Extraction–Pyrolytic Method for TiO2 Polymorphs Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Precursors
2.1.1. Preparation of Aqueous Solution of Titanium (III) Chloride TiCl3
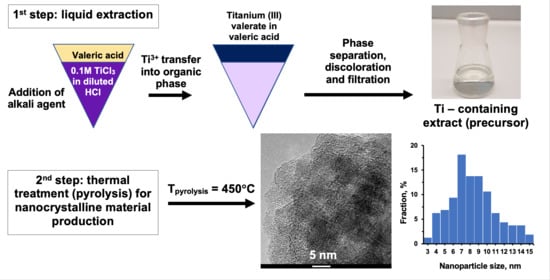
2.1.2. Preparation of Titanium-Containing Precursors (E) via Liquid–Liquid Extraction
2.1.3. Preparation of Titanium-Containing Precursor (P) via Precipitation
2.2. Thermal Treatment of Precursors
2.3. Characterization Methods
3. Results
3.1. Precursors Characterization
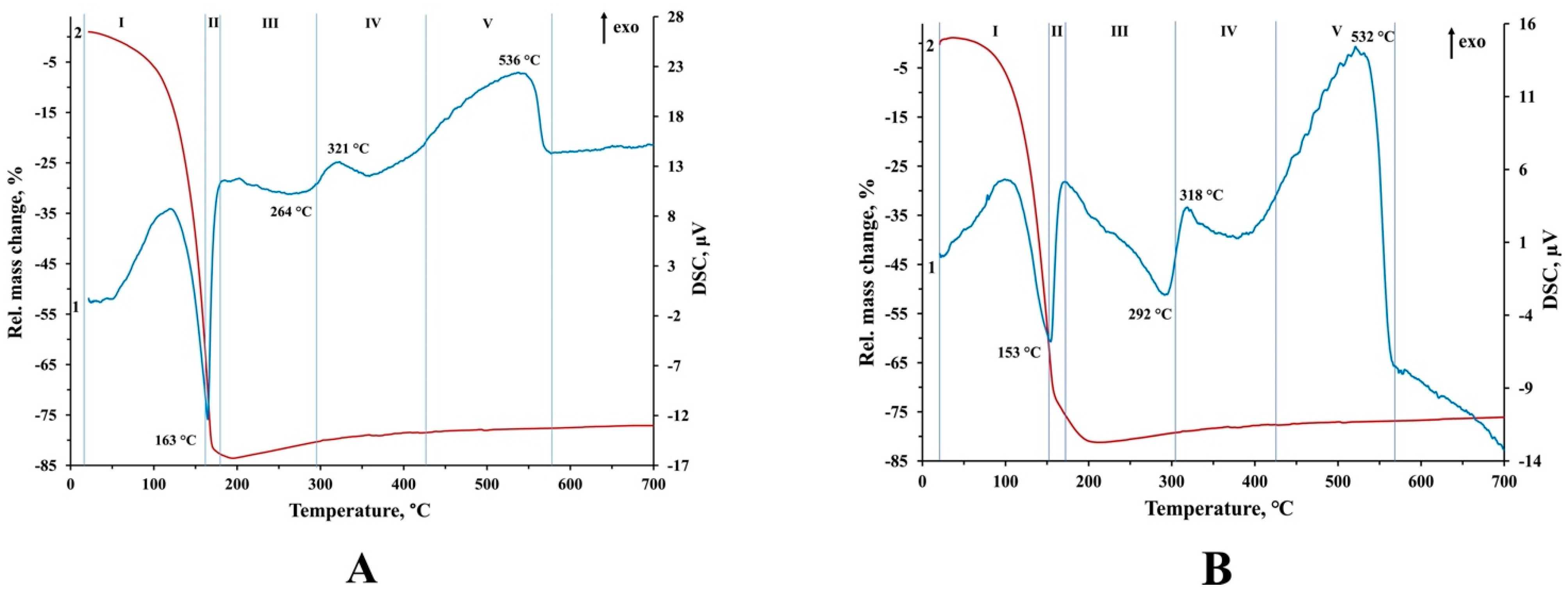
3.2. Thermal Behavior of Precursors E1, E2, and P
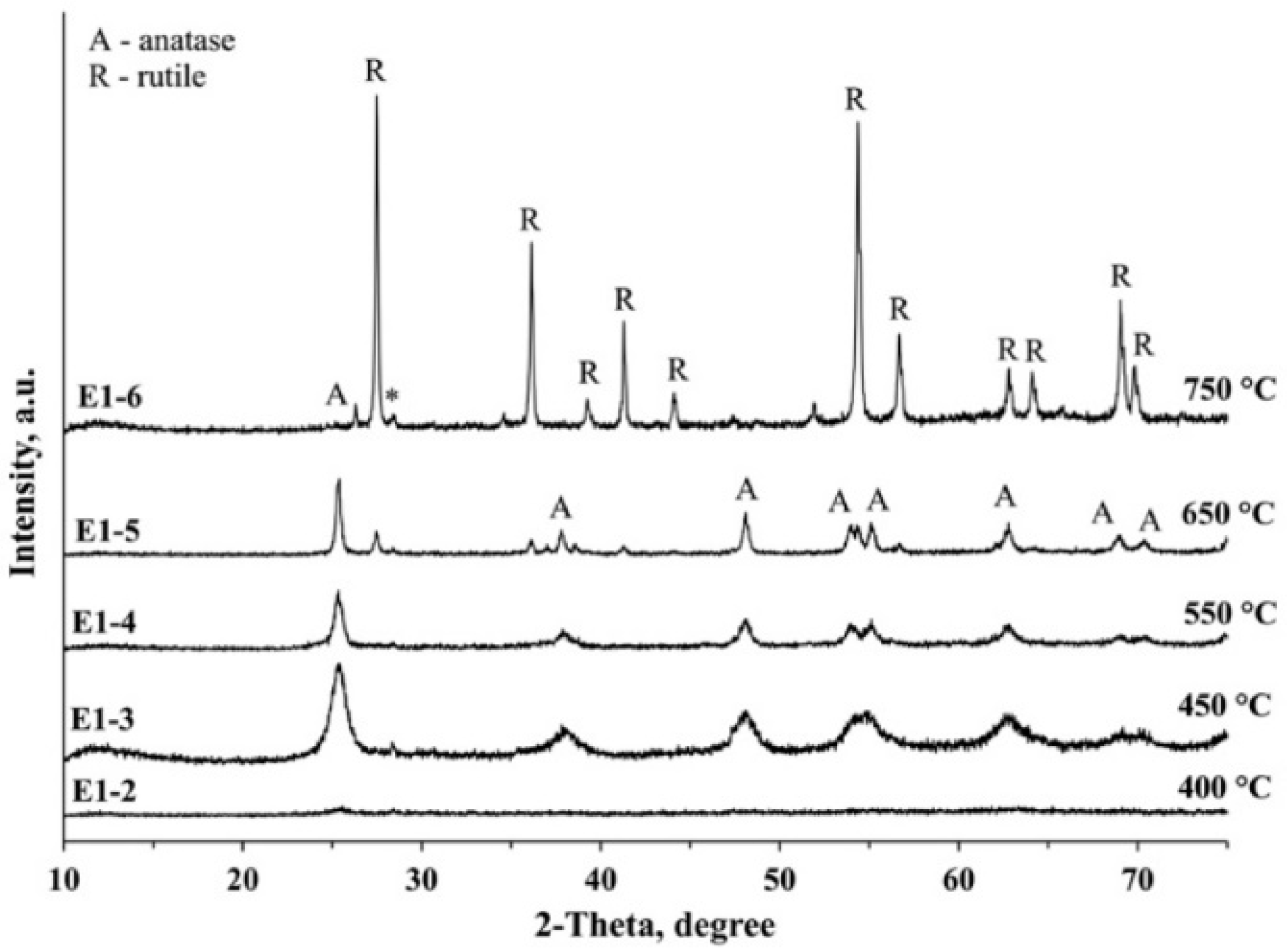
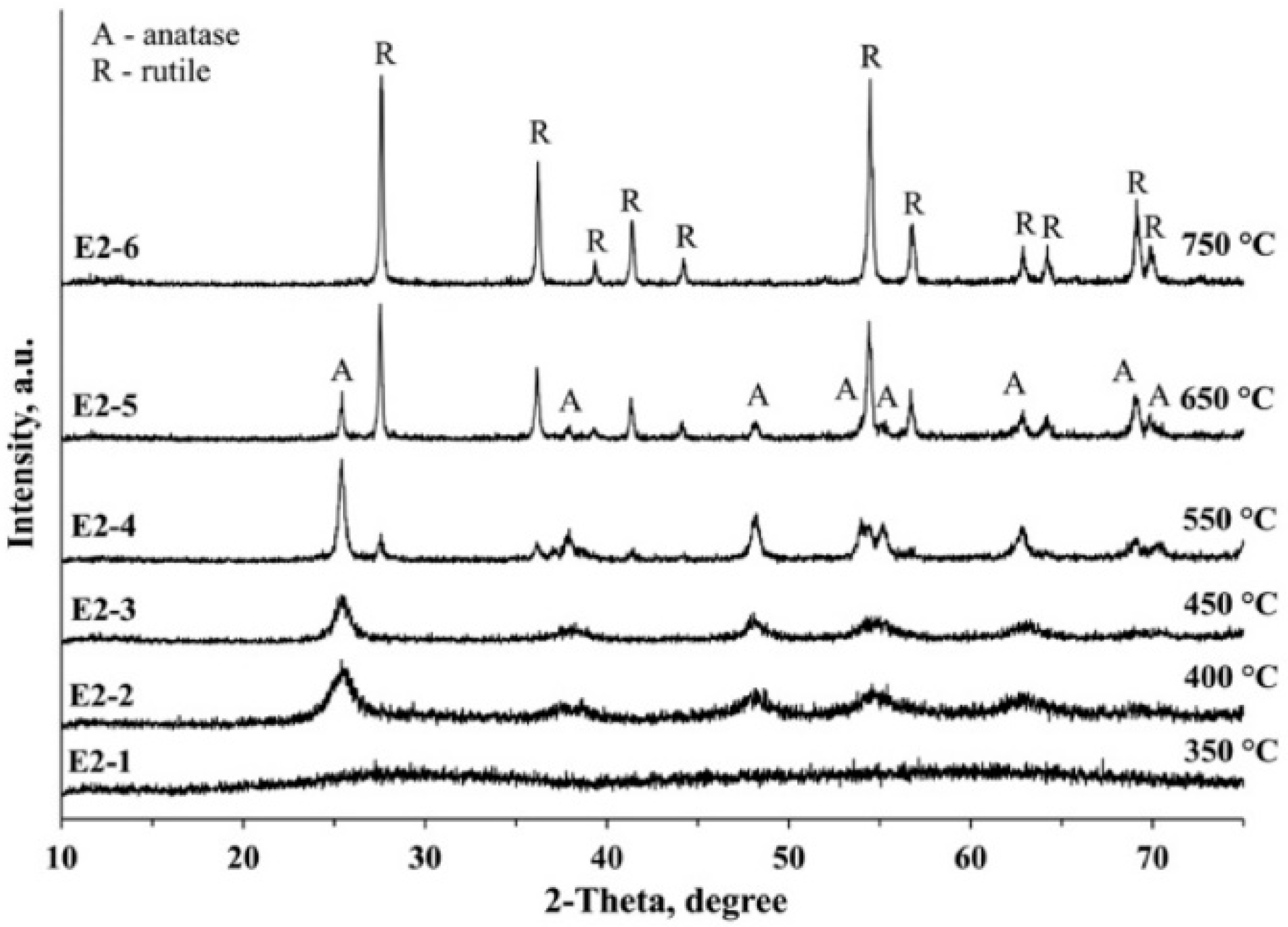
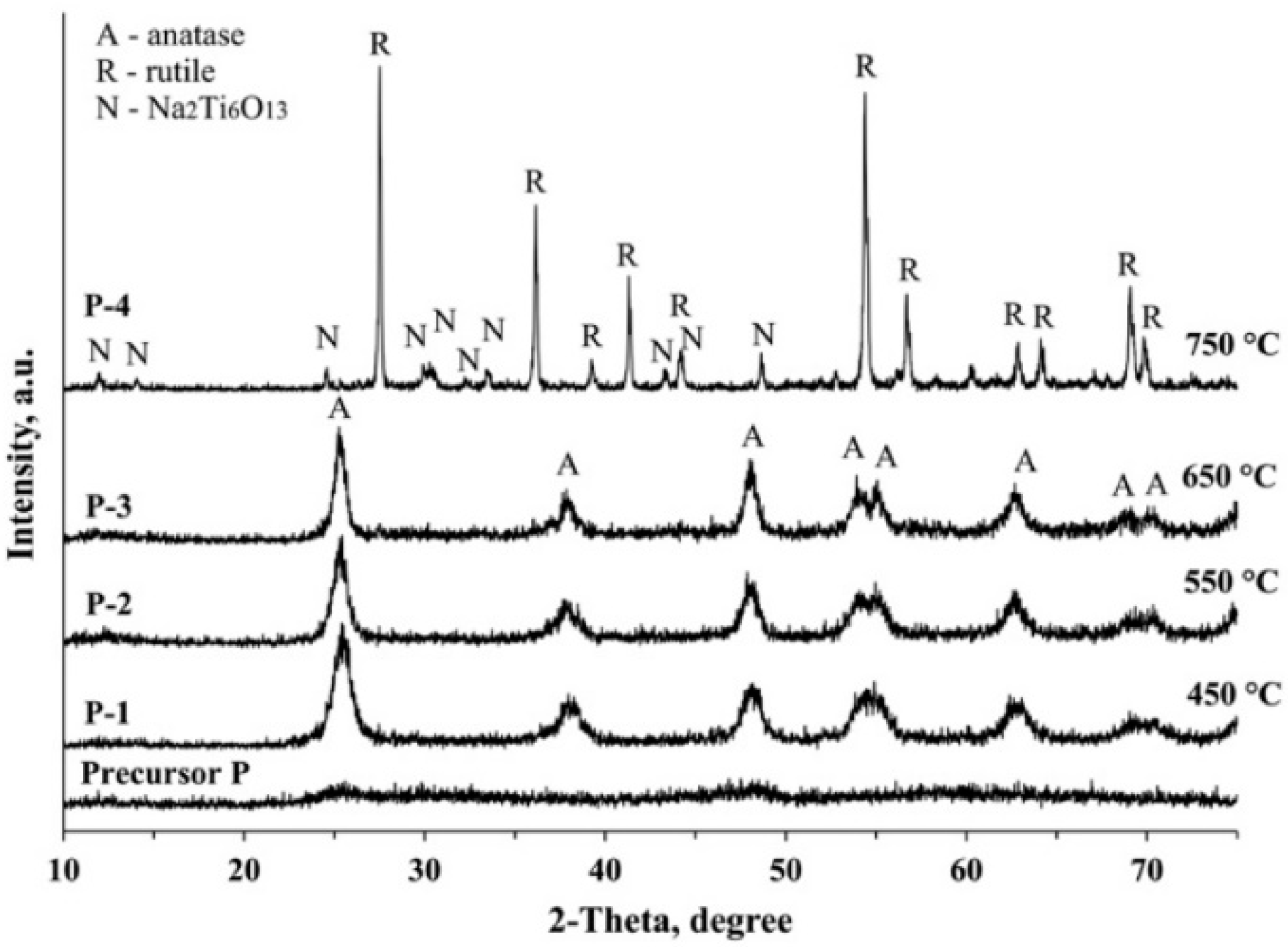
3.3. XRD Analysis
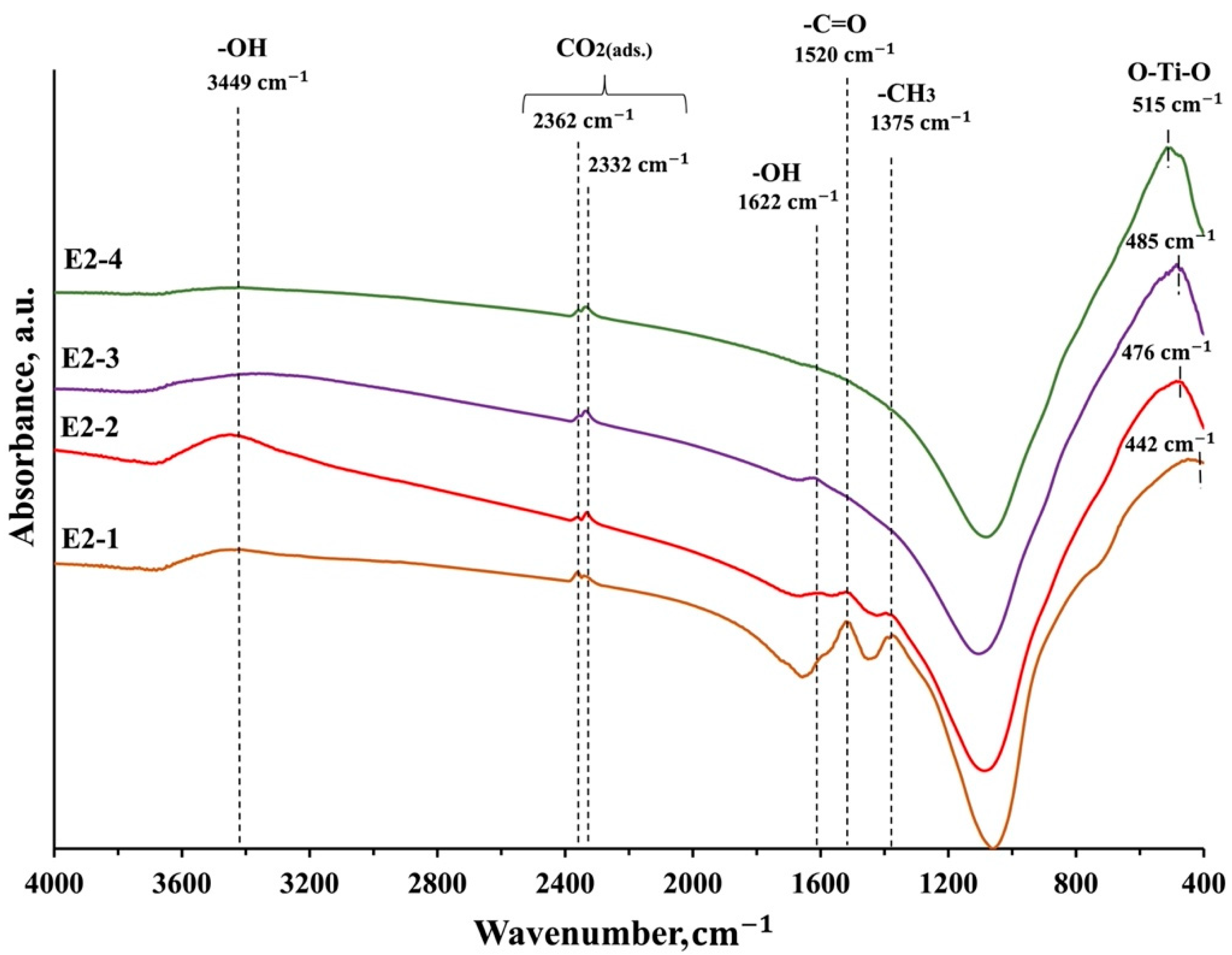
3.4. FTIR Spectroscopy
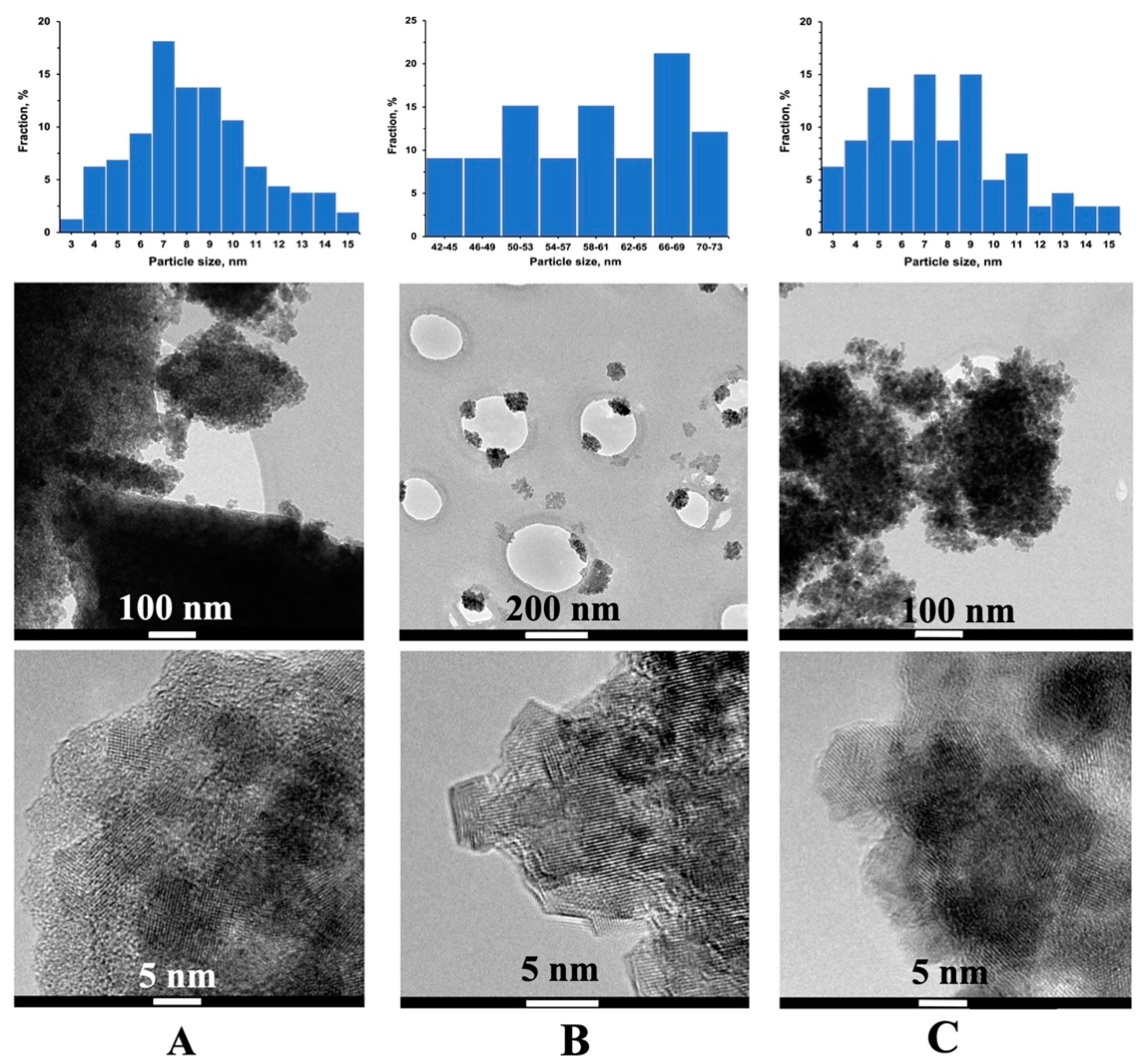
3.5. Transmission Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type synthesis and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, I.; Yadav, H.; Park, J.; Jo, J.W.; Yim, S.; Lee, J.-J. Non-hydrolytic sol-gel route to synthesize TiO2 nanoparticles under ambient condition for highly efficient and stable perovskite solar cells. Sol. Energy 2019, 185, 307–314. [Google Scholar] [CrossRef]
- Lingaraju, K.; Basavaraj, R.B.; Jayanna, K.; Bhavana, S.; Devaraja, S.; Kumar Swamy, H.M.; Nagaraju, G.; Nagabhushan, H.; Raja Naika, H. Biocompatible fabrication of TiO2 nanoparticles: Antimicrobial, anticoagulant, antiplatelet, direct hemolytic and cytotoxicity properties. Inorg. Chem. Commun. 2021, 127, 10850. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Haider, A.J.; AL-Anbari, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential environmental applications of TiO2 nanoparticles. Energy Procedia 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and characterization of TiO2 nanoparticles for the reduction of water pollutants. Materials 2017, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chen, C.C.; Chen, S.H. A review on production, characterization, and photocatalytic applications of TiO2 nanoparticles and nanotubes. Curr. Nanosci. 2017, 13, 373–393. [Google Scholar] [CrossRef]
- Wang, S.; Yu, H.; Yuan, S.; Zhao, Y.; Wang, Z.; Fang, J.; Zhang, M.; Shi, L. Synthesis of triphasic, biphasic, and monophasic TiO2 nanocrystals and their photocatalytic degradation mechanisms. Res. Chem. Intermed. 2016, 42, 3775–3788. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the application of stoichiometric and non-stoichiometric titanium oxides for the design of sensors for the determination of gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Zhukovskii, Y.F.; Piskunov, S.; Lisovski, O.; Bocharov, D.; Evarestov, R.A. Doped 1D nanostructures of transition-metal oxides: First-principles evaluation of photocatalytic suitability. Isr. J. Chem. 2017, 57, 461–476. [Google Scholar] [CrossRef]
- Sidaraviciute, R.; Kavaliunas, V.; Puodziukynas, L.; Guobiene, A.; Martuzevicius, D.; Andrulevicius, M. Enhancement of photocatalytic pollutant decomposition efficiency of surface mounted TiO2 via lithographic surface patterning. Environ. Technol. Innov. 2020, 19, 100983. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Tamm, A.; Seinberg, L.; Kozlova, J.; Link, J.; Pikma, P.; Stern, R.; Kukli, K. Quasicubic α-Fe2O3 nanoparticles embedded in TiO2 thin films grown by atomic layer deposition. Thin Solid Films 2016, 612, 445–449. [Google Scholar] [CrossRef]
- Rempel, A.A.; Kuznetsova, Y.V.; Dorosheva, I.B.; Valeeva, A.A.; Weinstein, I.A.; Kozlova, E.A.; Saraev, A.A.; Selishchev, D.S. High Photocatalytic Activity Under Visible Light of Sandwich Structures Based on Anodic TiO2/CdS Nanoparticles/Sol–Gel TiO2. Top. Catal. 2020, 63, 130–138. [Google Scholar] [CrossRef]
- Tuckute, S.; Varnagiris, S.; Urbonavicius, M.; Lelis, M.; Sakalauskaite, S. Tailoring of TiO2 film crystal texture for higher photocatalysis efficiency. Appl. Surf. Sci. 2019, 489, 576–583. [Google Scholar] [CrossRef]
- Kenmoe, S.; Lisovski, O.; Piskunov, S.; Bocharov, D.; Zhukovskii, Y.F.; Spohr, E. Water adsorption on clean and defective anatase TiO2 (001) nanotube surfaces: A surface science approach. J. Phys. Chem. B 2018, 122, 5432–5440. [Google Scholar] [CrossRef]
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. Electronic properties of Nano-porous TiO2- and ZnO- thin films—Comparison of simulations and experiments. J. Ceram. Process. Res. 2004, 5, 343–354. [Google Scholar]
- Knoks, A.; Kleperis, J.; Grinberga, L. Raman spectral identification of phase distribution in anodic titanium dioxide coating. Proc. Estonian Acad. Sci. 2017, 66, 422–429. [Google Scholar] [CrossRef]
- Brik, M.G.; Antic, Ž.M.; Vukovic, K.; Dramicanin, M.D. Judd-Ofelt analysis of Eu3+ emission in TiO2 anatase nanoparticles. Mater. Trans. 2015, 56, 1416–1418. [Google Scholar] [CrossRef]
- Nishioka, S.; Yanagisawa, K.; Lu, D.; Vequizo, J.J.M.; Yamakata, A.; Kimoto, K.; Inada, M.; Maeda, K. Enhanced water splitting through two-step photoexcitation by sunlight using tantalum/nitrogen-codoped rutile titania as a water oxidation photocatalyst. Sustain. Energy Fuels 2019, 3, 2337–2346. [Google Scholar] [CrossRef]
- Kavaliunas, V.; Krugly, E.; Sriubas, M.; Mimura, H.; Laukaitis, G.; Hatanaka, Y. Influence of Mg, Cu, and Ni dopants on amorphous TiO2 thin films photocatalytic activity. Materials 2020, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Hu, X.; Fan, J.; Sun, T.; Kang, L.; Hou, W.; Zhu, C.; Liu, H. Photocatalytic activity of Ag/TiO2 nanotube arrays enhanced by surface plasmon resonance and application in hydrogen evolution by water splitting. Plasmonics 2013, 8, 501–508. [Google Scholar] [CrossRef]
- Linitis, J.; Kalis, A.; Grinberga, L.; Kleperis, J. Photo-activity research of nano-structured TiO2 layers. IOP Conf. Ser. Mater. Sci. Eng. 2011, 23, 012010. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, D.; Kenzhina, I.; Boretskiy, O.; Zdorovets, M. Study of the effect of low-energy irradiation with O2+ ions on radiation hardening and modification of the properties of thin TiO2 films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 790–801. [Google Scholar] [CrossRef]
- Mattsson, M.S.M.; Azens, A.; Niklasson, G.A.; Granqvist, C.G.; Purans, J. Li intercalation in transparent Ti-Ce oxide films: Energetics and ion dynamics. J. Appl. Phys. 1997, 81, 6432–6437. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Kozlovskiy, A.; Kenzhina, I.; Berguzinov, A.; Zdorovets, M. Study of the effect of irradiation with Fe7+ ions on the structural properties of thin TiO2 foils. Mater. Res. Express 2019, 6, 046309. [Google Scholar] [CrossRef]
- Kiisk, V.; Akulitš, K.; Kodu, M.; Avarmaa, T.; Mändar, H.; Kozlova, J.; Eltermann, M.; Puust, L.; Jaaniso, R. Oxygen-sensitive photoluminescence of rare earth ions in TiO2 thin films. J. Phys. Chem. C 2019, 123, 17908–17914. [Google Scholar] [CrossRef]
- Milovanov, Y.S.; Gavrilchenko, I.V.; Gayvoronsky, V.Y.; Kuznetsov, G.V.; Skryshevsky, V.A. Impact of Nanoporous Metal Oxide Morphology on Electron Transfer Processes in Ti–TiO2–Si Heterostructures. J. Nanoelectron. Optoelectron. 2014, 9, 432–436. [Google Scholar] [CrossRef]
- Reklaitis, I.; Radiunas, E.; Malinauskas, T.; Stanionytė, S.; Juška, G.; Ritasalo, R.; Pilvi, T.; Taeger, S.; Strassburg, M.; Tomašiūnas, R. A comparative study on atomic layer deposited oxide film morphology and their electrical breakdown. Surf. Coat. Technol. 2020, 399, 126123. [Google Scholar] [CrossRef]
- Luchinsky, G.P. Chemistry of the Titanium; Khimija: Moskow, Russia, 1971. (In Russian) [Google Scholar]
- Gribb, A.A.; Banfield, J.F. Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Amer. Miner. 1997, 82, 717–728. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Kharat, A.N.; Fatholahi, L.; Beiranvand, Z.M. A review on synthesis of nano-TiO2 via different methods. J. Nanostruct. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cao, X.; Wu, S.; Liu, C.; Li, G.; Jiang, W.; Wang, H.; Wang, N.; Ding, W. Preparation and characterization of TiO2 nanoparticles by two different precipitation methods. Ceram. Int. 2020, 46, 15333–15341. [Google Scholar] [CrossRef]
- Wategaonkar, S.B.; Pawar, R.P.; Parale, V.G.; Nade, D.P.; Sargar, B.M.; Mane, R.K. Synthesis of rutile TiO2 nanostructures by single step hydrothermal route and its characterization. Mater. Today Proc. 2020, 23, 444–451. [Google Scholar] [CrossRef]
- Kusior, A.; Banas, J.; Trenczek-Zajac, A.; Zubrzycka, P.; Micek-Ilnicka, A.; Radecka, M. Structural properties of TiO2 nanomaterials. J. Mol. Struct. 2018, 1157, 327–336. [Google Scholar] [CrossRef]
- Sharma, A.; Karn, R.K.; Pandiyan, S.K. Synthesis of TiO2 nanoparticles by sol-gel method and their characterization. J. Basic Appl. Eng. Res. 2014, 1, 1–5. [Google Scholar]
- Toygun, S.; Konecoglu, G.; Kalpakli, Y. General principles of sol-gel. J. Eng. Nat. Sci. 2013, 31, 456–476. [Google Scholar]
- Khol’kin, A.I.; Patrusheva, T.N. Extraction-Pyrolytic Method: Fabrication of Functional Oxide Materials; KomKniga: Moskow, Russian, 2006; ISBN 548-400-582-5. (In Russian) [Google Scholar]
- Patrusheva, T.N.; Popov, V.S.; Prabhu, G.; Popov, A.V.; Ryzhenkov, A.V.; Snezhko, N.Y.; Morozchenko, D.A.; Zaikovskii, V.D.; Khol’kin, A.I. Preparation of a photoanode with a multilayer structure for solar cells by extraction pyrolysis. Theor. Found. Chem. Eng. 2014, 48, 454–460. [Google Scholar] [CrossRef]
- Popov, A.I.; Shirmane, L.; Pankratov, V.; Lushchik, A.; Kotlov, A.; Serga, V.E.; Kulikova, L.D.; Chikvaidze, G.; Zimmermann, J. Comparative study of the luminescence properties of macro- and nanocrystalline MgO using synchrotron radiation. Nucl. Instrum. Methods Phys. Res. B 2013, 310, 23–26. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudzius, R.; Zarkov, A.; Kareiva, A.; Popov, A. Impact of gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef] [PubMed]
- Burve, R.; Serga, V.; Krumina, A.; Poplausks, R. Preparation and characterization of nanocrystalline gadolinium oxide powders and films. Key Eng. Mater. 2020, 850, 267–272. [Google Scholar] [CrossRef]
- Gindin, L.M. Extraction Processes and Its Application; Nauka: Moskow, Russia, 1984. (In Russian) [Google Scholar]
- Sharlo, G. Quantitative Analysis of the Inorganic Compounds. In Methods of the Analytical Chemistry; Lur’e, Y.Y., Ed.; Himija: Moskow, Russia, 1969; Volume 2, ISBN 978-544-584-821-9. (In Russian) [Google Scholar]
- Drozdov, A.A.; Zlomanov, G.N.; Mazo, G.N.; Spiridinov, F.M. Chemistry of the Transition Elements. In Inorganic Chemistry; Tretyakov, Y.D., Ed.; Akademija: Moskow, Russia, 2008; Volume 3, Part 1; pp. 56–99. ISBN 576-952-532-0. (In Russian) [Google Scholar]
- Mehrotra, R.C.; Bohra, R. Metal Carboxylates; Academic Press: London, UK, 1983; ISBN 978-012-488-160-0. [Google Scholar]
- Patil, K.C.; Chandrashekhar, G.V.; George, M.V.; Rao, C.N.R. Infrared spectra and thermal decompositions of metal acetates and dicarboxylates. Can. J. Chem. 1968, 46, 257–265. [Google Scholar] [CrossRef]
- Smith, B.C. A process for successful infrared spectral interpretation. Spectroscopy 2016, 31, 14–21. [Google Scholar]
- Stuart, B. Analytical techniques in the sciences. In Infrared Spectroscopy: Fundamentals and Applications; Ando, D.J., Ed.; John Wiley&Sons: Chichester, UK, 2004; ISBN 978-047-085-427-3. [Google Scholar]
- Smith, B.C. The carbonyl group, part V: Carboxylates-coming clean. Spectroscopy 2018, 33, 20–23. [Google Scholar]
- Nyquist, R.A.; Kagel, R.O. Infrared spectra of inorganic compounds. In Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts, 1st ed.; Acad. Press: London, UK, 1971; pp. 1–18. ISBN 978-008-087-852-2. [Google Scholar]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 2 November 2020).
- Ocana, M.; Fornés, V.; García Ramos, J.V.; Serna, C.J. Factors affecting the infrared and raman spectra of rutile powders. J. Solid State Chem. 1988, 75, 364–372. [Google Scholar] [CrossRef]
- Savchyn, P.; Karbovnyk, I.; Vistovskyy, V.; Voloshinovskii, A.; Pankratov, V.; Cestelli Guidi, M.; Mirri, C.; Myahkota, O.; Riabtseva, A.; Mitina, N. Vibrational properties of LaPO4 nanoparticles in mid-and far-infrared domain. J. Appl. Phys. 2012, 112, 124309. [Google Scholar] [CrossRef]
- Balasubramanian, C.; Bellucci, S.; Cinque, G.; Marcelli, A.; Guidi, M.C.; Piccinini, M.; Popov, A.; Soldatov, A.; Onorato, P. Characterization of aluminium nitride nanostructures by XANES and FTIR spectroscopies with synchrotron radiation. J. Phys. Condens. Matter 2006, 18, S2095–S2104. [Google Scholar] [CrossRef]
- Bellucci, S.; Popov, A.I.; Balasubramanian, C.; Cinque, G.; Marcelli, A.; Karbovnyk, I.; Savchyn, V.; Krutyak, N. Luminescence, vibrational and XANES studies of AlN nanomaterials. Radiat. Meas. 2007, 42, 708–711. [Google Scholar] [CrossRef]

| Sample Nr. | Production Conditions | XRD Analysis Results | |||
|---|---|---|---|---|---|
| Precursor | Pyrolysis Temperature T, °C | Phase Composition | d, nm | W, % | |
| E1-1 | E1 | 350 | Amorphous | - | - |
| E2-1 | E2 | ||||
| E1-2 | E1 | 400 | Amorphous | - | - |
| E2-2 | E2 | Anatase | 5 | 100 | |
| E1-3 | E1 | 450 | Anatase | 8 | 100 |
| E2-3 | E2 | Anatase | 9 | 100 | |
| P-1 | P | Anatase | 9 | 100 | |
| E1-4 | E1 | 550 | Anatase | 15 | 100 |
| E2-4 | E2 | Anatase Rutile | 20 ~30 | 87.7 12.3 | |
| P2 | P | Anatase | 10 | 100 | |
| E1-5 | E1 | 650 | Anatase Rutile | 30 ~40 | 80.9 19.1 |
| E2-5 | E2 | Anatase Rutile | ~35 45 | 20.6 79.4 | |
| P3 | P | Anatase Rutile | 14 Discerned | 96.4 3.6 | |
| E1-6 | E1 | 750 | Anatase Rutile | Discerned 65 | 1.1 98.9 |
| E2-6 | E2 | Rutile | 53 | 100 | |
| P-4 | P | Rutile Na2Ti6O13 | 68 - | 100 - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. https://doi.org/10.3390/cryst11040431
Serga V, Burve R, Krumina A, Romanova M, Kotomin EA, Popov AI. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals. 2021; 11(4):431. https://doi.org/10.3390/cryst11040431
Chicago/Turabian StyleSerga, Vera, Regina Burve, Aija Krumina, Marina Romanova, Eugene A. Kotomin, and Anatoli I. Popov. 2021. "Extraction–Pyrolytic Method for TiO2 Polymorphs Production" Crystals 11, no. 4: 431. https://doi.org/10.3390/cryst11040431
APA StyleSerga, V., Burve, R., Krumina, A., Romanova, M., Kotomin, E. A., & Popov, A. I. (2021). Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals, 11(4), 431. https://doi.org/10.3390/cryst11040431