Crystal Structures of Antiarrhythmic Drug Disopyramide and Its Salt with Phthalic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Crystallization
2.2.1. DPA
2.2.2. DPA_PA Salt for Single X-ray Crystal Structure Analysis
2.2.3. DPA_PA Salt for Characterization (PXRD, DSC, IR, and TG)
2.3. Single-Crystal X-ray Diffraction
2.4. Powder X-ray Diffraction (PXRD)
2.5. Differential Scanning Calorimetry (DSC) and Thermogravimetric (TG) Measurements
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
3. Results and Discussion
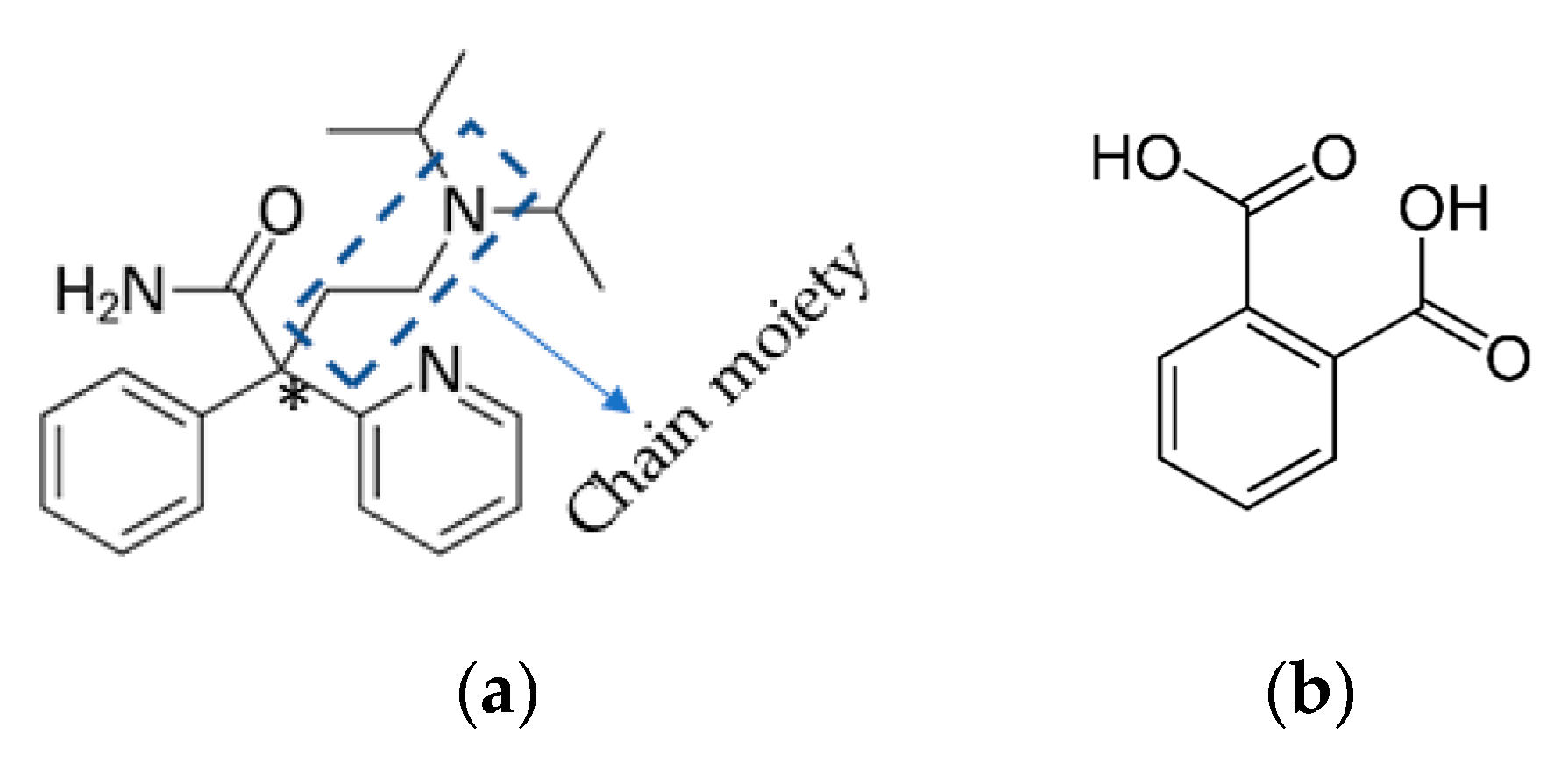
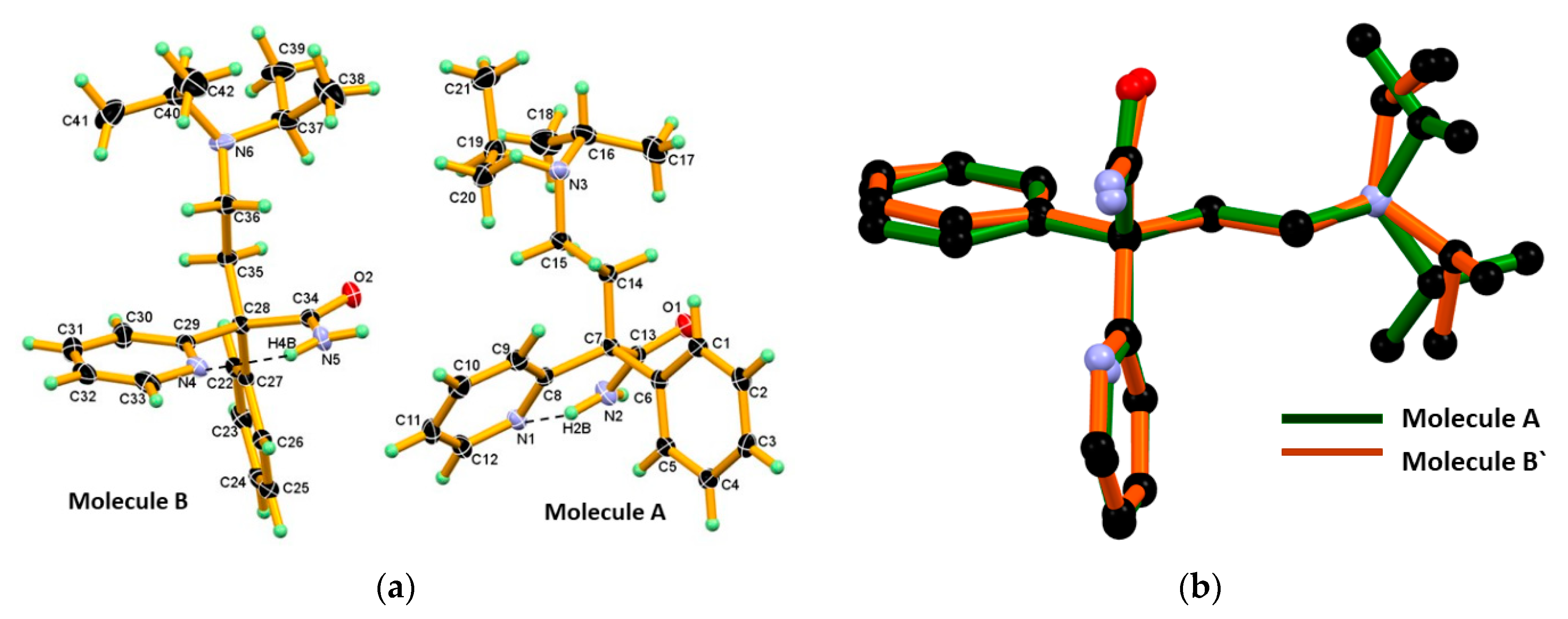
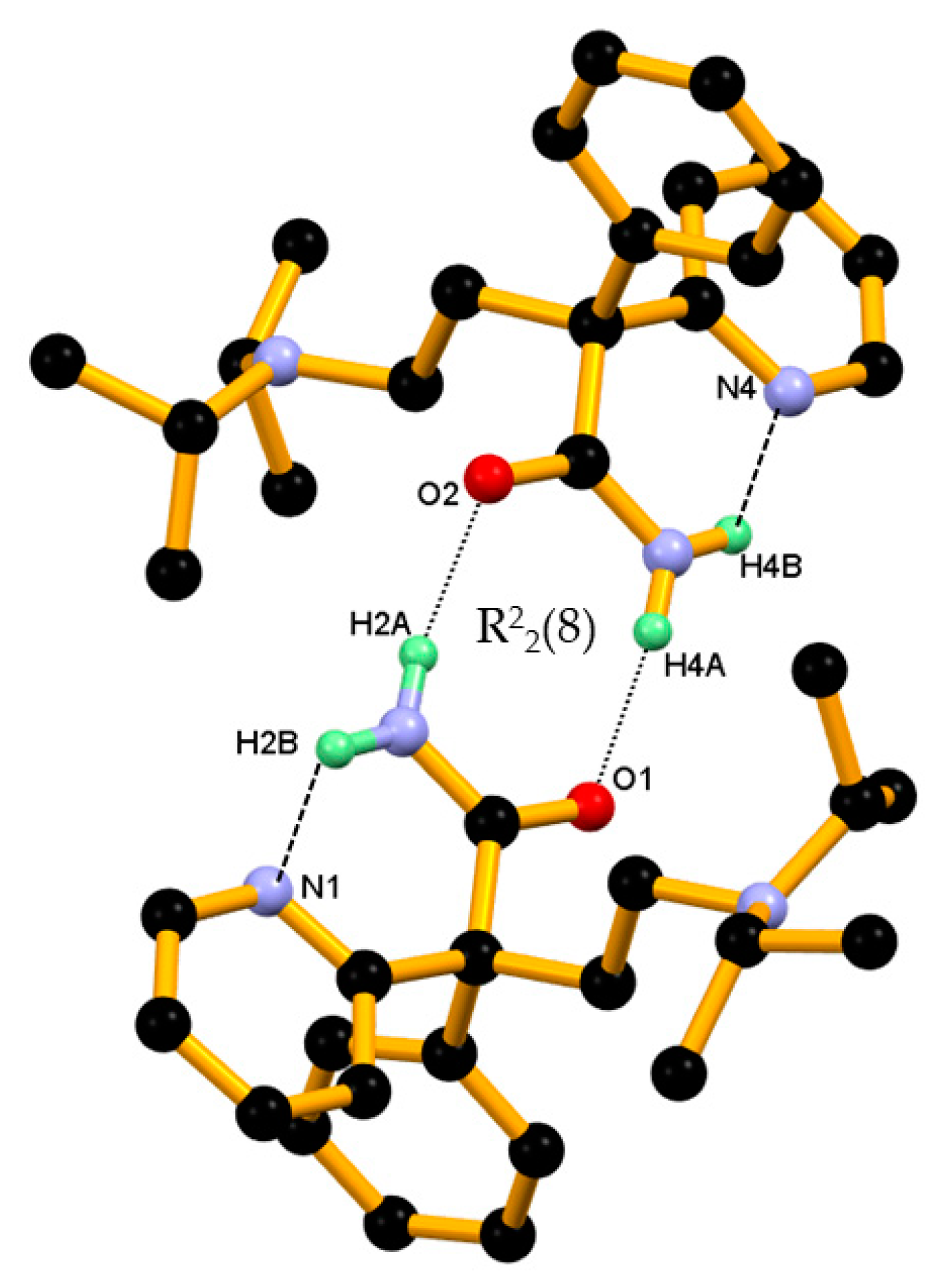
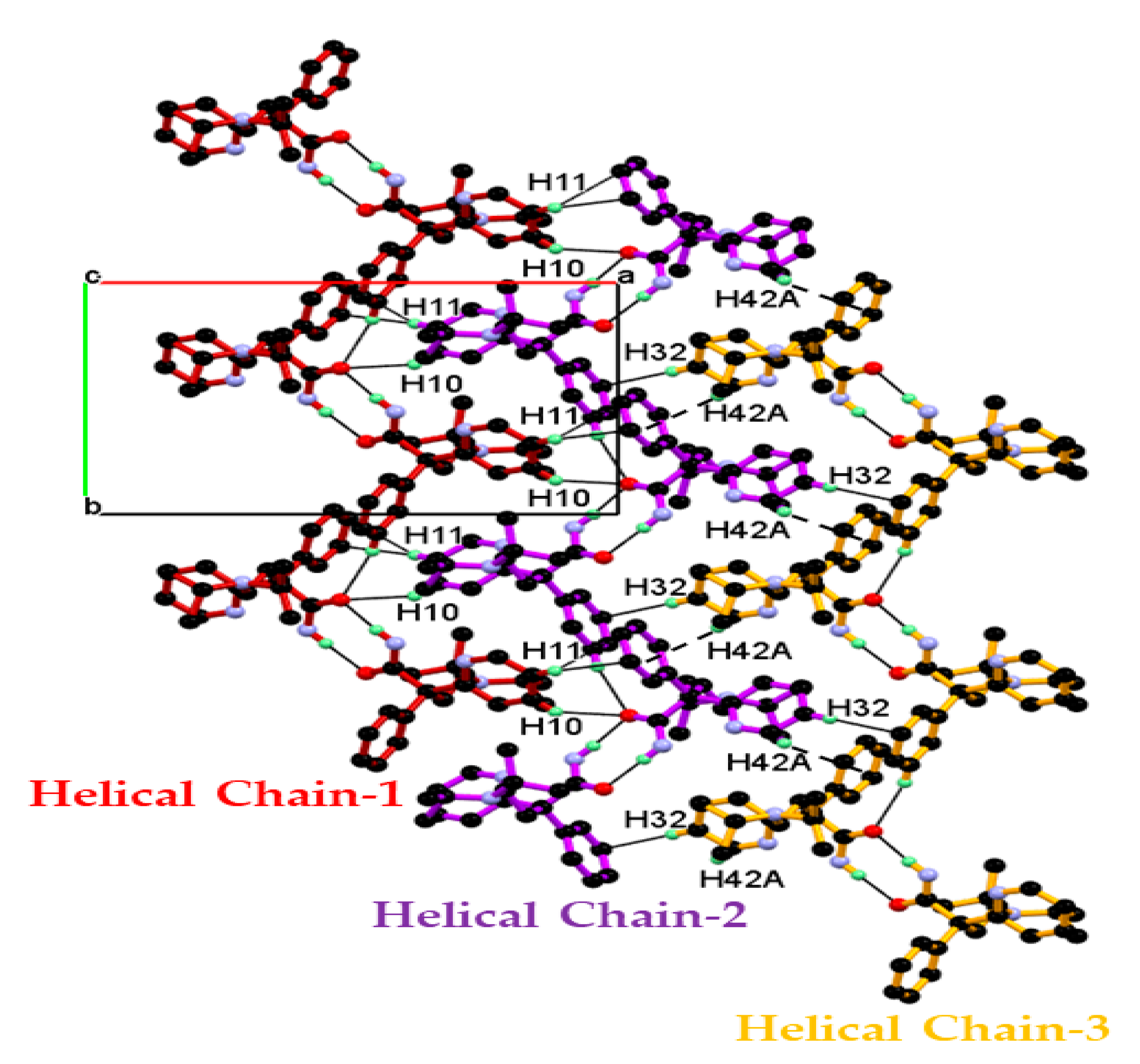
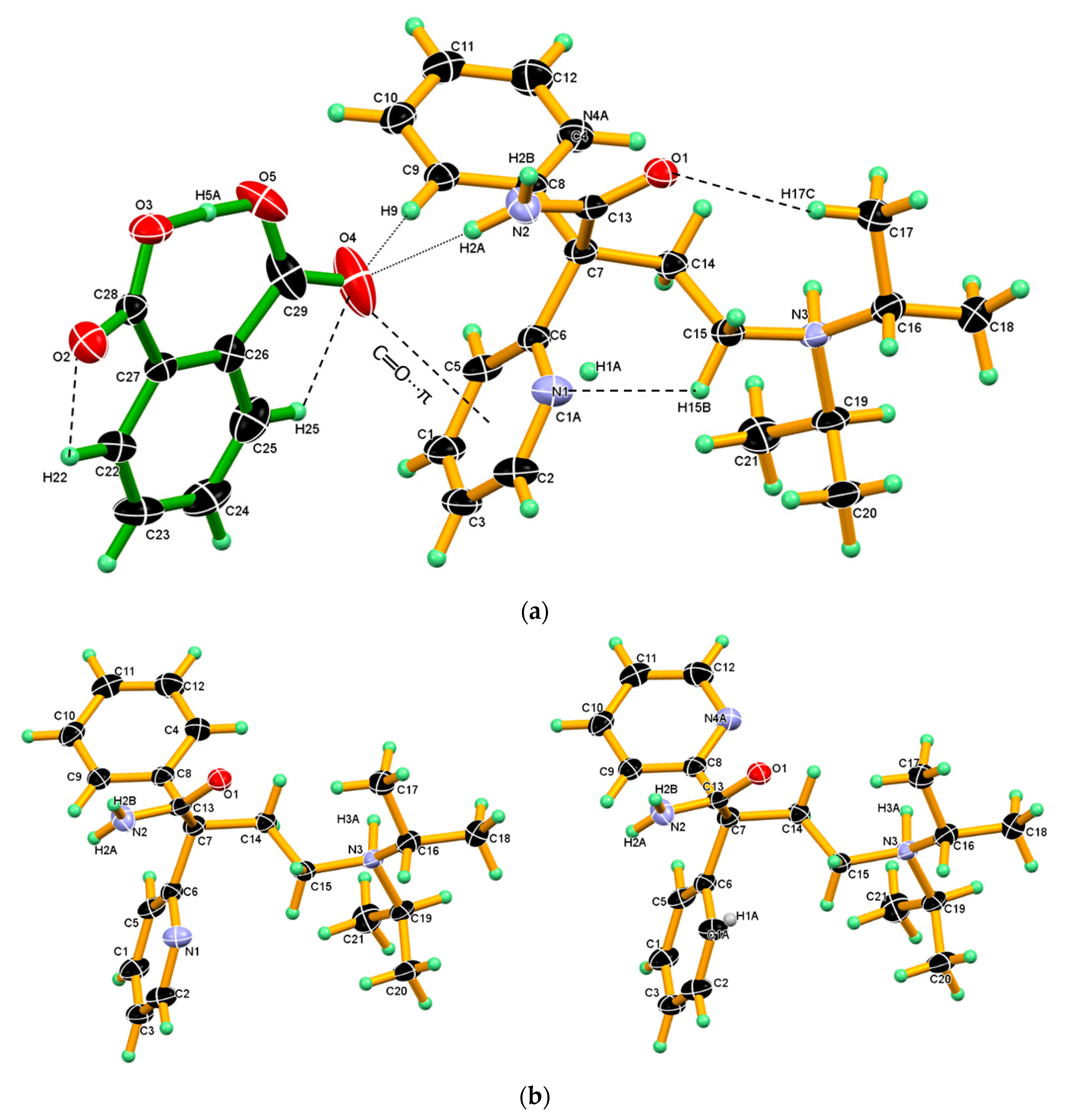
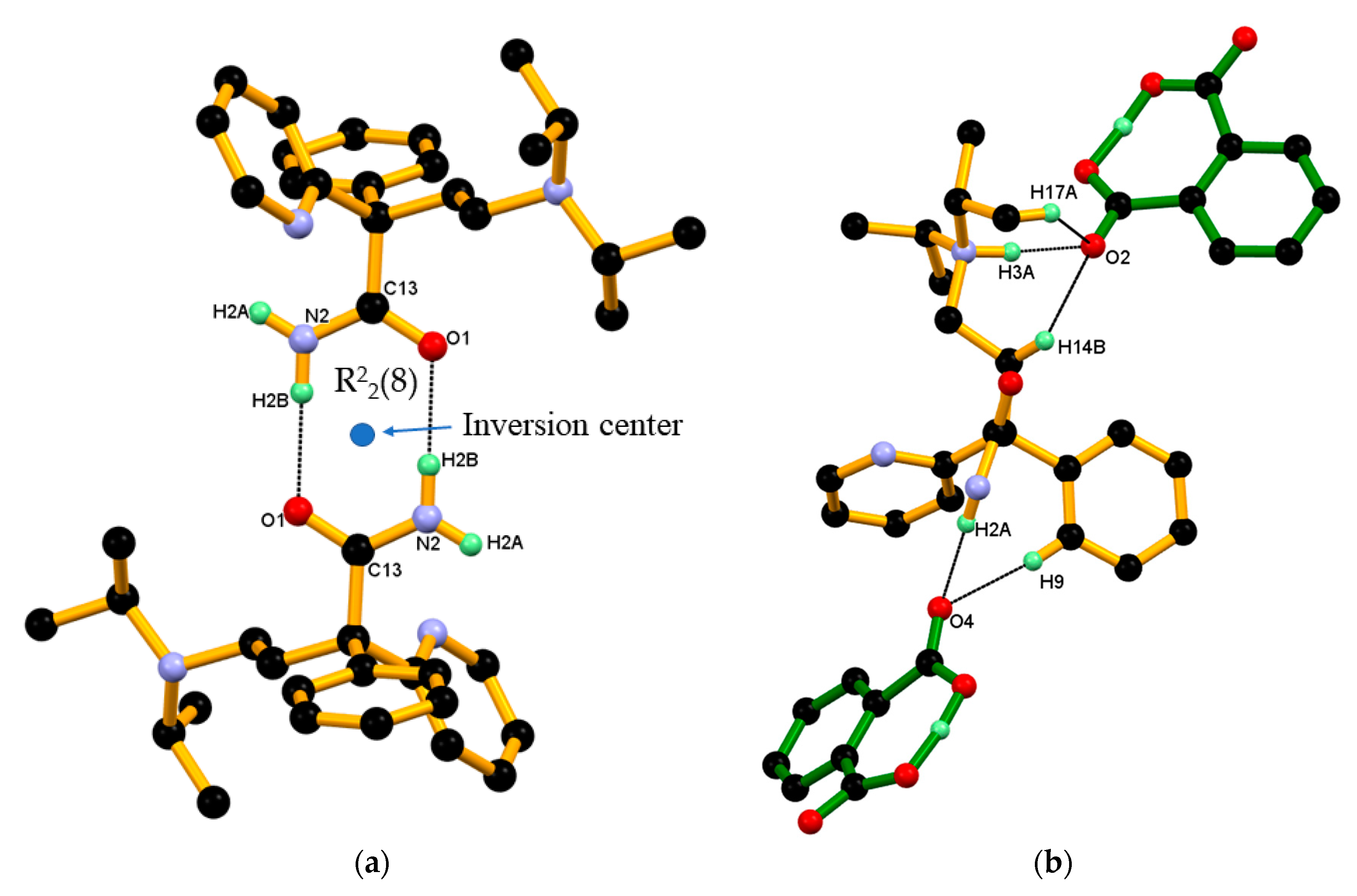
3.1. Crystal Structure of DPA
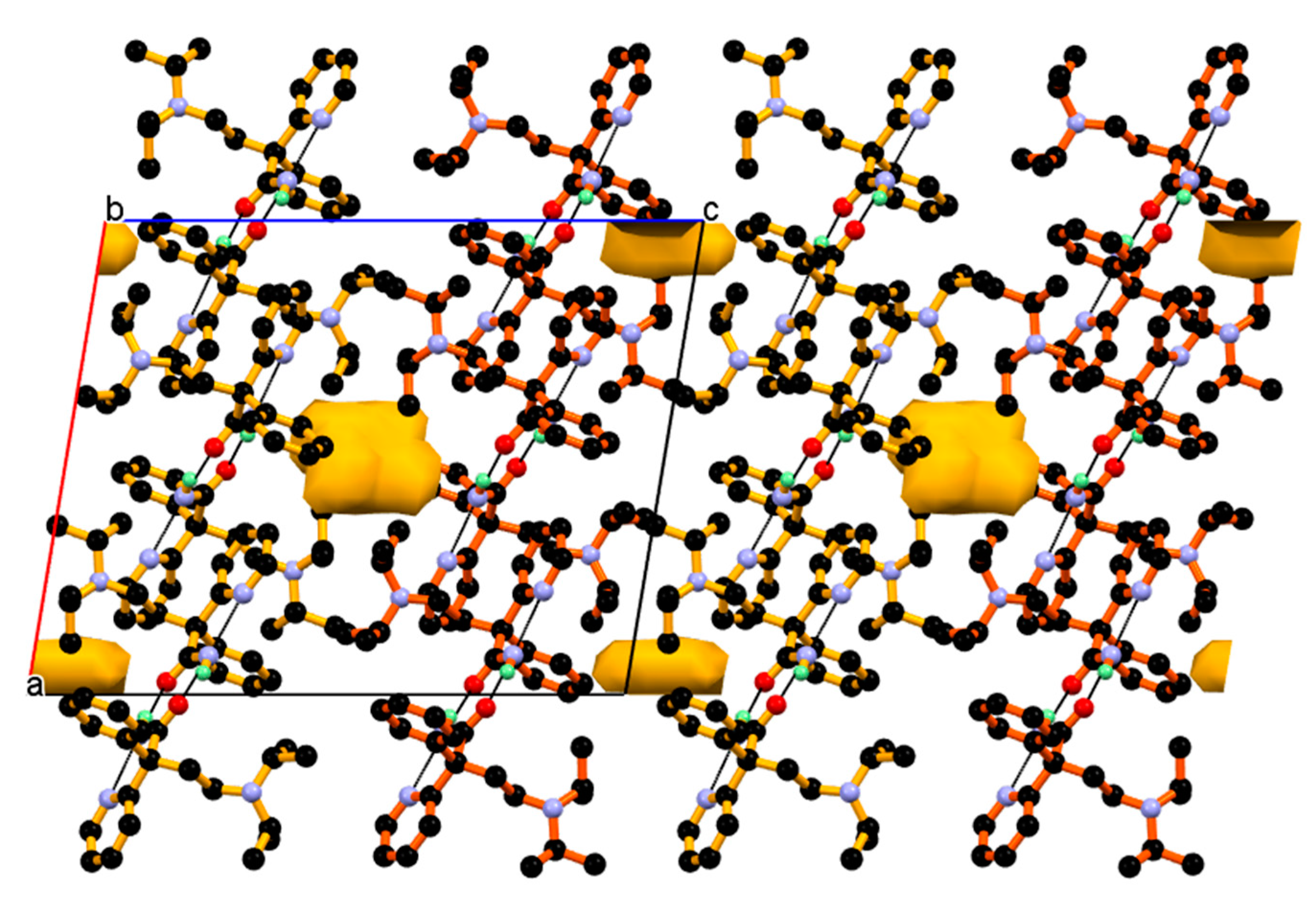
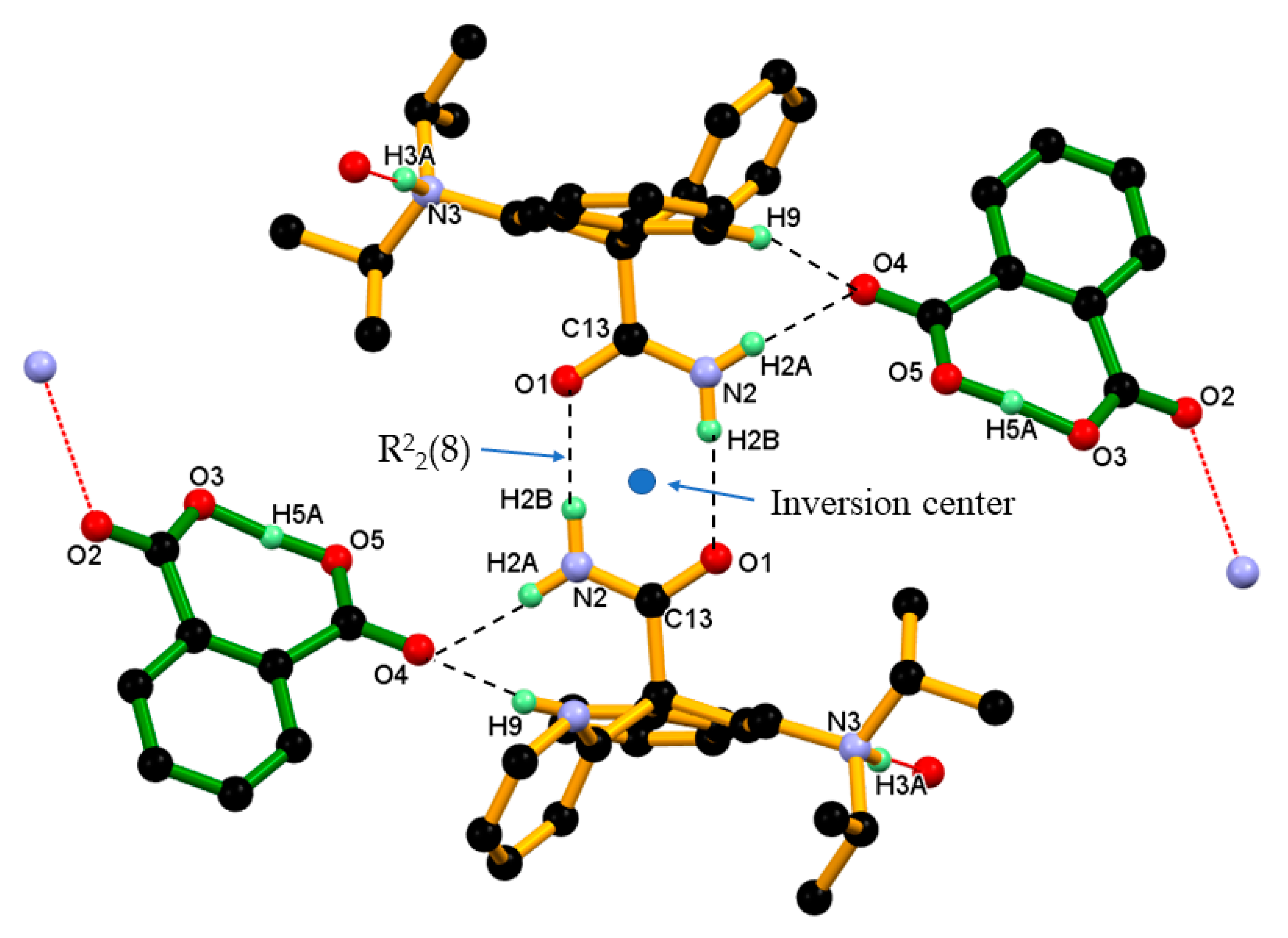
3.2. Crystal Structure of DPA_PA Salt
3.3. Structural Overlay
3.4. Characterization of DPA and DPA_PA Salt
3.4.1. PXRD
3.4.2. FT-IR Spectrum
3.4.3. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brittain, H.G. Polymorphism in Pharmaceutical Solids; Taylor & Francis: London, UK, 1999. [Google Scholar]
- Hilfiker, R.; von Raumer, M. Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- SeethaLekshmi, S.; Guru Row, T.N. Conformational Polymorphism in a Non-steroidal Anti-inflammatory Drug, Mefenamic Acid. Cryst. Growth Des. 2012, 12, 4283–4289. [Google Scholar] [CrossRef]
- Babu, N.J.; Cherukuvada, S.; Thakuria, R.; Nangia, A. Conformational and Synthon Polymorphism in Furosemide (Lasix). Cryst. Growth Des. 2010, 10, 1979–1989. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.H.; Diao, K.S. Improving the Solubility and Bioavailability of Pemafibrate via a New Polymorph Form II. ACS Omega 2020, 5, 26245–26252. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.A.E.; Vangala, V.R. Pharmaceutical Cocrystals: Molecules, Crystals, Formulations, Medicines. Cryst. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Gunnam, A.; Nangia, A.K. High-Solubility Salts of the Multiple Sclerosis Drug Teriflunomide. Cryst. Growth Des. 2019, 19, 5407–5417. [Google Scholar] [CrossRef]
- Bezerra, B.P.; Pogoda, D.; Perry, M.L.; Vidal, L.M.T.; Zaworotko, M.J.; Ayala, A.P. Cocrystal Polymorphs and Solvates of the Anti-Trypanosoma cruzi Drug Benznidazole with Improved Dissolution Performance. Cryst. Growth Des. 2020, 20, 4707–4718. [Google Scholar] [CrossRef]
- Berziņš, A.; Skarbulis, E.; Rekis, T.; Actiņš, A. On the Formation of Droperidol Solvates: Characterization of Structure and Properties. Cryst. Growth Des. 2014, 14, 2654–2664. [Google Scholar] [CrossRef]
- Zvoníček, V.; Skořepová, E.; Dušek, M.; Babor, M.; Žvátora, P.; Šoóš, M. First Crystal Structures of Pharmaceutical Ibrutinib: Systematic Solvate Screening and Characterization. Cryst. Growth Des. 2017, 17, 3116–3127. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Zhang, L.; Ren, G.; Zhang, S. Hydrates and Solvates of Acotiamide Hydrochloride: Crystallization, Structure, Stability, and Solubility. Cryst. Growth Des. 2018, 19, 768–779. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Sun, C.C. Structural Insights into the Distinct Solid-State Properties and Interconversion of Celecoxib N-Methyl-2-pyrrolidone Solvates. Cryst. Growth Des. 2020, 21, 277–286. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of PoorlyWater-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Bhatt, P.M.; Ravindra, N.V.; Desiraju, G.R. Saccharin Salts of Active Pharmaceutical Ingredients, Their Crystal Structures, and Increased Water Solubilities. Cryst. Growth Des. 2005, 5, 2299–2309. [Google Scholar] [CrossRef]
- Shan, N.; Perry, M.L.; Weyna, D.R.; Zaworotko, M.J. Impact of pharmaceutical cocrystals: The effects on drug pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1255–1271. [Google Scholar] [CrossRef]
- Swapna, B.; Maddileti, D.; Nangia, A. Cocrystals of the Tuberculosis Drug Isoniazid: Polymorphism, Isostructurality, and Stability. Cryst. Growth Des. 2014, 14, 5991–6005. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q.; Zhu, B.; Zhang, Z.; Bao, J.; Ding, Q.; Ren, G.; Mei, X. Pharmaceutical Cocrystals of Nicorandil with Enhanced Chemical Stability and Sustained Release. Cryst. Growth Des. 2020, 20, 6995–7005. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Reddy, I.R.; Tarafder, K.; Trivedi, D.R. Salt/Cocrystal of Anti-Fibrinolytic Hemostatic Drug Tranexamic acid: Structural, DFT, and Stability Study of Salt/Cocrystal with GRAS Molecules. Cryst. Growth Des. 2018, 19, 347–361. [Google Scholar] [CrossRef]
- Thakur, T.S.; Thakuria, R. Crystalline Multicomponent Solids: An Alternative for Addressing the Hygroscopicity Issue in Pharmaceutical Materials. Cryst. Growth Des. 2020, 20, 6245–6265. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Yao, J.; Ma, Y.-Y.; Chen, J.-M.; Lu, T.-B. Improving the Solubility and Bioavailability of Apixaban via Apixaban–Oxalic Acid Cocrystal. Cryst. Growth Des. 2016, 16, 2923–2930. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.; Wang, J.-R.; Mei, X. Cocrystals of Baicalein with Higher Solubility and Enhanced Bioavailability. Cryst. Growth Des. 2017, 17, 1893–1901. [Google Scholar] [CrossRef]
- Mannava, M.K.C.; Suresh, K.; Nangia, A. Enhanced Bioavailability in the Oxalate Salt of the Anti-Tuberculosis Drug Ethionamide. Cryst. Growth Des. 2016, 16, 1591–1598. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Corpinot, M.K.; Bučar, D.-K. A Practical Guide to the Design of Molecular Crystals. Cryst. Growth Des. 2018, 19, 1426–1453. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.C.B.; Sardo, M.; Santos, S.M.; Fernandes, A.; Antunes, A.; André, V.; Mafra, L.; Duarte, M.T. Packing Interactions and Physicochemical Properties of Novel Multicomponent Crystal Forms of the Anti-Inflammatory Azelaic Acid Studied by X-ray and Solid-State NMR. Cryst. Growth Des. 2015, 16, 154–166. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Wang, M.; Wang, Y.; Du, S.; Gong, J.; Wu, S. Intermolecular Interactions and Solubility Behavior of Multicomponent Crystal Forms of Orotic Acid: Prediction and Experiments. Cryst. Growth Des. 2021. [Google Scholar] [CrossRef]
- Rajput, L.; Sanphui, P.; Desiraju, G.R. New Solid Forms of the Anti-HIV Drug Etravirine: Salts, Cocrystals, and Solubility. Cryst. Growth Des. 2013, 13, 3681–3690. [Google Scholar] [CrossRef]
- George, C.P.; Thorat, S.H.; Shaligram, P.S.; Gonnade, R.G. Drug–drug cocrystals of anticancer drugs erlotinib–furosemide and gefitinib–mefenamic acid for alternative multi-drug treatment. Cryst. Eng. Comm. 2020, 22, 6137–6151. [Google Scholar] [CrossRef]
- Nangia, A.K.; Desiraju, G.R. Crystal Engineering: An Outlook for the Future. Angew. Chem. Int. Ed. Engl. 2019, 58, 4100–4107. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Strategy and Methodology in the Synthesis of Multicomponent Molecular Solids: The Quest for Higher Cocrystals. Acc. Chem. Res. 2019, 52, 2210–2220. [Google Scholar] [CrossRef]
- Sarkar, N.; Sinha, A.S.; Aakeröy, C.B. Systematic investigation of hydrogen-bond propensities for informing co-crystal design and assembly. Cryst. Eng. Comm. 2019, 21, 6048–6055. [Google Scholar] [CrossRef]
- Chu, Q.; Duncan, A.J.E.; Papaefstathiou, G.S.; Hamilton, T.D.; Atkinson, M.B.J.; Mariappan, S.V.S.; MacGillivray, L.R. Putting Cocrystal Stoichiometry to Work: A Reactive Hydrogen-Bonded “Superassembly” Enables Nanoscale Enlargement of a Metal-Organic Rhomboid via a Solid-State Photocycloaddition. J. Am. Chem. Soc. 2018, 140, 4940–4944. [Google Scholar] [CrossRef]
- Ericson, D.P.; Zurfluh-Cunningham, Z.P.; Groeneman, R.H.; Elacqua, E.; Reinheimer, E.W.; Noll, B.C.; MacGillivray, L.R. Regiocontrol of the [2 + 2] Photodimerization in the Solid State Using Isosteric Resorcinols: Head-to-Tail Cyclobutane Formation via Unexpected Embraced Assemblies. Cryst. Growth Des. 2015, 15, 5744–5748. [Google Scholar] [CrossRef]
- Katz, M.J.; Meyer, C.E.; El-Etr, A.; Slodki, S.J. Clinical evaluation of a new anti-arrhythmic agent, SC-7031. Curr. Ther. Res. Clin. Exp. 1963, 5, 343–350. [Google Scholar]
- Rizos, I.; Brachmann, J.; Lengfelder, W.; Schmitt, C.; von Olshausen, K.; Kubler, W.; Senges, J. Effects of intravenous disopyramide and quinidine on normal myocardium and on the characteristics of arrhythmias: Intraindividual comparison in patients with sustained ventricular tachycardia. Eur. Heart J. 1987, 8, 154–163. [Google Scholar] [CrossRef]
- Kim, S.Y.; Benowitz, N.L. Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide. Drug Saf. 1990, 5, 393–420. [Google Scholar] [CrossRef]
- Gunning, S.R.; Freeman, M.; Stead, J.A. Polymorphism of disopyramide. J. Pharm. Pharmacol. 1976, 28, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Hirayama, N. Crystal structure of α-diisopropylaminoethyl-α-phenylpyridine-2-Acetamide phosphate, [C21H30N3O] [H2PO4]. Z. Krist. N. Cryst. Struct. 2011, 226, 479. [Google Scholar] [CrossRef]
- Burke, T.R., Jr.; Nelson, W.L.; Mangion, M.; Hite, G.J.; Mokler, C.M.; Ruenitz, P.C. Resolution, absolute configuration, and antiarrhythmic properties of the enantiomers of disopyramide, 4-(diisopropylamino)-2-(2-pyridyl)-2-phenylbutyramide. J. Med. Chem. 1980, 23, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Putra, O.D.; Pettersen, A.; Nilsson Lill, S.O.; Umeda, D.; Yonemochi, E.; Nugraha, Y.P.; Uekusa, H. Capturing a new hydrate polymorph of amodiaquine dihydrochloride dihydrate via heterogeneous crystallisation. Cryst. Eng. Comm. 2019, 21, 2053–2057. [Google Scholar] [CrossRef]
- Putra, O.D.; Furuishi, T.; Yonemochi, E.; Terada, K.; Uekusa, H. Drug–Drug Multicomponent Crystals as an Effective Technique to Overcome Weaknesses in Parent Drugs. Cryst. Growth Des. 2016, 16, 3577–3581. [Google Scholar] [CrossRef]
- Nagase, H.; Kobayashi, M.; Ueda, H.; Furuishi, T.; Gunji, M.; Endo, T.; Yonemochi, E. Crystal Structure of an Epalrestat Dimethanol Solvate. X Ray Struct. Anal. Online 2016, 32, 7–9. [Google Scholar] [CrossRef][Green Version]
- Putra, O.D.; Umeda, D.; Nugraha, Y.P.; Furuishi, T.; Nagase, H.; Fukuzawa, K.; Uekusa, H.; Yonemochi, E. Solubility improvement of epalrestat by layered structure formation via cocrystallization. Cryst. Eng. Comm. 2017, 19, 2614–2622. [Google Scholar] [CrossRef]
- Hata, N.; Furuishi, T.; Tamboli, M.I.; Ishizaki, M.; Umeda, D.; Fukuzawa, K.; Yonemochi, E. Crystal Structural Analysis of DL-Mandelate Salt of Carvedilol and Its Correlation with Physicochemical Properties. Crystals 2020, 10, 53. [Google Scholar] [CrossRef]
- Higashi, T. Calculated Using ABSCOR. Empirical Absorption Correction Based on Fourier Series Approximation; Rigaku: The Woodland, TX, USA, 1994. [Google Scholar]
- Messerschmidt, A.; Schneider, M.; Huber, R. ABSCOR: A scaling and absorption correction program for the FAST area detector diffractometer. J. Appl. Crystallogr. 1990, 23, 436–439. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Crystallogr. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallographica. Sect. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0–New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Mittapalli, S.; Mannava, M.K.C.; Sahoo, R.; Nangia, A. Cocrystals, Salts, and Supramolecular Gels of Nonsteroidal Anti-Inflammatory Drug Niflumic Acid. Cryst. Growth Des. 2018, 19, 219–230. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Walker, G.; Lusi, M. Graph-Set Analysis Helps to Understand Charge Transfer in a Novel Ionic Cocrystal When the ΔpKa Rule Fails. Cryst. Growth Des. 2019, 19, 5308–5313. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Deng, Z.; Zhang, H. Ketoconazole: Solving the Poor Solubility via Cocrystal Formation with Phenolic Acids. Cryst. Growth Des. 2020, 20, 6973–6982. [Google Scholar] [CrossRef]
- Wickman, A.; Finnegan, P. Disopyramide Phosphate. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: New York, NY, USA, 1984; Volume 13, pp. 183–209. [Google Scholar]
- Brittain, H.G. Vibrational Spectroscopic Studies of Cocrystals and Salts. 1. The Benzamide−Benzoic Acid System. Crystal Growth Des. 2009, 9, 2492–2499. [Google Scholar] [CrossRef]
- Brittain, H.G. Vibrational Spectroscopic Studies of Cocrystals and Salts. 2. The Benzylamine−Benzoic Acid System. Cryst. Growth Des. 2009, 9, 3497–3503. [Google Scholar] [CrossRef]
- Smith, B.C. Organic nitrogen compounds V: Amine salts. Spectroscopy 2019, 34, 30–37. [Google Scholar]
- Kundu, S.; Kumari, N.; Soni, S.R.; Ranjan, S.; Kumar, R.; Sharon, A.; Ghosh, A. Enhanced Solubility of Telmisartan Phthalic Acid Cocrystals within the pH Range of a Systemic Absorption Site. ACS Omega 2018, 3, 15380–15388. [Google Scholar] [CrossRef] [PubMed]

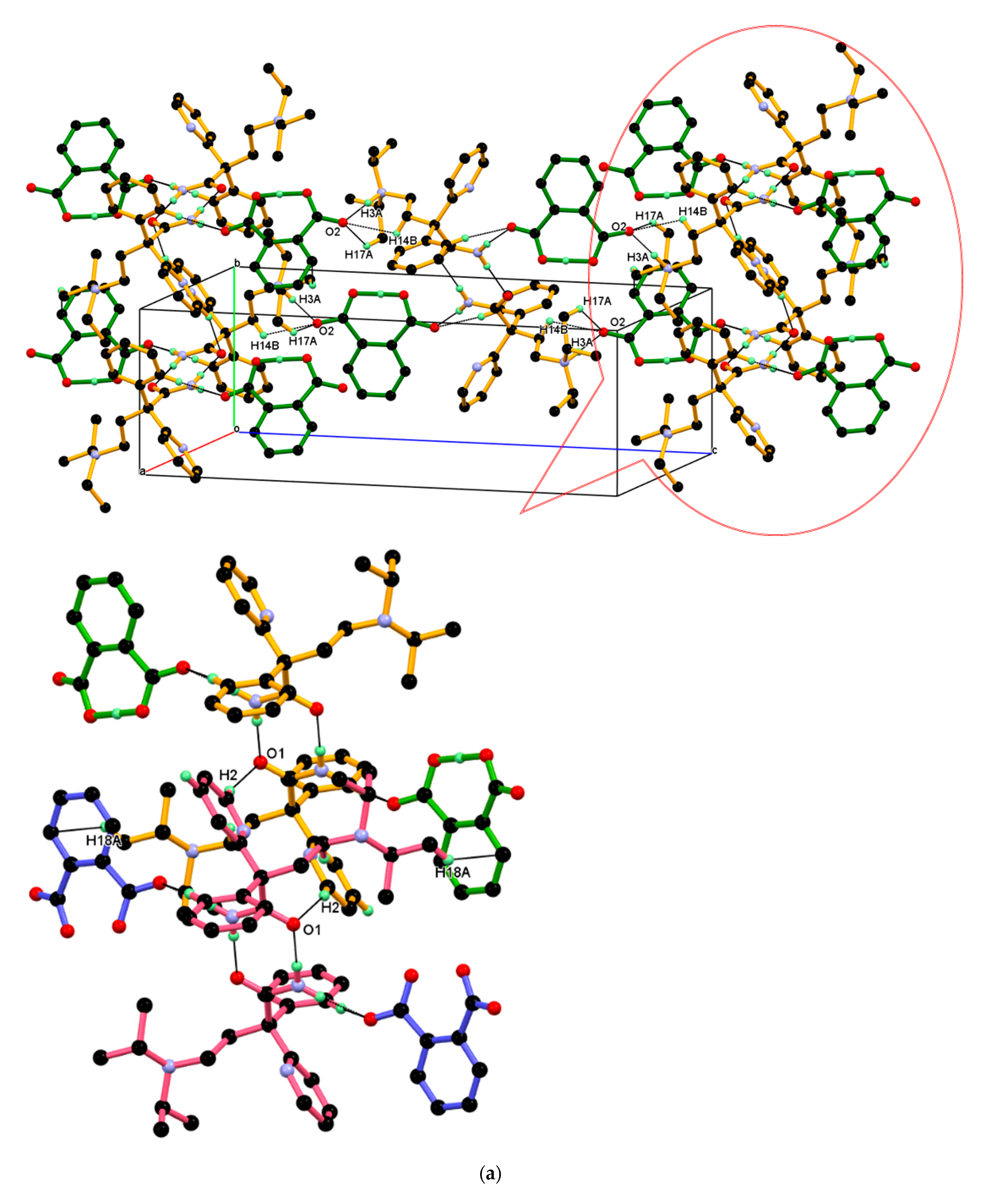
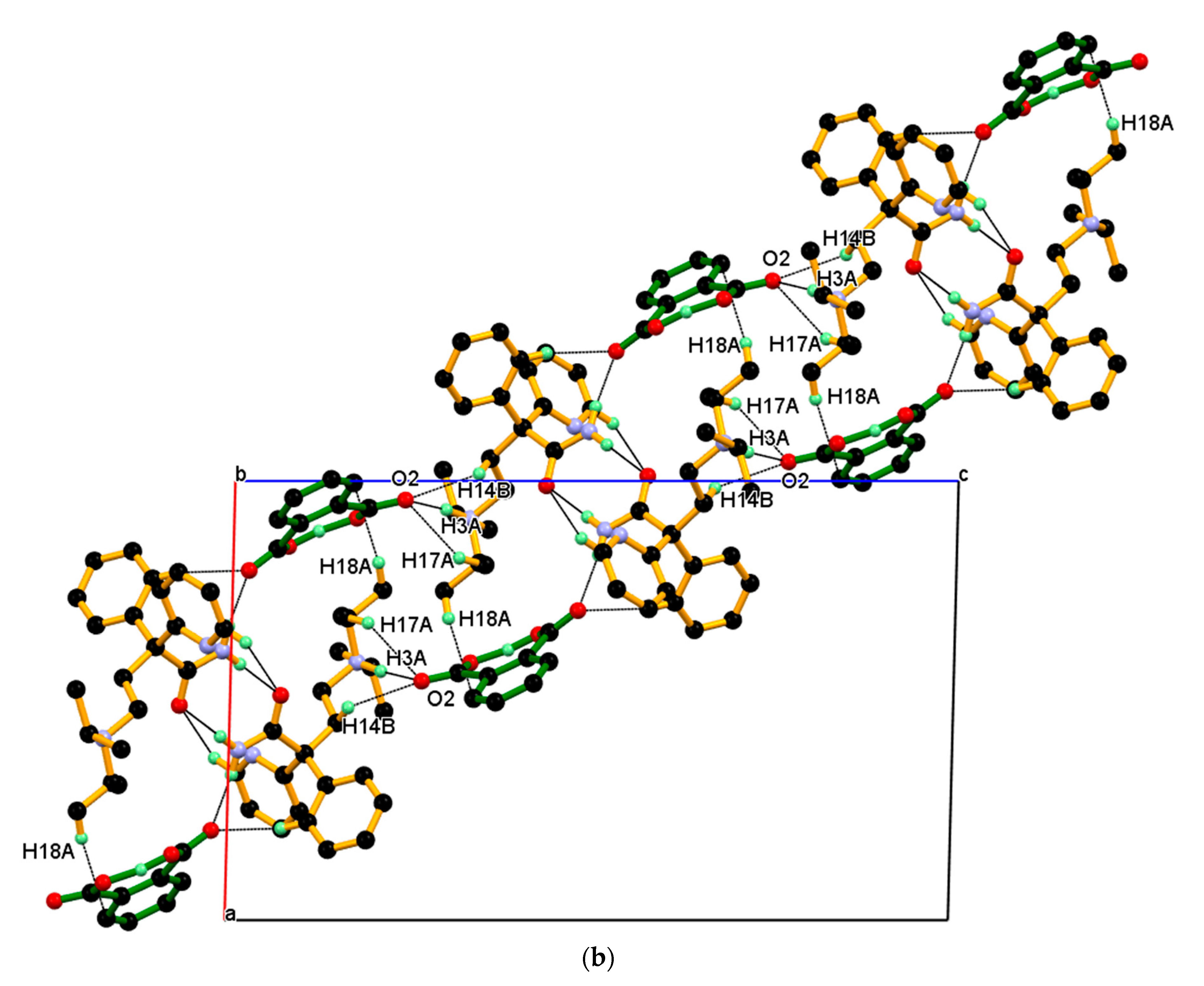
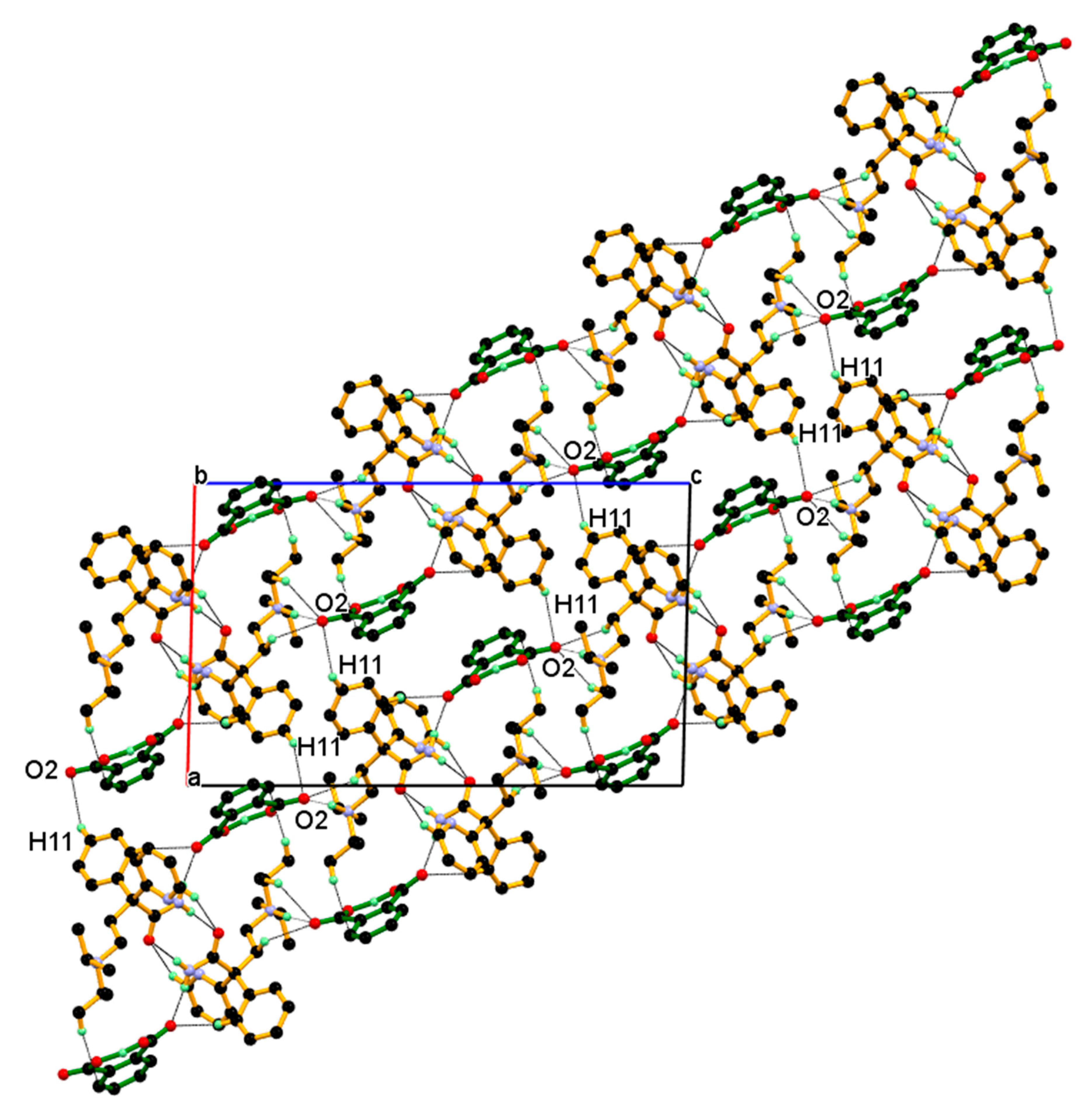
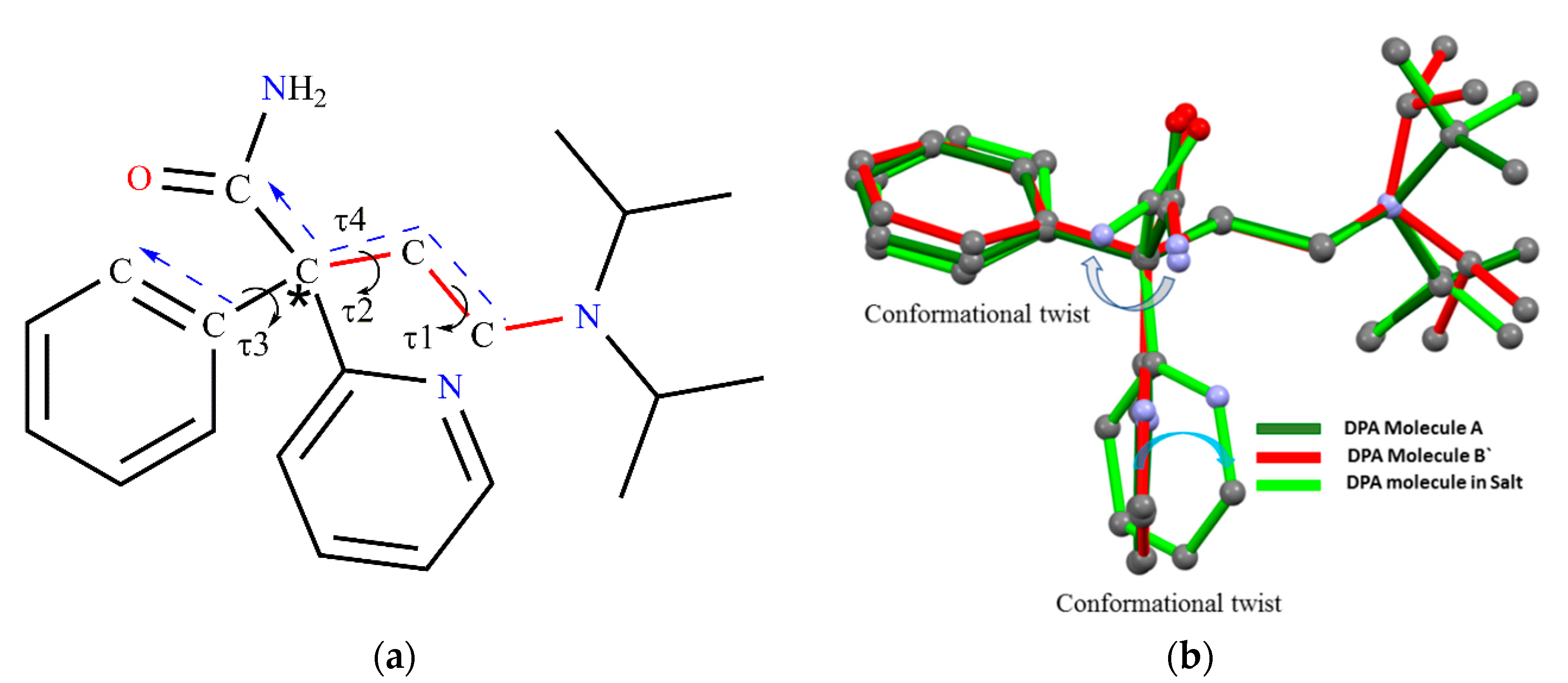
| Parameters | DPA | DPA_PA |
|---|---|---|
| Empirical formula | C21H29N3O | C29H35N3O5 |
| Formula weight | 339.47 | 505.60 |
| Temperature [K] | 93(2) | 93(2) |
| Wavelength [Å] | 1.54187 | 1.54187 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n |
| Unit cell dimensions | ||
| a [Å] b [Å] c [Å] α [°] β [°] γ [°] | 17.2970 (3) 10.7861 (2) 21.4831 (4) 90 99.385 (7) 90 | 14.2741 (4) 7.8827 (2) 23.5355 (7) 90 91.428 (6) 90 |
| Volume[Å3] | 3954.39 (15) | 2647.35 (13) |
| Z and Z’ | 4, 2 | 4, 1 |
| Density (calculated) [g/cm3] | 1.140 | 1.269 |
| Absorption coefficient [mm−1] | 0.552 | 0.705 |
| F (000) | 1472 | 1080.0 |
| Crystal size [mm x mm x mm] | 0.247 × 0.231 × 0.221 | 0.49 × 0.47 × 0.23 |
| Theta range for data collection [°] | 3.048 to 68.192 | 3.582 to 68.188 |
| Index ranges | −20 <= h <= 20, −12 <= k <= 12, −25 <= l <= 25 | −17 <=h <= 17, −9<= k <=9, −28 <=l <= 28 |
| Reflections collected | 44379 | 29013 |
| Independent reflections | 7200 [Rint = 0.0215, Rsigma = 0.0129] | 4847 [Rint = 0.0412, Rsigma = 0.0326] |
| Completeness to theta = 67.687° | 99.9% | 100% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.873 and 0.711 | 0.850, 0.513 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 7200/0/475 | 4847/0/350 |
| Goodness-of-fit on F2 | 1.052 | 1.079 |
| Final R indices [I>2sigma(I)] | R1 = 0.0416, wR2 = 0.1024 | R1 = 0.0434, wR2 = 0.1123 |
| R indices (all data) | R1 = 0.0435, wR2 = 0.1040 | R1 = 0.0489, wR2 = 0.11659 |
| Δρmax, Δρmin (e·Å–3) | 0.424 and −0.215 | 0.265/−0.171 |
| D-H···A | D-H (Å) | H···A (Å) | D···A (Å) | D-H···A (°) | Symmetry Codes |
|---|---|---|---|---|---|
| DPA | |||||
| N2-H2B∙∙∙N1 | 0.887 (17) | 1.965 (17) | 2.7006 (16) | 139.5 (14) | Intramolecular |
| N2-H2A∙∙∙O2 | 0.890 (16) | 2.025 (16) | 2.8959 (15) | 166.2 (14) | −x + 3/2, y − 1/2, −z+1/2 |
| N5-H4B∙∙∙N4 | 0.887 (17) | 1.970 (17) | 2.7035 (16) | 139.1 (14) | Intramolecular |
| N5-H4A∙∙∙O1 | 0.890 (17) | 2.046 (17) | 2.9286 (14) | 171.1 (14) | −x + 3/2, y + 1/2, −z+1/2 |
| C2-H2∙∙∙O2 | 0.95 | 2.677 | 3.605 | 165.71 | −x + 3/2, y − 1/2, −z+1/2 |
| C10-H10∙∙∙O2 | 0.95 | 2.62 | 3.3855(15) | 138 | x, y, z |
| C11-H11∙∙∙Cg4 | 0.95 | 2.98 | 3.7910(13) | 145 | x, y, z |
| C32-H32∙∙∙Cg2 | 0.95 | 2.90 | 3.7631(13) | 152 | −1 + x, y, z |
| C42-H42A∙∙∙Cg4 | 0.98 | 2.93 | 3.6320(18) | 129 | 1/2 − x, 1/2 + y, 1/2 − z |
| Cg2 centroid of the ring (C1-C2-C3-C4-C5-C6), Cg4 centroid of the ring (C22-C23-C24-C25-C26-C27) of molecule A and molecule B of DPA respectively | |||||
| DPA_PA salt | |||||
| N2-H2A∙∙∙O4 | 0.88 (2) | 2.02 (2) | 2.895 (2) | 174 (19) | x, y, z |
| N2-H2B∙∙∙O1 | 0.920 (18) | 2.071 (18) | 2.9910 (17) | 177.8 (18) | −x, 2 − y, 1 − z |
| N3-H3A∙∙∙O2 | 1.00 | 1.72 | 2.7159 (16) | 173 | −1/2 + x, 3/2 − y, 1/2 +z |
| O5-H5A∙∙∙O3 | 1.11(2) | 1.30 (2) | 2.4047 (19) | 172 (2) | Intramolecular |
| C2-H2∙∙∙O1 | 0.95 | 2.52 | 3.4269 (19) | 159 | −x, 1 − y, 1 − z |
| C9-H9∙∙∙O4 | 0.95 | 2.36 | 3.294 (2) | 167 | x, y, z |
| C15-H15B∙∙∙N1 | 0.99 | 2.57 | 3.078 (2) | 112 | Intramolecular |
| C17-H17C∙∙∙O1 | 0.98 | 2.54 | 3.4952 (19) | 166 | Intramolecular |
| C22-H22∙∙∙O2 | 0.95 | 2.33 | 2.674 (2) | 101 | Intramolecular |
| C25-H25∙∙∙O4 | 0.95 | 2.33 | 2.688 (2) | 101 | Intramolecular |
| C17-H17A∙∙∙O2 | 0.98 | 2.685 | 3.359 (2) | 126.23 | −1/2 + x, 3/2 − y,1/2 + z |
| C14-H14B∙∙∙O2 | 0.99 | 2.603 | 3.0812(18) | 109.73 | −1/2 + x,3/2 − y, 1/2 + z |
| C11–H11∙∙∙O2 | 0.95 | 2.711 | 3.519 | 143.35 | 1 − x, 2 − y, 1 − z |
| C18-H18A∙∙∙Cg5 | 0.98 | 2.93 | 3.6222(18) | 129 | −x, 1 − y, 1 − z |
| C29–O4∙∙∙ Cg2 | 3.4808 (16) | 146. 59 (14) | x, y, z | ||
| C29–O4∙∙∙ Cg3 | 3.4808 (16) | 146.59 (14) | x, y, z | ||
| Cg2 centroid of the ring (C1-C3-C2-C1A-C6-C5), Cg3 centroid of the ring (N1-C2-C3-C1-C5-C6) of disordered protonated DPA, Cg5 centroid of the ring (C22-C23-C24-C25-C26-C27) of PA¯ anion in the DPA_PA salt | |||||
| τ1 ° | τ2° | τ3 ° | τ4° | Dihedral Angle ° | |
|---|---|---|---|---|---|
| DPA Molecule A | −179.78 | 177.67 | −1.01 | 63.83 | 87.16(6) |
| DPA Molecule B` | −171.37 | 177.17 | 3.12 | 63.90 | 79.83(6) |
| DPA from Salt | −177.60 | −175.45 | −16.81 | 69.39 | 59.43(7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamboli, M.I.; Okamoto, Y.; Utsumi, Y.; Furuishi, T.; Wang, S.; Umeda, D.; Putra, O.D.; Fukuzawa, K.; Uekusa, H.; Yonemochi, E. Crystal Structures of Antiarrhythmic Drug Disopyramide and Its Salt with Phthalic Acid. Crystals 2021, 11, 379. https://doi.org/10.3390/cryst11040379
Tamboli MI, Okamoto Y, Utsumi Y, Furuishi T, Wang S, Umeda D, Putra OD, Fukuzawa K, Uekusa H, Yonemochi E. Crystal Structures of Antiarrhythmic Drug Disopyramide and Its Salt with Phthalic Acid. Crystals. 2021; 11(4):379. https://doi.org/10.3390/cryst11040379
Chicago/Turabian StyleTamboli, Majid Ismail, Yushi Okamoto, Yohei Utsumi, Takayuki Furuishi, Siran Wang, Daiki Umeda, Okky Dwichandra Putra, Kaori Fukuzawa, Hidehiro Uekusa, and Etsuo Yonemochi. 2021. "Crystal Structures of Antiarrhythmic Drug Disopyramide and Its Salt with Phthalic Acid" Crystals 11, no. 4: 379. https://doi.org/10.3390/cryst11040379
APA StyleTamboli, M. I., Okamoto, Y., Utsumi, Y., Furuishi, T., Wang, S., Umeda, D., Putra, O. D., Fukuzawa, K., Uekusa, H., & Yonemochi, E. (2021). Crystal Structures of Antiarrhythmic Drug Disopyramide and Its Salt with Phthalic Acid. Crystals, 11(4), 379. https://doi.org/10.3390/cryst11040379









