Synchrotron Microbeam Diffraction Studies on the Alignment within 3D-Printed Smectic-A Liquid Crystal Elastomer Filaments during Extrusion
Abstract
1. Introduction
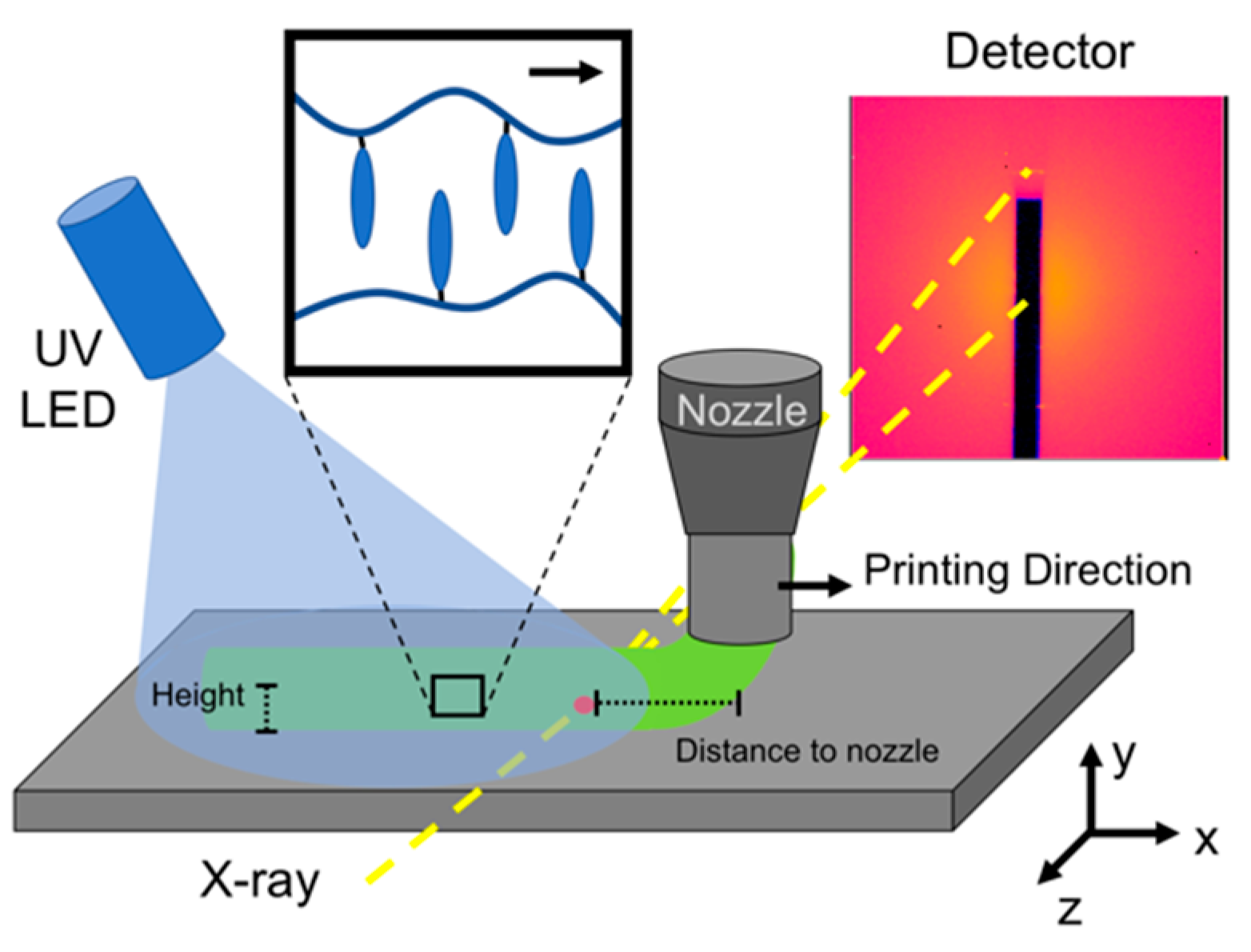
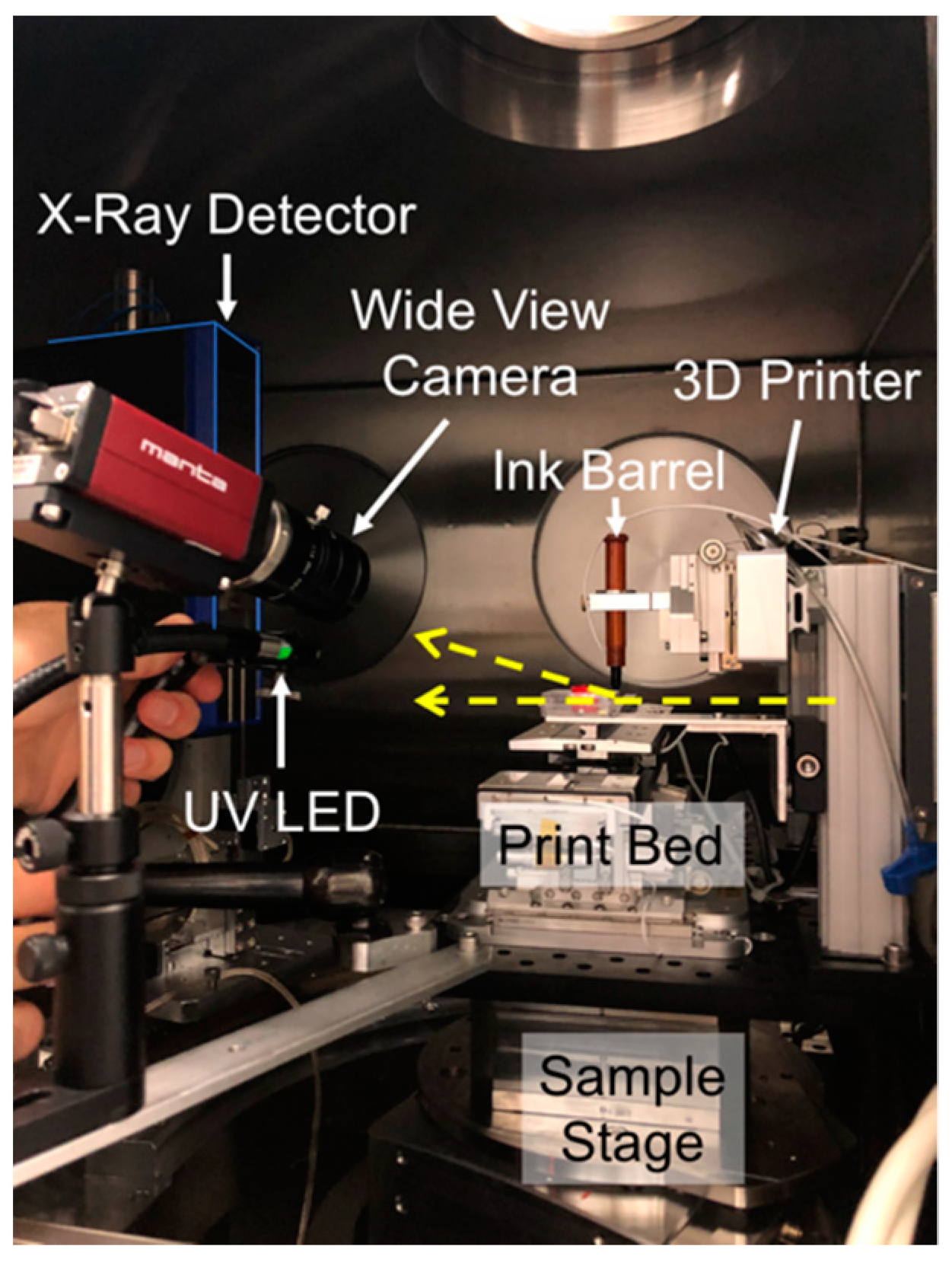
2. Materials and Methods
Synchrotron µ-SAXD
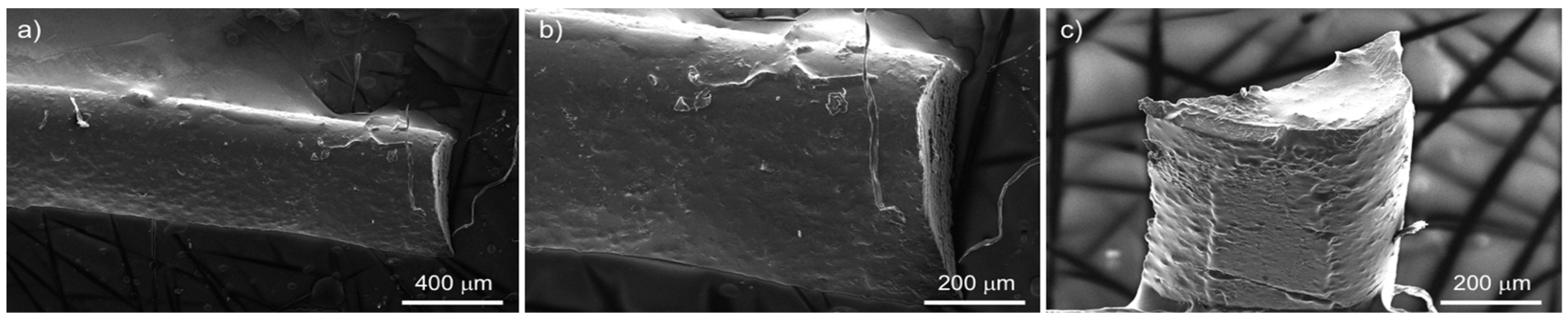
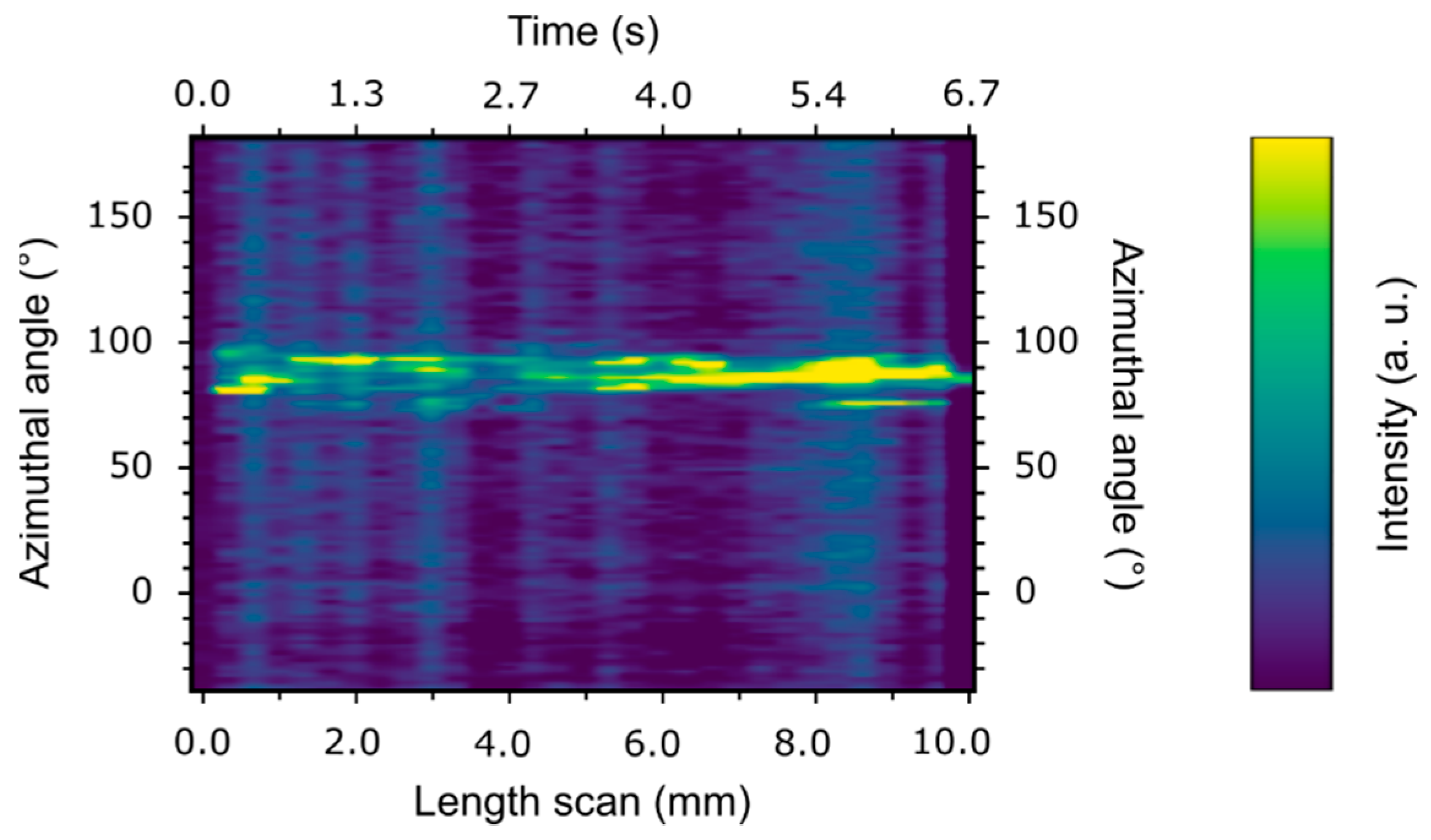
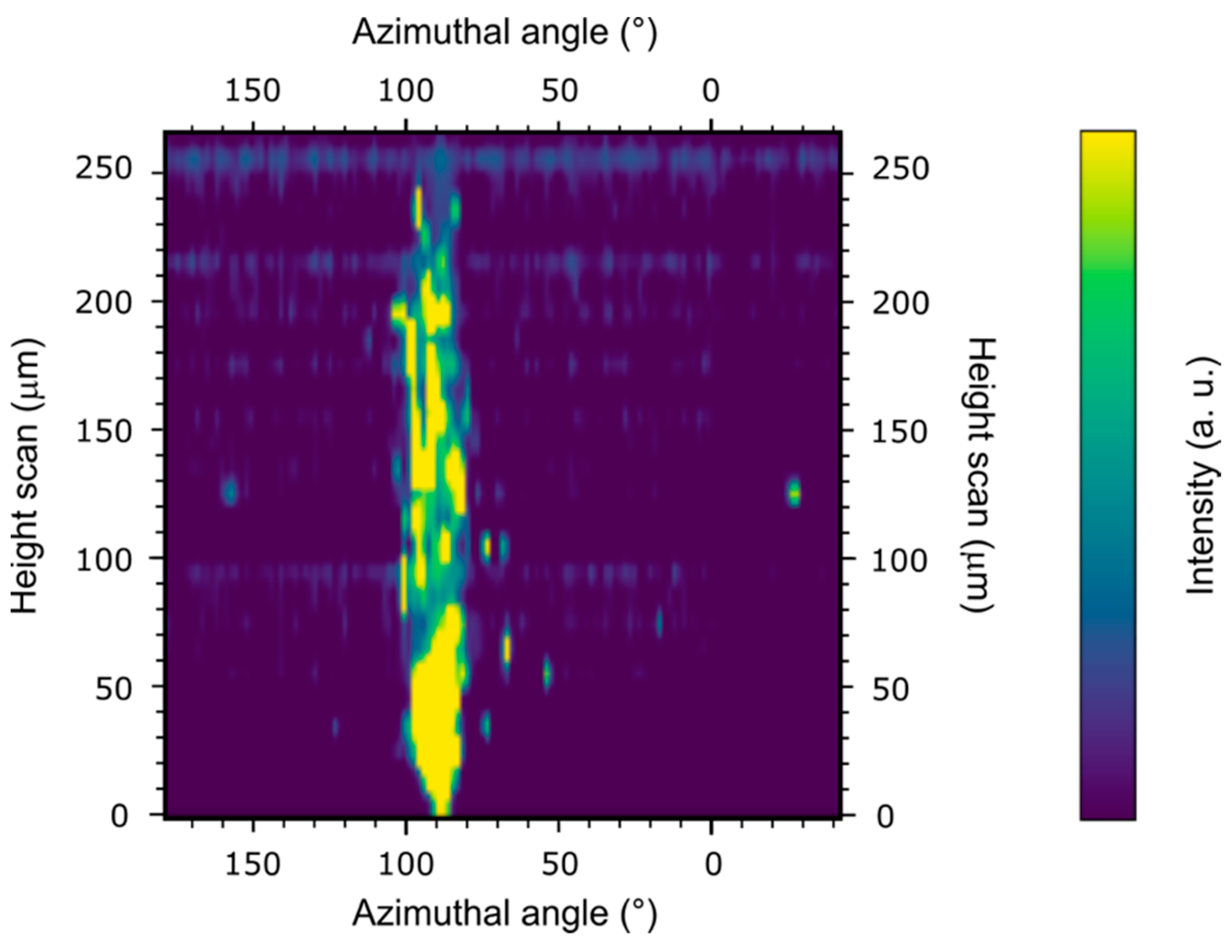
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iida, A.; Noma, T.; Miyata, H.; Mirano, K. Micro x-ray diffraction technique for analysis of the local layer structure in the ferroelectric liquid crystal. Rev. Sci. Instrum. 1995, 66, 1373–1375. [Google Scholar] [CrossRef]
- Takahashi, Y.; Iida, A.; Takanishi, Y.; Ogasawara, T.; Ishikawa, K.; Takezoe, H. Dynamic behaviour of the local layer structure of antiferroelectric liquid crystals under a high electric field measured by time-resolved synchrotron x-ray microbeam diffraction. Jpn. J. Appl. Phys. 2001, 40, 3294–3300. [Google Scholar] [CrossRef]
- Kang, S.; Tokita, M.; Takanishi, Y.; Takezoe, H.; Watanabe, J. Structure of a B6-like phase formed from bent-core liquid crystals determined by microbeam x-ray diffraction. Phys. Rev. E 2007, 76, 042701. [Google Scholar] [CrossRef]
- Yoon, D.; Yoon, J.; Kim, Y.H.; Choi, M.C.; Kim, J.; Sakata, O.; Kimura, S.; Kim, M.W.; Smalyukh, I.I.; Clark, N.A.; et al. Liquid-crystal periodic zigzags from geometrical and surface-anchoring-induced confinement: Origin and internal structure from mesoscopic scale to molecular level. Phys. Rev. E 2010, 82, 041705. [Google Scholar] [CrossRef]
- Feng, Z.; Perera, A.D.L.C.; Fukuda, A.; Vij, J.K.; Ishikawa, K.; Iida, A.; Takanishi, Y. Definite existence of subphases with eight- and ten-layer unit cells as studied by complementary methods, electric-field-induced birefringence and microbeam resonant x-ray scattering. Phys. Rev. E 2017, 96, 012701. [Google Scholar] [CrossRef]
- Nakata, M.; Zanchetta, G.; Chapman, B.D.; Jones, C.D.; Cross, J.O.; Pindak, R.; Bellini, T.; Clark, N.A. End-to-end stacking and liquid crystal condensation of 6–to-20–base pair DNA duplexes. Science 2007, 318, 1276–1279. [Google Scholar] [CrossRef]
- Yevdokimov, Y.M.; Sonin, A.S. DNA nanoscience: From prebiotic origins to emerging nanotechnology, by Kenneth Douglas, Boca Raton, CRC Press, 2016, 424 p. Liq. Cryst. Today 2016, 25, 82–84. [Google Scholar] [CrossRef]
- Tran, V.; Wen, X. Rapid prototyping technologies for tissue regeneration. In Rapid Prototyping of Biomaterials—Principles and Applications; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 97–155. [Google Scholar]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Xu, T.; Rodriguez-Devora, J.I.; Reyna-Soriano, D.; Mohammod, B.; Zhu, L.; Wang, K.; Yuan, Y. Bioprinting for constructing microvascular systems for organs. In Rapid Prototyping of Biomaterials—Principles and Applications; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 201–220. [Google Scholar]
- Truby, R.L.; Lewis, J.A. Printing Soft Matter in Three Dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Mackay, M.E. The Importance of Rheological Behavior in the Additive Manufacturing Technique Material Extrusion. J. Rheol. 2018, 62, 1549–1561. [Google Scholar] [CrossRef]
- Shmueli, Y.; Jiang, J.; Zhou, Y.; Xue, Y.; Chang, C.; Yuan, G.; Satija, S.K.; Lee, S.; Nam, C.; Kim, T.; et al. Simultaneous in Situ X‑ray Scattering and Infrared Imaging of Polymer Extrusion in Additive Manufacturing. ACS Appl. Polym. Mater. 2019, 1, 1559–1567. [Google Scholar] [CrossRef]
- Nogales, A.; Gutiérrez-Fernández, E.; García-Gutiérrez, M.-C.; Ezquerra, T.A.; Rebollar, E.; Šics, I.; Malfois, M.; Gaidukovs, S.; Gēcis, E.; Celms, K.; et al. Structure Development in Polymers during Fused Filament Fabrication (FFF): An in Situ Small- and Wide-Angle X‑ray Scattering Study Using Synchrotron Radiation. Macromolecules 2019, 52, 9715–9723. [Google Scholar] [CrossRef]
- Wiegart, L.; Doerk, G.S.; Fukuto, M.; Lee, S.; Li, R.; Marom, G.; Noack, M.M.; Osuji, C.O.; Rafailovich, M.H.; Sethian, J.A.; et al. Instrumentation for In Situ/Operando X-Ray Scattering Studies of Polymer Additive Manufacturing Processes. Synchrotron Radiat. News 2019, 32, 20–27. [Google Scholar] [CrossRef]
- Yavitt, B.M.; Wiegart, L.; Salatto, D.; Huang, Z.; Endoh, M.K.; Poeller, S.; Petrash, S.; Koga, T. Structural Dynamics in UV Curable Resins Resolved by In Situ 3D Printing X‑ray Photon Correlation Spectroscopy. ACS Appl. Polym. Mater. 2020, 2, 4096–4108. [Google Scholar] [CrossRef]
- Torres Arango, M.A.; Zhang, Y.; Li, R.; Doerk, G.; Fluerasu, A.; Wiegart, L. In-Operando Study of Shape Retention and Microstructure Development in a Hydrolyzing Sol−Gel Ink during 3D-Printing. ACS Appl. Mater. Interfaces 2020, 12, 51044–51056. [Google Scholar] [CrossRef]
- Bartolo, P.; Domingos, M.; Gloria, A.; Ciurana, J. BioCell Printing: Integrated Automated Assembly System for Tissue Engineering Constructs. CIRP Annals 2011, 60, 271–274. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Ustunel, S.; Hegmann, E. Liquid Crystal Elastomers—A Path to Biocompatible and Biodegradable 3D-LCE Scaffolds for Tissue Regeneration. Materials 2018, 11, 377. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Zheng, Y.; He, Q.; Wang, Y.; Cai, S. Three-dimensional printing of functionally graded liquid crystal elastomer. Sci. Adv. 2020, 6, eabc0034. [Google Scholar] [CrossRef]
- Ambulo, C.; Burroughs, J.J.; Boothby, J.M.; Kim, H.; Shankar, M.R.; Ware, T.H. Four-dimensional Printing of Liquid Crystal Elastomers. ACS Appl. Mater. Interfaces 2017, 9, 37332. [Google Scholar] [CrossRef]
- Jang, K.-S.; Johnson, C.J.; Hegmann, T.; Hegmann, E.; Korley, L.T.J. Biphenyl-based liquid crystals for elevated temperature processing with polymers. Liq. Cryst. 2014, 41, 1473–1482. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Nemati, A.; Cull, T.R.; Hegmann, E.; Hegmann, T. A zero-power, ppt-to ppm-level toxic gas and vapor sensor with image, text, and analytical capabilities. Adv. Mater. Technol. 2020, 5, 2000058. [Google Scholar] [CrossRef]
- Van Oosten, C.L.; Bastiaansen, C.W.M.; Broer, D.J. Printed artificial cilia from liquid-crystal network actuators modularly driven by light. Nat. Mater. 2009, 8, 677–682. [Google Scholar] [CrossRef]
- Kularatne, R.S.; Kim, H.; Boothby, J.M.; Ware, T.H. Liquid Crystal Elastomer Actuators: Synthesis, Alignment, and Applications. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 395–411. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Applications of Liquid Crystalline Elastomers. In Liquid Crystal Elastomers: Materials and Applications. Advances in Polymer Science; De Jeu, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 250. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Bergquist, L.E.; Sharma, A.; Mori, T.; Gao, Y.; Bera, T.; Zhu, C.; Leslie, M.T.; Cukelj, R.; Korley, L.T.J.; et al. New developments in 3D liquid crystal elastomers scaffolds for tissue engineering: From physical template to responsive substrate. In Proceedings of the SPIE Organic Photonics + Electronics, San Diego, CA, USA, 6–10 August 2017; Volume 103610T, pp. 1–11. [Google Scholar] [CrossRef]
- Ustunel, S.; Prévôt, M.E.; Clements, R.J.; Hegmann, E. Cradle-to-Cradle: Designing Biomaterials to Fit as Truly Biomimetic Cell Scaffolds—A Review. Liq. Cryst. Today 2020, 29, 40. [Google Scholar] [CrossRef]
- Martella, D.; Pattelli, L.; Matassini, C.; Ridi, F.; Bonini, M.; Paoli, P.; Baglioni, P.; Wiersma, D.; Parmeggiani, C. Liquid crystal induced myoblast alignment. Adv. Healthc. Mater. 2019, 8, 1801489. [Google Scholar] [CrossRef] [PubMed]
- Babakhanova, G.; Krieger, J.; Li, B.-X.; Turiv, T.; Kim, M.-H.; Lavrentovich, O.D. Cell alignment by smectic liquid crystal elastomer coatings with nanogrooves. J. Biomed. Mater. Res. Part A 2020, 108, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, N.A.; Mohr, J.C.; Ji, L.; Murphy, C.J.; Palecek, S.P.; de Pablo, J.J.; Abbott, N.L. Thermotropic liquid crystals as substrates for imaging the reorganization of matrigel by human embryonic stem cells. Adv. Funct. Mater. 2006, 16, 618–624. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Ustunel, S.; Bergquist, L.E.; Cukelj, R.; Gao, Y.; Mori, T.; Pauline, L.; Clements, R.J.; Hegmann, E. Synthesis of Biocompatible Liquid Crystal Elastomer Foams as Cell Scaffolds for 3D Spatial Cell Cultures. J. Vis. Exp. 2017, 122, e55452. [Google Scholar] [CrossRef]
- Dhakal, N.P.; Jiang, J.; Guo, Y.; Peng, C. Self-Assembly of Aqueous Soft Matter Patterned by Liquid-Crystal Polymer Networks for Controlling the Dynamics of Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 13680–13685. [Google Scholar] [CrossRef]
- Turiv, T.; Koizumi, R.; Thijssen, K.; Genkin, M.M.; Yu, H.; Peng, C.; Wei, Q.-H.; Yeomans, J.M.; Aranson, I.S.; Doostmohammadi, A.; et al. Polar jets of swimming bacteria condensed by a patterned liquid crystal. Nat. Phys. 2020, 16, 481–487. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D Cell Culture: A Review of Current Approaches and Techniques. Methods in Molecular Biology 3D Cell Culture; Humana Press: Totowa, NJ, USA, 2010; pp. 1–15. [Google Scholar] [CrossRef]
- Liu, X.H.; Jin, X.B.; Ma, P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011, 10, 398–406. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paul, S.F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Sakr, M.A.; Seo, J.; Bandaru, P.; Arneri, A.; Bersini, S.; Zare-Eelanjegh, E.; Jalilian, E.; Cha, B.-H.; Antona, S.; et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017, 11, 044109. [Google Scholar] [CrossRef]
- Degennes, P.G. One type of nematic polymers. Comptes Rendus Hebd. Seances Acad. Sci. Ser. B 1975, 281, 101–103. [Google Scholar]
- Schneider, A.; Muller, S.; Finkelmann, H. Lyotropic mesomorphism of AB block copolymers in nematic solvents. Macromol. Chem. Phys. 2000, 201, 184–191. [Google Scholar] [CrossRef]
- Broemmel, F.; Kramer, D.; Finkelmann, H. Preparation of liquid crystalline elastomers. In Liquid Crystal Elastomers: Materials and Applications; De Jeu, W.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 250, pp. 1–48. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Saed, M.; Nair, D.P.; Gong, T.; Reed, S.M.; Bowman, C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol–acrylate reaction. RSC Adv. 2015, 5, 18997–19001. [Google Scholar] [CrossRef]
- He, Q.; Wang, Z.; Wang, Y.; Minori, A.; Tolley, M.T.; Cai, S. Electrically controlled liquid crystal elastomer–based soft tubular actuator with multimodal actuation. Sci. Adv. 2019, 5, eaax5746. [Google Scholar] [CrossRef]
- Kotikian, A.; Truby, R.L.; Boley, J.W.; White, T.J.; Lewis, J.A. 3D Printing of Liquid Crystal Elastomeric Actuators with Spatially Programed Nematic Order. Adv. Mater. 2018, 30, 1706164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mori, T.; Mahnen, C.J.; Everson, H.R.; Leslie, M.T.; Nielsen, A.D.; Lussier, L.; Zhu, C.; Malcuit, C.; Hegmann, T.; et al. Effects of structural variations on the cellular response and mechanical properties of biocompatible, biodegradable, and porous smectic liquid crystal elastomers. Macromol. Biosci. 2017, 17, 1600278. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Ustunel, S.; Freychet, G.; Webb, C.R.; Zhernenkov, M.; Pindak, R.; Clements, R.J.; Hegmann, E. Physical models from physical templates using biocompatible liquid crystal elastomers as new morphologically programmable inks for 3D printing. J. Appl. Phys. 2021. in peer review. [Google Scholar]
- Sharma, A.; Neshat, A.; Mahnen, C.J.; Nielsen, A.d.; Snyder, J.; Stankovich, T.L.; Daum, B.G.; LaSpina, E.M.; Beltrano, G.; Gao, Y.; et al. Biocompatible, Biodegradable and Porous Liquid Crystal Elastomer Scaffolds for Spatial Cell Cultures. Macromol. Biosci. 2015, 15, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Zhernenkov, M.; Canestrari, N.; Chubar, O.; DiMasi, E. Soft matter interfaces beamline at NSLS-II: Geometrical ray-tracing vs. wavefront propagation simulations. In Proceedings of the SPIE Optical Engineering + Applications 2014, San Diego, CA, USA, 18–21 August 2014; Volume 9209, p. 92090G. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Andro, H.; Alexander, S.L.M.; Ustunel, S.; Zhu, C.; Nikolov, Z.; Rafferty, S.T.; Brannum, M.T.; Kinsel, B.; Korley, L.T.J.; et al. Liquid crystal elastomer foams with elastic properties specifically engineered as biodegradable brain tissue scaffolds. Soft Matter 2018, 14, 354–360. [Google Scholar] [CrossRef]
- Pandolfi, R.J.; Allan, D.B.; Arenholz, E.; Barroso-Luque, L.; Campbell, S.I.; Caswell, T.A.; Blair, A.; De Carlo, F.; Fackler, S.; Fournier, A.P.; et al. Xi-cam: A versatile interface for data visualization and analysis. J. Synchrotron Radiat. 2018, 25, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prévôt, M.E.; Ustunel, S.; Yavitt, B.M.; Freychet, G.; Webb, C.R.; Zhernenkov, M.; Hegmann, E.; Pindak, R. Synchrotron Microbeam Diffraction Studies on the Alignment within 3D-Printed Smectic-A Liquid Crystal Elastomer Filaments during Extrusion. Crystals 2021, 11, 523. https://doi.org/10.3390/cryst11050523
Prévôt ME, Ustunel S, Yavitt BM, Freychet G, Webb CR, Zhernenkov M, Hegmann E, Pindak R. Synchrotron Microbeam Diffraction Studies on the Alignment within 3D-Printed Smectic-A Liquid Crystal Elastomer Filaments during Extrusion. Crystals. 2021; 11(5):523. https://doi.org/10.3390/cryst11050523
Chicago/Turabian StylePrévôt, Marianne E., Senay Ustunel, Benjamin M. Yavitt, Guillaume Freychet, Caitlyn R. Webb, Mikhail Zhernenkov, Elda Hegmann, and Ron Pindak. 2021. "Synchrotron Microbeam Diffraction Studies on the Alignment within 3D-Printed Smectic-A Liquid Crystal Elastomer Filaments during Extrusion" Crystals 11, no. 5: 523. https://doi.org/10.3390/cryst11050523
APA StylePrévôt, M. E., Ustunel, S., Yavitt, B. M., Freychet, G., Webb, C. R., Zhernenkov, M., Hegmann, E., & Pindak, R. (2021). Synchrotron Microbeam Diffraction Studies on the Alignment within 3D-Printed Smectic-A Liquid Crystal Elastomer Filaments during Extrusion. Crystals, 11(5), 523. https://doi.org/10.3390/cryst11050523





