A Review of Pharmaceutical Nano-Cocrystals: A Novel Strategy to Improve the Chemical and Physical Properties for Poorly Soluble Drugs
Abstract
1. Introduction
2. Pharmaceutical Cocrystal
2.1. Definition of Cocrystal and Nanocrystal
2.2. Application of Pharmaceutical Nano-Cocrystal
2.2.1. Drug Delivery
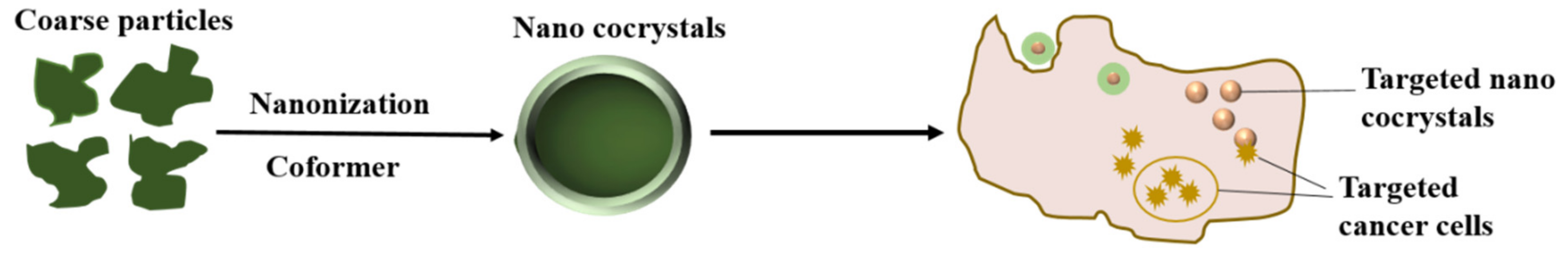
2.2.2. Targeted Nanocrystals
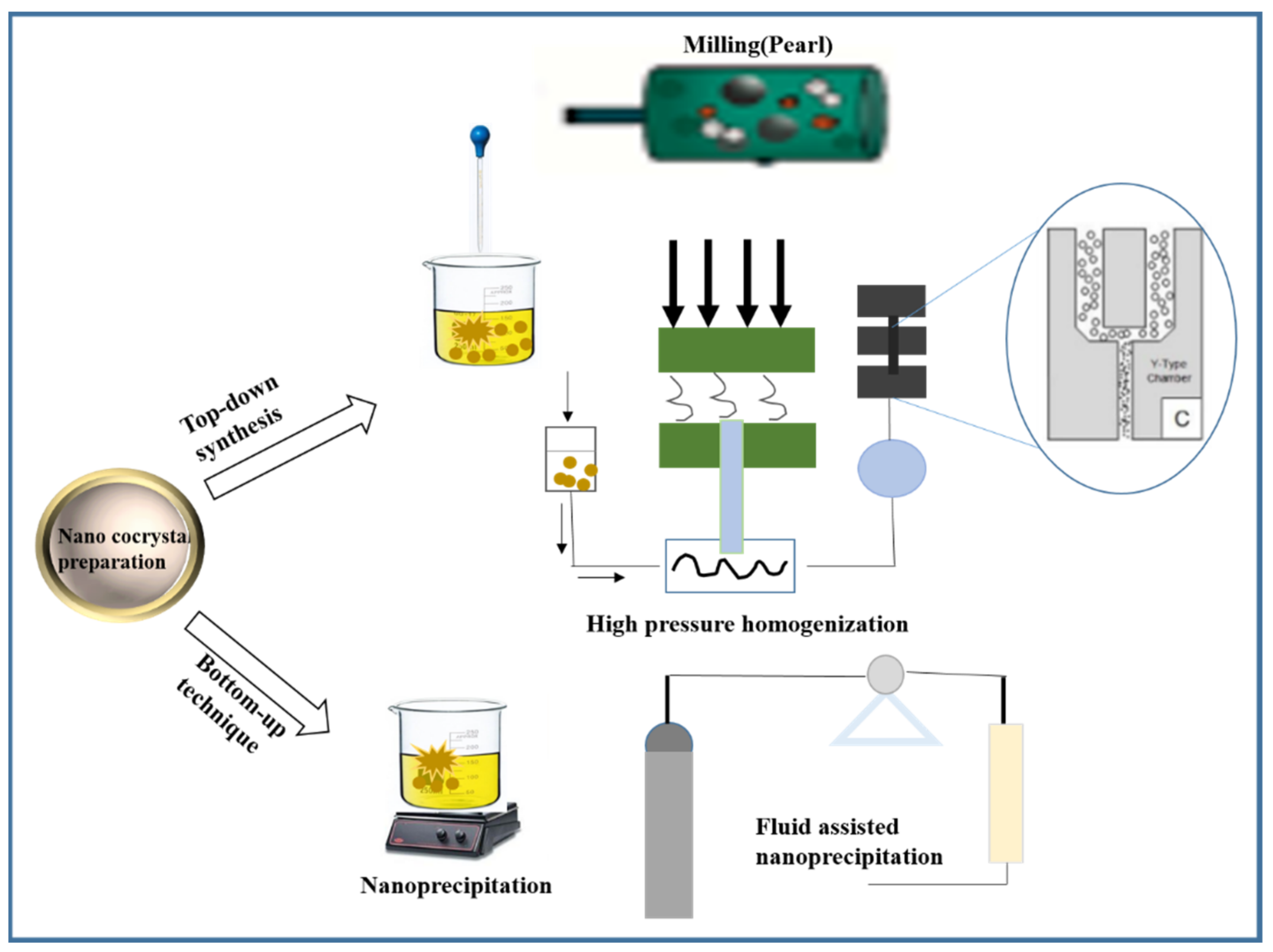
3. Preparation Method of Pharmaceutical Nano-Cocrystals
3.1. Top-Down Synthesis
3.1.1. High Pressure Homogenization
3.1.2. Milling
3.2. Bottom-Up Technique
3.3. Electrohydrodynamic Atomization (EHDA) and Spray Drying Method (SD)
4. Advanced Characterization Techniques
4.1. X-ray Diffraction (PXRD, SCXRD)
4.2. Thermal Analysis
4.3. Molecular Vibration Spectroscopy (IR, Raman)
4.4. Microscopy Techniques (TEM, AFM)
4.5. Solid-State NMR Spectroscopy
5. Conclusions and Prospect
- Structural analysis. Study of the molecular conformation and functional groups of drug molecules can provide assistance in the design and preparation of specific cocrystals, which has always been a huge challenge due to the complexity of the chemical structure of APIs.
- Selection of co-formers. When preparing the cocrystals, the selection of co-formers is an important step. Usually, medicinal excipients, common salt-forming ligands, food additives, and other active pharmaceutical ingredients are the preferred co-formers.
- Development of advanced analytical techniques. Analytical technique is an effective means to detect the internal structure of drug molecules. Appropriate characterization may provide practical guidelines for the preparation of new nano-cocrystals, as well as aiding in studying the interaction mechanism of nano-cocrystals.
Author Contributions
Funding
Conflicts of Interest
References
- Almeida e Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Supersaturation Potential of Salt, Co-Crystal, and Amorphous Forms of a Model Weak Base. Cryst. Growth Des. 2016, 16, 737–748. [Google Scholar] [CrossRef]
- Saini, A.; Chadha, R.; Gupta, A.; Singh, P.; Bhandari, S.; Khullar, S.; Mandal, S.; Jain, D.S. New conformational polymorph of hydrochlorothiazide with improved solubility. Pharm. Dev. Technol. 2016, 21, 611–618. [Google Scholar] [CrossRef]
- Basavoju, S.; Boström, D.; Velaga, S.P. Pharmaceutical Cocrystal and Salts of Norfloxacin. Cryst. Growth Des. 2006, 6, 2699–2708. [Google Scholar] [CrossRef]
- Billot, P.; Hosek, P.; Perrin, M.-A. Efficient Purification of an Active Pharmaceutical Ingredient via Cocrystallization: From Thermodynamics to Scale-Up. Org. Process Res. Dev. 2013, 17, 505–511. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, K.; Yamamoto, M.; Karashima, M.; Hohokabe, M.; Takeda, J.; Yamamoto, K.; Ikeda, Y. Pharmaceutical Cocrystal Development of TAK-020 with Enhanced Oral Absorption. Crystals 2020, 10, 211. [Google Scholar] [CrossRef]
- Shaikh, R.; Walker, G.M.; Croker, D.M. Continuous, simultaneous cocrystallization and formulation of Theophylline and 4-Aminobenzoic acid pharmaceutical cocrystals using twin screw melt granulation. Eur. J. Pharm. Sci. 2019, 137, 104981. [Google Scholar] [CrossRef] [PubMed]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Kuminek, G.; Rodríguez-Hornedo, N.; Siedler, S.; Rocha, H.V.A.; Cuffini, S.L.; Cardoso, S.G. How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chem. Commun. 2016, 52, 5832–5835. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Wang, S.; Li, W.; Kebebe, D.; Zhang, Y.; Zhang, B.; Qi, D.; Guo, P.; Li, N.; Liu, Z. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J. Pharm. Sci. 2019, 14, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Aakeroy, C. Is there any point in making co-crystals? Acta Crystallogr. Sect. B 2015, 71, 387–391. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef]
- Mazzeo, P.P.; Carraro, C.; Monica, A.; Capucci, D.; Pelagatti, P.; Bianchi, F.; Agazzi, S.; Careri, M.; Raio, A.; Carta, M.; et al. Designing a Palette of Cocrystals Based on Essential Oil Constituents for Agricultural Applications. ACS Sustain. Chem. Eng. 2019, 7, 17929–17940. [Google Scholar] [CrossRef]
- Capucci, D.; Balestri, D.; Mazzeo, P.P.; Pelagatti, P.; Rubini, K.; Bacchi, A. Liquid Nicotine Tamed in Solid Forms by Cocrystallization. Cryst. Growth Des. 2017, 17, 4958–4964. [Google Scholar] [CrossRef]
- Bacchi, A.; Capucci, D.; Giannetto, M.; Mattarozzi, M.; Pelagatti, P.; Rodriguez-Hornedo, N.; Rubini, K.; Sala, A. Turning Liquid Propofol into Solid (without Freezing It): Thermodynamic Characterization of Pharmaceutical Cocrystals Built with a Liquid Drug. Cryst. Growth Des. 2016, 16, 6547–6555. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- De Smet, L.; Saerens, L.; De Beer, T.; Carleer, R.; Adriaensens, P.; Van Bocxlaer, J.; Vervaet, C.; Remon, J.P. Formulation of itraconazole nanococrystals and evaluation of their bioavailability in dogs. Eur. J. Pharm. Biopharm. 2014, 87, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Karashima, M.; Kimoto, K.; Yamamoto, K.; Kojima, T.; Ikeda, Y. A novel solubilization technique for poorly soluble drugs through the integration of nanocrystal and cocrystal technologies. Eur. J. Pharm. Biopharm. 2016, 107, 142–150. [Google Scholar] [CrossRef]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-J.; Wu, F.-L.L. Nanomedicines in renal transplant rejection--focus on sirolimus. Int. J. Nanomed. 2007, 2, 25–32. [Google Scholar] [CrossRef]
- Gupta, A.D. A review on recent advancement of cancer therapy using nanoparticles. Biochem. Mol. Biol. Lett. 2017, 3, 104. [Google Scholar]
- Ali, H.S.; York, P.; Ali, A.M.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Ghosh, I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur. J. Pharm. Sci. 2013, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Yue, P.-F.; Hu, P.-Y.; Wu, Z.-F.; Yang, M.; Yuan, H.-L. A novel high-pressure precipitation tandem homogenization technology for drug nanocrystals production–a case study with ursodeoxycholic acid. Pharm. Dev. Technol. 2014, 19, 662–670. [Google Scholar] [CrossRef]
- Li, H.; An, C.; Guo, W.; Geng, X.; Wang, J.; Xu, W. Preparation and performance of nano HMX/TNT cocrystals. Propellants Explos. Pyrotech. 2015, 40, 652–658. [Google Scholar] [CrossRef]
- Salem, A.; Takácsi-Nagy, A.; Nagy, S.; Hagymási, A.; Gősi, F.; Vörös-Horváth, B.; Balić, T.; Pál, S.; Széchenyi, A. Synthesis and Characterization of Nano-Sized 4-Aminosalicylic Acid–Sulfamethazine Cocrystals. Pharmaceutics 2021, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Kanswami, N.; Lakshmi, P. Nano Co-crystal Engineering Technique to Enhance the Solubility of Ezetimibe. J. Young Pharm. 2020, 12, S10. [Google Scholar] [CrossRef]
- Witika, B.A.; Smith, V.J.; Walker, R.B. Top-Down Synthesis of a Lamivudine-Zidovudine Nano Co-Crystal. Crystals 2021, 11, 33. [Google Scholar] [CrossRef]
- Sander, J.R.; Bučar, D.K.; Henry, R.F.; Zhang, G.G.; MacGillivray, L.R. Pharmaceutical nano-cocrystals: Sonochemical synthesis by solvent selection and use of a surfactant. Angew. Chem. Int. Ed. 2010, 49, 7284–7288. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, D.; Risse, B.; Schnell, F.; Pichot, V.; Klaumünzer, M.; Schaefer, M. Continuous engineering of nano-cocrystals for medical and energetic applications. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.-M.; Lai, Z.-H.; Wu, J.; Lu, T.-B.; Chen, J.-M. Phenazopyridine-phthalimide nano-cocrystal: Release rate and oral bioavailability enhancement. Eur. J. Pharm. Sci. 2017, 109, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Junghanns, J. Drug nanocrystals/nanosuspensions for the delivery of poorly soluble drugs. Nanopart. Drug Carr. 2006, 1, 307–328. [Google Scholar]
- Rabinow, B.; Kipp, J.; Papadopoulos, P.; Wong, J.; Glosson, J.; Gass, J.; Sun, C.-S.; Wielgos, T.; White, R.; Cook, C. Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat. Int. J. Pharm. 2007, 339, 251–260. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wang, L.; Xu, Y.; Cheng, X.; Wei, P. Formulation and pharmacokinetic evaluation of a paclitaxel nanosuspension for intravenous delivery. Int. J. Nanomed. 2011, 6, 1497. [Google Scholar]
- Ganta, S.; Paxton, J.W.; Baguley, B.C.; Garg, S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int. J. Pharm. 2009, 367, 179–186. [Google Scholar] [CrossRef]
- Zirar, S.B.; Astier, A.; Muchow, M.; Gibaud, S. Comparison of nanosuspensions and hydroxypropyl-β-cyclodextrin complex of melarsoprol: Pharmacokinetics and tissue distribution in mice. Eur. J. Pharm. Biopharm. 2008, 70, 649–656. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, D.; Chen, M.; Zheng, T.; Wang, S. Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement. Drug Dev. Ind. Pharm. 2007, 33, 1332–1339. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Sun, M.; Guo, C.; Yu, A.; Xi, Y.; Cui, J.; Lou, H.; Zhai, G. Preparation and characterization of intravenously injectable curcumin nanosuspension. Drug Deliv. 2011, 18, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E.; Rittenhouse, K.D.; Wilson, C.G.; Weber, D.A.; Kuppermann, B.D. Ophthalmic drug delivery systems for the treatment of retinal diseases: Basic research to clinical applications. Investig. Ophth. Vis. Sci. 2010, 51, 5403–5420. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.; Rahman, A.A.; Ghorab, M.; Ahmed, M.; Khalil, R. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007, 340, 126–133. [Google Scholar] [CrossRef]
- Baba, K.; Nishida, K. Steroid nanocrystals prepared using the nano spray dryer B-90. Pharmaceutics 2013, 5, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Samanta, M.K.; Raichur, A.M. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS Pharmscitech. 2010, 11, 322–335. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Devesa, S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 2001, 37, 4–66. [Google Scholar] [CrossRef]
- Talekar, M.; Kendall, J.; Denny, W.; Jamieson, S.; Garg, S. Development and evaluation of PIK75 nanosuspension, a phosphatidylinositol-3-kinase inhibitor. Eur. J. Pharm. Sci. 2012, 47, 824–833. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, Y.; Zhang, D.; Liu, Z.; Duan, C.; Jia, L.; Wang, F.; Liu, Y.; Liu, G.; Hao, L.; et al. In vitro antitumor activity of silybin nanosuspension in PC-3 cells. Cancer Lett. 2011, 307, 158–164. [Google Scholar] [CrossRef]
- Shegokar, R.; Singh, K.K. Surface modified nevirapine nanosuspensions for viral reservoir targeting: In vitro and in vivo evaluation. Int. J. Pharm. 2011, 421, 341–352. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Ramírez Rigo, M.V.; Gonzalez Vidal, N.L. Praziquantel systems with improved dissolution rate obtained by high pressure homogenization. Mater. Sci. Eng. C 2018, 93, 28–35. [Google Scholar] [CrossRef]
- Trask, A.V.; Jones, W. Crystal engineering of organic cocrystals by the solid-state grinding approach. Org. Solid State React. 2005, 41–70. [Google Scholar]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P.A. Grinding as solvent-free green chemistry approach for cyclodextrin inclusion complex preparation in the solid state. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hong, C.; Li, G.; Ma, P.; Xie, Y. The generation of myricetin–nicotinamide nanococrystals by top down and bottom up technologies. Nanotechnology 2016, 27, 395601. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Motherwell, W.S.; Trask, A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef]
- Shan, N.; Toda, F.; Jones, W. Mechanochemistry and co-crystal formation: Effect of solvent on reaction kinetics. Chem. Commun. 2002, 20, 2372–2373. [Google Scholar] [CrossRef]
- Lien Nguyen, K.; Friščić, T.; Day, G.M.; Gladden, L.F.; Jones, W. Terahertz time-domain spectroscopy and the quantitative monitoring of mechanochemical cocrystal formation. Nat. Mater. 2007, 6, 206–209. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Sun, W.-J.; Lu, T.; Tong, H.H.Y.; Sun, C.C.; Zheng, Y. Resveratrol cocrystals with enhanced solubility and tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018, 8, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-R.; Kwon, E.; Oikawa, H.; Kasai, H.; Nakanishi, H. Effect of solvent on organic nanocrystal growth using the reprecipitation method. J. Cryst. Growth 2006, 294, 459–463. [Google Scholar] [CrossRef]
- Thakor, P.; Yadav, B.; Modani, S.; Shastri, N.R. Preparation and optimization of nano-sized cocrystals using a quality by design approach. CrystEngComm 2020, 22, 2304–2314. [Google Scholar] [CrossRef]
- Arzi, R.S.; Sosnik, A. Electrohydrodynamic atomization and spray-drying for the production of pure drug nanocrystals and co-crystals. Adv. Drug Deliv. Rev. 2018, 131, 79–100. [Google Scholar] [CrossRef]
- Peltonen, L.; Valo, H.; Kolakovic, R.; Laaksonen, T.; Hirvonen, J. Electrospraying, spray drying and related techniques for production and formulation of drug nanoparticles. Expert Opin. Drug Deliv. 2010, 7, 705–719. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Clasen, C.; Van den Mooter, G. Pharmaceutical applications of electrospraying. J. Pharm. Sci. 2016, 105, 2601–2620. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A. Production of drug-loaded polymeric nanoparticles by electrospraying technology. J. Biomed. Nanotechnol. 2014, 10, 2200–2217. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997. [Google Scholar]
- Jaworek, A. Micro-and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Patil, S.; Kulkarni, J.; Mahadik, K. Exploring the potential of electrospray technology in cocrystal synthesis. Ind. Eng. Chem. Res. 2016, 55, 8409–8414. [Google Scholar] [CrossRef]
- Wang, M.; Rutledge, G.C.; Myerson, A.S.; Trout, B.L. Production and characterization of carbamazepine nanocrystals by electrospraying for continuous pharmaceutical manufacturing. J. Pharm. Sci. 2012, 101, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Calvo, A.; Davila, J.; Barrero, A. Current and droplet size in the electrospraying of liquids. Scaling laws. J. Aerosol Sci. 1997, 28, 249–275. [Google Scholar] [CrossRef]
- Vega-Mercado, H.; Góngora-Nieto, M.M.; Barbosa-Cánovas, G.V. Advances in dehydration of foods. J. Food Eng. 2001, 49, 271–289. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; Velaga, S.P. Formation of cocrystals from stoichiometric solutions of incongruently saturating systems by spray drying. Cryst. Growth Des. 2010, 10, 3302–3305. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Kaialy, W.; Buckton, G.; Gill, H.; Nokhodchi, A.; Velaga, S.P. Theophylline cocrystals prepared by spray drying: Physicochemical properties and aerosolization performance. AAPS Pharmscitech 2013, 14, 265–276. [Google Scholar] [CrossRef]
- Baba, K.; Nishida, K. Calpain inhibitor nanocrystals prepared using Nano Spray Dryer B-90. Nanoscale Res. Lett. 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Prinn, K.B.; Costantino, H.R.; Tracy, M. Statistical modeling of protein spray drying at the lab scale. AAPS Pharmscitech 2002, 3, 32–39. [Google Scholar] [CrossRef]
- Jaywant, N.P.; Purnima, D.A. Development of efavirenz cocrystals from stoichiometric solutions by spray drying technology. Mater. Today Proc. 2016, 3, 1742–1751. [Google Scholar] [CrossRef]
- Harris, K.D.; Tremayne, M.; Kariuki, B.M. Contemporary advances in the use of powder X-ray diffraction for structure determination. Angew. Chem. Int. Ed. 2001, 40, 1626–1651. [Google Scholar] [CrossRef]
- Tremayne, M. The impact of powder diffraction on the structural characterization of organic crystalline materials. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2691–2707. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics 2020, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Buol, X.; Robeyns, K.; Caro Garrido, C.; Tumanov, N.; Collard, L.; Wouters, J.; Leyssens, T. Improving nefiracetam dissolution and solubility behavior using a cocrystallization approach. Pharmaceutics 2020, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, A.; Śniechowska, J.; Kaźmierski, S.; Wielgus, E.; Bujacz, G.D.; Mlostoń, G.; Chworos, A.; Suwara, J.; Potrzebowski, M.J. Application of 1-Hydroxy-4, 5-Dimethyl-Imidazole 3-Oxide as Coformer in Formation of Pharmaceutical Cocrystals. Pharmaceutics 2020, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Gabbott, P. Principles and Applications of Thermal Analysis; John Wiley Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Höhne, G.W.H.; Hemminger, W.; Flammersheim, H.-J. Theoretical fundamentals of differential scanning calorimeters. In Differential Scanning Calorimetry; Springer: Berlin/Heidelberg, Germany, 2003; pp. 31–63. [Google Scholar]
- Lu, J.; Rohani, S. Preparation and characterization of theophylline−nicotinamide cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Stuart, B. Infrared spectroscopy. In Analytical Techniques in Forensic Science; John Wiley Sons: Hoboken, NJ, USA, 2021; pp. 145–160. [Google Scholar]
- Guo, M.; Fu, Q.; Wu, C.; Guo, Z.; Li, M.; Sun, J.; He, Z.; Yang, L. Rod shaped nanocrystals exhibit superior in vitro dissolution and in vivo bioavailability over spherical like nanocrystals: A case study of lovastatin. Colloids Surf. B Biointerfaces 2015, 128, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Wijayasinghe, R.; Vasiljevic, T.; Chandrapala, J. Water-lactose behavior as a function of concentration and presence of lactic acid in lactose model systems. J. Dairy Sci. 2015, 98, 8505–8514. [Google Scholar] [CrossRef]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chen, D.; Singh, D.; Sirkar, K.K.; Pfeffer, R. Continuous preparation of polymer coated drug crystals by solid hollow fiber membrane-based cooling crystallization. Int. J. Pharm. 2016, 499, 395–402. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Lodge, T.P.; Hillmyer, M.A. Detection of pharmaceutical drug crystallites in solid dispersions by transmission electron microscopy. Mol. Pharm. 2015, 12, 983–990. [Google Scholar] [CrossRef]
- Hübner, J.; Deckert-Gaudig, T.; Glorian, J.; Deckert, V.; Spitzer, D. Surface characterization of nanoscale co-crystals enabled through tip enhanced Raman spectroscopy. Nanoscale 2020, 12, 10306–10319. [Google Scholar] [CrossRef] [PubMed]
- Pinon, A.C.; Rossini, A.J.; Widdifield, C.M.; Gajan, D.; Emsley, L. Polymorphs of theophylline characterized by DNP enhanced solid-state NMR. Mol. Pharm. 2015, 12, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
| Nano CoCrystals | Preparation | Nano Size | Advantages | Ref. |
|---|---|---|---|---|
| 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane (HMX) and 2,4,6-trinitrotoluene (TNT) | Spray drying method | 50 nm to 200 nm | High energy and low sensitivity | [29]. |
| Baicalein-nicotinamide (BE-NCT) | High pressure homogenization | 251.53 nm | Improving dissolution rate (2.54-fold) and bioavailability(6.02-fold) of BE | [12]. |
| 4-aminosalysilic acid and sulfamethazine | High-pressure homogenization (HPH) and high-power ultrasound | - | Enhanced the dissolution of sulfamethazine; stable for 6 months when stored at room temperature | [30]. |
| Ezetimibe | Solvent evaporation method and anti-solvent method | 226.4 ± 53 nm | Showed 18.8-fold increase in the dissolution efficiency | [31]. |
| Lamivudine (3TC) and zidovudine (AZT) | Wet media milling top-down approach | 271.0 ± 92.0 nm | Nanosuspensions exhibit a reduced side effect; increased adherence to therapy and reduced resistance to therapy | [32]. |
| Caffeine and 2,4-dihydroxybenzoic acid | Using “top-down” media milling | <100 nm | Enhanced dissolution rates; improved bioavailability and efficacy of medication | [33]. |
| Caffeine/Oxalic acid 2:1 and Caffeine/Glutaric acid 1:1 cocrystals | Spray flash evaporation process | About 60 nm | Enhanced stability, high solubility, high bioavailability, optimized drug up-take | [34]. |
| Hydroxypropyl methylcellulose and sodium dodecyl sulfate | Wet milling | <300 nm | Improve the absorption of poorly soluble drugs | [22]. |
| Characterization Techniques | Analysis Object | Advantages |
|---|---|---|
| SCXRD | Analysis of internal structure of crystals, such as unit cell dimensions, bond lengths, angles, and details of crystal packing); analysis of the charge density; chirality test of molecules; real-time monitoring | Maintain the pristine structure of samples; easy sample preparation; rapid test technique. |
| PXRD | Analysis of phase purity and crystallinity | High sensitivity; easy sample preparation; rapid test technique; maintain the pristine structure of samples. |
| DSC,TGA | Test solid state properties | Simple and fast; has excellent accuracy; easy to operate; wide range of temperatures; real-time process monitoring. |
| IR, Raman | Test the properties of solid state | Easy to operate; provide information on chemical structure and interactions. |
| SEM, TEM,AFM | Characterization of particle shape, morphology, as well as the particle size | Easy to operate; provide available information on shape and morphology of samples, even in a nanometer scale. |
| NMR | Solid state properties | Important method for analysis and determination of crystal forms in multiply crystals system; analysis of the dynamics behavior and chemical environment of atom. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Liu, J.; Ran, L. A Review of Pharmaceutical Nano-Cocrystals: A Novel Strategy to Improve the Chemical and Physical Properties for Poorly Soluble Drugs. Crystals 2021, 11, 463. https://doi.org/10.3390/cryst11050463
Tan J, Liu J, Ran L. A Review of Pharmaceutical Nano-Cocrystals: A Novel Strategy to Improve the Chemical and Physical Properties for Poorly Soluble Drugs. Crystals. 2021; 11(5):463. https://doi.org/10.3390/cryst11050463
Chicago/Turabian StyleTan, Jianbing, Jianhao Liu, and Liling Ran. 2021. "A Review of Pharmaceutical Nano-Cocrystals: A Novel Strategy to Improve the Chemical and Physical Properties for Poorly Soluble Drugs" Crystals 11, no. 5: 463. https://doi.org/10.3390/cryst11050463
APA StyleTan, J., Liu, J., & Ran, L. (2021). A Review of Pharmaceutical Nano-Cocrystals: A Novel Strategy to Improve the Chemical and Physical Properties for Poorly Soluble Drugs. Crystals, 11(5), 463. https://doi.org/10.3390/cryst11050463





