Potato Chip-Like 0D Interconnected ZnCo2O4 Nanoparticles for High-Performance Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
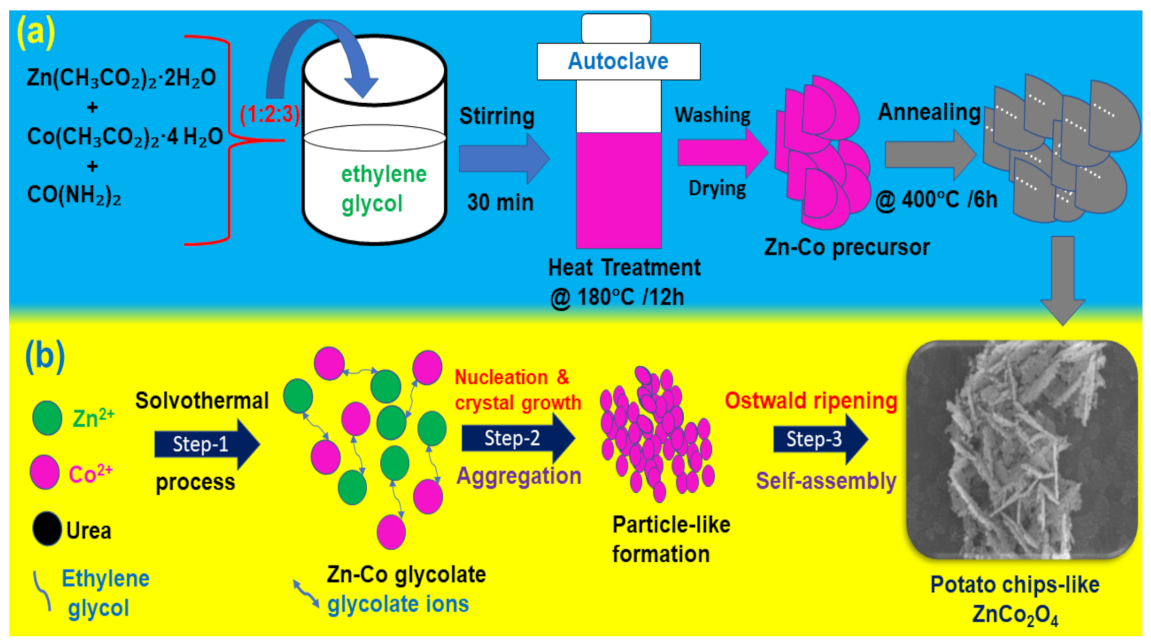
2.1. Solvothermal Synthesis of PIZCON
2.2. Characterization
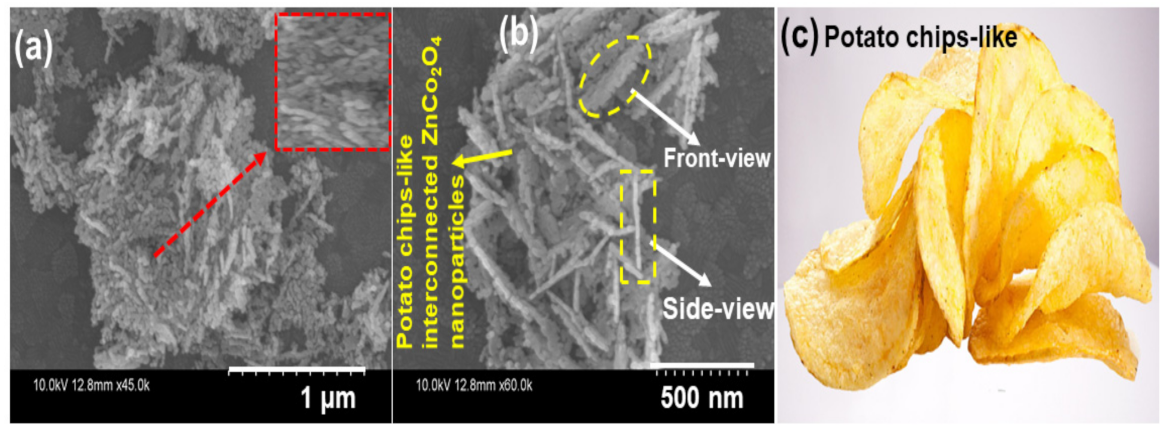
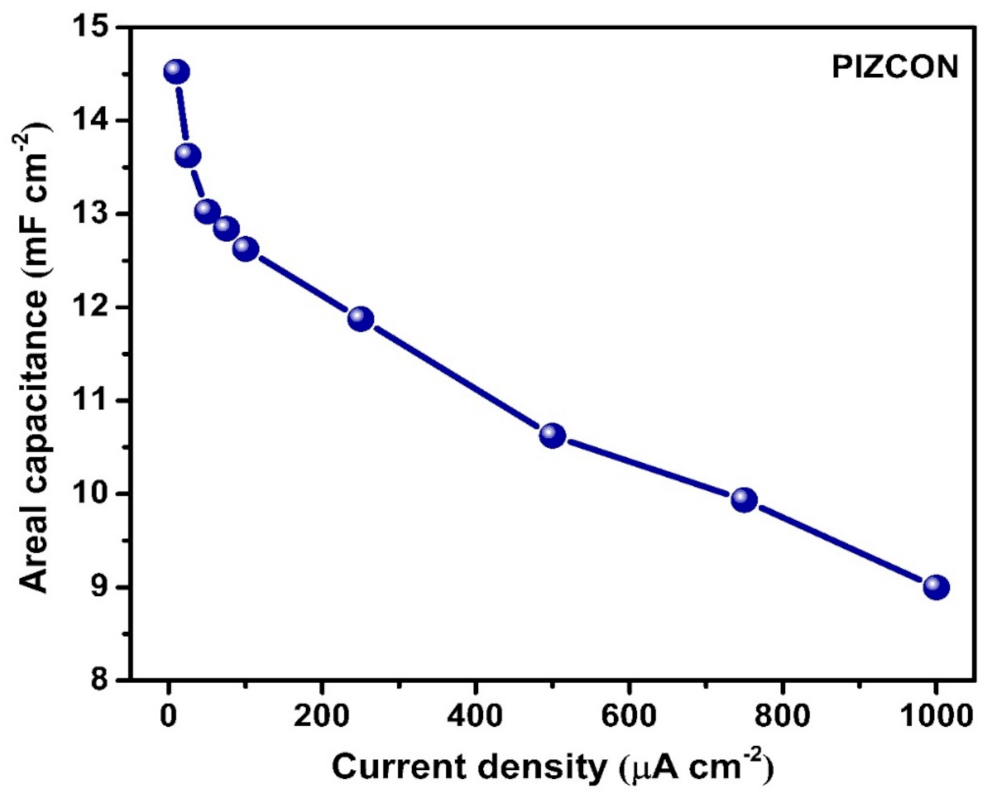
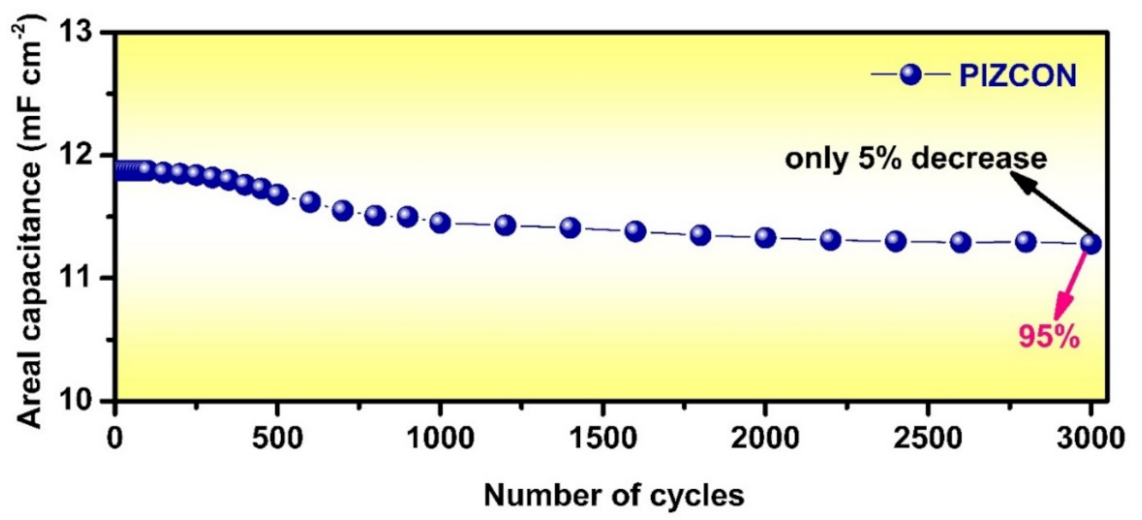
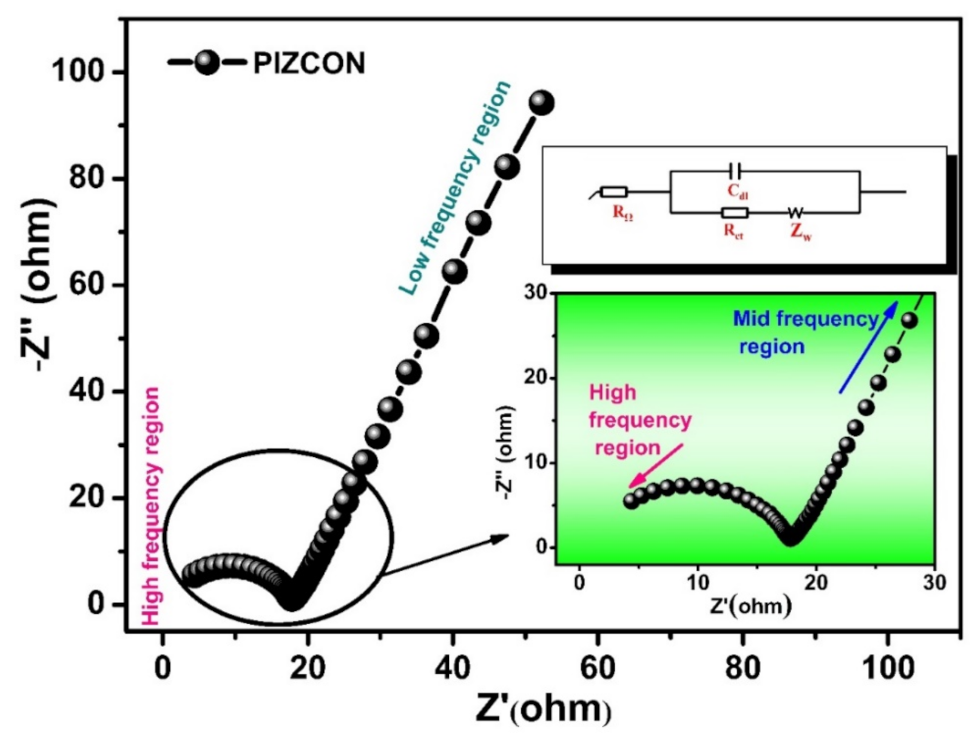
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Li, C.; Zhang, Q.; Zhang, J.; Wang, X.; Lin, Z.; Wang, J.; Lv, W.; Lu, C.; Wong, C.; et al. An all-solid-state, lightweight, and flexible asymmetric supercapacitor based on cabbage-like ZnCo2O4 and porous VN nanowires electrode materials. J. Mater. Chem. A 2017, 5, 6928–6936. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Gutturu, R.R.; TVM, S.; Rajavaram, R.; Borelli, D.P.R.; GR, D.; PC, N. Effect of reaction time and PVP contents on morphologies of hierarchical 3D flower-like ZnCo2O4 microstructures for energy storage devices. Int. J. Energy Res. 2020, 44, 11233–11247. [Google Scholar] [CrossRef]
- Gai, Y.; Shang, Y.; Gong, L.; Su, L.; Hao, L.; Dong, F.; Li, J. A self-template synthesis of porous ZnCo2O4 microspheres for high-performance quasi-solid-state asymmetric supercapacitors. RSC Adv. 2017, 7, 1038–1044. [Google Scholar] [CrossRef]
- Song, D.; Zhu, J.; Li, J.; Pu, T.; Huang, B.; Zhao, C.; Xie, L.; Chen, L. Free-standing two-dimensional mesoporous ZnCo2O4 thin sheets consisting of 3D ultrathin nanoflake array frameworks for high performance asymmetric supercapacitor. Electrochim. Acta 2017, 257, 455–464. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.R.; Dillip, G.R.; Sreekanth, T.V.M.; Rajavaram, R. Mechanistic investigation of defect-engineered, non-stoichiometric, and microstructures and their applications toward high-performance supercapacitors. Appl. Surf. Sci. 2020, 529, 147123. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Wu, L.; Fu, Q.; Gao, H. Annealing temperature dependent ZnCo2O4 nanosheet arrays supported on Ni foam for high-performance asymmetric supercapacitor. J. Alloys Compd. 2019, 773, 367–375. [Google Scholar] [CrossRef]
- Prasad, K.; Reddy, G.R.; Rajesh, M.; Babu, P.R.; Shanmugam, G.; Sushma, N.J.; Reddy, M.S.P.; Raju, B.D.P.; Mallikarjuna, K. Electrochemical performance of 2D-hierarchical sheet-Like ZnCo2O4 microstructures for supercapacitor applications. Crystals 2020, 10, 566. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Han, X.; Liao, F.; Zhang, Y.; Gao, L.; Xu, C. Facile synthesis of mesoporous ZnCo2O4 hierarchical microspheres and their excellent supercapacitor performance. Ceram. Int. 2019, 45, 8577–8584. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Attia, S.Y.; Allam, N.K. One-step, calcination-free synthesis of zinc cobaltite nanospheres for high-performance supercapacitors. Mater. Today Energy 2017, 4, 97–104. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, H.; Zhang, M.; Li, L.; Wang, G.; Shan, X. Three-dimensional porous ZnCo2O4 sheet array coated with Ni(OH )2 for high-performance asymmetric supercapacitor. J. Colloid Interface Sci. 2017, 497, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tomboc, G.M.; Jadhav, H.S.; Kim, H. PVP assisted morphology-controlled synthesis of hierarchical mesoporous ZnCo2O4 nanoparticles for high-performance pseudocapacitor. Chem. Eng. J. 2017, 308, 202–213. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L.; Sun, L.; Liu, Y.; Jiao, L. Facile synthesis of hierarchical porous ZnCo2O4 microspheres for high-performance supercapacitors. J. Mater. Chem. A 2014, 3, 982–985. [Google Scholar] [CrossRef]
- Reddy, G.R.; Dillip, G.R.; Sreekanth, T.V.M.; Rajavaram, R.; Prasad, B.D.; Nagajyothi, P.C.; Shim, J. In situ engineered 0D interconnected network-like CNS decorated on Co-rich ZnCo2O4 2D nanosheets for high-performance supercapacitors. J. Taiwan Inst. Chem. Eng. 2020, 113, 155–164. [Google Scholar] [CrossRef]
- Sun, J.; Zan, P.; Ye, L.; Yang, X.; Zhao, L. Superior performance of ZnCo2O4/ZnO@multiwall carbon nanotubes with laminated shape assembled as highly practical all-solid-state asymmetric supercapacitors. J. Mater. Chem. 2017, 5, 9815–9823. [Google Scholar] [CrossRef]
- Juliet, A.A.; Mary, C.; Bose, A.C. Hydrothermal synthesis of Mn-doped ZnCo2O4 electrode material for high-performance supercapacitors. Appl. Surf. Sci. 2017, 425, 201–211. [Google Scholar]
- Prasad, K.; Reddy, G.R.; Raju BD, P. Surfactant assisted morphological transformation of rod-like ZnCo2O4 into hexagonal-like structures for high-performance supercapacitors. Indian J. Sci. Technol. 2021, 14, 676–689. [Google Scholar] [CrossRef]
- Wu, H.; Lou, Z.; Yang, H.; Shen, G. A flexible spiral-type supercapacitor based on ZnCo2O4 nanorod electrodes. Nanoscale 2015, 7, 1921–1926. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Double hydroxide mediated synthesis of nanostructured ZnCo2O4 as high-performance electrode material for supercapacitor applications. Chem. Eng. J. 2017, 321, 474–483. [Google Scholar] [CrossRef]
- Reddy, G.R.; Kumar, N.S.; Deva, B.; Raju, P. Enhanced Supercapacitive Performance of Higher-Ordered 3D-Hierarchical Structures of Hydrothermally Obtained ZnCo2O4 for Energy Storage Devices. Nanomaterials 2020, 10, 1206. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Liu, W.; Xu, L.; Lei, F.; Xie, J.; Zhao, Y.; Huang, Y.; Guan, M.; Li, H. ZnCo2O4 ultrathin nanosheets towards the high performance of flexible supercapacitors and bifunctional electrocatalysis. J. Alloys Compd. 2018, 764, 565–573. [Google Scholar] [CrossRef]
- Ramachandran, T.; Hamed, F. Electrochemical performance of plate-like zinc cobaltite electrode material for supercapacitor applications. J. Phys. Chem. Solids 2018, 121, 93–101. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H. Reagents assisted ZnCo2O4 nanomaterial for supercapacitor application. Electrochim. Acta 2019, 330, 135261. [Google Scholar] [CrossRef]
- Du, C.; Han, E.; Sun, L.; Qiao, S.; Li, L. Template agent for assisting in the synthesis of ZnCo2O4 on Ni foam for high-performance supercapacitors. Ionics 2019, 26, 383–391. [Google Scholar] [CrossRef]
- Chen, H.; Du, X.; Sun, J.; Mao, H.; Wu, R.; Xu, C. Simple preparation of ZnCo2O4 porous quasi-cubes for high performance asymmetric supercapacitors. Appl. Surf. Sci. 2020, 515, 146008. [Google Scholar] [CrossRef]
- Kumar, V.; Mariappan, C.R.; Azmi, R.; Moock, D.; Indris, S.; Bruns, M.; Ehrenberg, H.; Prakash, G.V. Pseudocapacitance of Mesoporous Spinel-Type MCo2O4 (M = Co, Zn, and Ni) Rods Fabricated by a Facile Solvothermal Route. ACS Omega 2017, 2, 6003–6013. [Google Scholar] [CrossRef] [PubMed]
- Bhagwan, J.; Hussain, S.K.; Yu, J.S. Aqueous asymmetric supercapacitors based on ZnCo2O4 nanoparticles via facile combustion method. J. Alloys Compd. 2019, 815, 152456. [Google Scholar] [CrossRef]
- Venkata, T.; Sree, M.; Chidanandha, P. Urea assisted ceria nanocubes for efficient removal of malachite green organic dye from aqueous system. Sci. Rep. 2019, 9, 14477. [Google Scholar]
- Etacheri, V.; Seisenbaeva, G.A.; Caruthers, J.M.; Daniel, G.; Nedelec, J.-M.; Kessler, V.G.; Pol, V.G. Ordered Network of Interconnected SnO2 Nanoparticles for Excellent Lithium-Ion Storage. Adv. Energy Mater. 2015, 5, 1401289. [Google Scholar] [CrossRef]
- Xing, Z.; Liu, Q.; Asiri, A.M.; Sun, X. Closely Interconnected Network of Molybdenum Phosphide Nanoparticles: A Highly Efficient Electrocatalyst for Generating Hydrogen from Water. Adv. Mater. 2014, 26, 5702–5707. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, M.; Ning, M.; Wu, K.; Li, H.; Hussain, S.; Zhao, S. Facile ordered ZnCo2O4@MnO2 nanosheet arrays for superior-performance supercapacitor electrode. Solid State Sci. 2018, 84, 51–56. [Google Scholar] [CrossRef]




| Material | Synthesis Method | Morphology | Areal Capacitance @ Current Density | Ref |
|---|---|---|---|---|
| ZnCo2O4 | Hydrothermal | Flake-like | 2.72 F cm−2 @ 2.02 mA cm−2 | [5] |
| ZnCo2O4 | Hydrothermal | Nanosheet array | 3.19 F cm−2 @ 2 mA cm−2 | [8] |
| ZnCo2O4 | Hydrothermal | Sheet-like | 16.13 mF cm−2 @ 10 μA cm−2 | [9] |
| ZnCo2O4 | Hydrothermal | Hexagonal-like | 41.43 mF cm−2 @ 10 μA cm−2 | [18] |
| ZnCo2O4 | Hydrothermal | Rod-like | 31 mF cm−2 @ 10 μA cm−2 | |
| ZnCo2O4 (PIZCON) | Solvothermal | Potato chip-like | 14.52 mF cm−2 @ 10 μA cm−2 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallem, S.P.R.; Koduru, M.; Chandrasekhar, K.; Prabhakar Vattikuti, S.V.; Manne, R.; Reddy, V.R.; Lee, J.-H. Potato Chip-Like 0D Interconnected ZnCo2O4 Nanoparticles for High-Performance Supercapacitors. Crystals 2021, 11, 469. https://doi.org/10.3390/cryst11050469
Mallem SPR, Koduru M, Chandrasekhar K, Prabhakar Vattikuti SV, Manne R, Reddy VR, Lee J-H. Potato Chip-Like 0D Interconnected ZnCo2O4 Nanoparticles for High-Performance Supercapacitors. Crystals. 2021; 11(5):469. https://doi.org/10.3390/cryst11050469
Chicago/Turabian StyleMallem, Siva Pratap Reddy, Mallikarjuna Koduru, Kuppam Chandrasekhar, S. V. Prabhakar Vattikuti, Ravi Manne, V. Rajagopal Reddy, and Jung-Hee Lee. 2021. "Potato Chip-Like 0D Interconnected ZnCo2O4 Nanoparticles for High-Performance Supercapacitors" Crystals 11, no. 5: 469. https://doi.org/10.3390/cryst11050469
APA StyleMallem, S. P. R., Koduru, M., Chandrasekhar, K., Prabhakar Vattikuti, S. V., Manne, R., Reddy, V. R., & Lee, J.-H. (2021). Potato Chip-Like 0D Interconnected ZnCo2O4 Nanoparticles for High-Performance Supercapacitors. Crystals, 11(5), 469. https://doi.org/10.3390/cryst11050469








