Abstract
The ferrite aluminate cement (FAC) could rapidly lose fluidity or workability due to its excessive hydration rate, and greatly reduce the construction performance. Chemical admixtures are commonly used to provide the workability of cement-based materials. In this study, to ensure required fluidity of FAC, chemically different water reducing agents are incorporated into the FAC pastes. The experiments are performed with aliphatic water reducing agent (AP), polycarboxylic acid water reducing agent (PC) and melamine water reducing agent (MA), respectively. Influence of the water reducing agents on fluidity, setting time, hydration process, hydration product and zeta potential of the fresh cement pastes is investigated. The results show that PC has a better dispersion capacity compared to AP and MA. Besides decreasing water dosage, PC also acts as a retarder, significantly increasing the setting times, delaying the hydration rate and leading to less ettringite in the hydration process of FAC particles. The water reducing agents molecules are adsorbed on the surface of positively charged minerals and hydration products, however, for PC, steric hindrance from the long side chain of PC plays a critical role in dispersing cement particles, whereas AP and MA acting through an electrostatic repulsion force.
1. Introduction
As the most important building material, Portland cement has been widely used in engineering construction worldwide. However, in the manufacturing process of Portland cement clinker, the decarbonation and calcination process produce approximately 5–8% of global anthropogenic CO2 emissions [1,2,3,4,5]. One of the ways to reduce CO2 emission from Portland cement production is to replace Portland cement with Calcium sulfoaluminate. Compared with Portland cement clinker, the CO2 emissions are relatively lower owing to the lower production temperature and lime saturation factor of Calcium sulfoaluminate clinker [6,7,8,9].
Calcium sulfoaluminate cement is often used for emergency repairing including bridge decks, airport runways, municipal roads and so on, due to its better performances than PC, such as rapid setting, negative hardening temperature, high early strength, small volume expansion and anti-permeability [10,11,12]. Therefore, Calcium sulfoaluminate cement is considered as an alternative binder to PC [13]. However, despite the increasing interests and excellent advantages of this cement, the depletion of alumina-bearing raw materials such as bauxite is also the challenging issure of the Calcium sulfoaluminate-based materials, which limits its industrial-scale production and wider application [8,14]. Therefore, lowering the consumption of aluminum material and seeking alternative alumina sources are inevitable and essential requirements in the Calcium sulfoaluminate clinker production. In recent years, even though red mud and fly ash, which are Al-rich industrial wastes, have been successfully used to produce Calcium sulfoaluminate clinker in the laboratory, drawback of these industrial wastes is their low Al2O3 content, owing to the high content of SiO2 [15,16,17,18,19]. These industrial wastes still can not meet the requirement of aluminum materials in Calcium sulfoaluminate raw meal [14]. To reduce the demand for bauxite, another strategy is to partially substitute the aluminum material in Calcium sulfoaluminate cement with ferrite material [20,21,22]. The ferrite aluminate cement (FAC) is prepared by partially replacing Al2O3 in Calcium sulfoaluminate clinker with Fe2O3, and the substitution level depends on the F/A ratio of the raw mixtures [23].
The FAC paste will rapidly lose fluidity or workability due to its excessive hydration rate, and greatly reduce its construction performance. Water reducing agents is a kind of important chemical admixture for Portland cement-based materials [24,25,26,27]. They can increase the workability of fresh concrete without additional water, or reduce the amount of water for a given fluidity [28,29,30,31]. At present, very few researches concerning the effect of water reducing agents on the early performance of FAC paste are reported, and how the water reducing agents regulate the hydration process of FAC paste is still not clear. In the present work, the impact of water reducing agents on the early properties of FAC is investigated by evaluating the fluidity, setting time, hydration process, hydration product and zeta potential of fresh pastes. The resultings data of chemically different water reducing agents in FAC can provide a fundamental understanding for the industrial application.
2. Experimental Section
2.1. Materials
Water Reducing Agent. Aliphatic water reducing agent (AP) and polycarboxylic acid water reducing agent (PC), melamine water reducing agent (MA), were used to investigate the compatibility with ferrite aluminate cement (FAC), respectively. The AP (powder) and PC (solution, solid content: 50.5%), MA (powder) were purchased from BASF Advanced Chemicals Co., Ltd., Shanghai, China.
Cement. The FAC used in the experiment was obtained from Yicheng Anda Special Cement Co., Ltd., Yicheng, Hubei, China. And its chemical composition is shown in Table 1.

Table 1.
The Chemical Composition of FAC.
2.2. Characterization and Test
X-ray Diffraction (XRD): After curing to the test age, the hydration of FAC pastes was stopped by submerging the crushed pastes in anhydrous ethanol for 24 h. Then, the sample was ground in agate mortar. Crystal phases were characterized by XRD (Supernova, Japan, Cu Kα) in the range from 5° to 60°, with operating voltage of 40 kV, working current of 40 mA, and step size of 0.02°.
Fluidity: 300 g of FAC, water reducing agent and water were mixed by sterring at low speed for 2 min in a mixer. After that, the FAC mixture was allowed to rest for 15 s and then stirred at high speed for 2 min. After mixing, the mixture was transferred into a mini-slump cone open at both ends (upper diameter of 36 mm, bottom diameter of 60 mm, and height of 60 mm) to measure the fluidity. For all samples, the ratio of water to FAC (Water/Cement) was 0.29.
Setting Time: After fluidity test, the pastes were placed into a standard curing cabinet to study the effect of water reducing agents on the setting time of FAC, and Vicat apparatus was used according to Chinese National Standard GB/T 1346-2011. The temperature was 20 ± 1 °C, and the relative humidity was 95 ± 1% in the standard curing cabinet.
Calorimetric Measurements: An isothermal calorimetry (TAM Air, TA instruments, USA) was performed to determine how water reducing agents influence FAC hydration kinetics with Water/Cement ratio of 0.29. The tests of hydration exothermic rate and hydration heat during the first 30 h were executed at 25 °C. After initial FAC and water reducing agent solution contact in ampoule, the ampoule (11.61 g FAC paste) was closed and placed into the instrument as soon as possible. After 30 h, the measurement was stopped, and the hydration exothermic rate and hydration heat was obtained. Furthermore, a reference sample without water reducing agent was simultaneously prepared and measured.
Zeta potential: Zeta potential of FAC pastes with different water reducing agents was determined by a zeta-potential instrument (NanoZS90, Malvern Instruments, Ltd., Malvern, UK) with a fixed. The Water/Cement ratio was fixed at 50. The FAC was added to water containing a certain amount of water reducing agents, and the suspension was poured into the sample cell. Then, zeta potential tests were conducted..
3. Results and Discussion
3.1. Minerals of FAC Particles
It could be seen from Table 1 and Figure 1 that a FAC particle contained cement minerals and gypsum in interstitial phase. The cement minerals contained calcium sulphoaluminate (C4A3ŝ) and belite (C2S), and Al was partially replaced by Fe () in C4A3ŝ.
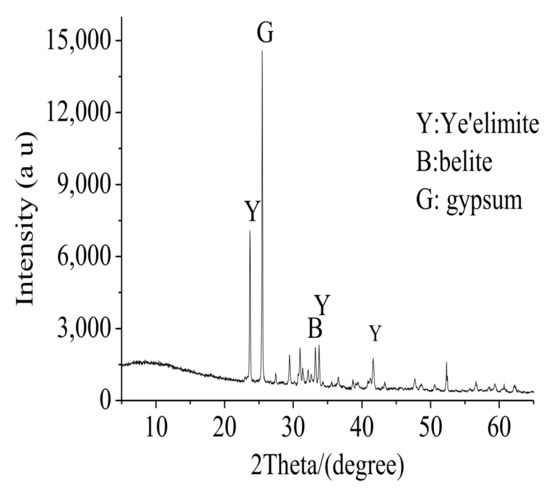
Figure 1.
FAC minerals.
3.2. Structure of Water Reducing Agents
AP was an anionic surfactant with chain-like molecular structure, containing carboxylate, sulfonate and hydroxyl active groups.The second employed polymer was comb-shaped PC, which consisted of main backbone with carboxylate groups and side chains. The third polymer-based water reducing agent used was MA, which was a water-soluble melamine sulfonate formaldehyde polymer with strong anions. The molecular structure of AP, PC and MA were presented in Figure 2, respectively.
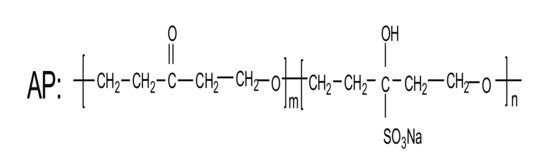
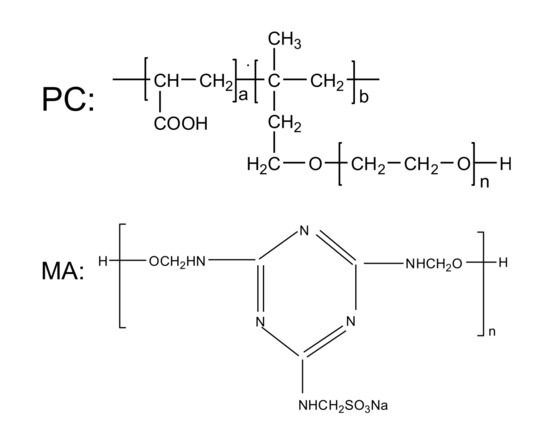
Figure 2.
Molecular structure of three water reducing agents.
3.3. Flow Test of FAC
In measuring process of dispersing properties for the water reducing agents, proportions of water reducing agents added into the samples were investigated for a given fluidity (180 mm, 240 mm, 270 mm, respectively). Figure 3 exhibited the fraction variation of water reducing agents with fluidity of FAC paste. The FAC paste almost could not flow when the water reducing agents were not added, and the fluidity value was too small to be recorded. It could be seen from Figure 3 that addition of the water reducing agents indeed improved the fluidity of FAC paste. When the fluidity was 180 mm, dosages of the water reducing agents were 5‰ (AP), 0.84‰ (PC) and 4‰ (MA), respectively. An increased dosage was observed for higher fluidity. The dosages increased to 5.5‰ (AP), 1.5‰ (PC), 5‰ (MA) and 7‰ (AP), 2.5‰ (PC), 6.5‰ (MA), when fluidity increased to 240 mm and 270 mm, respectively. For a given fluidity (180 mm, 240 mm and 270 mm), dosage of the water reducing agents was in the following order: AP > MA > PC. In other words, compared with AP and MA, PC had a better dispersion capacity.
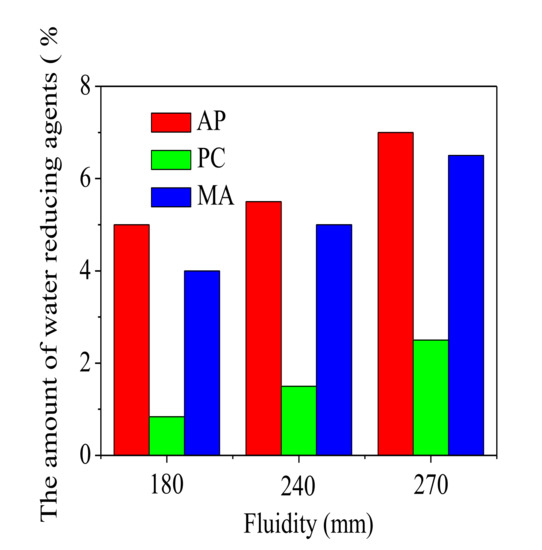
Figure 3.
Flow test of FAC pastes.
3.4. Setting Time of FAC
Setting time of the FAC pastes mixed with different water reducing agents was presented in Figure 4. The figure indicated that the initial and final setting times of blank FAC paste (without water reducing agents) were 1.05 h and 1.41 h, respectively. It could be clearly seen that both initial and final setting times of FAC pastes increased with the water reducing agents added. The setting time of AP modified FAC pastes increased slightly when the AP water reducing agent dosage increased from 0‰ to 7‰. For a given fluidity (180 mm, 240 mm and 270 mm), setting time of the FAC pastes mixed with MA water reducing agent was longer than that with AP. However, initial and final setting times of the pastes increased significantly with increasing dosage of the PC water reducing agent. When the content of PC water reducing agent was 2.5‰, initial and final setting times of the FAC paste were 480% (6.08 h) and 400% (7.16 h) higher than those of blank FAC paste.
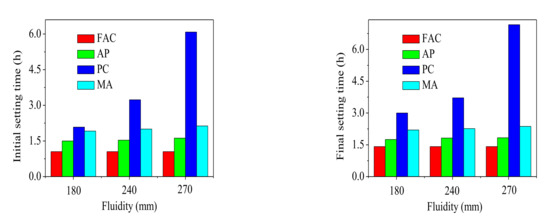
Figure 4.
The effect of water reducing agents on setting times of the FAC pastes.
3.5. Early Hydration Kinetics of FAC
Hydration kinetic comparisons of the FAC pastes with different water reducing agents at the fluidity of 240 mm was conducted. Hydration exothermic rate and accumulative heat of these FAC pastes with ongoing hydration process were shown in Figure 5 and Figure 6, respectively. As shown in Figure 5, the hydration process of FAC particles could be divided into five stages: the initial reaction period firstly after adding water, then transited to the induction period, then entered the acceleration period, finally gradually shifted into the deceleration period and the stable period, which was similar to that of Portland cement. However, unlike Portland cement, multiple consecutive exothermic peaks were presented due to the formation of ettringite in the hydration process of FAC particles.
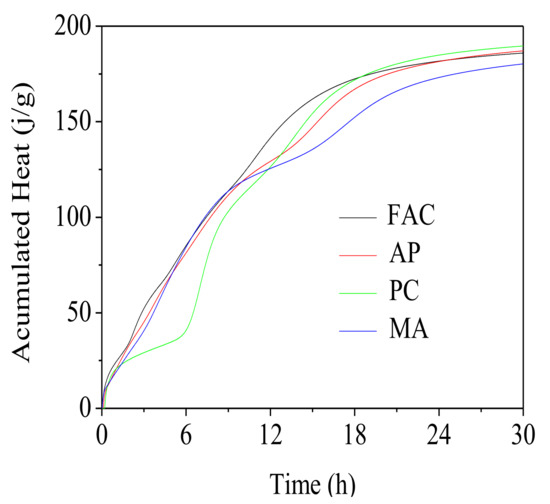
Figure 5.
Heat flow curves for FAC pastes from 0 to 30 h.
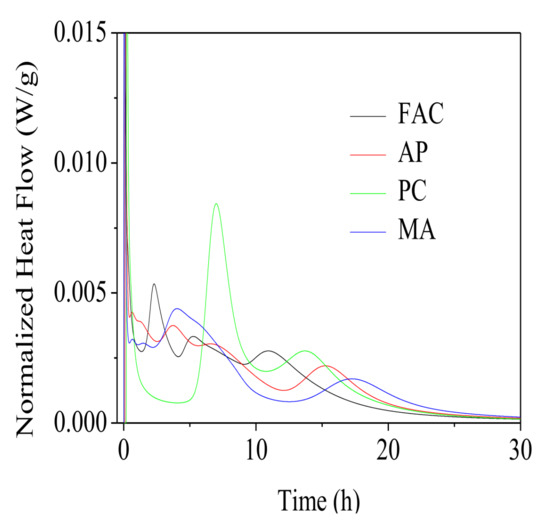
Figure 6.
Cumulative heat curves for FAC pastes from 0 to 30 h.
As Figure 5 and Figure 6 illustrated, a change in the hydration induction period of FAC pastes was observed for three types of water reducing agents used. Presence of these water reducing agents retarded occurrence of the heat evolution peak. Time for dissolution of FAC particles was increased by 1.5 h for paste mixed with AP, 4.7 h for PC, and 1.8 h for MA. Duration of the induction period for PC modified paste was prolonged significantly. Obviously, in comparison with the reference paste, the hydration heat released from PC modified paste before 6 h lowered down greatly, due to its longer setting time. However, the released hydration heat of AP modified paste and MA modified paste before 6 h was not affected. These results were in accordance with the setting times. After 24 h, cumulative heat for the reference paste, and AP, PC, MA modified pastes was 181.68 J/g, 181.49 J/g, 184.83 J/g and 173.14 j/g, separately. It indicated that the water reducing agents did not affect hydration degree of FAC particles within 24 h except for MA.
3.6. XRD Analysis
As water reducing agents were observed to have a significant retarding effect on the hydration kinetics of FAC (as illustrated in Figure 5 and Figure 6), the effect of water reducing agents on phase development at the fluidity of 240 mm was further investigated through XRD studies. XRD patterns for the samples containing water reducing agents hydrated for different curing ages were illustrated in Figure 7. It could be seen from Figure 7 that at the initial stage of hydration (within 5 h), in contrast to FAC material, a large amount of Ye’elimite and gypsum were dissolved in the blank paste, and a small amount of ettringite formed, of which the reaction mechanism was shown in Equation (1). After 11 h, peak intensity of the hydration product increased, indicating that more ettringite formed. While, because Al(OH)3 and Fe(OH)3 gels were poorly crystallized or amorphous, they were not detected in the pastes. It was observed that the incorporation of PC significantly lowered the characteristic peak of ettringite compared with the blank FAC sample at the ages of 5 h and 11 h, which is consistent with the setting time and hydration heat tests. However, for AP and MA modified pastes, there was an increasing trend for the diffraction peak intensity of ettringite, because of their less effect on the setting time.
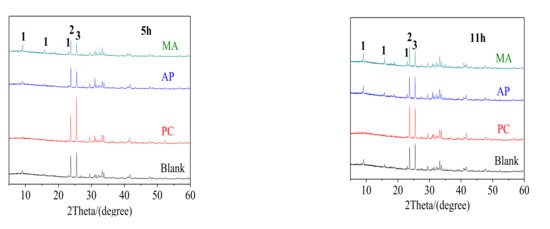
Figure 7.
XRD patterns of samples hydrated for 5 h and 11 h (1-ettringite, 2-Ye’elimite, and 3-gypsum).
3.7. Zeta Potential of the Cement Suspension
In order to analyze the interaction mechanisms between FAC particle and water reducing agents, zeta potential of various FAC pastes was tested. Effects of the water reducing agents on the zeta potential of the FAC pastes were presented in Table 2. As we could see from Table 2, the zeta potential of FAC particles had a positive value (+0.53 mv) due to partial dissolution of C4A3ŝ, and formation of ettringite. When the water reducing agents were added, these negatively charged molecules were adsorbed on surface of the positively charged C4A3ŝ, and ettringite, and then zeta potentials of all the FAC samples were negative. In the meantime, adsorption of water reducing agents reduced the interactions between the FAC particles, so that the whole paste system became more stable.

Table 2.
Effect of Water Reducing Agents on Zeta Potential of the Cement Suspension.
With PC added, zeta potentials (between −0.74 mV and −1.28 mV) of the FAC pastes slightly decreased. However, when MA was added, it induced lower potentials between −12.1 mV and −13.9 mV significantly. The effect of AP was similar to that of MA. Zeta potential values (between −15.50 mV and −17.40 mV) of the AP modified FAC pastes were slightly lower than that of MA modified FAC pastes. Therefore, for AP and MA, the strong decline of zeta potential to more negative values indicated that electrostatic repulsion was the main force for dispersion. While the slightly decreased zeta potential of the PC modified FAC particles indicated that steric repulsion caused by the PC side chains was the key factor for dispersion (Figure 8).
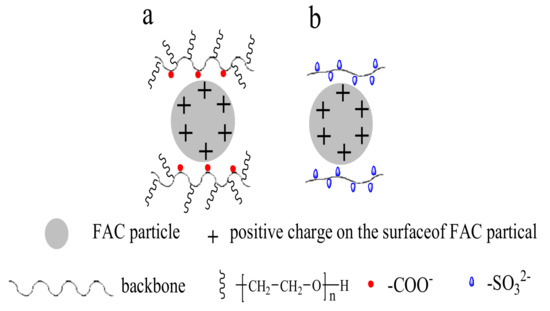
Figure 8.
Schematic illustration for the adsorption of water reducing agents on FAC particle surfaces: (a) PC water reducing agent, (b) AP and MA water reducing agent.
4. Conclusions
In this paper, three types of water reducing agents as dispersants were incorporated into ferrite aluminate cement to increase its fluidity. The effect of water reducing agents on the early hydration of ferrite aluminate cement was studied using isothermal calorimetry, XRD, and zeta potential analysis. The results showed that PC was much more efficient for increasing fluidity of the FAC pastes than AP and MA. However, in comparison with AP and MA, PC water reducing agent increased significantly setting times of the pastes, and delayed immensely hydration rate and formation of hydration product ettringite in the hydration process of FAC particles. The action mechanism of PC water reducing agent was confirmed to be mainly steric resistance, whereas AP and MA acted through an electrostatic repulsion mechanism.
Author Contributions
J.Z. and Y.W. designed the experiments and reviewed the original draft; C.H. performed the experiments and wrote the original draft; Z.C. and J.P. polished the English wrighting. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2019YFC1905103), the Postdoctoral Science Foundation of China (2020M672970) and the Fundamental Research Funds for the Central Universities (20lgpy157).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mugahed, A.; Gunasekaran, M.; Roman, F.; Nikolai, V.; Yuriy, V.; Hakim, A. Palm oil fuel ash-based eco-efficient concrete: A critical review of the short-term properties. Materials 2021, 14, 3–32. [Google Scholar]
- Zhao, J.H.; Tong, L.Y.; Li, B.E.; Chen, T.H.; Wang, C.P.; Yang, G.Q.; Zheng, Y. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Huang, C.L.; Wang, D.M. Surface modification of nano-SiO2 particles with polycarboxylate ether-based superplasticizer under microwave irradiatio. ChemistrySelect 2017, 2, 9349–9354. [Google Scholar] [CrossRef]
- Huang, C.L.; Wang, Y.R.; Zhao, J.H.; Wang, D.M. Potential effect of surface modified nano-SiO2 with PDDA on the cement paste early hydration. ChemistrySelect 2020, 5, 3159–3163. [Google Scholar] [CrossRef]
- Basto, P.D.; Savastano, H.; Neto, A.A.D. Characterization and pozzolanic properties of sewage sludge ashes (SSA) by electrical conductivity. Cem. Concr. Compos. 2019, 104, 103410. [Google Scholar] [CrossRef]
- Hanein, T.; Galvez-Martos, J.L.; Bannerman, M.N. Carbon footprint of calcium sulfoaluminate clinker production. J. Clean. Prod. 2018, 172, 2278–2287. [Google Scholar] [CrossRef] [Green Version]
- Borstnar, M.; Daneu, N.; Dolenec, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Ceram. Int. 2020, 46, 29421–29428. [Google Scholar] [CrossRef]
- Gao, D.Y.; Meng, Y.; Yang, L.; Tang, J.Y.; Lv, M.Y. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Xu, J.T.; Chen, J.W.; Lu, D.Y.; Xu, Z.Z.; Hooton, R.D. Effect of dolomite powder on the hydration and properties of calcium sulfoaluminate cements with different gypsum contents. Constr. Build. Mater. 2019, 225, 302–310. [Google Scholar] [CrossRef]
- Garcia-Mate, M.; De la Torre, A.G.; Leon-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Hu, C.; Hou, D.S.; Li, Z.J. Micro-mechanical properties of calcium sulfoaluminate cement and the correlation with microstructure. Cem. Concr. Compos. 2017, 80, 10–16. [Google Scholar] [CrossRef]
- Li, G.X.; Liu, Q.F.; Niu, M.D.; Cao, L.; Nan, B.; Shi, C. Characteristic of silica nanoparticles on mechanical performance and microstructure of sulphoaluminate cement/ordinary Portland cement binary blends. Constr. Build. Mater. 2020, 242, 118158. [Google Scholar] [CrossRef]
- Zou, D.H.; Wang, K.; Li, H.Y.; Guan, X.M. Effect of LiAl-layered double hydroxides on hydration of calcium sulfoaluminate cement at low temperature. Constr. Build. Mater. 2019, 223, 910–917. [Google Scholar] [CrossRef]
- Huang, Y.B.; Pei, Y.; Qian, J.S.; Gao, X.; Liang, J.; Duan, G.B.; Zhao, P.Q.; Lu, L.C.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Yang, L.; Yan, Y.; Hu, Z.H.; Xie, X.L. Utilization of phosphate fertilizer industry waste for belite-ferroaluminate cement production. Constr. Build. Mater. 2013, 38, 8–13. [Google Scholar] [CrossRef]
- Pace, M.L.; Telesca, A.; Marroccoli, M.; Valenti, G.L. Use of industrial byproducts as alumina sources for the synthesis of calcium sulfoaluminate cements. Environ. Sci. Technol. 2011, 45, 6124–6128. [Google Scholar] [CrossRef]
- Rungchet, A.; Chindaprasirt, P.; Wansom, S.; Pimraksa, K. Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J. Clean. Prod. 2016, 115, 273–283. [Google Scholar] [CrossRef]
- Da Costa, E.B.; Rodríguez, E.D.; Berna, S.A.; Provis, J.L.; Gobbo, L.A.; Kirchheim, A.P. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr. Build. Mater. 2016, 122, 373–383. [Google Scholar] [CrossRef]
- Rungchet, A.; Poon, C.S.; Chindaprasirt, P.; Pimraksa, K. Synthesis of low-temperature calcium sulfoaluminate-belite cements from industrial wastes and their hydration: Comparative studies between lignite fly ash and bottom ash. Cem. Concr. Compos. 2017, 83, 10–19. [Google Scholar] [CrossRef]
- Idrissi, M.; Diouri, A.; Damidot, D.; Greneche, J.M.; AlamiTalbi, M.; Taibi, M. Characterisation of iron inclusion during the formation of calcium sulfoaluminate phase. Cem. Concr. Res. 2010, 40, 1314–1319. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Niu, X.; Song, L. Influence of minor oxides on formation and decomposition of mineral calcium sulfoaluminate. Mater. Res. Innov. 2007, 11, 92–94. [Google Scholar] [CrossRef]
- Lelkhadiri, I.; Diouri, A.; Boukhari, A.; Puertas, F.; Vázquez, T. Obtaining a sulfoaluminate belite cement by industrial waste. Mater. Constr. 2003, 53, 57–69. [Google Scholar]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Wang, B.M.; Pang, B. Mechanical property and toughening mechanism of water reducing agents modified graphene nanoplatelets reinforced cement composites. Constr. Build. Mater. 2019, 226, 699–711. [Google Scholar] [CrossRef]
- Schmid, M.; Plank, J. Dispersing performance of different kinds of polycarboxylate (PCE) superplasticizers in cement blended with a calcined clay. Constr. Build. Mater. 2020, 258, 119576. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Hong, W.L.; Shui, L.L.; Wang, X.F.; Wang, H.R.; Peng, L. Effects of polycarboxylate superplasticisers with various functional groups on the pore structure of cement mortar. Adv. Cem. Res. 2020, 32, 510–518. [Google Scholar] [CrossRef]
- Sheridan, J.; Sonebi, M.; Taylor, S.; Amziane, S. The effect of a polyacrylic acid viscosity modifying agent on the mechanical, thermal and transport properties of hemp and rapeseed straw concrete. Constr. Build. Mater. 2020, 235, 117536. [Google Scholar] [CrossRef]
- Silva, B.; Pinto, A.P.F.; Gomes, A.; Candeias, A. Fresh and hardened state behaviour of aerial lime mortars with superplasticizer. Constr. Build. Mater. 2019, 225, 1127–1139. [Google Scholar] [CrossRef]
- Ma, B.G.; Qi, H.H.; Tan, H.B.; Su, Y.; Li, X.G.; Liu, X.H.; Li, C.B.; Zhang, T. Effect of aliphatic-based superplasticizer on rheological performance of cement paste plasticized by polycarboxylate superplasticizer. Constr. Build. Mater. 2020, 233, 117181. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.H.; Choi, B.I. Influence of pumping pressure on the viscosity curve and rheological stability of mortar incorporating polycarboxylate. Cem. Concr. Compos. 2020, 105, 103419. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, K.; Guan, X.M. Properties of sulfoaluminate cement-based grouting materials modified with LiAl-layered double hydroxides in the presence of PCE superplasticizer. Constr. Build. Mater. 2019, 226, 399–405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).