Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications
Abstract
1. Introduction
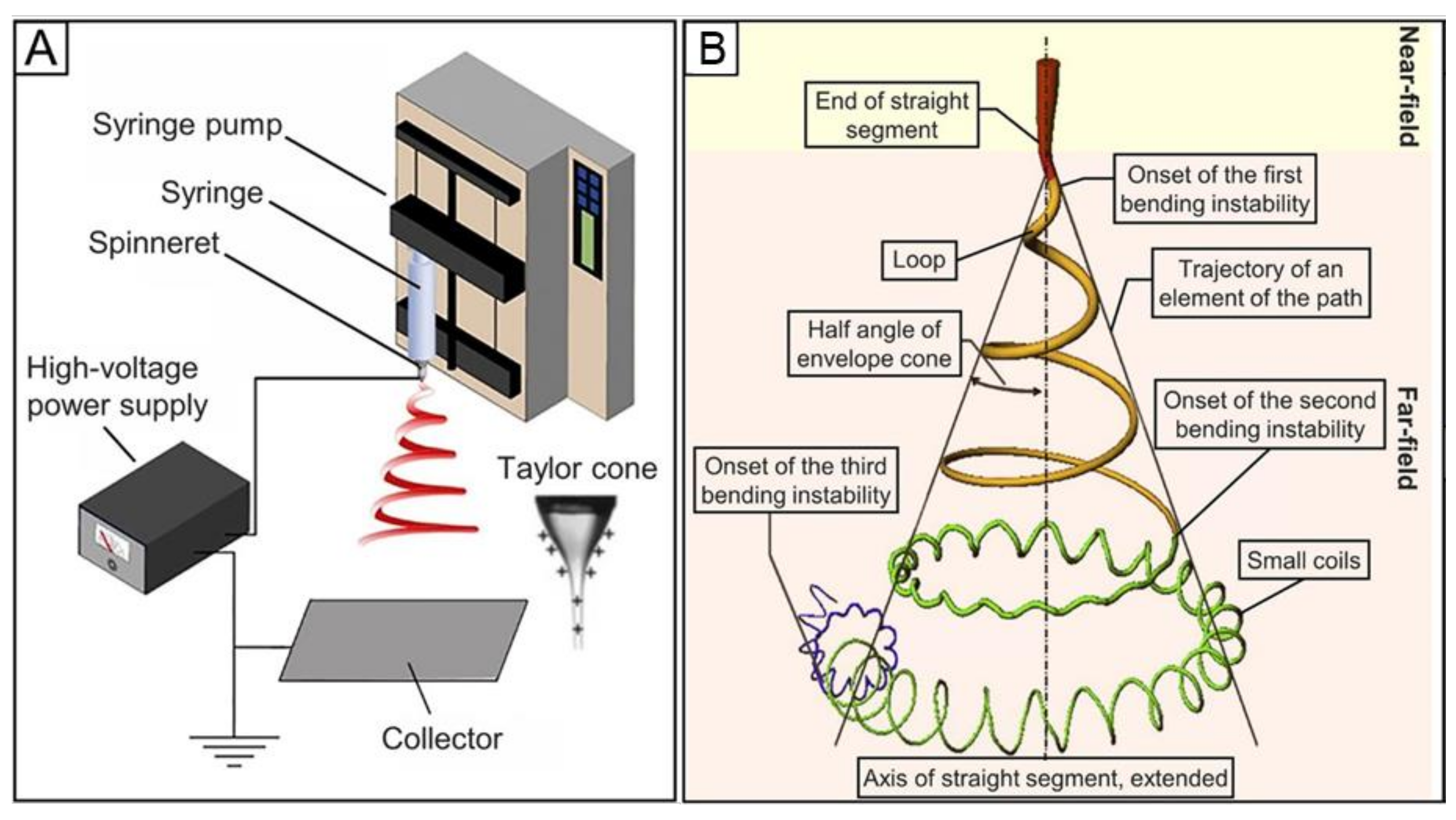
2. Fundamental Principles of Electrospinning
3. Key Parameters for Electrospinning
3.1. Solution Parameters
3.2. Instrumental Parameters
3.3. Environmental Parameters
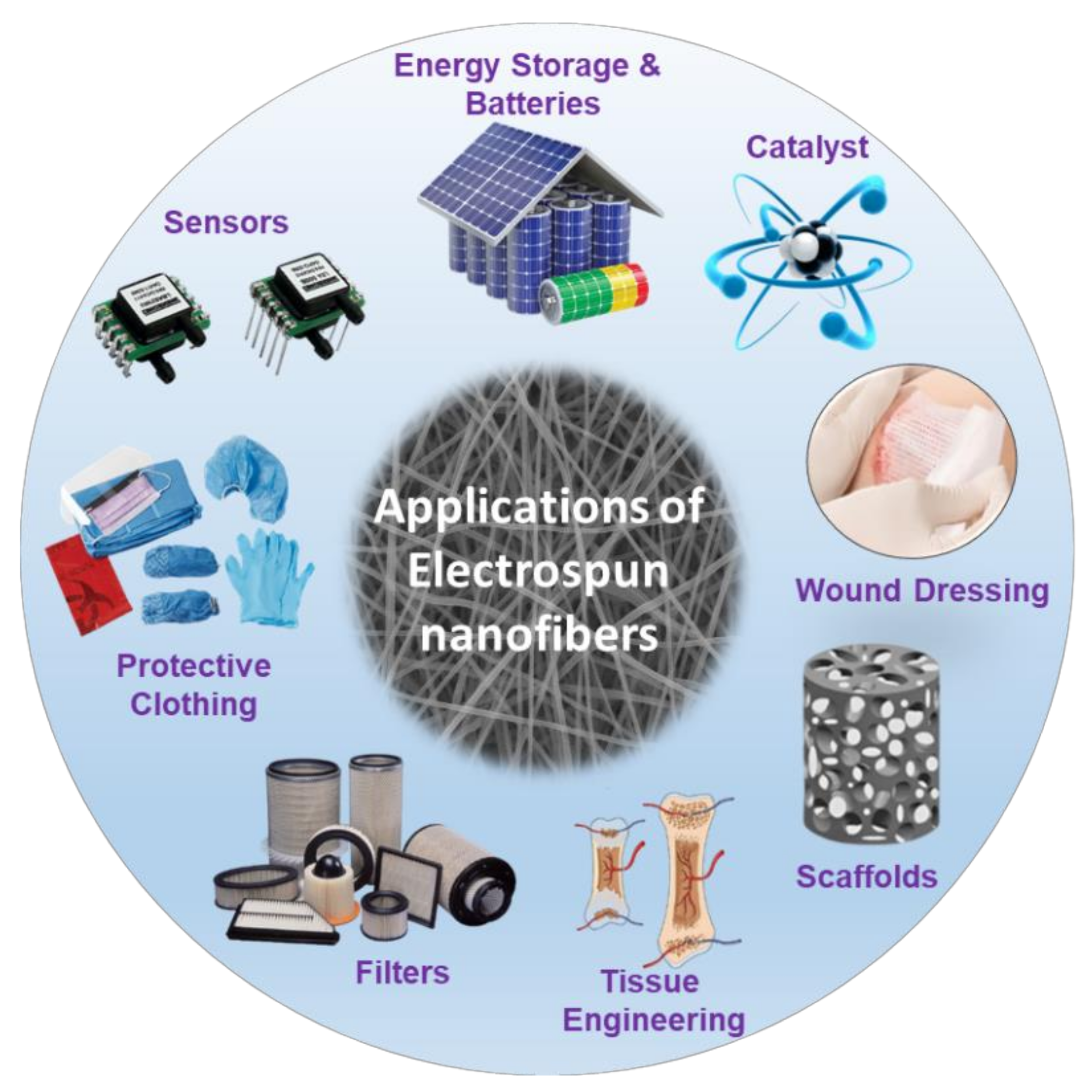
4. Possible Applications of Electrospun Nanofibers
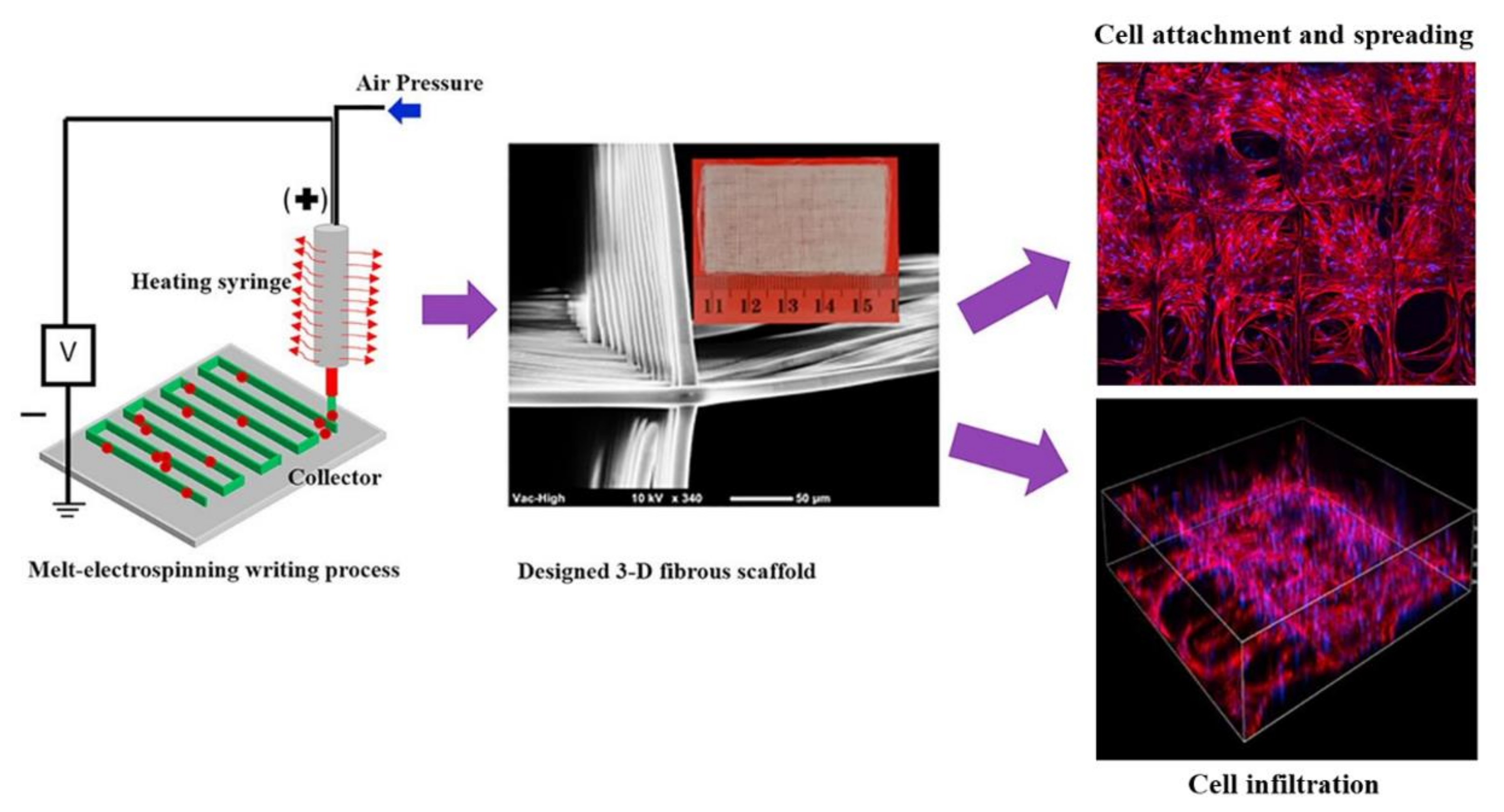
4.1. Tissue Engineering Applications of Electrospun Nanofibers
4.2. Other Applications of Electrospun Nanofibers
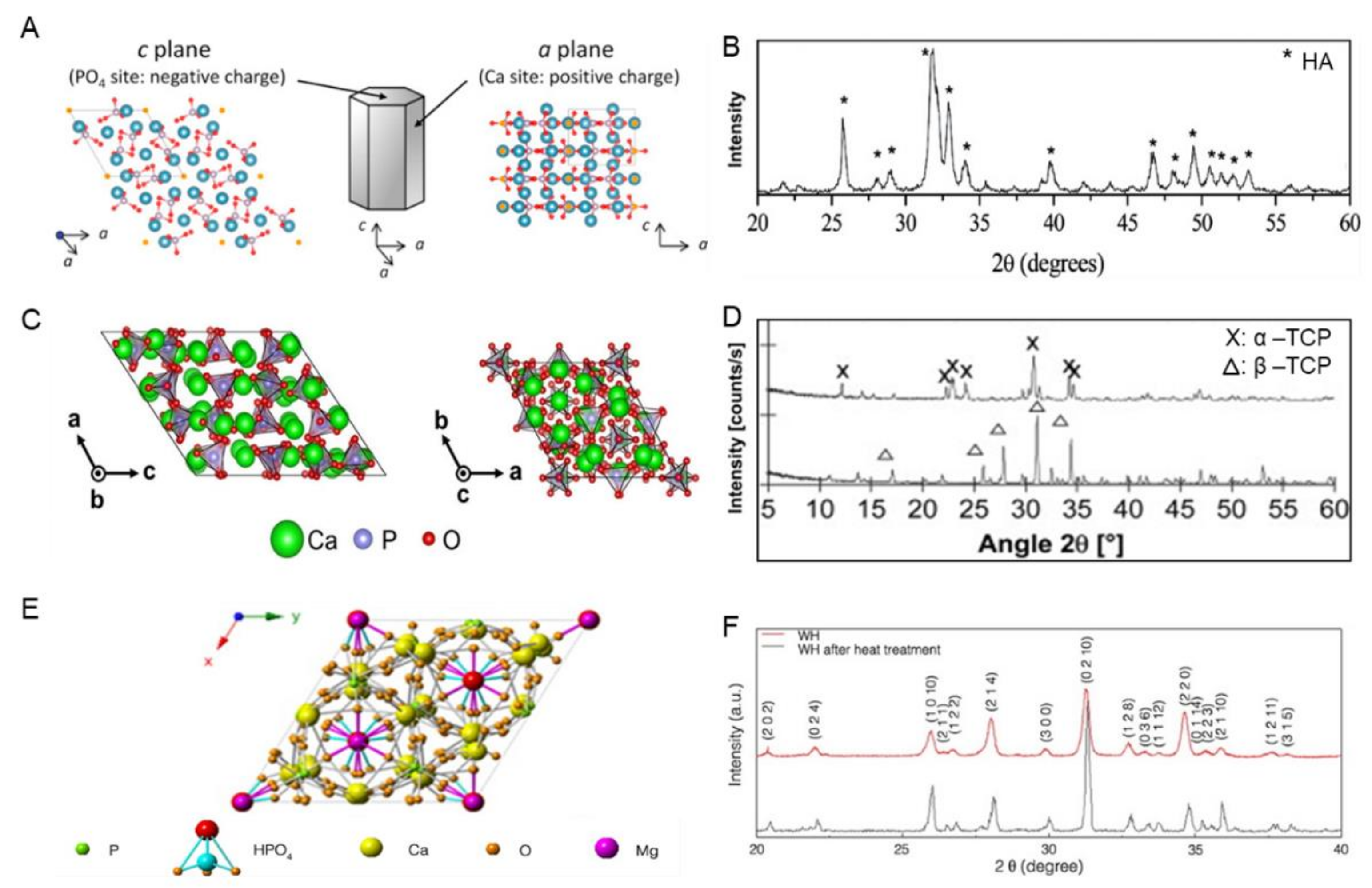
5. Encapsulation of Hydroxyapatite (HA) on Electrospun Nanofibers
5.1. Polycaprolactone/Hydroxyapatite (PCL/HA) Nanocomposited Electrospun Fibers
5.2. Polylactic or Poly(L-lactic-co-glycolic) Acid/Hydroxyapatite (PLA or PLGA/HA) Nanocomposited Electrospun Fibers
5.3. Polyhydroxyalkanoates/Hydroxyapatite (PHA/HA) Nanocomposited Electrospun Fibers
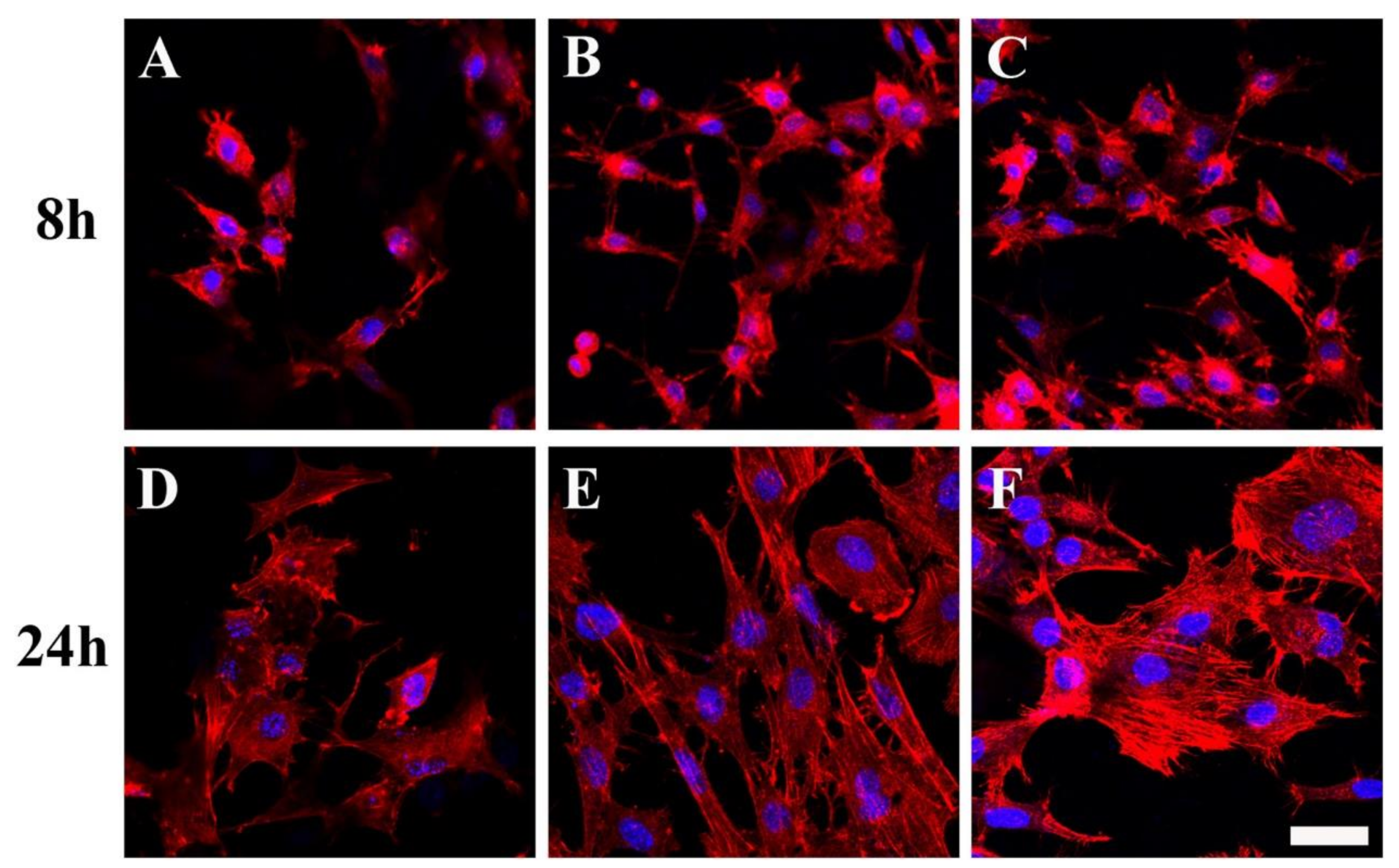
5.4. Chitosan/Hydroxyapatite (CS/HA) Nanocomposited Electrospun Fibers
5.5. Other Polymer/HA Nanocomposited Electrospun Fibers
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Sakamaki, I.; Nishida, E.; Kanemoto, Y.; Miyaji, H. In Vitro and in Vivo Analysis of Mineralized Collagen-Based Sponges Prepared by a Plasma- and Precursor-Assisted Biomimetic Process. ACS Appl. Mater. Interfaces 2017, 9, 22185–22194. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Mahanti, M.; Koga, K.; Shitomi, K.; Miyaji, H. Rapid and area-specific coating of fluoride-incorporated apatite layers by a laser-assisted biomimetic process for tooth surface functionalization. Acta Biomater. 2018, 79, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, B.; Wu, H.; Zheng, L.; Zhao, J. Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Mater. Sci. Eng. C 2017, 70, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Barbieri, D.; Luo, X.; Weng, J.; De Bruijn, J.D.; Yuan, H. Submicron-surface structured tricalcium phosphate ceramic enhances the bone regeneration in canine spine environment. J. Orthop. Res. 2016, 34, 1865–1873. [Google Scholar] [CrossRef]
- Chiba, S.; Anada, T.; Suzuki, K.; Saito, K.; Shiwaku, Y.; Miyatake, N.; Baba, K.; Imaizumi, H.; Hosaka, M.; Itoi, E.; et al. Effect of resorption rate and osteoconductivity of biodegradable calcium phosphate materials on the acquisition of natural bone strength in the repaired bone. J. Biomed. Mater. Res. Part A 2016, 104, 2833–2842. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Abbasi, N.; Gwiazda, M.; Hamlet, S.; Ivanovski, S. Novel polycaprolactone/hydroxyapatite nanocomposite fibrous scaffolds by direct melt-electrospinning writing. Eur. Polym. J. 2018, 105, 257–264. [Google Scholar] [CrossRef]
- Rogina, A.; Rico, P.; Ferrer, G.G.; Ivankovic, M.; Ivankovic, H. Effect of in situ formed hydroxyapatite on microstructure of freeze-gelled chitosan-based biocomposite scaffolds. Eur. Polym. J. 2015, 68, 278–287. [Google Scholar] [CrossRef]
- Jang, H.L.; Jin, K.; Lee, J.; Kim, Y.; Nahm, S.H.; Hong, K.S.; Nam, K.T. Revisiting Whitlockite, the Second Most Abundant Biomineral in Bone: Nanocrystal Synthesis in Physiologically Relevant Conditions and Biocompatibility Evaluation. ACS Nano 2014, 8, 634–641. [Google Scholar] [CrossRef]
- Groza, A.; Dreghici, D.B.; Ganciu, M. Calcium Phosphate Layers Deposited on Thermal Sensitive Polymer Substrates in Radio Frequency Magnetron Plasma Discharge. Coatings 2019, 9, 709. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Okada, M.; Matsumoto, T. Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn. Dent. Sci. Rev. 2015, 51, 85–95. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Hong, S.I.; Mangalaraj, D.; Ponpandian, N.; Chen, P.C. Template-Free Growth of Novel Hydroxyapatite Nanorings: Formation Mechanism and Their Enhanced Functional Properties. Cryst. Growth Des. 2012, 12, 3565–3574. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kubota, T.; Toyoura, K.; Nakamura, A. First-principles calculations of divalent substitution of Ca 2+ in tricalcium phosphates. Acta Biomater. 2015, 23, 329–337. [Google Scholar] [CrossRef]
- Galea, L.G.; Bohner, M.; Lemaître, J.; Kohler, T.; Müller, R. Bone substitute: Transforming β-tricalcium phosphate porous scaffolds into monetite. Biomaterials 2008, 29, 3400–3407. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Bat, E.; Zhang, Z.; Feijen, J.; Grijpma, D.W.; Poot, A.A. Biodegradable elastomers for biomedical applications and regenerative medicine. Regen. Med. 2014, 9, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Goreninskii, S.I.; Bogomolova, N.N.; Malchikhina, A.I.; Golovkin, A.; Bolbasov, E.N.; Safronova, T.; Putlyaev, V.I.; Tverdokhlebov, S. Biological Effect of the Surface Modification of the Fibrous Poly(L-lactic acid) Scaffolds by Radio Frequency Magnetron Sputtering of Different Calcium-Phosphate Targets. BioNanoScience 2017, 7, 50–57. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Boys, C.V. On the Production, Properties, and some suggested Uses of the Finest Threads. Proc. Phys. Soc. Lond. 1887, 9, 8–19. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, D.; Jing, D.; Liu, H.; Liu, L.; Jia, Y.; Gao, M.; Guo, L.; Huo, Z. Enhanced photodynamic therapy of mixed phase TiO2(B)/anatase nanofibers for killing of HeLa cells. Nano Res. 2014, 7, 1659–1669. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Chai, Y.; Wang, Y. Synthesis and characterization of Zn 2+ and SeO 3 2− co-substituted nano-hydroxyapatite. Adv. Powder Technol. 2016, 27, 1857–1861. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Feng, Y.; Liu, J.; Yu, X. Synthesis and characterization of ultralong nanofibrillar and hydroxyapatite powder. Adv. Powder Technol. 2015, 26, 428–433. [Google Scholar] [CrossRef]
- Xu, Y.; An, L.; Chen, L.; Xu, H.; Zeng, D.; Wang, G. Controlled hydrothermal synthesis of strontium-substituted hydroxyapatite nanorods and their application as a drug carrier for proteins. Adv. Powder Technol. 2018, 29, 1042–1048. [Google Scholar] [CrossRef]
- Strnad, G.; Petrovan, C.; Russu, O.; Jakab-Farkas, L. TiO2nanostructured surfaces for biomedical applications developed by electrochemical anodization. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012051. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Rajzer, I. Fabrication of bioactive polycaprolactone/hydroxyapatite scaffolds with final bilayer nano-/micro-fibrous structures for tissue engineering application. J. Mater. Sci. 2014, 49, 5799–5807. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Wang, X. Biomedical applications of nano-titania in theranostics and photodynamic therapy. Biomater. Sci. 2016, 4, 40–54. [Google Scholar] [CrossRef]
- Bancelin, S.; Aimé, C.; Gusachenko, I.; Kowalczuk, L.; Latour, G.; Coradin, T.; Schanne-Klein, M.-C. Determination of collagen fibril size via absolute measurements of second-harmonic generation signals. Nat. Commun. 2014, 5, 4920. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2012, 21, 1–16. [Google Scholar] [CrossRef]
- Dias, J.; Granja, P.; Bártolo, P. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Bonadies, I.; Maglione, L.; Ambrogi, V.; Paccez, J.D.; Zerbini, L.F.; E Silva, L.F.R.; Picanço, N.S.; Tadei, W.P.; Grafova, I.; Grafov, A.; et al. Electrospun core/shell nanofibers as designed devices for efficient Artemisinin delivery. Eur. Polym. J. 2017, 89, 211–220. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R.; Kumar, P.S.; Sridhar, R.; Sundaramurthy, J.; Venugopal, J.R.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun composite nanofibers and their multifaceted applications. J. Mater. Chem. 2012, 22, 12953–12971. [Google Scholar] [CrossRef]
- Chinnappan, A.; Baskar, C.; Baskar, S.; Ratheesh, G.; Ramakrishna, S. An overview of electrospun nanofibers and their application in energy storage, sensors and wearable/flexible electronics. J. Mater. Chem. C 2017, 5, 12657–12673. [Google Scholar] [CrossRef]
- Han, W.; Wang, Y.; Su, J.; Xin, X.; Guo, Y.; Long, Y.-Z.; Ramakrishna, S. Fabrication of nanofibrous sensors by electrospinning. Sci. China Ser. E: Technol. Sci. 2019, 62, 886–894. [Google Scholar] [CrossRef]
- Aliheidari, N.; Aliahmad, N.; Agarwal, M.; Dalir, H. Electrospun Nanofibers for Label-Free Sensor Applications. Sensors 2019, 19, 3587. [Google Scholar] [CrossRef]
- Li, Y.; Mohammed, A.; Tang, L.; Li, D.; Wang, L. Electrospun Nanofibers for Sensors. In Electrospinning: Nanofabrication and Applications; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 571–601. [Google Scholar]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of Nanofibers for Energy Applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Liu, M.; Chen, Y.; Hu, X.; Ye, Z.; Dong, D. Study on Nanofibrous Catalysts Prepared by Electrospinning for Methane Partial Oxidation. Catalysts 2019, 9, 479. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.-H.; Lee, J.; Chung, I.-M.; Karvembu, R.; Kim, I.S. Noble metal/functionalized cellulose nanofiber composites for catalytic applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, N.; Maeda, T. Statins and bone metabolism. Oral Dis. 2006, 12, 85–101. [Google Scholar] [CrossRef]
- Papapoulos, S.E. Bisphosphonates: How do they work? Best Pr. Res. Clin. Endocrinol. Metab. 2008, 22, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhu, G.; Wang, L.; An, G.; Shi, X.; Wang, Y. Assembling of electrospun meshes into three-dimensional porous scaffolds for bone repair. Biofabrication 2017, 9, 015018. [Google Scholar] [CrossRef] [PubMed]
- Stastna, E.; Částková, K.; Ráheľ, J. Influence of Hydroxyapatite Nanoparticles and Surface Plasma Treatment on Bioactivity of Polycaprolactone Nanofibers. Polymers 2020, 12, 1877. [Google Scholar] [CrossRef]
- Hassan, M.I.; Sun, T.; Sultana, N. Fabrication of Nanohydroxyapatite/Poly(caprolactone) Composite Microfibers Using Electrospinning Technique for Tissue Engineering Applications. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Jabur, A.R.; Al-Hassani, E.S.; Al-Shammari, A.M.; Najim, M.A.; Hassan, A.A.; Ahmed, A.A. Evaluation of Stem Cells’ Growth on Electrospun Polycaprolactone (PCL) Scaffolds Used for Soft Tissue Applications. Energy Procedia 2017, 119, 61–71. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Dai, N.-T.; Williamson, M.; Khammo, N.; Adams, E.; Coombes, A. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, B.; Zhang, W.; Yan, C.; Yao, Q.; Shao, C.; Yu, F.; Li, F.; Fu, Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17, 3717–3726. [Google Scholar] [CrossRef]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; De Cassan, D.; Menzel, H.; Hoheisel, A.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Wellmann, M.; et al. In vivo analysis of vascularization and biocompatibility of electrospun polycaprolactone fibre mats in the rat femur chamber. J. Tissue Eng. Regen. Med. 2019, 13, 1190–1202. [Google Scholar] [CrossRef]
- Gredes, T.; Schönitz, S.; Gedrange, T.; Stepien, L.; Kozak, K.; Kunert-Keil, C. In vivo analysis of covering materials composed of biodegradable polymers enriched with flax fibers. Biomater. Res. 2017, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, C.; Jin, X.; Ke, Q. An electrospun poly(ε-caprolactone) nanocomposite fibrous mat with a high content of hydroxyapatite to promote cell infiltration. RSC Adv. 2018, 8, 25228–25235. [Google Scholar] [CrossRef]
- Deliormanl, A.M.; Konyalı, R. Preparation and characterization of electrospun poly (ε-caprolactone)-bioactive glass/HA biocomposite nanofibers. J. Aust. Ceram. Soc. 2019, 55, 247–256. [Google Scholar] [CrossRef]
- Nazarnezhad, S.; Baino, F.; Shin, H.W.; Webster, T.J.; Kargozar, S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials 2020, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced osteogenesis and anewgiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Sultana, N.; Hamdan, S. Bioactivity Assessment of Poly(ɛ-caprolactone)/Hydroxyapatite Electrospun Fibers for Bone Tissue Engineering Application. J. Nanomater. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Keivani, F.; Shokrollahi, P.; Zandi, M.; Irani, S.; Shokrollahi, P.; Khorasani, S. Engineered electrospun poly(caprolactone)/polycaprolactone-g-hydroxyapatite nano-fibrous scaffold promotes human fibroblasts adhesion and proliferation. Mater. Sci. Eng. C 2016, 68, 78–88. [Google Scholar] [CrossRef]
- Hanas, T.; Kumar, T.S.; Perumal, G.; Doble, M.; Ramakrishna, S. Electrospun PCL/HA coated friction stir processed AZ31/HA composites for degradable implant applications. J. Mater. Process. Technol. 2018, 252, 398–406. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef]
- Johari, N.; Fathi, M.; Fereshteh, Z.; Kargozar, S.; Samadikuchaksaraei, A. The electrospun poly(ε-caprolactone)/fluoridated hydroxyapatite nanocomposite for bone tissue engineering. Polym. Adv. Technol. 2019, 31, 1019–1026. [Google Scholar] [CrossRef]
- Rivero, G.; Aldana, A.A.; Lopez, Y.R.F.; Liverani, L.; Boccacini, A.R.; Bustos, D.M.; Abraham, G.A. 14-3-3ε protein-immobilized PCL-HA electrospun scaffolds with enhanced osteogenicity. J. Mater. Sci. Mater. Med. 2019, 30, 99. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Health Mater. 2018, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; He, W.; Huang, Q.; Zhang, R.; Feng, Q. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2018, 106, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lawrence, J.G.; Bhaduri, S.B. Fabrication aspects of PLA-CaP/PLGA-CaP composites for orthopedic applications: A review. Acta Biomater. 2012, 8, 1999–2016. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z. Fabrication and Characterization of Electrospun PLGA/MWNTs/ Hydroxyapatite Biocomposite Scaffolds for Bone Tissue Engineering. J. Bioact. Compat. Polym. 2010, 25, 241–259. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Zhu, J.; Niu, Z.; Wang, Y.; Li, J.; Yang, X.-Y.; Bai, Y. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS ONE 2017, 12, e0188352. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, W.; Azhar, U.; Tabassum, S.; Jamal, M.; Siddiqi, S.A.; Tariq, M.; Muhammad, N.; Asif, A.; Chaudhry, A.A.; Sharif, F. Doping and Incorporation of Hydroxyapatite in Development of PU-PLA Electrospun Osteogenic Membranes. J. Polym. Environ. 2020, 28, 2988–3002. [Google Scholar] [CrossRef]
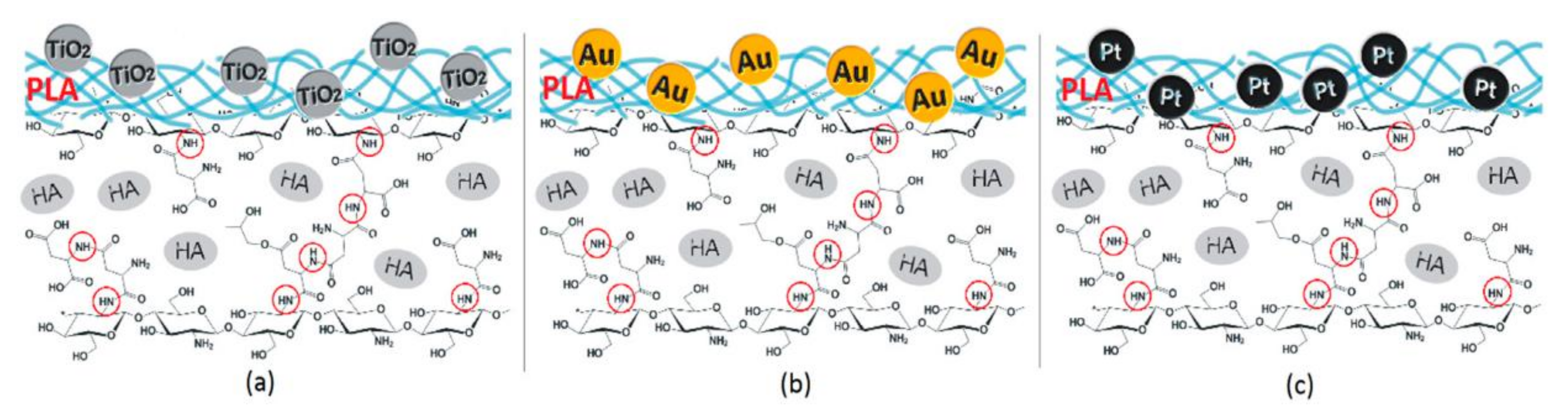
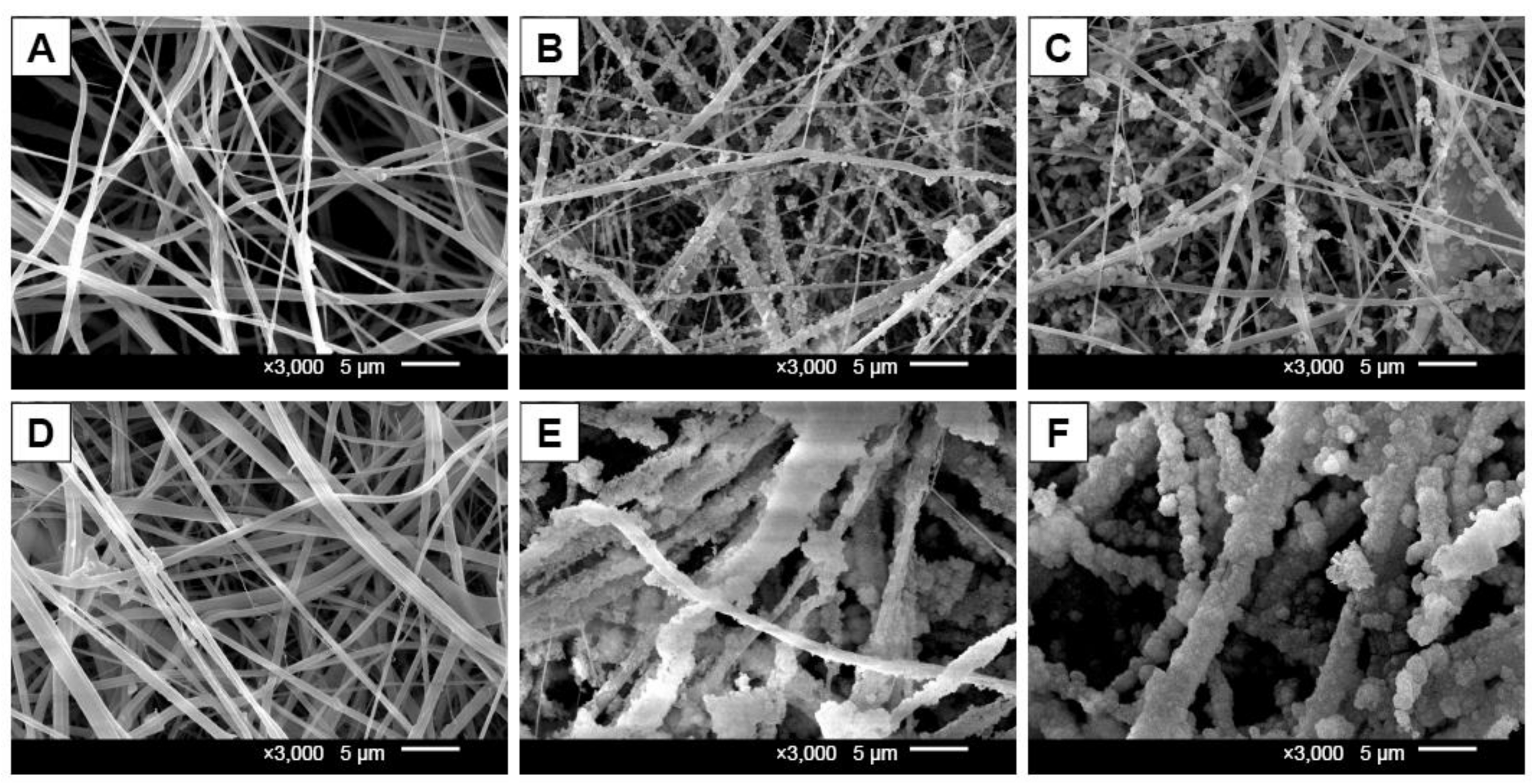
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matýsek, D. 3D Hierarchical, Nanostructured Chitosan/PLA/HA Scaffolds Doped with TiO2/Au/Pt NPs with Tunable Properties for Guided Bone Tissue Engineering. Polymers 2020, 12, 792. [Google Scholar] [CrossRef]
- Magiera, A.; Markowski, J.; Menaszek, E.; Pilch, J.; Blazewicz, S. PLA-Based Hybrid and Composite Electrospun Fibrous Scaffolds as Potential Materials for Tissue Engineering. J. Nanomater. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Kareem, M.M.; Tanner, K.E. Optimising micro-hydroxyapatite reinforced poly(lactide acid) electrospun scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.-K. Micro/Nano Multilayered Scaffolds of PLGA and Collagen by Alternately Electrospinning for Bone Tissue Engineering. Nanoscale Res. Lett. 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-W.; Zi, Y.-P.; Xu, W.; Zhou, R.; Cai, Z.-Y.; Zheng, W.-J.; Chen, F.; Qian, Q.-R. Electrospinning of calcium phosphate-poly (d,l-lactic acid) nanofibers for sustained release of water-soluble drug and fast mineralization. Int. J. Nanomed. 2016, 11, 5087–5097. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef]
- Williams, S.F.; Martin, D.P. Applications of Polyhydroxyalkanoates (PHA) in Medicine and Pharmacy. Biopolym. Online 2002. [CrossRef]
- Hazer, D.B.; Kılıçay, E.; Hazer, B. Poly(3-hydroxyalkanoate)s: Diversification and biomedical applications. Mater. Sci. Eng. C 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Sadat-Shojai, M. Electrospun Polyhydroxybutyrate/Hydroxyapatite Nanohybrids: Microstructure and Bone Cell Response. J. Mater. Sci. Technol. 2016, 32, 1013–1020. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Chen, X.; Gandhi, M.R.; Ko, F.K.; Lelkes, P.I. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 2006, 79, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, M. In vitro evaluation of hydroxyapatite reinforced polyhydroxybutyrate composite. Mater. Sci. Eng. C 2002, 20, 101–109. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.K.; Viezzer, M.M.D.C.F.C.; Berutti, A.K.A.F.A.; Bergmann, C.P. Effect of Electrospun Phb and Hap-Phb Composite Scaffolds Characteristics on Mesenchymal Stem Cell Growth Viability. MOJ Appl. Bionics Biomech. 2017, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Keikhaei, S.; Mohammadalizadeh, Z.; Karbasi, S.; Salimi, A. Evaluation of the effects of β-tricalcium phosphate on physical, mechanical and biological properties of Poly (3-hydroxybutyrate)/chitosan electrospun scaffold for cartilage tissue engineering applications. Mater. Technol. 2019, 34, 615–625. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Boonyagul, S. Physical properties and morphology of electrospun composite fiber mats of polyhydroxyalkanoate containing nanoclay and tricalcium phosphate additives. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 012013. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Xu, R.; Wu, G.; Zhao, X.; Yu, D.; Cui, F.; Chen, D.; Tian, W. Composition-graded Films of Fluoroapatite/PHB Fabricated via Electrospinning for Tissue Engineering. J. Bioact. Compat. Polym. 2007, 22, 379–393. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.; Mamba, B. Fundamentals of chitosan for biomedical applications. Chitosan Based Biomater. 2017, 1, 3–30. [Google Scholar] [CrossRef]
- Hu, L.; Meng, X.; Xing, R.; Liu, S.; Chen, R.; Qin, Y.; Yu, H.; Li, P. Design, synthesis and antimicrobial activity of 6-N-substituted chitosan derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 4548–4551. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Alves, C.M.; Mano, J.F.; Reis, R.L. Production and Characterization of Chitosan Fibers and 3-D Fiber Mesh Scaffolds for Tissue Engineering Applications. Macromol. Biosci. 2004, 4, 811–819. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.-J.; Lee, J.-Y.; Park, Y.-J.; Lee, Y.-M.; -Ku, Y.; Rhyu, I.-C.; Lee, S.-J.; Han, S.-B.; Chung, C.-P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Topsakal, A.; Uzun, M.; Ugar, G.; Ozcan, A.; Altun, E.; Oktar, F.N.; Ikram, F.; Ozkan, O.; Sasmazel, H.T.; Gunduz, O. Development of Amoxicillin-Loaded Electrospun Polyurethane/Chitosan/ ββ -Tricalcium Phosphate Scaffold for Bone Tissue Regeneration. IEEE Trans. NanoBiosci. 2018, 17, 321–328. [Google Scholar] [CrossRef]
- Liverani, L.; Abbruzzese, F.; Mozetic, P.; Basoli, F.; Rainer, A.; Trombetta, M. Electrospinning of hydroxyapatite-chitosan nanofibers for tissue engineering applications. Asia-Pacific J. Chem. Eng. 2014, 9, 407–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
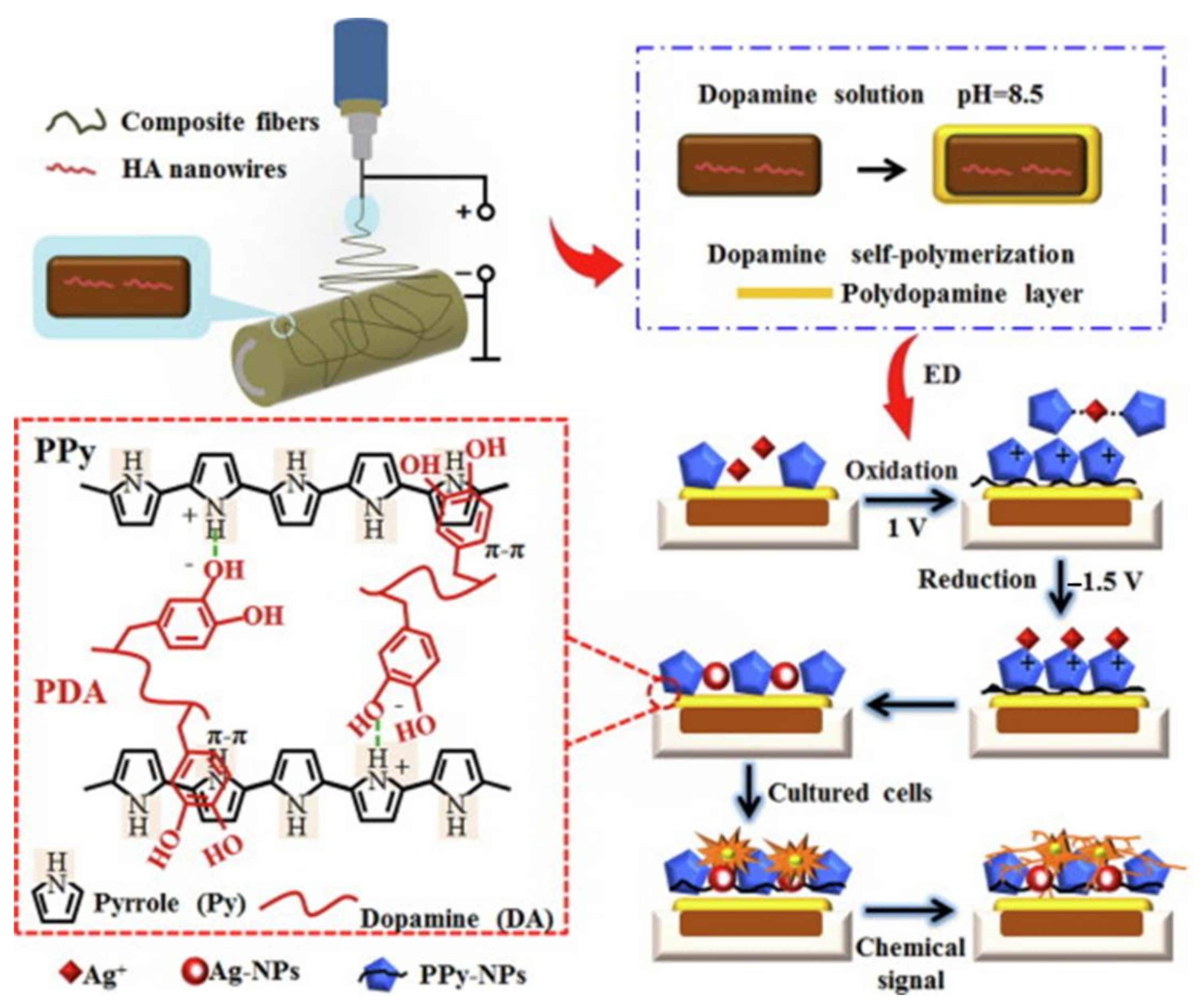
- Jin, S.; Li, J.; Wang, J.; Jiang, J.; Zuo, Y.; Li, Y.; Yang, F. Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int. J. Nanomed. 2018, 13, 4591–4605. [Google Scholar] [CrossRef] [PubMed]
- Hezma, A.M.; El-Rafei, A.M.; El-Bahy, G.S.; Abdelrazzak, A.B. Electrospun Hydroxyapatite Containing Polyvinyl Alcohol Nanofibers Doped with Nanogold for Bone Tissue Engineering. Interceram—Int. Ceram. Rev. 2017, 66, 96–100. [Google Scholar] [CrossRef]
- Nahavandizadeh, N.; Rezaei, M. Preparation of Shape Memory Polyurethane/Hydroxyapatite Nanocomposite Scaffolds by Electrospinning Method and Investigation of Their Microstructure and Physical-Mechanical Properties. Polym. Technol. Mater. 2020, 59, 1562–1573. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gualandi, C.; Fiorani, A.; Bracci, B.; Focarete, M.L.; Bigi, A. Fast Coprecipitation of Calcium Phosphate Nanoparticles inside Gelatin Nanofibers by Tricoaxial Electrospinning. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-J. Hydroxyapatite nanofibers fabricated through electrospinning and sol–gel process. Ceram. Int. 2014, 40, 3361–3369. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Azami, M.; Majidi, R.F. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Kwon, G.-W.; Gupta, K.C.; Jung, K.-H.; Kang, I.-K. Lamination of microfibrous PLGA fabric by electrospinning a layer of collagen-hydroxyapatite composite nanofibers for bone tissue engineering. Biomater. Res. 2017, 21, 11. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Chen, T.; Zhang, N.; Wei, Q.; Tian, J.; Wang, Y.; Ma, C.; Lu, Y. Hydroxyapatite/silver electrospun fibers for anti-infection and osteoinduction. J. Adv. Res. 2020, 21, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.X.; Zhao, M.L.; Yin, M.; Huang, D.; Wang, N.; Wei, Y.; Hu, Y.; Lian, X.; Chen, W. Mineralized Polyamide66/Calcium Chloride Nanofibers for Bone Tissue Engineering. Tissue Eng. Part C Methods 2020, 26, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Raic, A.; Friedrich, F.; Kratzer, D.; Bieback, K.; Lahann, J.; Lee-Thedieck, C. Potential of electrospun cationic BSA fibers to guide osteogenic MSC differentiation via surface charge and fibrous topography. Sci. Rep. 2019, 9, 20003–20015. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nathanael, A.J.; Oh, T.H. Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications. Crystals 2021, 11, 199. https://doi.org/10.3390/cryst11020199
Nathanael AJ, Oh TH. Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications. Crystals. 2021; 11(2):199. https://doi.org/10.3390/cryst11020199
Chicago/Turabian StyleNathanael, Arputharaj Joseph, and Tae Hwan Oh. 2021. "Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications" Crystals 11, no. 2: 199. https://doi.org/10.3390/cryst11020199
APA StyleNathanael, A. J., & Oh, T. H. (2021). Encapsulation of Calcium Phosphates on Electrospun Nanofibers for Tissue Engineering Applications. Crystals, 11(2), 199. https://doi.org/10.3390/cryst11020199





