Abstract
White button mushroom or (Agaricus bisporus) is known as a healthy foodstuff with several nutrients, polyphenols, proteins, and dietary fibers. Mushrooms have a short shelf-life, approximately three to four days at commercial storage and about eight days under chilling conditions. In the current study, titanium dioxide nanoparticles and chitosan films were used as novel active coating materials with the addition of thymol and tween (T and T) as food preservatives to prolong mushroom shelf life up to 12 days. Chitosan, Chitosan-Nano, and Chitosan-Nano/TT were used as coating materials, while water was used as control. Chitosan-Nano/TT film reported the lowest peroxidase activity (0.005 U kg−1 FW) and the highest superoxide dismutase activity (4.033 U kg−1 FW), while catalase activity in Chitosan-Nano film was (0.45 U kg−1 FW). Chitosan-Nano film enhanced the reactive oxygen species production levels, DPPH radicals (74.70%), and malondialdehyde content (1.68 µmol kg−1FW). Chitosan-Nano/TT film preserved the respiration rates (O2 consumption −0.026 mmol s−1kg−1, CO2 production −0.004 mg CO2 kg−1s−1) and increased the phenolic contents (0.38 g kg−1). The results suggested that nano-coating films can increase the oxidation processes which enhanced the quality of the mushrooms.
Keywords:
Agaricus bisporus; coatings; film; storage; reactive oxygen species; total phenols; antioxidant; enzymes 1. Introduction
The natural bioactive compounds and oxidation processes research has increased tremendously due to the safety and health-promoting properties of the compounds [1,2,3,4]. Agaricus bisporus, the commercially cultivated button mushrooms, are known worldwide for their anticancer therapies, antitumor, pharmacological, cholesterol-lowering, immunostimulating, antimicrobial, anti-inflammatory, and antioxidant activities [5,6]. Mushrooms were used as folk medicines, they act as a valuable non-toxic medicine against several diseases such cirrhosis, Alzheimer’s, atherosclerosis, Parkinsonism, diabetes, and hypertension [7]. Mushroom methanolic extracts can capture minerals, reduce lipoxygenase, cell death, tissue damage, and remove free radical intermediates [8]. In addition, ease of harvesting, high nutrients, and lower prices are the major reasons for the common cultivation [9]. Mushrooms have an extremely short shelf life, approximately 3 to 4 days at commercial storage and about eight days under chilling conditions due to high respiration rate, water loss, mechanical damage, loss of turgor, and microbial spoilage which can negatively affect the marketability and customer’s acceptance. Oxidative reactions during the storage period can lower the quality of the mushrooms such as enzymatic browning, off-flavor, cap opening, and senescence [10]. Various techniques have been applied to manage quality during storage such as chilling conditions, chemical washing, high-pressure, argon, UV-c, γ-irradiation, pulsed light, ultrasound waves, gaseous ozone, and active and modified atmosphere packaging, though undesirable changes in the mushroom quality and appearance can occur [11]. One such technique is coating with chitosan, apple peel, aloe vera gel, peppermint oils, and nano-coating [12,13,14,15,16]. Titanium dioxide nanoparticles with low concentrations of less than 1% by weight of the food is non-toxic. It is well known for the chemical stability and suitable cost according to Food and Drug Administration (FDA) recommendations [17]. The nano-titanium dioxide is safe, non-toxic, has high rigidity and strength with a white color, and it can be colored with all colors to suit all food products. Chitosan can enhance the gas barrier by the interaction in-between chitosan chains and other chemical substances such as polysaccharides. Thymol and tween are considered effective antimicrobial agents for microbial contamination [5,6]. The combination between nano-titanium dioxide, chitosan, thymol, and tween-80 can be used in a lot of fields such as antireflection coating and the packaging industry against the permeation of gases that prevent spoilage, reduce the volatile compounds, and preserve the sensory evaluations.
For that reason, the current research work aims to evaluate the effect of coating on white button mushrooms quality characteristics such as antioxidant activities, browning enzymes, reactive oxygen species, respiration rates, malondialdehyde, and phenolic contents during storage to prolong the shelf-life.
2. Materials and Methods
2.1. Materials
Titanium dioxide nanoparticles (15 nm with purity more than 99 wet %), chitosan (85% deacetylation), thymol and tween-80, Folin–Ciocalteu, and other reagents were from Sigma, Co., St. Louis, MO, USA.
2.2. Coating Film Preparations
Chitosan 1% (w/v) was prepared by mixing acetic acid 1% (v/v) and glycerol 0.5% (v/v) and stirring overnight at 300 rpm. Approximately 1% of titanium dioxide nanoparticles was added and named with Chitosan-Nano. Thymol and tween-80 1% (w/v) were added to Chitosan-Nano as antimicrobial preservatives and named with Chitosan-Nano/TT.
2.3. Mushroom Samples and Treatments
Fresh white button mushrooms were from the Food Science and Nutrition Department, Taif University, Taif City with closed caps (diameter of 4 ± 00 cm), uniform size, mature sporophores, and free from any sign of physical weaknesses. They were immediately pre-cooled for 12 h at 4 °C. A total number of 120 mushroom caps with gills (thirtypieceper basket, three baskets per group). Four groups were prepared and then coated as follows: Control (distilled water), Chitosan, Chitosan Nano, and Chitosan-Nano/TT were prepared for coating treatments. Mushroom samples were spray-coated continually for five minutes [5,6]. Figure 1 presents film preparation and mushroom treatments. Mushrooms were allowed to dry for 1 h by using an industrial fan at ambient temperature. Mushroom samples were placed in 0.05 mm thickness bags, sealed, and stored at 4 ± 1 °C for 12 days. Evaluations including browning enzyme activities, total phenols, malondialdehyde, antioxidant activities, reactive oxygen species production, O2 consumption, and CO2 production rates in all the mushroom treatments were carried out at 0, 3, 6, 9, and 12 days of storage under chilling.

Figure 1.
Film preparation and mushroom treatments.
2.4. Determination of Browning Enzyme Activities
Enzyme activities are responsible for browning during the storage period as they catalyze the polyphenolic matric to create dyes. The determination method for peroxidase, superoxide dismutase activities measurements were reported from Sami et al. [18] and Mirshekari et al. [12], and catalaze activity was measured by a titrimetric method reported according to Eissa [19]. Fresh mushrooms (10 g) were blended with 20 mL 50 mM mol phosphate buffer for the enzymatic measurements. The experiments were designed with three replications and expressed as U kg−1 fresh weight (FW).
2.5. Determination of Total Phenols
Mushroom samples (10 g) were taken and ground with 10 ml of 80% methanol (MeOH), centrifuged for 15 min at 2000 r/min, and then filtered [4,10]. The color was measured by blending 2.0 mL aliquot filtrate, 1 mL distilled water, 1 mL Folin–Ciocalteu, and 3 ml Na2CO3 (20 %). After 2 h, the absorbance was evaluated at 650 nm with the help of the spectrophotometer (Spectronic 20D, City, China). Total phenol concentration was measured according to the catechol concentrations (8–32 μg/mL) as a standard and expressed in g kg−1 FW.
2.6. Determination of Malondialdehyde Contents
The malondialdehyde contents were evaluated according to the described method in Eldib et al. [20]. Mushroom samples (2 g) were ground, homogenized with 5 mL of 5% trichloroacetic acid (TCA) using a mortar and pestle, cooled, and centrifuged at 8000 r/min for 10 min. Approximately 2 mL of the supernatant were blended with 2 mL of 0.67 % thiobarbituric acid, heated at 100 °C for 30 min, centrifuged again, and measured at 450 nm, 532 nm, and 600 nm, respectively:
MDA (µmol kg−1 FW) = 6.45 × (D532 − D600) − 0.56 × D450
2.7. Determination of Antioxidant Activities
2.7.1. Scavenging Effect of DPPH Radicals
A liquate of 1 ml of 0.5 mmol/L DPPH solution in MeOH, 0.05 mol/L acetate buffer (pH = 5.5), an equal volume of methanolic mushroom extracts were shaken and incubated in the dark for 30 minutes and 1 ml of MeOH was served as a standard [8,10]. The absorbance was evaluated at 517 nm and expressed as a percentage against various concentrations of stock solution of ascorbic acid (1, 0.5, 0.25, 0.125, and 0.062).
2.7.2. Scavenging Effect of ABTS Radicals
Antioxidant activity evaluated by using the ABTS radicals was reported by Rokayya et al. [3]. Mushroom extracts (10 μL) were blended with 90 μL of 7 mM ABTS solution, evaluated at 734 nm, and expressed as a percentage against MeOH as a blank.
2.8. Measurements of Respiration Rates
Mushroom samples were kept in glass jars with 2 valves and a rubber septum. O2 consumption and CO2 production rates were evaluated by the gas analyzer (PBI Dansensor Checkmate, 9900) during the whole storage time. The rates were evaluated according to the following equations [8,21]:
where yi O2, yi CO2, y O2, and y CO2 are, respectively, the O2 and CO2 concentrations at the initial time ti(hour) and at time t (hour). RO2 and RCO2 are the respiration rates and W is the weight, and Vf is the free volume.
2.9. Determination of Reactive Oxygen Species Productions
Hydrogen peroxide content was evaluated according to the described method [8,10]. Mushroom samples (1 g) were blended with 5 mL of 0.1% TCA and centrifuged at 10,000 r/min for 15 min. The supernatant (1 mL) was added to 1 mL of 10 mmol/L potassium phosphate buffers (pH = 7) and 1 mL of 10 mol L−1 potassium iodide, evaluated at 390 nm after incubation for 1 hour and expressed as μmol g−1 (FW).
Hydroxyl radical was evaluated according to the described method [8,10]. Mushroom samples (50 mg) were blended with 1 mL of 10 mmol L−1 Na-phosphate buffer (pH = 7.4) with 15 mmol L−1 2-deoxy-d-ribose. After incubation at 37 °C, 0.7 mL of that solution was added to 3 mL of 0.5% TBA, 1 mL of glacial acetic acid, evaluated at 532 nm, and expressed as μmol g−1 (FW).
2.10. Statistical Analyses
All of the results were expressed as mean (+/−) standard deviation. One-way ANOVA (parametric data) with three replications was used. Duncan’s test as a posthoc was used as multiple ranges at a significant level p-value < 0.05.
3. Results and Discussion
3.1. Browning Enzyme Activities
Antioxidant enzymes such as peroxidase, superoxide dismutase, and catalase play a vital role in the defense of the mushroom oxidation [22,23]. The mushroom quality started to deteriorate after the third day of storage due to oxidative enzymes that negatively influence taste, color, and smell. Figure 2 presents the quality characteristics of the chemical constituents during the storage period. Clear differences were noticed between various coating films. In Figure 2a, the highest activity of peroxidase was for the control samples (0.010 U kg−1 FW) and followed by Chitosan as (0.008 U kg−1 FW), while Chitosan-Nano/TT film reported the lowest activity (0.005 U kg−1 FW) on day 12. Sami et al. [18] and Li et al. [24] reported that oxygen is required for the oxidation activities’ occurrence; thus, the use of nano-films on blueberries could explain the peroxidase activity reduction.
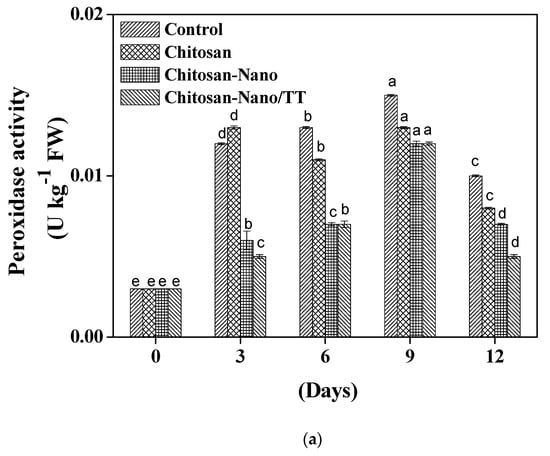

Figure 2.
Effect oxidoreductase enzyme activities measured in mushroom during storage at 4 °C; peroxidase (a), superoxide dismutase (b), and catalase (c) activities; different small letters a;b mean significant differences between treatments at p ≤ 0.05.
As shown in Figure 2b, a minor increase in superoxide dismutase level was detected in treated mushrooms with Chitosan-Nano and Chitosan-Nano/TT films after three days of the storage period. Chitosan-Nano/TT mushrooms exhibited the highest superoxide dismutase activity (4.033 U kg−1 FW) (p < 0.05). High superoxide dismutase activity can decrease the free radical aggregation by hydrogen peroxide formation as the superfluous hydrogen peroxide could convert into non-toxic molecules. Nano-coating with the addition of the antimicrobial agents can effectively catalyze the dismutation of oxygen to produce hydrogen peroxide contents [8,10,25].
Catalase activity can decrease the oxidative damage caused by hydrogen peroxide contents in mushroom samples [8,10,26]. In Figure 2c, catalase activity in Chitosan-Nano (0.45 U kg−1 FW) and Chitosan (0.44 U kg−1 FW) films reported similar values at the end of the storage period. In general, chitosan combination with titanium dioxide nanoparticles coating films can fill the groove between hydrogen bonds and Π–sigma, or even Π–Π interactions which extend the shelf-life of mushroom samples during storage [6,18,23,24]. In addition, many indexes such as season, type, maturation, and environmental conditions can affect the browning enzyme activities [6,24,27].
3.2. Total Phenol and Malondialdehyde Contents
Figure 3a shows that the total phenol content of mushroom samples reported an increasing trend during the storage period after coating treatments, while the control samples were stable during the third day of the entire storage (0.38 g kg−1). Chitosan-Nano/TT treated mushrooms had a significantly increased total phenol content (p < 0.05) at the end of the storage period. Phenylalanine ammonia-lyase enzymes in mushrooms have an important role in raising the phenolic contents [10,28]. Cold conditions also help in raising the total phenol content by changing the phenolic metabolism [6]. Results recommend that Chitosan-Nano/TT treatment may maintain the total phenol contents’ accumulation in the mushroom that can alleviate the browning processes.
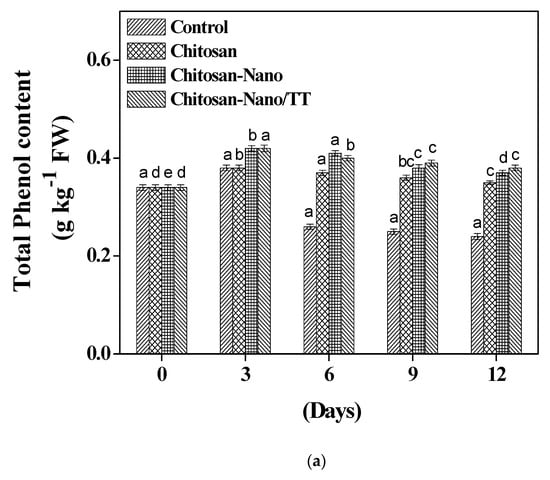

Figure 3.
Effect of total phenol (a) and malondialdehyde (b) contents on mushrooms during storage; * Different small letters a;b;c;d mean significant differences between treatments at p ≤ 0.
Malondialdehyde accumulation content has been considered as a key index for the degree of membrane polyunsaturated fatty acid oxidation [8,27,29]. In Figure 3b, malondialdehyde contents increased continuously during the storage for 12 days in mushroom samples at 4 °C, which indicates a cell membrane integrity reduction [8,25]. In addition, coating with Chitosan-Nano film resulted in significantly (p < 0.05) lower malondialdehyde contents formation (1.68 µmol kg−1 FW) compared to the untreated mushrooms after 12 days of storage. Nano-coating probably preserved the mushrooms from peroxidation by reactive oxygen species and lipoxygenase [9,10,27]. The difference in the malondialdehyde contents may be due to the dissolution power and polarity of the coating materials [20,27]. The current research indicated that nano-coating treatment decreased the membrane lipid peroxidation, in that manner prolonging the shelf life and delaying the aging processes [30,31].
3.3. Antioxidant Activities
The scavenging effect of mushroom extracts on DPPH radicals is presented in (Table 1). Chitosan-Nano (74.70%) followed by Chitosan-Nano/TT (72.60%) exhibited a significantly different (p < 0.05). Cheung et al. [32] reported lower DPPH activity results (37.9%, 55.4 %) for mushrooms.

Table 1.
Effect of the different coatings on antioxidant activities in mushrooms during storage.
The rapid accumulation of antioxidant capacity might be attributed to cold conditions, DPPH radical scavenging capacity, and physically powerful maintenance of phenolic compounds of nano-coated mushroom samples that reached the saturation level in Agaricus bisporus bodies.
Antioxidant activity on ABTS radical results of coated mushrooms is expressed as a percentage in (Table 1). The scavenging effect of mushroom extracts was between 45.55% for control and 70.39% for Chitosan-Nano/TT mushrooms at the end of the whole storage period. The greatest impact of the ABTS radical enhancement was due to the presence of nano-coating with the addition of the antimicrobial agents, which possibly reduce the oxidoreductase enzyme activities and damage on tissues [10,33].
3.4. Respiration Rates
The reduced respiratory metabolism increases the shelf-life of the stored products. The changes in respiration rates after coating treatments are presented in Figure 4. Figure 3a,b show that O2 consumption and CO2 production rates increased in response to the storage period at 4 °C. The effect was further noticed with the increase of the storage time. The O2 consumption rate continued to increase especially for uncoated samples and Chitosan films to reach (0.071, 0.058 mmol s−1kg−1, respectively), while Chitosan-Nano/TT and Chitosan-Nano films remarkably reached the lowest values (0.026, 0.035 mmol s−1kg−1, respectively) at the end of the storage period. Mild heat was recently used for decreasing the O2 production rate on fresh-cut Agaricus bisporus mushrooms [34].
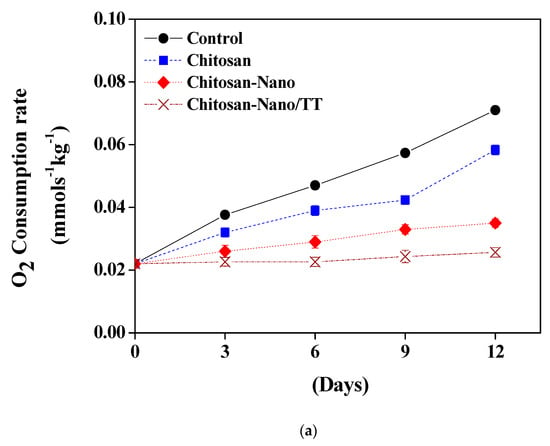

Figure 4.
Effect of respiration rates on mushrooms during storage; O2 consumption (a) and CO2 production rates (b).
CO2 production rates were raised conversely and Chitosan-Nano/TT film recorded the lowest CO2 rate (0.004 mg CO2 kg−1s−1) on the 12th day of the storage time. Hu et al. [35] reported that the high respiration rate can be due to the anaerobic respiration confirmed by high respiratory quotient values on uncoated samples and Chitosan samples, while, for nano-coated samples, the anaerobic behavior was not reversed. Similar results for respiration rates have been reported for mushrooms that could be due to the microbial attack [6,10,11].
3.5. Reactive Oxygen Species
The reactive oxygen species production levels, hydrogen peroxide, and hydroxyl radical contents in mushroom samples are shown in Table 2. Only low quantities of hydrogen peroxide contents were observed for Chitosan-Nano (22.40 µmol g−1 FW), while the control was observed to be nearly double the content (41.23 µmol g−1 FW) at the end of the storage period. Khan et al. [36] reported that a high level of hydrogen peroxide content can oxidize the membrane lipids, which leads to cell integrity destruction and the aging of mushrooms.

Table 2.
Effect of the different coatings on hydrogen peroxide and hydroxyl radical levels in mushrooms during storage.
Hydroxyl radical contents play a vital role in the oxidation process [8,10]. In the current work, Chitosan-Nano mushroom samples achieved the lowest values (0.16 µmol g−1) followed by Chitosan-Nano/TT (0.19 µmol g−1), while Chitosan reported (0.21 µmol g−1). Chomkitichai et al. [37] reported a proportional relationship between the accumulation of reactive oxygen species and the browning degrees. Higher reactive oxygen species could disrupt cell membrane structures, browning, and quality during storage [37]. The decrease in oxygen production rate has been occurred in mushrooms treated with 4-methoxy cinnamic acid, while the browning increase may lead to hydrogen peroxide and hydroxyl radical increase [8,10]. In a word, nano-titanium with the combination of the chitosan coating film can reduce the oxidation processes of white button mushrooms by maintaining the lowest hydrogen peroxide and hydroxyl radical activities during the storage period by reducing the cell degradation and oxidation processes [18,21].
4. Conclusions
The current results present some insights into the oxidation processes and quality deterioration of white button mushrooms after the post-harvest methods to extend the shelf life. Reducing the oxidation is essential to provide more appropriate recommendations for the edible fungus industry with maintaining the sensory and nutritive values of fresh mushrooms by promoting antioxidant production. Coating nano-titanium with the combination of chitosan enhanced the color, reactive oxygen species, antioxidant activity, and malondialdehyde accumulation contents, while the addition of antimicrobial agents such as thymol and tween reduced the respiration rates and increased the phenolic contents. As a result, nano-coating with the combination of antimicrobial agents in food preservation is recommended for increasing the shelf-life of mushrooms with other fruits and vegetables.
Author Contributions
Funding acquisition, E.K. and R.S.; Methodology, E.K., R.S., M.H., N.B. and M.A.; Software, M.H., N.B., M.A. and M.S.A.; Visualization, E.K., R.S., M.H., N.B. and M.A.; Writing—review & editing, E.K., R.S., M.H., A.E., N.B., M.A. and M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided from the Deanship of Scientific Research by Taif University, award number (102-441-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request from the corresponding author.
Acknowledgments
Financial support was provided from the Deanship of Scientific Research by Taif University, award number (102-441-1).
Conflicts of Interest
The authors declared no conflicts of interest.
References
- Khojah, E.Y.; Sami, R. Fatty Acids Composition and Oxidative Stability of Peanut and Sesame Oils with the Sensory Evaluation of Mayonnaise Prepared by Different Oils. Assiut J. Agric. Sci. 2016, 47, 460–472. [Google Scholar]
- Wang, H.; Sun, Y.; Li, Y.; Tong, X.; Regenstein, J.M.; Huang, Y.; Ma, W.; Sami, R.; Qi, B.; Jiang, L. Effect of the condition of spray-drying on the properties of the polypeptide-rich powders from enzyme-assisted aqueous extraction processing. Dry. Technol. 2019, 37, 2105–2115. [Google Scholar] [CrossRef]
- Elhakem, A.H.; Benajiba, N.; Koko, M.Y.; Khojah, E.; Rok, A. DPPH, FRAP and TAEC Assays with Postharvest Cabbage (Brassica oleracea) Parameters During the Packaging Process. Pakistan J. Biol. Sci. 2021, 24, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.H.; Wenxin, D.; Zhigang, X.; Sami, R.; Khojah, E.; Amanullah, S. Antioxidant and Anti-Inflammatory Capacities of Pepper Tissues. Italian Journal of Food Science 2020, 32, 265–274. [Google Scholar]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Jing, J.; Helal, M. Effect of Titanium Dioxide Nanocomposite Material and Antimicrobial Agents on Mushrooms Shelf-Life Preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]
- Rokayya, S.; Khojah, E.; Elhakem, A.; Benajiba, N.; Chavali, M.; Vivek, K.; Iqbal, A.; Helal, M. Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage. Coatings 2021, 11, 44. [Google Scholar] [CrossRef]
- Parepalli, Y.; Chavali, M.; Sami, R.; Singh, M.; Sinha, S.; Touahra, F. Ganoderma Lucidum: Extraction and Characterization of Polysaccharides, Yields and their Bioapplications. Algerian J. Res. Technol. 2021, 5, 30–43. [Google Scholar]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Fikry, M.; Helal, M. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 C. Sci. Rep. 2021, 11, 6031. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 7915. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Abdelazez, A.; Helal, M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage. Coatings 2021, 11, 149. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Golding, J.B. Aloe vera gel treatment delays postharvest browning of white button mushroom (Agaricus bisporus). J. Food Meas. Charact. 2019, 13, 1250–1256. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Thakur, R.R.; Shahi, N.C.; Mangaraj, S.; Lohani, U.C.; Chand, K. Effect of apple peel based edible coating material on physicochemical properties of button mushrooms (Agaricus bisporus) under ambient condition. Int. J. Chem. Stud. 2020, 8, 2362–2370. [Google Scholar] [CrossRef]
- Qu, T.; Li, B.-B.; Huang, X.; Li, X.; Ding, Y.; Chen, J.; Tang, X.J.F.; Technology, B. Effect of Peppermint Oil on the Storage Quality of White Button Mushrooms (Agaricus bisporus). Food Bioprocess Technol. 2020, 13, 404–418. [Google Scholar] [CrossRef]
- E 21—Food And Drugs, Chapter I-Food and Drug Administration Department of Health And Human Services. Cfr—Code of Federal Regulations Title 21 3. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.480 (accessed on 1 April 2020).
- Rokayya, S.; Jia, F.; Li, Y.; Nie, X.; Xu, J.; Han, R.; Yu, H.; Amanullah, S.; Almatrafi, M.M.; Helal, M. Application of nano-titanum dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT 2021, 137, 110422. [Google Scholar] [CrossRef]
- Eissa, H.A. Effect of chitosan coating on shelf-life and quality of fresh-cut mushroom. J. Food Qual. 2008, 30, 623–645. [Google Scholar] [CrossRef]
- Eldib, R. Application of Nano-coating and Chitosan Combination Films on Cantaloupe Preservation. Pak. J. Biol. Sci. 2020, 23, 1037–1043. [Google Scholar] [CrossRef]
- Khojah, E.; Sami, R.; Helal, M.; Elhakem, A.; Benajiba, N.; Alkaltham, M.S.; Salamatullah, A.M. Postharvest Physicochemical Properties and Fungal Populations of Treated Cucumber with Sodium Tripolyphosphate/Titanium Dioxide Nanoparticles during Storage. Coatings 2021, 11, 613. [Google Scholar] [CrossRef]
- Eldib, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality. Coatings 2020, 10, 962. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W.; Rok, A. Effect of Chitosan/Nano-Titanium Dioxide/Thymol and Tween Films on Ready-to-Eat Cantaloupe Fruit Quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, Z.; Zhao, J.; Nie, Y.; Zhang, Y.; Sheng, J.; Meng, D.; Mao, H.; Tang, X. Effects of Postharvest Brassinolide Treatment on the Metabolism of White Button Mushroom (Agaricus bisporus) in Relation to Development of Browning During Storage. Food Bioprocess. Technol. 2016, 9, 1327–1334. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-M.; Xu, L.; Cui, Y.; Yu, X.-Y.; Gao, H.-J.; Wang, Q.; Liu, K.; Shi, Y.; Chen, Q.-X. Postharvest application of 4-methoxy cinnamic acid for extending the shelf life of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 104, 33–41. [Google Scholar] [CrossRef]
- Sami, R.; Almatrafi, M.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Helal, M. Effect of Nano Silicon Dioxide Coating Films on the Quality Characteristics of Fresh-Cut Cantaloupe. Membranes 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Elhakem, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Ahmed Mohamed, T.; Fikry, M.; Helal, M. In-Vitro Evaluation of the Antioxidant and Anti-Inflammatory Activity of Volatile Compounds and Minerals in Five Different Onion Varieties. Separations 2021, 8, 57. [Google Scholar] [CrossRef]
- Babalar, M.; Pirzad, F.; Sarcheshmeh, M.A.A.; Talaei, A.; Lessani, H. Arginine treatment attenuates chilling injury of pomegranate fruit during cold storage by enhancing antioxidant system activity. Postharvest Biol. Technol. 2018, 137, 31–37. [Google Scholar] [CrossRef]
- Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M.; Alhuthal, N.; Alzahrani, S.A.; Alharbi, M.; Chavali, M. Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life. Appl. Sci. 2021, 11, 3879. [Google Scholar] [CrossRef]
- Sami, R.; Soltane, S.; Helal, M. Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation. Membranes 2021, 11, 303. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Rokayya, S.; Li, C.J.; Zhao, Y.; Li, Y.; Sun, C.H. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2014, 14, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, S.; Wang, A.; Li, J.; Zong, W. Mild heat treatment inhibits the browning of fresh-cut Agaricus bisporus during cold storage. LWT Food Sci. Technol. 2017, 82, 104–112. [Google Scholar] [CrossRef]
- Hu, W.; Uchino, T.; Yasunaga, E.; Hamanaka, D.; Hori, Y. Respiration Rate of Shiitake Mushroom in Modified Atmosphere Packages. IFAC Proc. Vol. 2001, 34, 189–196. [Google Scholar] [CrossRef]
- Khan, Z.U.; Bu, J.; Khan, N.M.; Khan, R.U.; Jiang, Z.; Mou, W.; Luo, Z.; Mao, L.; Ying, T. Integrated Treatment of CaCl2, Citric Acid and Sorbitol Reduces Loss of Quality of Button Mushroom (Agaricus Bisporus) during Postharvest Storage. J. Food Proc. Preserv. 2015, 39, 2008–2016. [Google Scholar] [CrossRef]
- Chomkitichai, W.; Chumyam, A.; Rachtanapun, P.; Uthaibutra, J.; Saengnil, K. Reduction of reactive oxygen species production and membrane damage during storage of ‘Daw’ longan fruit by chlorine dioxide. Sci. Hortic. 2014, 170, 143–149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).