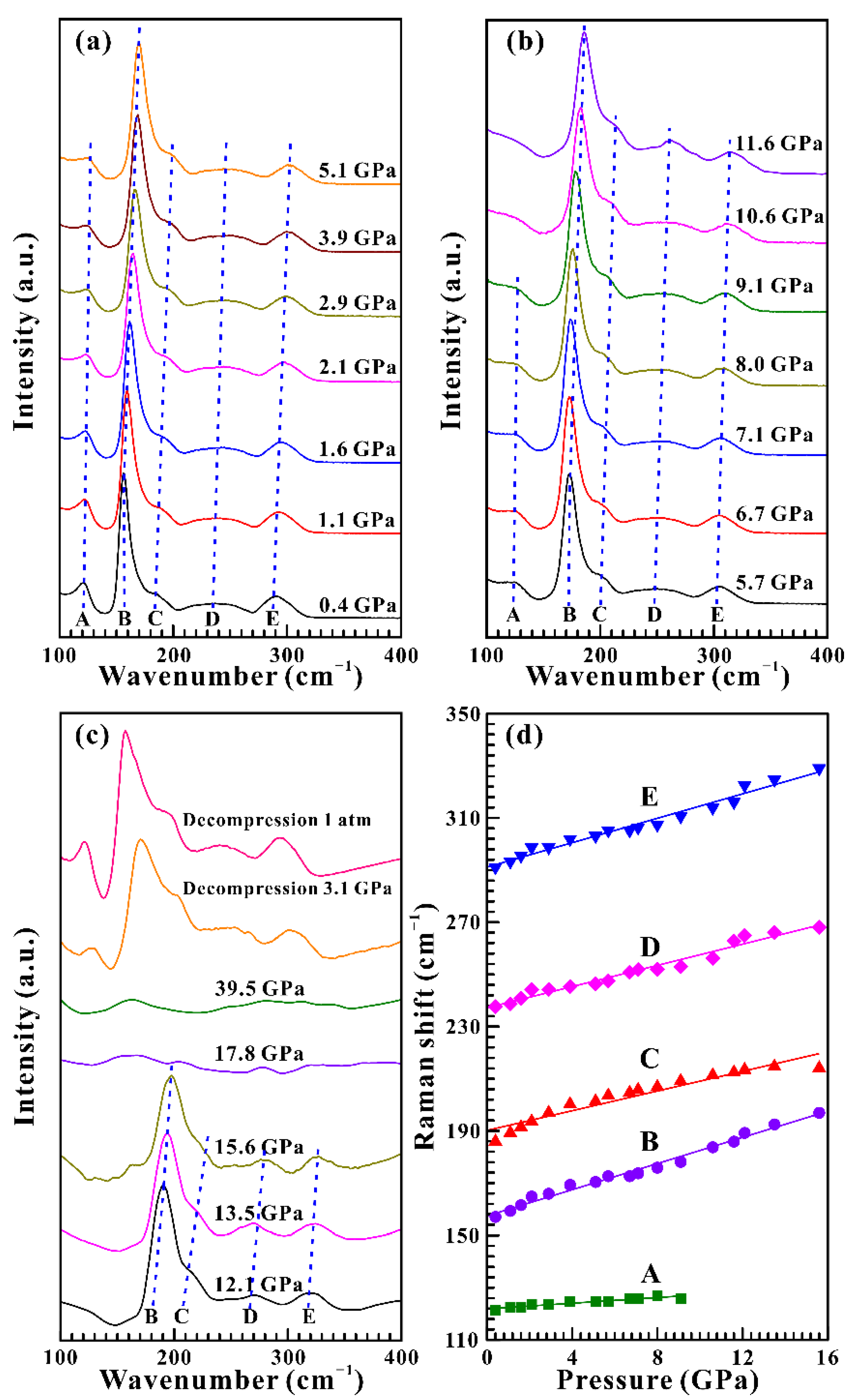


| Pressure Condition | Pressure (GPa) | ω (cm−1) | dω/dP (cm−1 GPa−1) | ω (cm−1) | dω/dP (cm−1 GPa−1) |
|---|---|---|---|---|---|
| Non-hydrostatic | 0.4–15.6 | 121.3 (A) | 0.54 | 157.0 (B) | 2.51 |
| 185.9 (C) | 1.89 | 237.6 (D) | 2.03 | ||
| 291.1 (E) | 2.34 | ||||
| Hydrostatic | 0.8–20.2 | 122.5 (A) | 0.45 | 157.0 (B) | 2.56 |
| 185.9 (C) | 2.17 | 237.6 (D) | 2.12 | ||
| 292.2 (E) | 2.30 |
| Pressure Condition | Pressure (GPa) | ω (cm−1) | dF/dP (cm−1 GPa−1) | ω (cm−1) | dF/dP (cm−1 GPa−1) | ω (cm−1) | dF/dP (cm−1 GPa−1) |
|---|---|---|---|---|---|---|---|
| Non-hydrostatic | 0.4–10.6 | 157.0 (B) | 0.39 | 237.6 (D) | –0.18 | 291.1 (E) | –0.031 |
| 10.6–15.6 | 183.7 (B) | 1.51 | 256.2 (D) | –13.87 | 313.9 (E) | –0.43 | |
| Hydrostatic | 0.8–13.2 | 157.0 (B) | 0.36 | 237.6 (D) | –1.34 | 292.2 (E) | –0.068 |
| 13.2–20.2 | 192.5 (B) | 0.87 | 262.1 (D) | –8.67 | 322.5 (E) | –1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Dai, L.; Hu, H.; Zhang, X. Pressure-Induced Structural Phase Transition and Metallization in Ga2Se3 Up to 40.2 GPa under Non-Hydrostatic and Hydrostatic Environments. Crystals 2021, 11, 746. https://doi.org/10.3390/cryst11070746
Hong M, Dai L, Hu H, Zhang X. Pressure-Induced Structural Phase Transition and Metallization in Ga2Se3 Up to 40.2 GPa under Non-Hydrostatic and Hydrostatic Environments. Crystals. 2021; 11(7):746. https://doi.org/10.3390/cryst11070746
Chicago/Turabian StyleHong, Meiling, Lidong Dai, Haiying Hu, and Xinyu Zhang. 2021. "Pressure-Induced Structural Phase Transition and Metallization in Ga2Se3 Up to 40.2 GPa under Non-Hydrostatic and Hydrostatic Environments" Crystals 11, no. 7: 746. https://doi.org/10.3390/cryst11070746
APA StyleHong, M., Dai, L., Hu, H., & Zhang, X. (2021). Pressure-Induced Structural Phase Transition and Metallization in Ga2Se3 Up to 40.2 GPa under Non-Hydrostatic and Hydrostatic Environments. Crystals, 11(7), 746. https://doi.org/10.3390/cryst11070746









