Single Crystal Growth and Physical Properties of Pyroxene CoGeO3
Abstract
1. Introduction
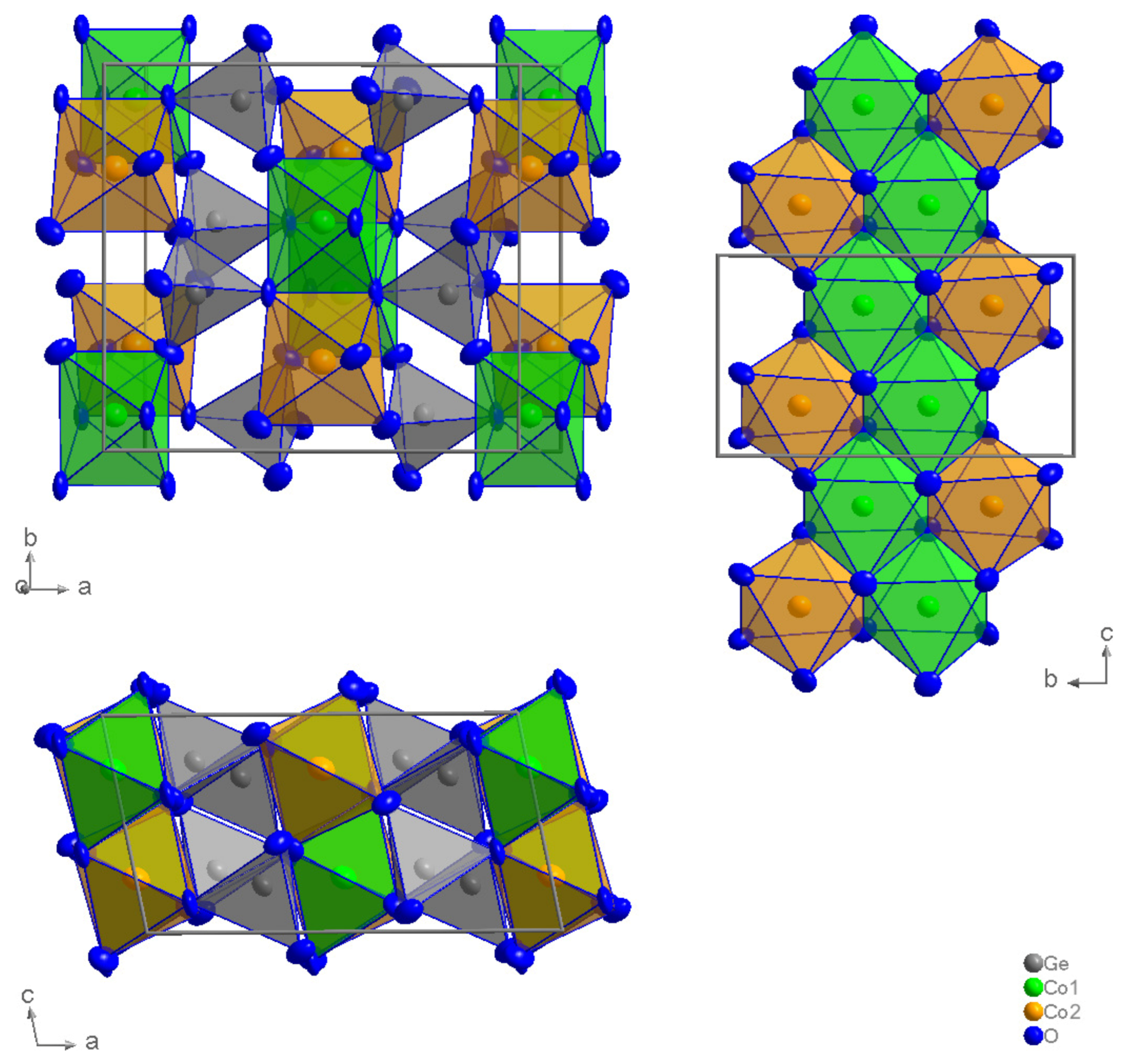
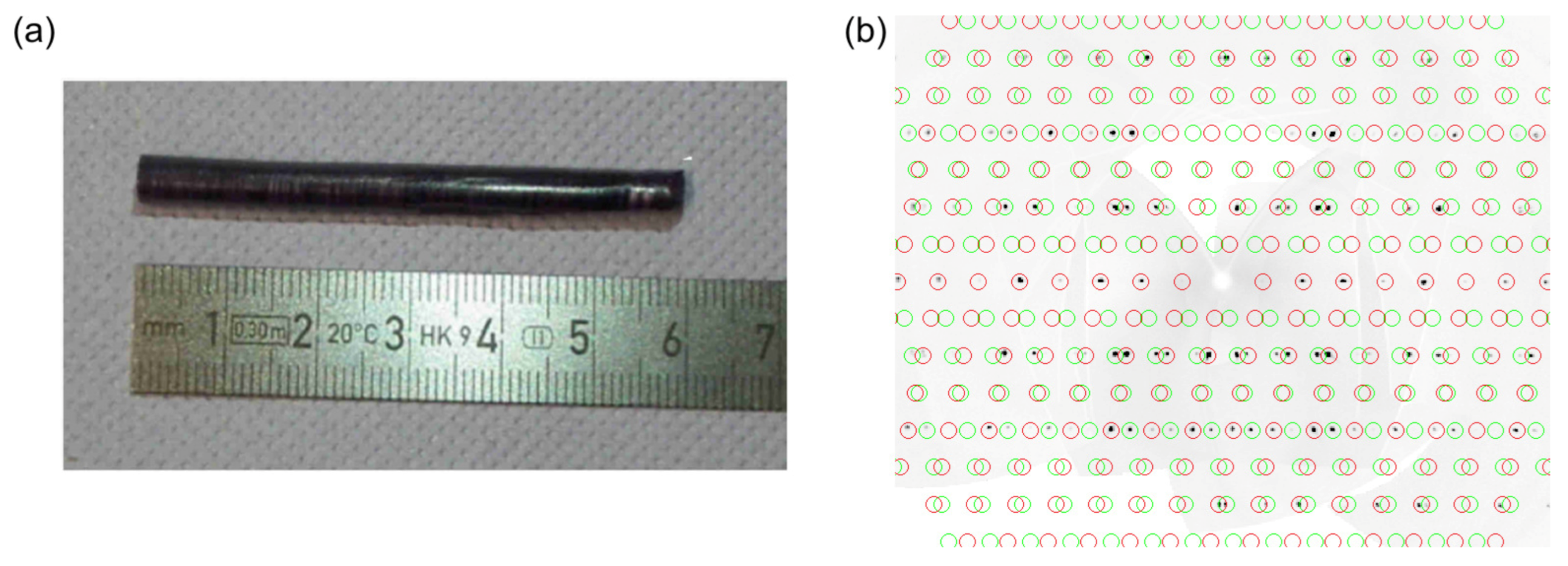
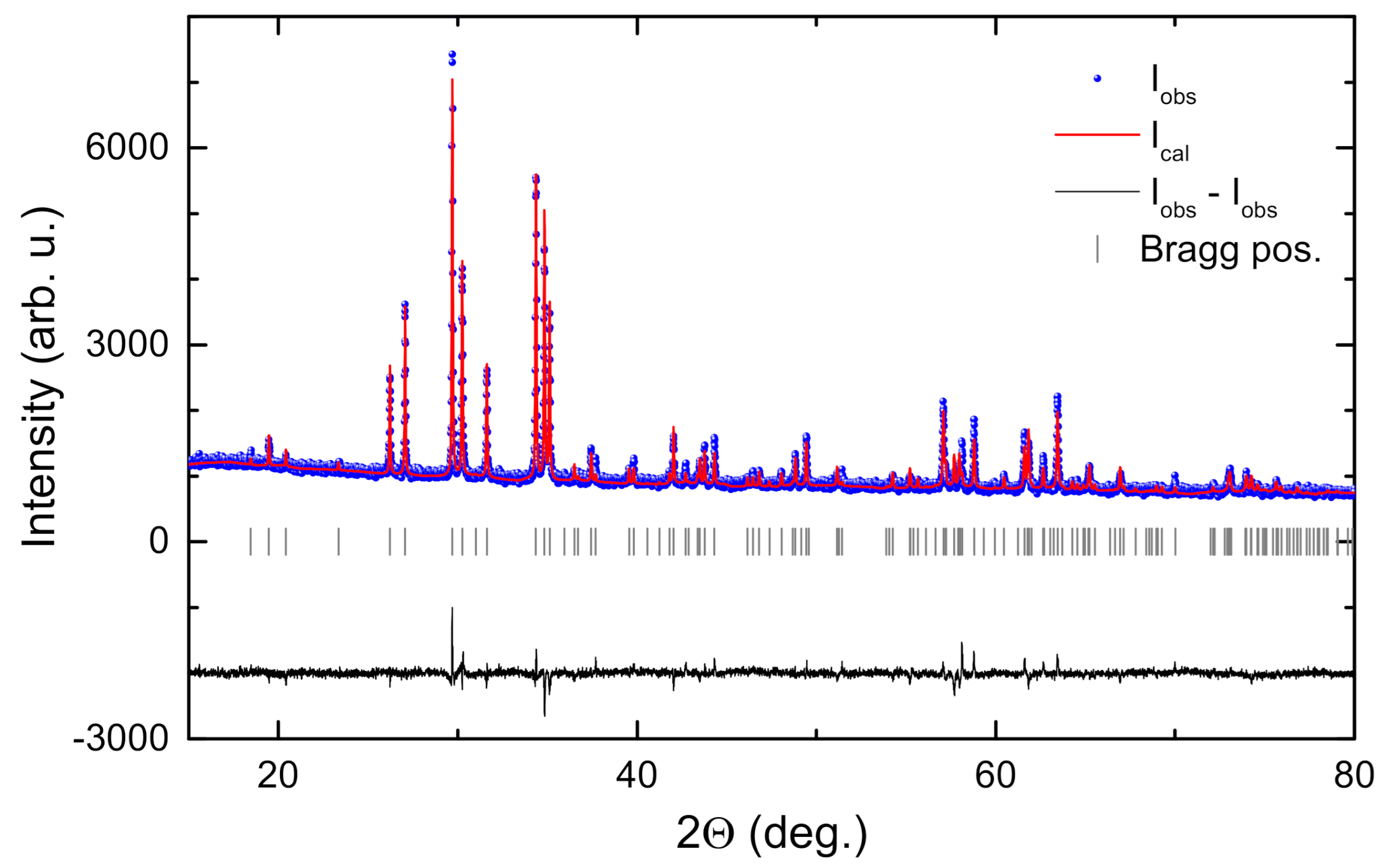
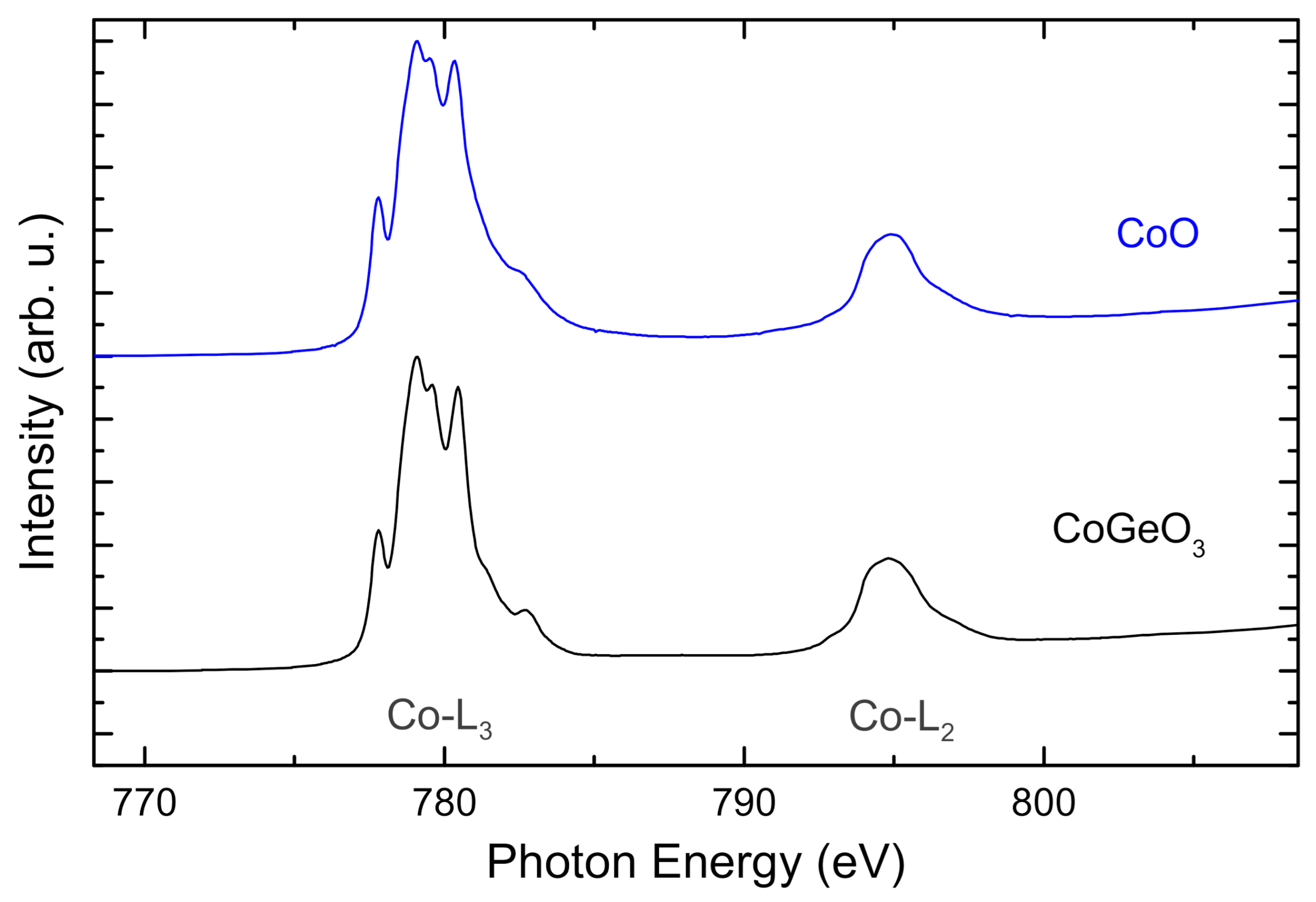
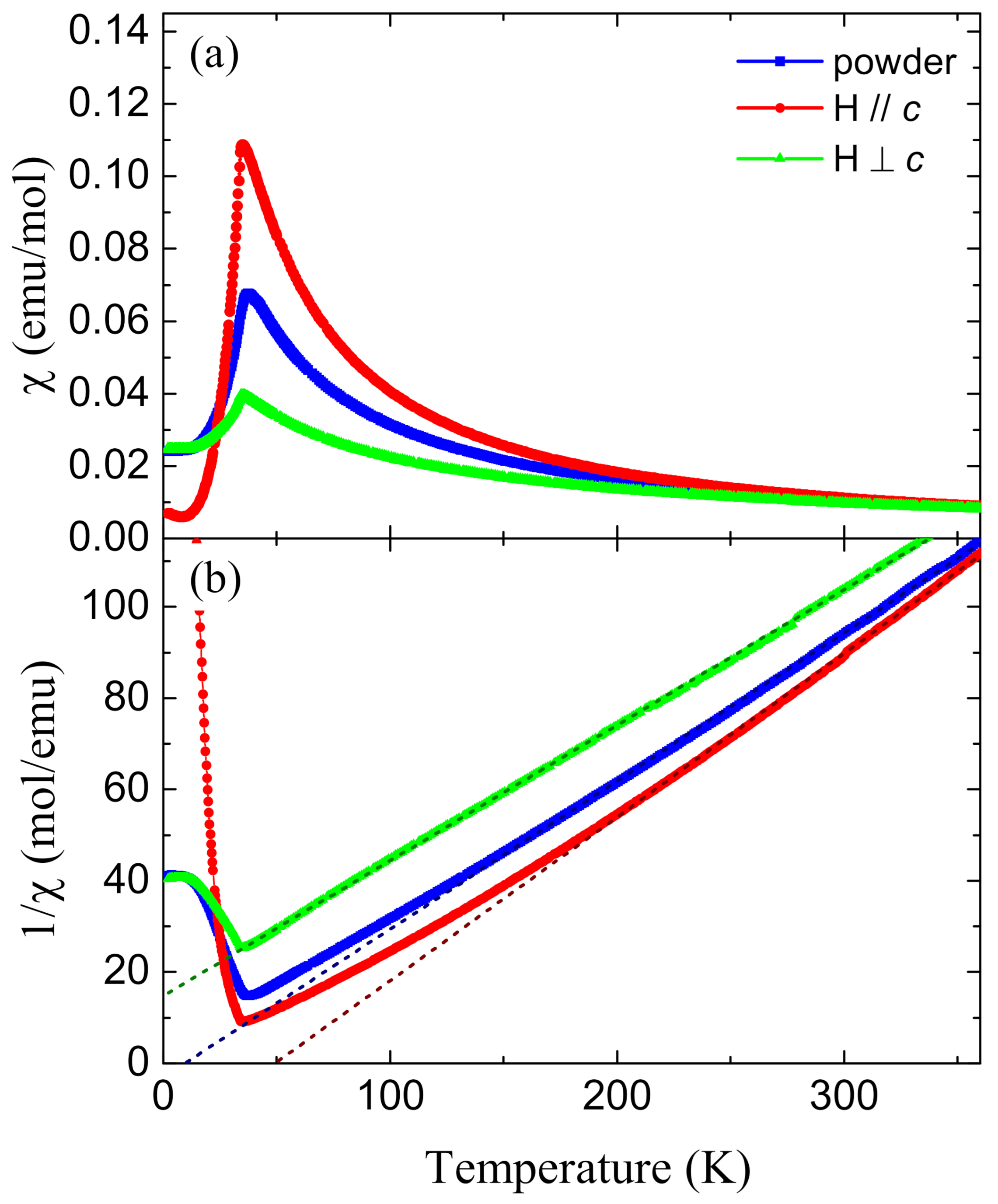
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J.; Aoki, K.; Gottardi, G. Nomenclature of pyroxenes. Am. Miner. 1988, 73, 1123–1133. [Google Scholar]
- Lindsley, D.H. Pyroxene thermometry. Am. Miner. 1983, 68, 477–493. [Google Scholar]
- Boyd, F. A pyroxene geotherm. Geochim. Cosmochim. Acta 1973, 37, 2533–2546. [Google Scholar] [CrossRef]
- Warren, J.M.; Hauri, E.H. Pyroxenes as tracers of mantle water variations. J. Geophys. Res. Solid Earth 2014, 119, 1851–1881. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Ignatchik, O.L.; Sokolov, A.N.; Hiroi, Z.; Isobe, M.; Ueda, Y. Long-range magnetic order in quasi-one-dimensional chromium-based (S = ) pyroxenes (Li, Na)Cr(Si, Ge)2O6. Phys. Rev. B 2005, 72, 012412. [Google Scholar] [CrossRef]
- Streltsov, S.V.; Popova, O.A.; Khomskii, D.I. Comment on “Sodium Pyroxene NaTiSi2O6: Possible Haldane Spin-1 Chain System”. Phys. Rev. Lett. 2006, 96, 249701. [Google Scholar] [CrossRef] [PubMed]
- Jodlauk, S.; Becker, P.; Mydosh, J.A.; Khomskii, D.I.; Lorenz, T.; Streltsov, S.V.; Hezel, D.C.; Bohatý, L. Pyroxenes: A new class of multiferroics. J. Phys. Condens. Matter 2007, 19, 432201. [Google Scholar] [CrossRef]
- Redhammer, G.J.; Senyshyn, A.; Tippelt, G.; Pietzonka, C.; Roth, G.; Amthauer, G. Magnetic and nuclear structure and thermal expansion of orthorhombic and monoclinic polymorphs of CoGeO3 pyroxene. Phys. Chem. Miner. 2010, 37, 311–332. [Google Scholar] [CrossRef]
- Tauber, A.; Kohn, J.A. Orthopyroxene and clinopyroxene polymorphs of CoGeO3. Am. Miner. 1965, 50, 13–21. [Google Scholar]
- Peacor, D.R. The crystal structure of CoGeO3*. Z. Krist. 1968, 126, 299–306. [Google Scholar] [CrossRef]
- Burnus, T.; Hu, Z.; Hsieh, H.H.; Joly, V.L.J.; Joy, P.A.; Haverkort, M.W.; Wu, H.; Tanaka, A.; Lin, H.J.; Chen, C.T.; et al. Local electronic structure and magnetic properties of LaMn0.5Co0.5O3 studied by x-ray absorption and magnetic circular dichroism spectroscopy. Phys. Rev. B 2008, 77, 125124. [Google Scholar] [CrossRef]
- Chang, C.F.; Hu, Z.; Wu, H.; Burnus, T.; Hollmann, N.; Benomar, M.; Lorenz, T.; Tanaka, A.; Lin, H.J.; Hsieh, H.H.; et al. Spin Blockade, Orbital Occupation, and Charge Ordering in La1.5Sr0.5CoO4. Phys. Rev. Lett. 2009, 102, 116401. [Google Scholar] [CrossRef] [PubMed]
- Burnus, T.; Hu, Z.; Wu, H.; Cezar, J.C.; Niitaka, S.; Takagi, H.; Chang, C.F.; Brookes, N.B.; Lin, H.J.; Jang, L.Y.; et al. X-ray absorption and x-ray magnetic dichroism study on Ca3CoRhO6 and Ca3FeRhO6. Phys. Rev. B 2008, 77, 205111. [Google Scholar] [CrossRef]
- Khomskii, D.I. Transition Metal Compounds; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. 2014, 229, 345. [Google Scholar]
- Rodrguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- De Groot, F. X-ray absorption and dichroism of transition metals and their compounds. J. Electron Spectrosc. Relat. Phenom. 1994, 67, 529–622. [Google Scholar] [CrossRef]
| Empirical formula | CoGeO |
| Formula weight (g/mol) | 179.5 |
| Temperature | room temperature |
| Wavelength | Mo K |
| Crystal system | monoclinic |
| Space group | C 2/c (15) |
| Unit cell dimensions | a = 9.6623(2) Å |
| b = 8.9928(2) Å | |
| c = 5.16980(10) Å | |
| = 101.2785(10) | |
| Volume | 440.535(16) Å |
| Z | 8 |
| Density (g/cm) | 5.4134 |
| Absorption coefficient | 20.861 |
| F(000) | 664 |
| Crystal size | ∼10–20 m |
| 2 | 106.58 |
| Index range | h: −21 → 21 |
| k: −19 → 20 | |
| l: −11 → 10 | |
| Reflections in total / independent | 13,219/2438 |
| Observed reflections / independent | 10,541/2078 |
| Internal R-value | 2.31% |
| Completeness up to 2 | 91.48% |
| Absorption correction | multi-scan |
| Min. / max. transmission | 0.3738 / 0.7505 |
| Refinement method | least squares on |
| Reflections threshold * | |
| Goodness of fit | 1.97 |
| R / R | 1.63/5.28% |
| Largest minima in Fourier difference | −3.10 e Å |
| Largest maxima in Fourier difference | 2.78 e Å |
| Atom | x | y | z | |
|---|---|---|---|---|
| Ge1 | 0.30104(2) | 0.09381(2) | 0.21471(4) | |
| Co1 | 0 | 0.09179(4) | 0.75 | |
| Co2 | 0 | 0.26966(4) | 0.25 | |
| O1 | 0.11779(15) | 0.09052(14) | 0.1358(3) | |
| O2 | 0.38225(14) | 0.24390(16) | 0.3830(3) | |
| O3 | 0.36047(15) | 0.06723(16) | 0.9099(3) | |
| atom | U (Å) | U (Å) | U (Å) | |
| Ge1 | 0.00347(12) | 0.00445(13) | 0.00401(12) | |
| Co1 | 0.00526(17) | 0.00499(19) | 0.00458(18) | |
| Co2 | 0.00622(16) | 0.00571(17) | 0.00483(16) | |
| O1 | 0.0020(5) | 0.0075(6) | 0.0067(5) | |
| O2 | 0.0074(5) | 0.0062(5) | 0.0059(5) | |
| O3 | 0.0077(6) | 0.0071(5) | 0.0052(5) | |
| atom | U (Å) | U (Å) | U (Å) | |
| Ge1 | −0.00022(5) | 0.00010(8) | −0.00009(5) | |
| Co1 | 0 | 0.00021(13) | 0 | |
| Co2 | 0 | 0.00010(12) | 0 | |
| O1 | 0.0003(4) | 0.0003(5) | −0.0003(4) | |
| O2 | −0.0025(4) | −0.0001(4) | −0.0012(4) | |
| O3 | −0.0019(5) | 0.0027(5) | −0.0014(4) |
| Atoms | Distance (Å) / BVS |
|---|---|
| Ge1-O1 | 1.7399(14) |
| Ge1-O2 | 1.7142(14) |
| Ge1-O3 | 1.7965(18) |
| Ge1-O3 | 1.7963(15) |
| BVS(Ge1) | 3.896(8) |
| Co1-O1 | 2.0959(15) |
| Co1-O1 | 2.0959(15) |
| Co1-O1 | 2.1458(15) |
| Co1-O1 | 2.1458(15) |
| Co1-O2 | 2.0649(16) |
| Co1-O2 | 2.0649(16) |
| BVS(Co1) | 1.999(3) |
| Co2-O1 | 2.1229(15) |
| Co2-O1 | 2.1229(15) |
| Co2-O2 | 2.0157(15) |
| Co2-O2 | 2.0157(15) |
| Co2-O3 | 2.2588(17) |
| Co2-O3 | 2.2588(17) |
| BVS(Co2) | 1.894(3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Hu, Z.; Guo, H.; Geibel, C.; Lin, H.-J.; Chen, C.-T.; Khomskii, D.; Tjeng, L.H.; Komarek, A.C. Single Crystal Growth and Physical Properties of Pyroxene CoGeO3. Crystals 2021, 11, 378. https://doi.org/10.3390/cryst11040378
Zhao L, Hu Z, Guo H, Geibel C, Lin H-J, Chen C-T, Khomskii D, Tjeng LH, Komarek AC. Single Crystal Growth and Physical Properties of Pyroxene CoGeO3. Crystals. 2021; 11(4):378. https://doi.org/10.3390/cryst11040378
Chicago/Turabian StyleZhao, Li, Zhiwei Hu, Hanjie Guo, Christoph Geibel, Hong-Ji Lin, Chien-Te Chen, Daniel Khomskii, Liu Hao Tjeng, and Alexander C. Komarek. 2021. "Single Crystal Growth and Physical Properties of Pyroxene CoGeO3" Crystals 11, no. 4: 378. https://doi.org/10.3390/cryst11040378
APA StyleZhao, L., Hu, Z., Guo, H., Geibel, C., Lin, H.-J., Chen, C.-T., Khomskii, D., Tjeng, L. H., & Komarek, A. C. (2021). Single Crystal Growth and Physical Properties of Pyroxene CoGeO3. Crystals, 11(4), 378. https://doi.org/10.3390/cryst11040378






