Processing and Optical Properties of Eu-Doped Chloroborate Glass-Ceramic
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization Methods
3. Results and Discussion
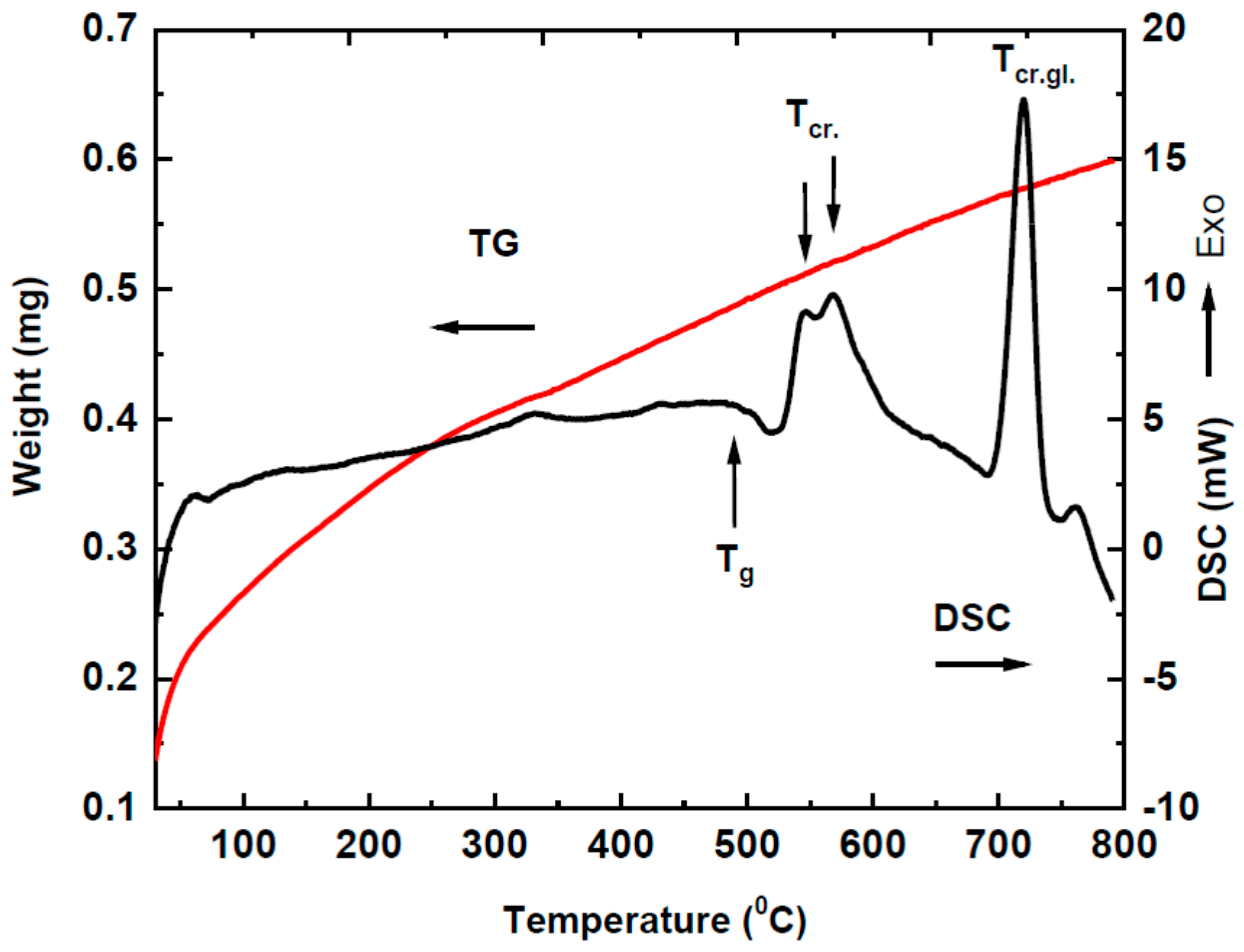
3.1. Thermal Analysis
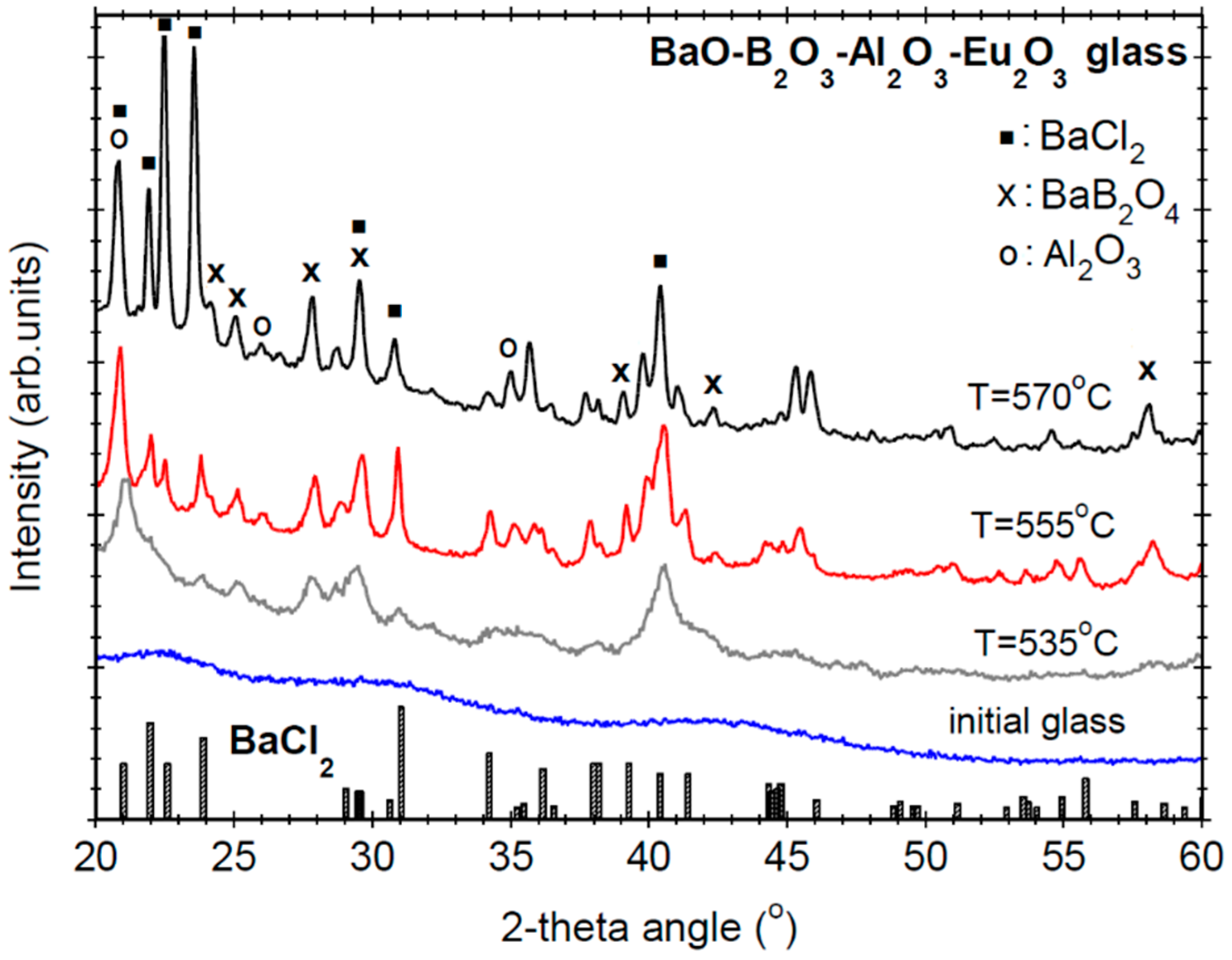
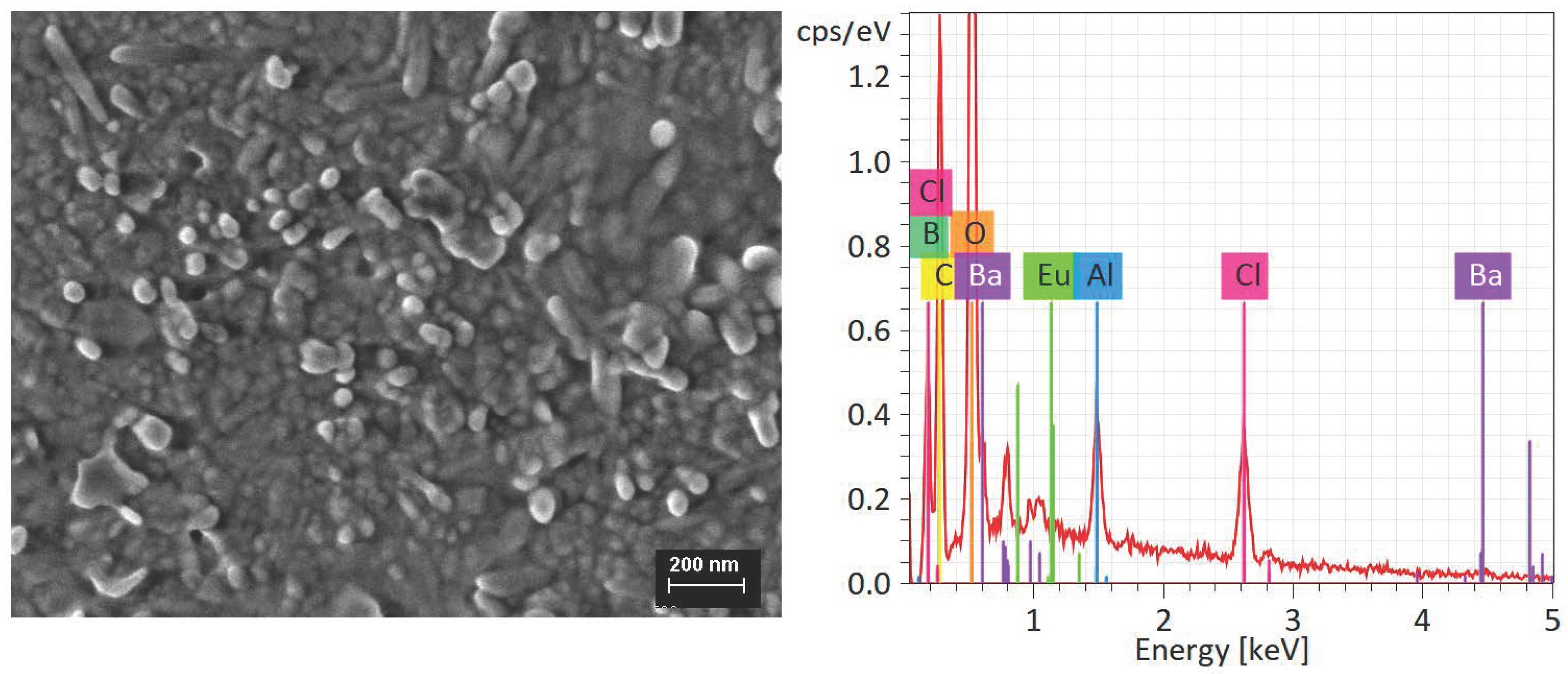
3.2. Structural and Morphological Characterization
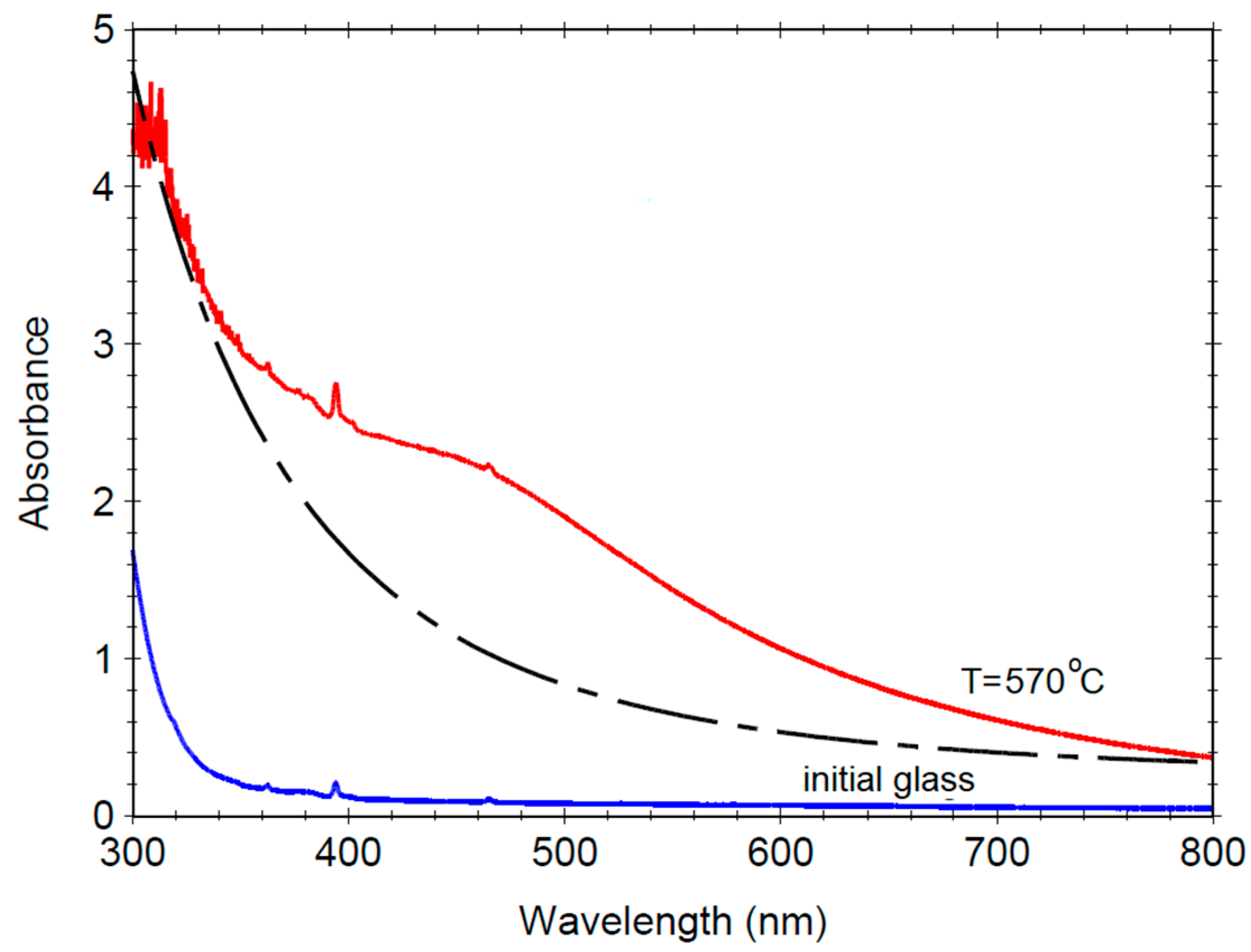
3.3. Optical Absorption UV–Vis
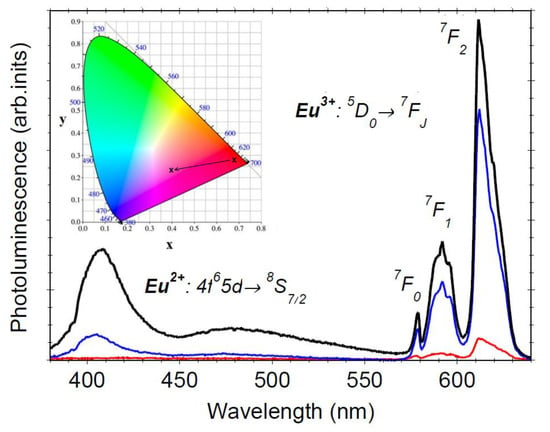
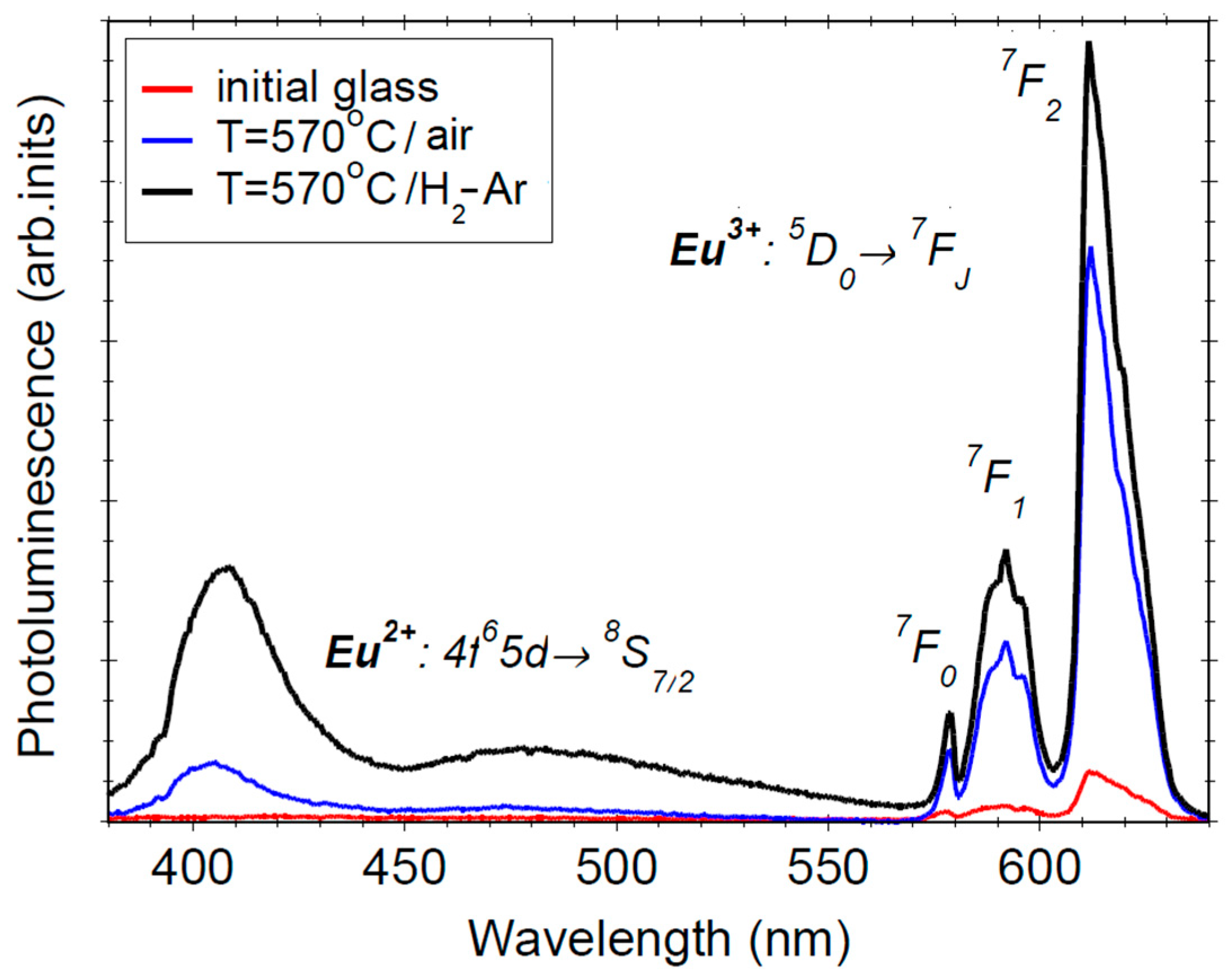
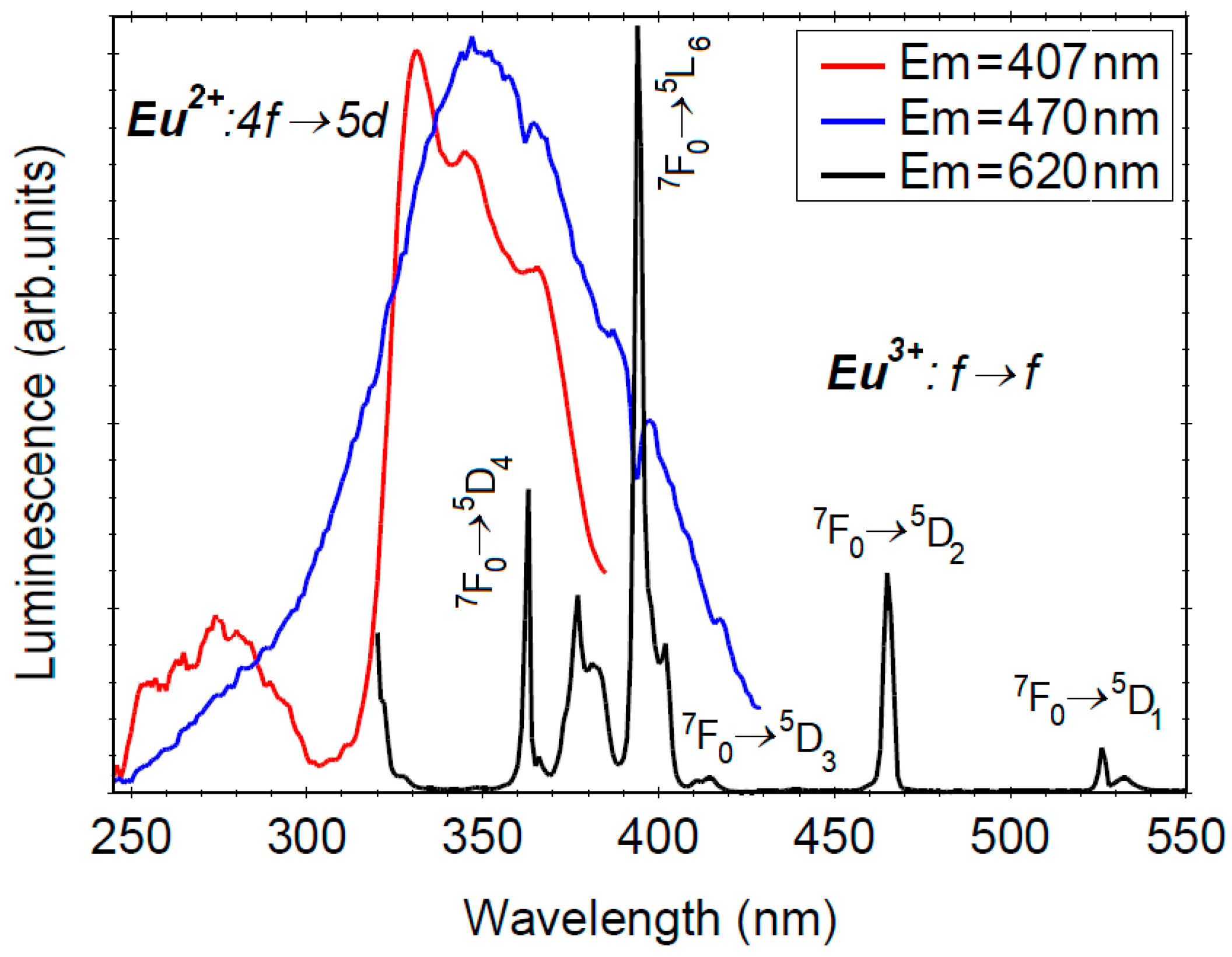
3.4. Photoluminescence Properties
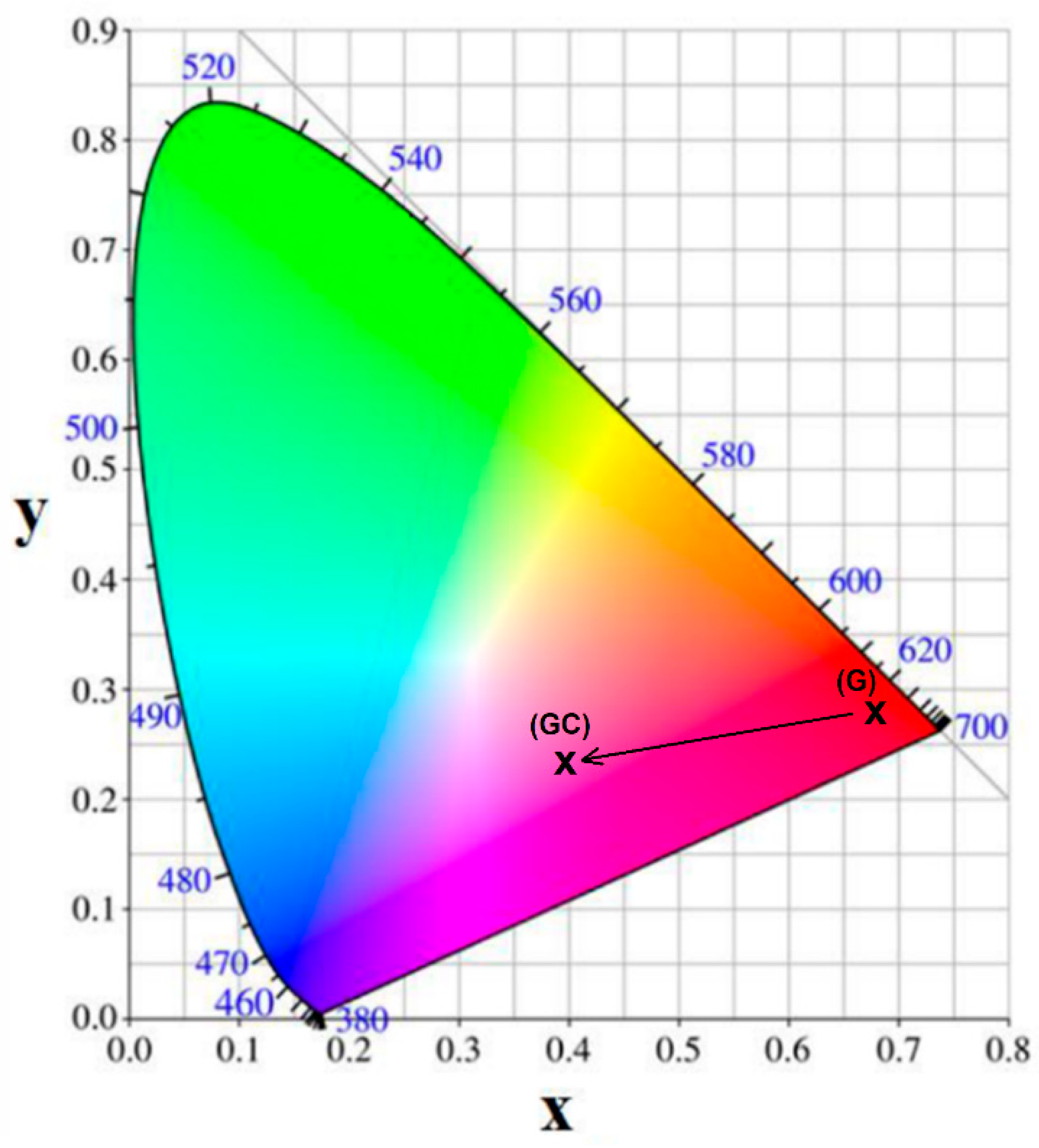
3.5. Colorimetry Analysis
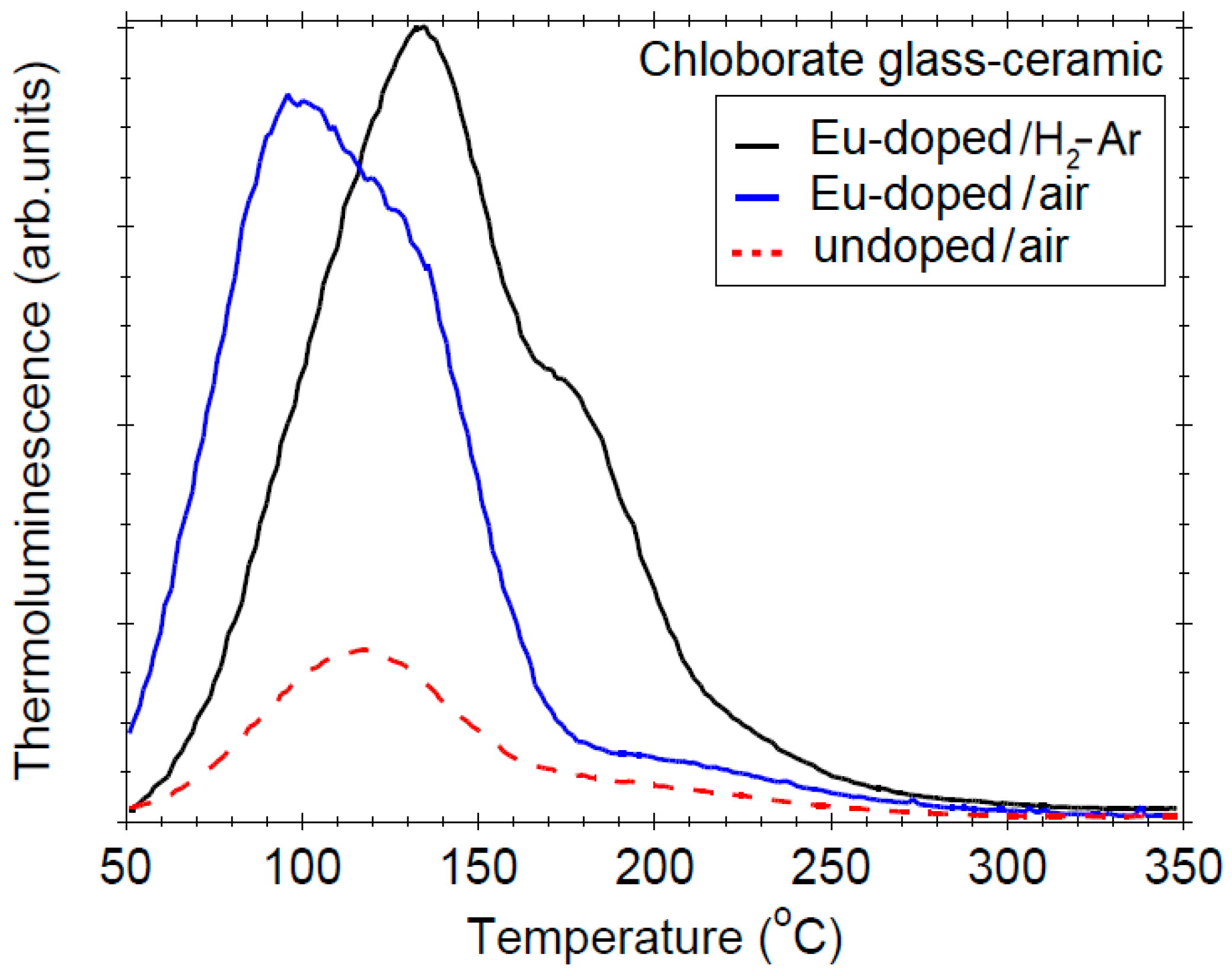
3.6. Thermoluminescence Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhou, J.; Zhou, S.; Yue, Y.; Qiu, J. Transparent glass-ceramics functionalized by dispersed crystals. Prog. Mater. Sci. 2018, 97, 38–96. [Google Scholar] [CrossRef]
- De Pablos-Martín, A.; Durán, A.; Pascual, M.J. Nanocrystallisation in oxyfluoride systems: Mechanisms of crystallisation and photonic properties. Int. Mater. Rev. 2018, 57, 165–186. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Popov, A.I. Transparent oxyfluoride glass ceramics. J. Fluor. Chem. 2015, 172, 22–50. [Google Scholar] [CrossRef]
- Paßlick, C.; Ahrens, B.; Henke, B.; Johnson, J.A.; Schweizer, S. Crystallization behavior of rare-earth doped fluorochlorozirconate glasses. J. Non-Cryst. Solids 2011, 357, 2450–2452. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.J.; Leonard, R.L.; Gray, S.K.; Johnson, J.A.; Petford, A.K. Structural and Kinetic Analysis of BaCl2 Nanocrystals in Fluorochlorozirconate Glass-Ceramics. J. Am. Ceram Soc. 2015, 98, 1099–1104. [Google Scholar] [CrossRef]
- Pfau, C.; Skrzypczak, U.; Ahrens, B.; Schweizer, S. Multiphonon relaxation of rare-earth ions in fluorozirconate-based glass ceramics. J. Phys. Condens. Matter 2014, 26, 025406. [Google Scholar] [CrossRef]
- Johnson, J.A.; Schweizer, S.; Henke, B.; Chen, G.; Woodford, J.; Newman, P.; MacFarlane, D. 2006 Eu-activated from fluorochlorozirconate glass-ceramic scintillators. J. Appl. Phys. 2006, 100, 034701. [Google Scholar] [CrossRef]
- Schweizer, S.; Hobbs, L.W.; Secu, M.; Spaeth, J.M.; Edgar, A.; Hamlin, J. Photostimulated luminescence fluorochlorozirconate glass ceramics and the effect of crystallite size. J. Appl. Phys. 2005, 97, 083522. [Google Scholar] [CrossRef]
- Pfau, C.; Paßlick, C.; Gray, S.K.; Johnson, J.A.; Johnson, C.E.; Schweizer, S. Mössbauer spectroscopy of europium-doped fluorochlorozirconate glasses and glass ceramics: Optimization of storage phosphors in computed radiography. J. Phys. Condens. Matter 2013, 25, 205402. [Google Scholar] [CrossRef]
- Yan, Z.; Gundiah, G.; Bizarri, G.A.; Samulon, E.C.; Derenzo, S.E.; Bourret-Courchesne, E.D. Eu2+ -activated BaCl2, BaBr2 and BaI2 scintillators revisited. Nucl. Instrum. Methods Phys. Res. Sect. A 2014, 735, 83–87. [Google Scholar] [CrossRef]
- Secu, M.; Kalchgruber, R.; Schweizer, S.; Spaeth, J.M.; Edgar, A. Photostimulated luminescence in BaX 2: Eu 2+(X=Br, Cl) X-ray storage phosphors. Radiat. Eff. Defects Solids 2002, 157, 957. [Google Scholar] [CrossRef]
- Margha, F.H.; Abdel-Hameed, S.A.M.; Ghonim, N.A.; Saad, E.A.; Satokawa, S.; Kojima, T. Crystallization behavior of new transparent glass-ceramics based on barium borate glasses. J. Ceram. Soc. Jpn. 2008, 116, 624–631. [Google Scholar] [CrossRef][Green Version]
- Steudel, F.; Rimbach, A.C.; Loos, S.; Ahrens, B.; Schweizer, S. Effect of induced crystallization in rare-earth doped lithium borate glass. Radiat. Meas. 2016, 90, 274–278. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rai, S.B. Blue and Red Luminescence from Europium-Doped Barium Tetra-Borate Crystals. J. Am. Ceram. Soc. 2010, 93, 727–731. [Google Scholar] [CrossRef]
- Kao, Y.H.; Yi, H.; Zheng, H.; Mackenzie, J.D.; Perry, K.; Bourhill, G.; Perry, J.W. Second harmonic generation in transparent barium borate glass-ceramics. J. Non-Cryst. Solids 1994, 167, 247–254. [Google Scholar] [CrossRef]
- Brekhovskikh, M.N.; Voronko, Y.u.K.; Dmitruk, L.N.; Moiseeva, L.V.; Petrova, O.B.; Popov, A.V.; Fedorov, V.A. Synthesis and glass formation in the BaO-B2O3-BaCl2 system. Inorg. Mater. 2010, 46, 1391–1395. [Google Scholar] [CrossRef]
- Appleby, G.A.; Edgar, A.; Williams, G.V.M.; Bos, A.J.J. Photostimulated luminescence from BaCl2:Eu2+ nanocrystals in lithium borate glasses following neutron irradiation. Appl. Phys. Lett. 2006, 89, 101902. [Google Scholar] [CrossRef]
- Krause, W.; Nolze, G. PowderCell a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Kapoor, S.; Bola, G.H.; Betzen, A.; Affatigato, M.; Feller, S. Physical Properties of Barium Borate Glasses determined over a Wide Range of Compositions. J. Non-Cryst. Solids 2000, 270, 215–222. [Google Scholar] [CrossRef]
- Hull, S.; Norberg, S.T.; Ahmed, I.; Eriksson, S.G.; Mohn, C.E. High temperature crystal structures and superionic properties of SrCl2, SrBr2, BaCl2 and BaBr2. J. Solid State Chem. 2011, 184, 2925–2935. [Google Scholar] [CrossRef]
- Secu, C.; Rostas, A.M. Investigations of BaCl2:Eu2+ nanophosphor using electron paramagnetic resonance, structural analysis and thermoluminescence. J. Alloy Compd. 2020, 815, 152400. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Schweizer, S.; Hobbs, L.W.; Secu, M.; Spaeth, J.-M.; Edgar, A.; Williams, G.V.M. Photostimulated luminescence in Eu-doped fluorochlorozirconate glass ceramics. Appl. Phys. Lett. 2003, 83, 449. [Google Scholar] [CrossRef]
- Aronne, A.S.; Esposito, S.; Pernice, P. FTIR and DTA study of Structural Transformations and Crystallisation in BaO–B2O3–TiO2 Glasses. Phys. Chem. Glasses 1999, 40, 63–68. [Google Scholar]
- Dejneka, M.; Snitzer, E.; Riman, R.E. Blue, green and red fluorescence and energy-transfer of Eu3+ in fluoride glasses. J. Lumin. 1995, 65, 227–245. [Google Scholar] [CrossRef]
- Edgar, A.; Williams, G.V.M.; Hamelin, J. Optical scattering in glass ceramics. Curr. Appl. Phys. 2006, 6, 355–358. [Google Scholar] [CrossRef]
- Secu, C.E.; Bartha, C.; Polosan, S.; Secu, M. Thermally activated conversion of a silicate gel to an oxyfluoride glass ceramic: Optical study using Eu3+ probe ion. J. Lumin. 2014, 146, 539–543. [Google Scholar] [CrossRef]
- Gorller-Walrand, C.; Binnemans, K. Spectral Intensities of f-f Transition. In Handbook on the Physics and Chemistry of Rare Earths; Gshneidner, K.A., Jr., Eyring, L., Eds.; Elsevier: North-Holland, Amsterdam, 1988; Volume 25, Chapter 167; pp. 101–264. [Google Scholar]
- Kodaira, C.A.; Brito, H.F.; Malta, O.L.; Serra, O.A. Luminescence and energy transfer of the europium(III) tungstate obtained via the Pechini method. J. Lumin. 2003, 101, 11–21. [Google Scholar] [CrossRef]
- Hreniak, D.; Strek, W.; Amami, J.; Guyot, Y.; Boulon, G.; Goutaudier, C.; Pazik, R. The size-effect on luminescence properties of BaTiO3:Eu3+ nanocrystallites prepared by the sol–gel method. J. Alloy Compd. 2004, 380, 348–351. [Google Scholar] [CrossRef]
- de Silva, G.F.; Malta, O.L.; Donega, C.M.; Simas, A.M.; Longo, R.L.; Santa-Cruz, P.A.; Silva, E.F., Jr. degradation temperatures for the lanthanide ion complexes PA. Coord. Chem. Rev. 2000, 196, 165–195. [Google Scholar]
- Wen, H.; Duan, C.; Jia, G.; Tanner, P.A.; Brik, M.G. Glass composition and excitation wavelength dependence of the luminescence of Eu3+ doped lead borate glass. J. Appl. Phys. 2011, 110, 033536. [Google Scholar] [CrossRef]
- Dorenbos, P. Energy of the first 4f7→ 4f65d transition of Eu2+ in inorganic compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]
- Pei, Z.W.; Su, Q.; Zhang, J.Y. The Valence Change from RE3+ to RE2+ (RE = Eu, Sm, Yb) in SrB4O7: RE Prepared in Air and the Spectral Properties of RE2+. J. Alloy Compd. 1993, 198, 51–53. [Google Scholar] [CrossRef]
- Pei, Z.; Zeng, Q.; Su, Q. The Application and a Substitution Defect Model for Eu3+-Eu2+ Reduction in Non Reducing Atmospheres in Borates Containing BO4 Anion Groups. J. Phys. Chem. Solids 2000, 61, 9–12. [Google Scholar] [CrossRef]
- Secu, M.; Secu, C.E.; Polosan, S.; Aldica, G.; Ghica, C. Crystallization and spectroscopic properties of Eu doped CaF2 nanocrystals in transparent oxyfluoride glass-ceramics. J. Non-Cryst. Solids 2009, 355, 1869–1872. [Google Scholar] [CrossRef]
- Gawande, A.B.; Sonekar, R.P.; Omanwar, S.K. Photoluminescence Properties of Eu2+ in BaB2O4. Elixir Chem. Phys. 2014, 75, 27993–27995. [Google Scholar]
- Kobayasi, K.; Mroczkowski, S.; Owen, J.F.; Brixner, L.H. Fluorescence Lifetime and Quantum Efficiency for 5d-4f Transitions in Eu2+ Doped Chloride and Fluoride Crystals. J. Lumin. 1980, 21, 247–257. [Google Scholar] [CrossRef]
- Secu, C.E.; Negrila, C.; Secu, M. Investigation of sol-gel derived BaCl2:Eu2+ luminescent nanophosphor and the corresponding PVP@ BaCl2:Eu2+ polymer nanocomposite. J. Phys. D Appl. Phys. 2018, 51, 305302. [Google Scholar] [CrossRef]
- McKeever, S.W.S. Thermoluminescence of Solids; Cambridge University Press: New York, NY, USA, 1985. [Google Scholar]
- Bos, A.J.J. Theory of thermoluminescence. Radiat Meas 2006, 41, 45–56. [Google Scholar] [CrossRef]
- Iflazoglu, S.; Yilmaz, A.A.; Bulur, E. Structural, photo, optical and thermal luminescent properties of beta barium metaborate. J. Alloy Compd. 2020, 829, 154430. [Google Scholar] [CrossRef]
- Robinson, J. A Photo and Thermally Stimulated Luminescence Study of BaCl2:Eu2+ with Application to Neutron Imaging. Master’s Thesis, Victoria University of Wellington, Wellington, New Zealand, 2008. [Google Scholar]
| Sample | a (Å) | b (Å) | c (Å) | Cell Volume (Å3) |
|---|---|---|---|---|
| Annealed in air | 7.903 | 4.751 | 9.422 | 353.9 |
| Annealed in H2-Ar | 7.906 | 4.752 | 9.426 | 354.2 |
| BaCl2 [20] | 7.8813 (2) | 4.7369 (1) | 9.4360 (2) | 352.27 |
| BaCl2:Eu2+ [21] | 7.878 (5) | 4.735 (8) | 9.428 (1) | 351.68 |
| Sample | Ω2 (×10−20 cm2) | Ω4 (×10−20 cm2) | PL Lifetime (ms) |
|---|---|---|---|
| Initial glass | 5.9 | 3.9 | 1.86 ± 0.02 |
| Annealed in air | 5.8 | 3.9 | 2.20 ± 0.02 |
| Annealed in H2-Ar | 5.1 | 3.9 | 2.20 ± 0.02 |
| Lead borate glass [32] | 6.03 | 4.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secu, M.; Secu, C.E. Processing and Optical Properties of Eu-Doped Chloroborate Glass-Ceramic. Crystals 2020, 10, 1101. https://doi.org/10.3390/cryst10121101
Secu M, Secu CE. Processing and Optical Properties of Eu-Doped Chloroborate Glass-Ceramic. Crystals. 2020; 10(12):1101. https://doi.org/10.3390/cryst10121101
Chicago/Turabian StyleSecu, Mihail, and Corina Elisabeta Secu. 2020. "Processing and Optical Properties of Eu-Doped Chloroborate Glass-Ceramic" Crystals 10, no. 12: 1101. https://doi.org/10.3390/cryst10121101
APA StyleSecu, M., & Secu, C. E. (2020). Processing and Optical Properties of Eu-Doped Chloroborate Glass-Ceramic. Crystals, 10(12), 1101. https://doi.org/10.3390/cryst10121101