One-Pot Microwave-Assisted Synthesis of Water-Soluble Pyran-2,4,5-triol Glucose Amine Schiff Base Derivative: XRD/HSA Interactions, Crystal Structure, Spectral, Thermal and a DFT/TD-DFT
Abstract
:1. Introduction
2. Experimental
2.1. Measurements
2.2. Computational
2.3. Crystal Data
2.4. GASB-1 Synthesis
3. Results and Discussion
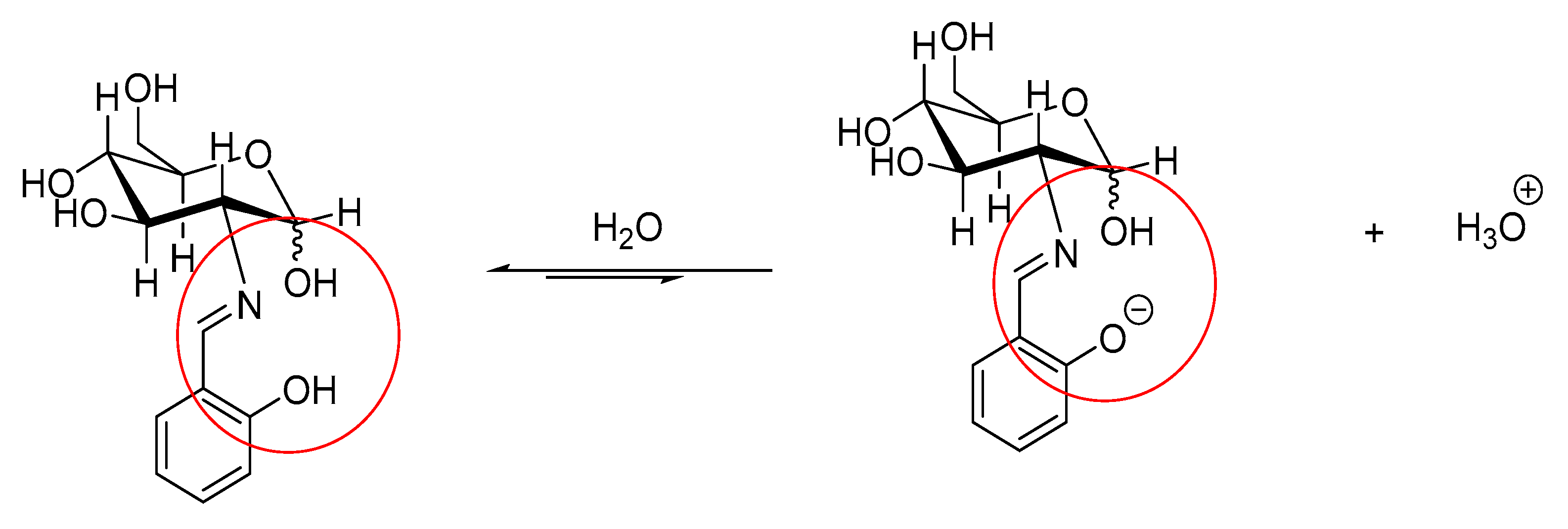
3.1. Microwave Synthesis
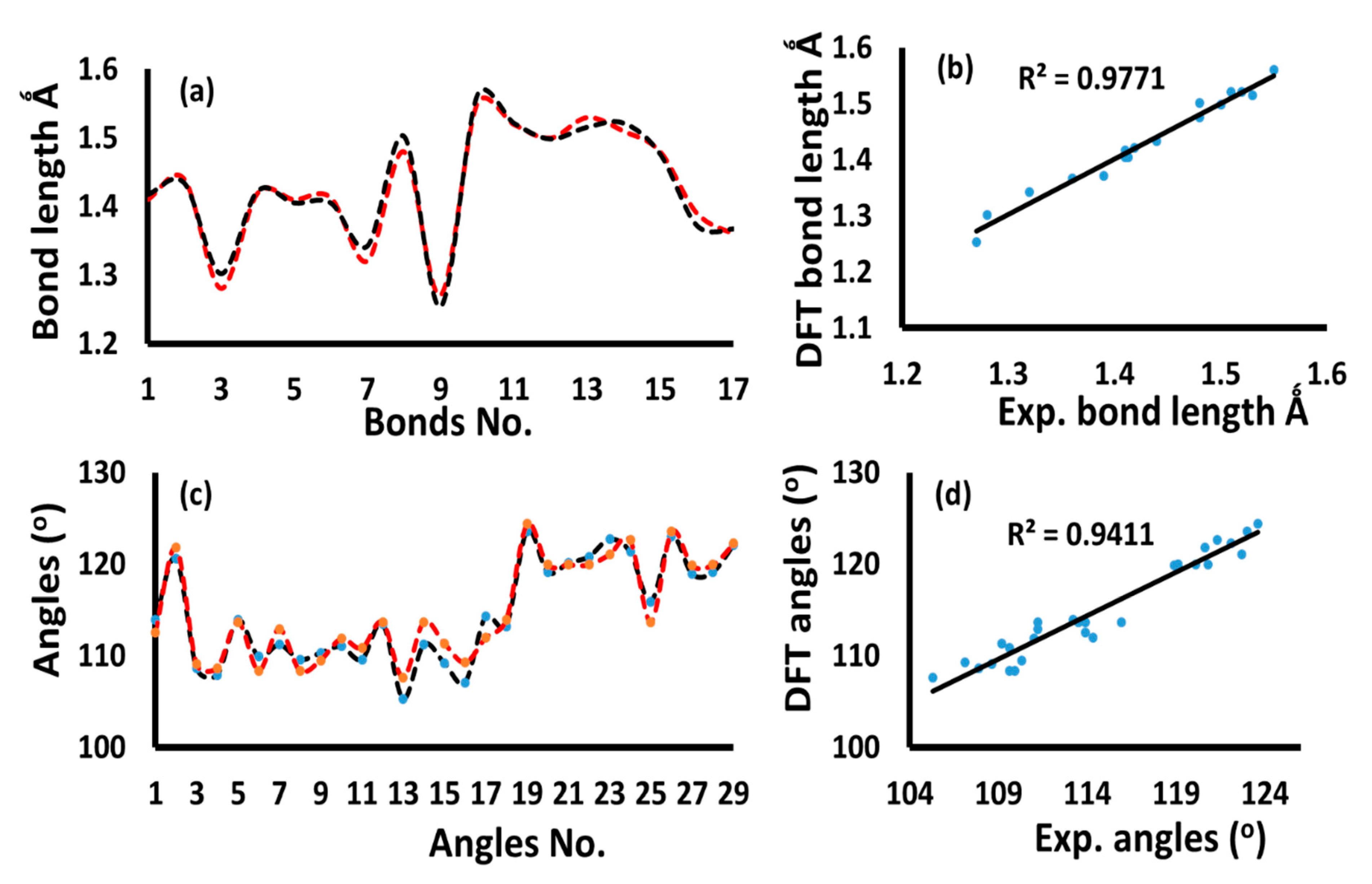
3.2. XRD and DFT
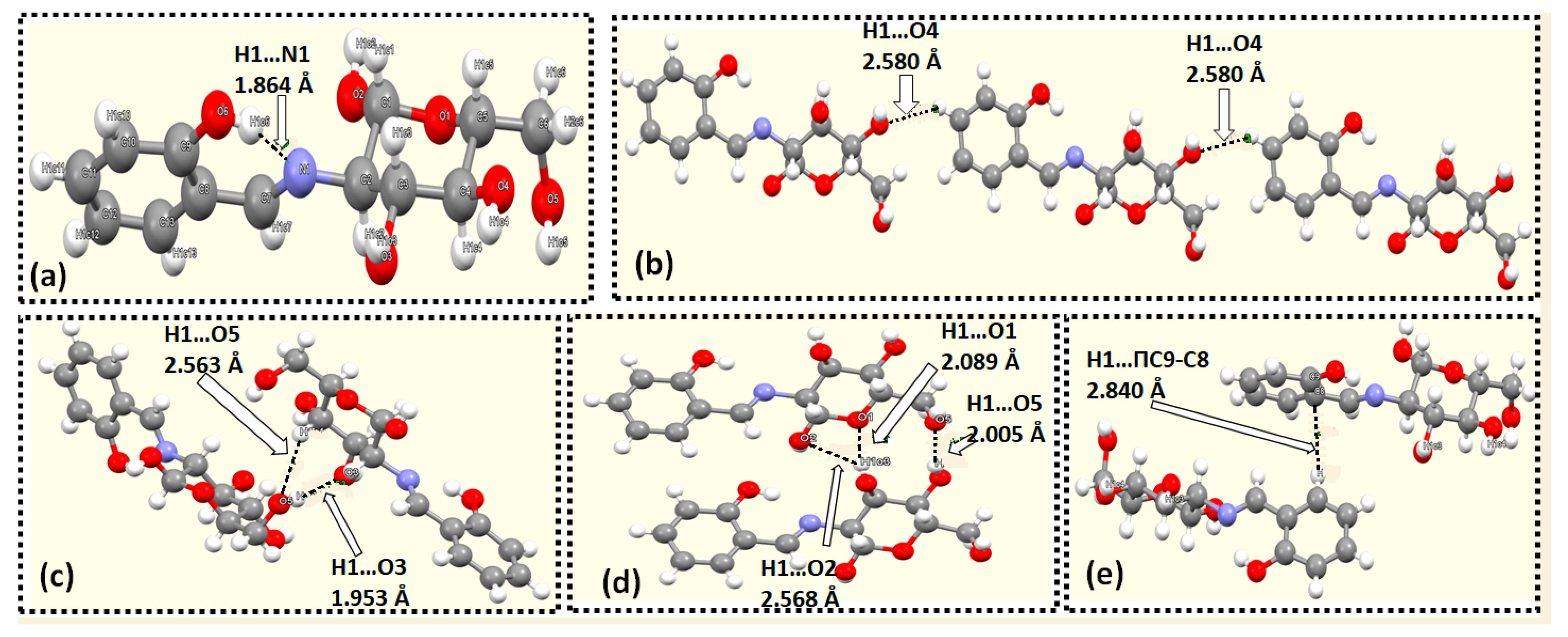
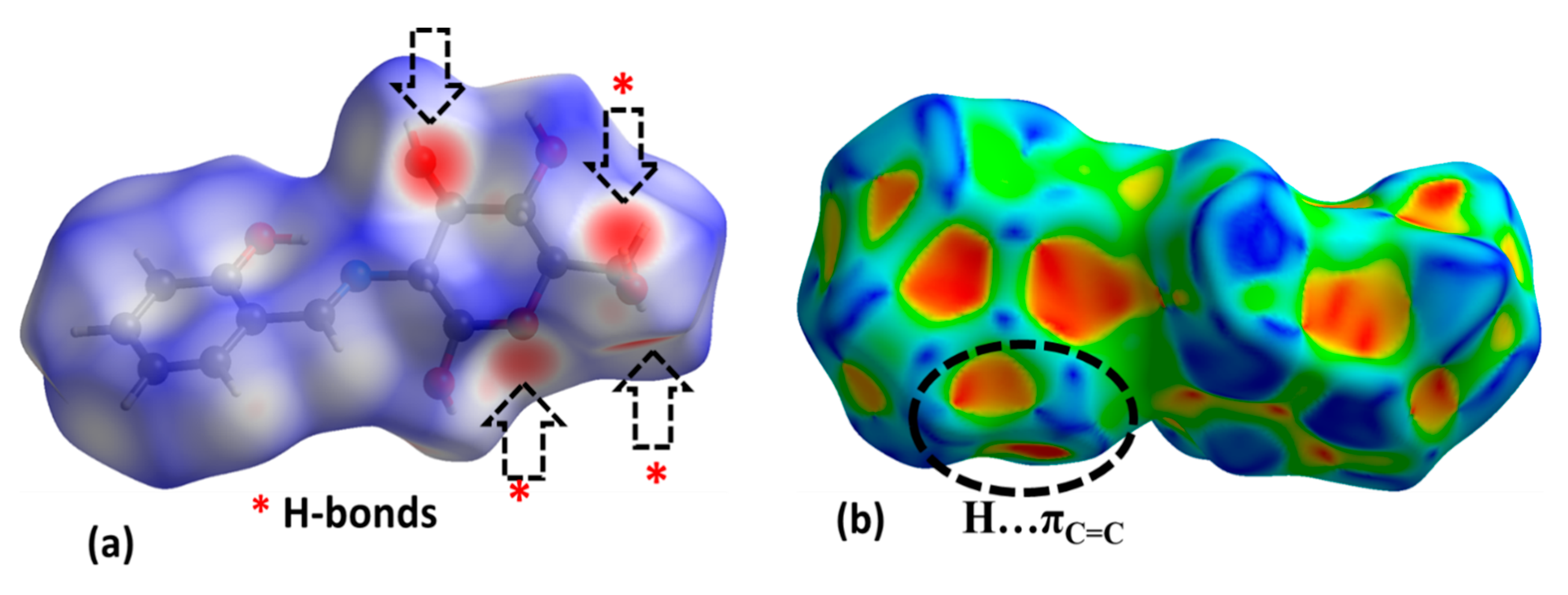
3.3. XRD Packing and HSA
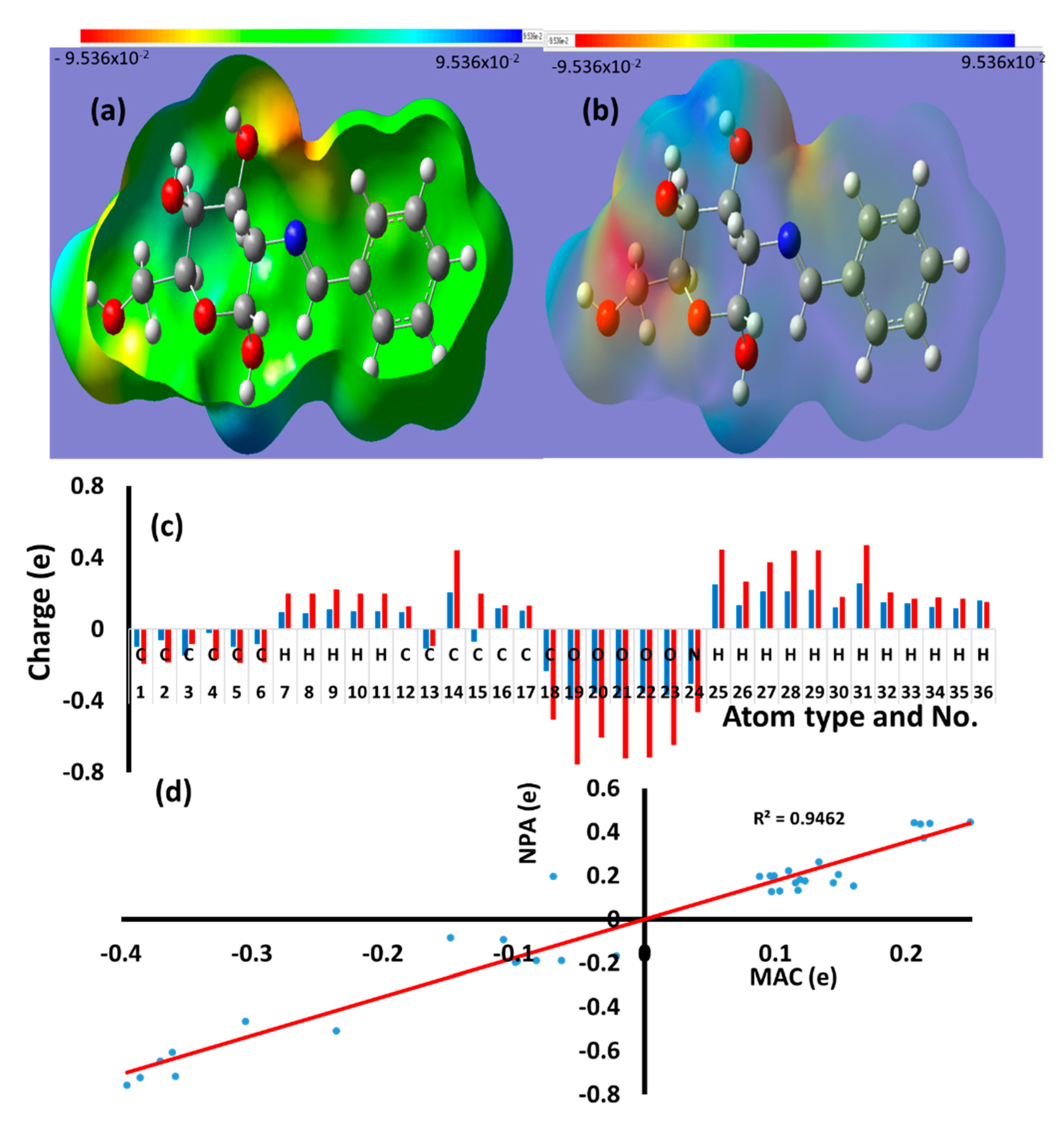
3.4. Molecular Electrostatic Potential (MEP), Mulliken Atomic Charge (MAC), and Natural Population Analysi (NPA)
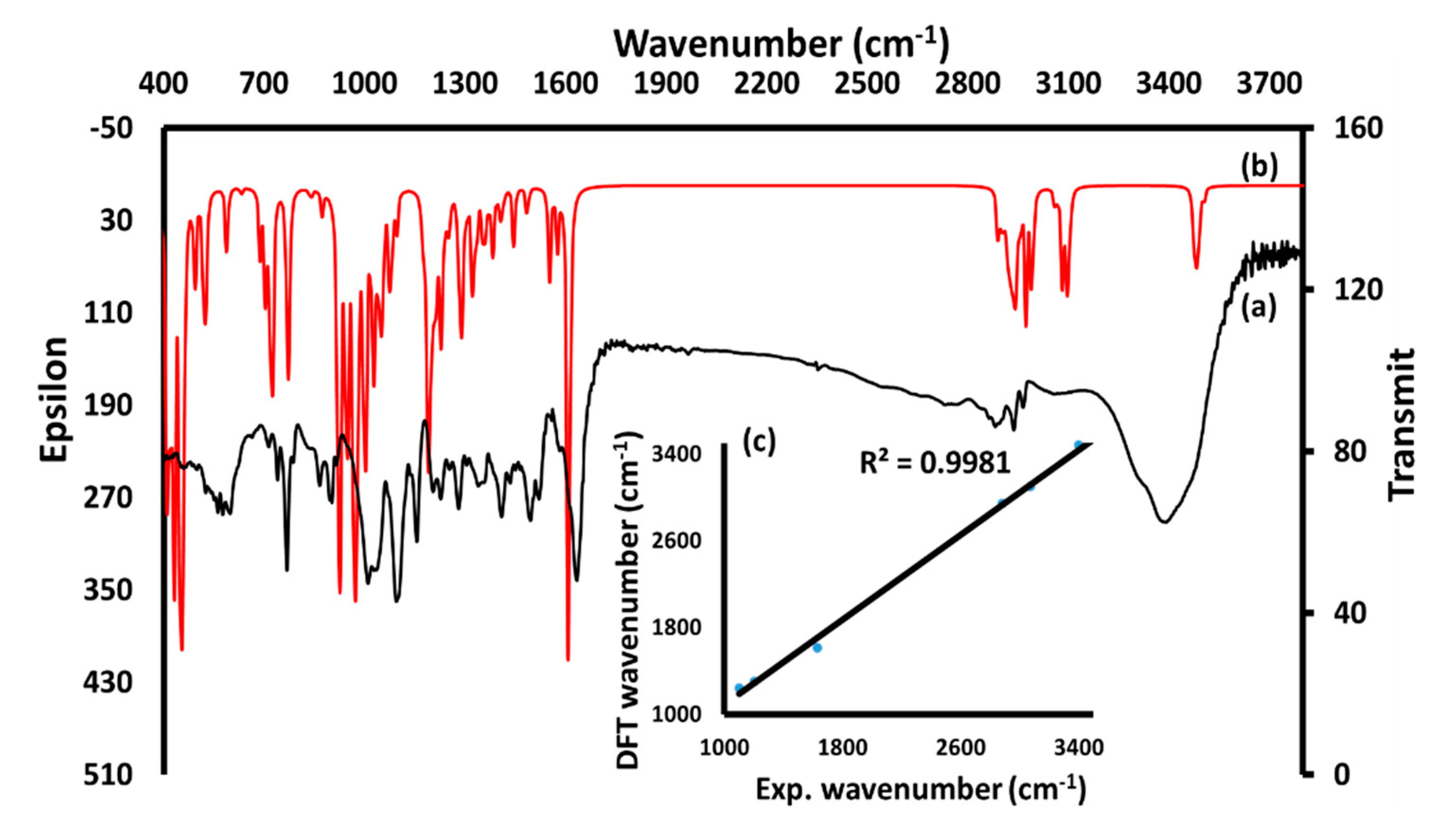
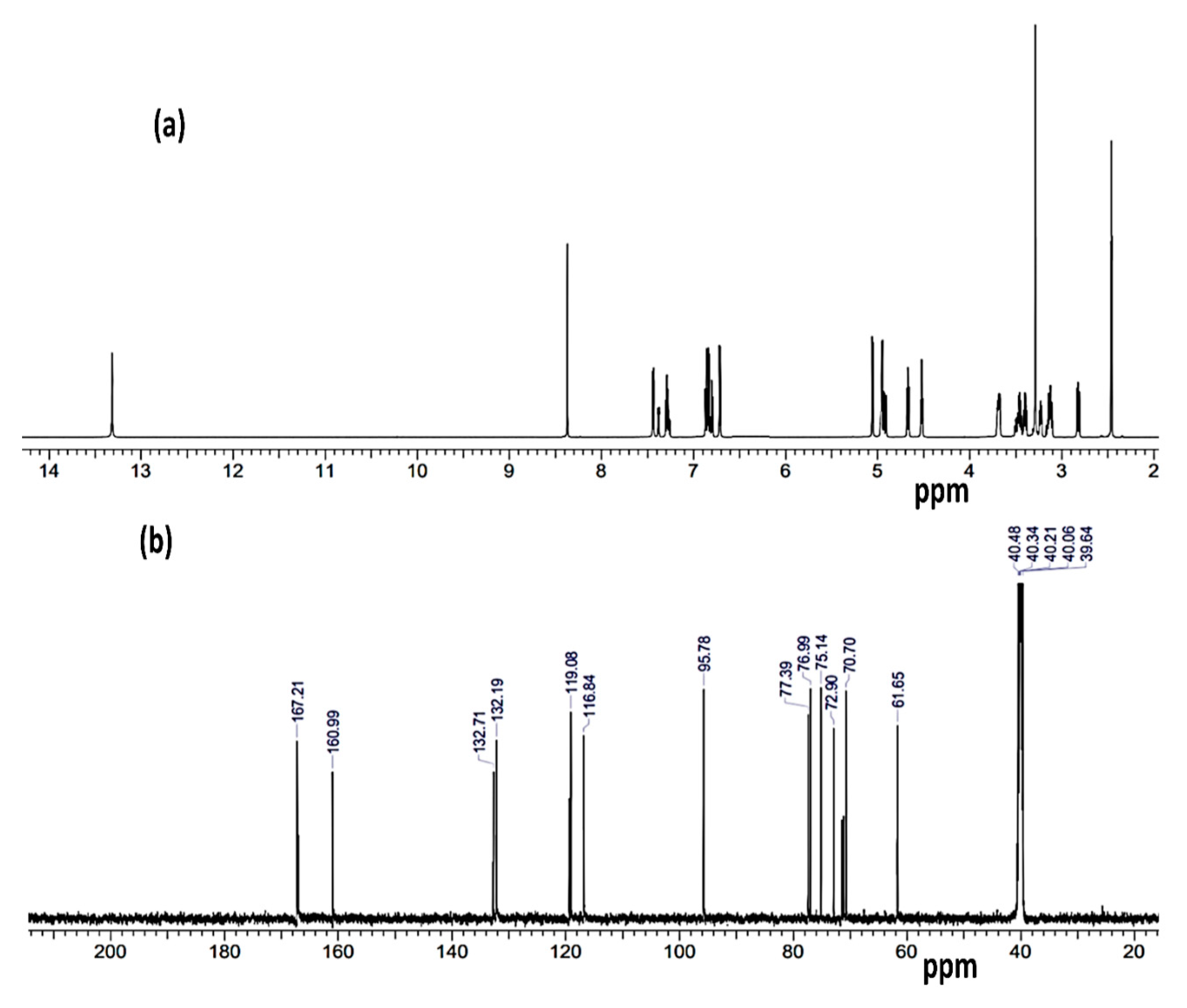
3.5. IR, B3LYP, and NMR Investigation
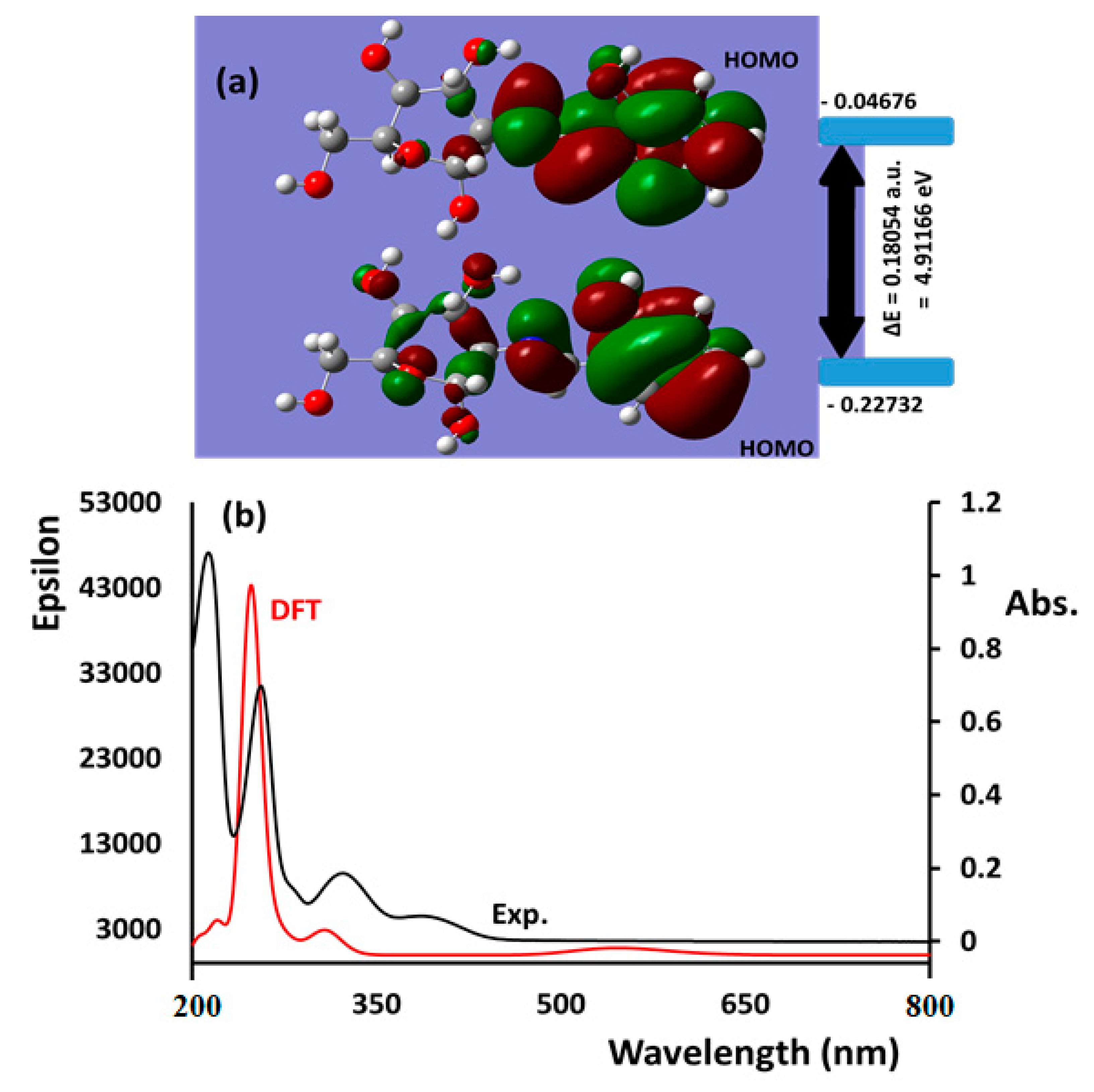
3.6. HOMO/LUMO and Absorption/TD-DFT
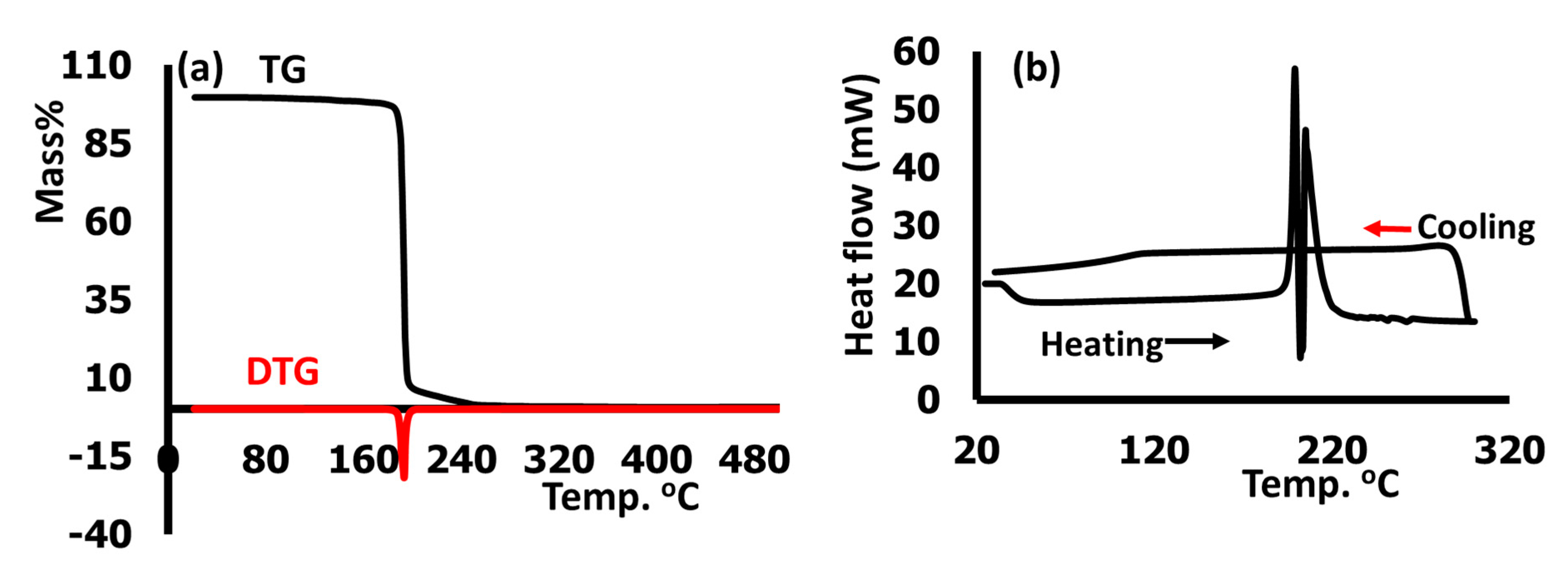
3.7. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weng, Q.; Yi, J.; Chen, X.; Luo, D.; Wang, Y.; Sun, W.; Kang, J.; Han, Z. Rising from the horizon: Three-dimensional functional architectures assembled with MXene nanosheets. ACS Omega 2020, 5, 24864. [Google Scholar] [CrossRef]
- Safoura, F. Novel Synthesis of Schiff bases Bearing Glucosamine Moiety. Res. J. Chem. Sci. 2014, 4, 25. [Google Scholar]
- Thanh, D.; van Quoc, N. Study on Synthesis of 2-(Substituted Benzylidene)amino-2-Deoxy-1,3,4,6-Tetra-O-Acetyl-β-D-Glucopyranoses from D-Glucosamine. Lett. Org. Chem. 2013, 10, 85. [Google Scholar] [CrossRef]
- Appelt, R.; Oliveira, S.; Santos. Synthesis and Antimicrobial Activity of Carbohydrate Based Schiff Bases: Importance of Sugar Moiety. V. Int. J. Carbohy. Chem. 2013, 2013, 320892. [Google Scholar] [CrossRef]
- Li, Y.; Liu, P. Synthesis of Novel D-glucosamine Schiff Bases. Chinese J. Synthetic Chem. 2006, 14, 523. [Google Scholar]
- Ghoneim, A.; El-Sherif, A. Review on Synthesis of N-glucosylamine Derivatives and Their Biological Activity. J. Chem. Pharm. Res. 2019, 11, 20. [Google Scholar]
- Garoufis, A.; Hadjikakou, K.; Hadjiliadis. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. N. Coordin. Chem. Rev. 2009, 253, 1384. [Google Scholar] [CrossRef]
- Rao, P.; Chinta, P.; Mitra, A. Selective Detection of Aromatic Alpha-Amino Acids and Derivatives Thereof. US20120091355A1, 19 October 2010. [Google Scholar]
- Arabahmadi, R. Amani, Azo Schiff bases as colorimetric and fluorescent sensors for recognition of F−, Cd2+ and Hg2+ ions. S. Supramol. Chem. 2014, 26, 321. [Google Scholar] [CrossRef]
- Mitra, A.; Ramanujam, B.; Rao, P. 1-(d-Glucopyranosyl-2-deoxy-2-iminomethyl)-2-hydroxynaphthalene as chemo-sensor for Fe3+ in aqueous HEPES buffer based on colour changes observable with the naked eye. Tetrahedron Lett. 2009, 50, 776. [Google Scholar] [CrossRef]
- Feng, J.; Le, F.; Luo, H.Q. Synthesis and Characterization of Cobalt(III) Zinc(II) and Copper(II) Complexes with N-Salicylaldehyde-D-Glucosamine and N,O-Vanillin-D-Glucosamine. Synth. React. Inorg. Met. Org. Chem. 1998, 28, 1105. [Google Scholar]
- Ye, Y.; Hu, J.; Feng, C.; Zeng, Y. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. Wuhan Univ. J. Nat. Sci. 1997, 2, 467. [Google Scholar]
- Gupta, C.; Sutar, K. Coordin. Coordination chemistry of some new Cu(II), Ni(II) and Co(II) macroacyclic (N2O4) Schiff base complexes: X-ray crystal structure of Cu(II) complex. Chem. Rev. 2008, 252, 1420. [Google Scholar]
- Bagherzadeh, M.; Amini, M.; Derakhshandeh, G.; Haghdoost. An efficient glucose-based ligand for Heck and Suzuki coupling reactions in aqueous media. A. J. Iran. Chem. Soc. 2014, 11, 441. [Google Scholar] [CrossRef]
- Vikneshvaran, S.; Velmathi, S. Impact of Halide-Substituted Chiral Schiff Bases on Corrosion Behaviour of Carbon Steel in Acidic Environment. J. Nanosci. Nanotechnol. 2019, 19, 4458. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, J.; Lillo, E.; Matsuhiro, B.; Noseda, D.; Villagrán, M. Ni(II) complexes with Schiff bases derived from amino sugars. Carbohydr. Res. 2003, 338, 1535. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Mazumder, M.A.J.; Quraishi, M.A.; Ansari, K.R.; Suleiman, R.K. Microwave-assisted synthesis of a new Piperonal-Chitosan Schiff base as a bio-inspired corrosion inhibitor for oil-well acidizing. Int. J. Biol. Macromol. 2020, 158, 231. [Google Scholar] [CrossRef]
- Canton-Díaz, A.M.; Munoz-Flores, B.M.; Moggio, I.; Arias, E.; Turlakov, G.; Angel-Mosqueda, C.D.; Ramirez-Montes, P.I.; Jimenez-Perez, V.M. Molecular structures, DFT studies and their photophysical properties in solution and solid state. Microwave-assisted multicomponent synthesis of organotin bearing Schiff bases. Mol. Struct. 2019, 1180, 642–650. [Google Scholar]
- Antony, R.; Arun, T.; Manickam, S.T. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 2.1; University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. A Library for Package-Independent Computational Chemistry Algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView Version; Semichem Inc.: Shawnee Mission, KS, USA, 2009; Volume 5. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L.; Kristallogr, Z. Crystallographic Computing System JANA2006: General features. Zeitschrift für Kristallographie-Crystalline Materials 2014, 229, 345–352. [Google Scholar]
- Bruker, APEX2, SAINT-Plus, XPREP and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004.
- Evans, G.G.; Boeyens, J.A. The linear space of ring conformations. Acta Cryst. 1989, 45, 581–590. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. General Definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXL-97; Universitat Geottingen: Geottingen, Germany, 1999. [Google Scholar]
- Tabti, S.; Djedouani, A.; Aggoun, D.; Warad, I.; Rahmouni, S.; Romdhane, S.; Fouzi, H. Crystal interactions, computational, spectral and thermal analysis of (E)-N’-(thiophen-2-ylmethylene)isonicotinohydrazide as O-N-S-tridentate schiff base ligand. J. Mol. Struct. 2018, 1155, 11. [Google Scholar] [CrossRef]
- Warad, I.; Abdoh, M.; al Ali, A.; Shivalingegowda, N.; Kumara, K.; Zarrouk, A.; Lokanath, N.K. Synthesis, spectra and X-ray crystallography of dipyridin-2-ylmethanone oxime and its CuX2(oxime)2 complexes: Thermal, Hirshfeld surface and DFT analysis. J. Mol. Struct. 2018, 1154, 619–625. [Google Scholar] [CrossRef]
- Warad, I.; Awwadi, F.F.; Al-Ghani, B.A.; Sawafta, A.; Shivalingegowda, N.; Lokanath, N.K.; Mubarak, M.S.; Hadda, T.B.; Zarrouk, A.; Al-Rimawi, F.; et al. Barghouthi, Ultrasound-assisted synthesis of two novel [CuBr(diamine)2·H2O]Br complexes: Solvatochromism, crystal structure, physicochemical, Hirshfeld surface thermal, DNA/binding, antitumor and antibacterial activities. S.A. Ultrason. Sonochem. 2018, 48, 1. [Google Scholar] [CrossRef] [PubMed]
- Aouad, M.R.; Messali, M.; Rezki, N.; Al-Zaqri, N.; Warad, I. Single proton intramigration in novel 4-phenyl-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)-1H-1,2,4-triazole-5(4H)-thione: XRD-crystal interactions, physicochemical, thermal, Hirshfeld surface, DFT realization of thiol/thione tautomerism. J. Mol. Liq. 2018, 264, 621. [Google Scholar] [CrossRef]
- Aouad, M.R.; Messali, M.; Rezki, N.; Said, M.A.; Lentz, D.; Zubaydi, L.; Warad, I. Synthesis and XRD of neutral NiL complex using unsymmetrical ONNO tetradentate schiff base: Hirschfeld, spectral, DFT and thermal analysis. J. Mol. Struct. 2019, 1180, 455. [Google Scholar] [CrossRef]
- Hijji, Y.; Benjamin, E.; Butcher, R.; Zarrouk, A.; Warad, I. Crystal structure, spectral, thermal and experimental/computational investigation of Anthracen-benzo [d] thiazol-2-amine new Schiff base derivative. J. Mol. Struct. 2021, 1229, 129824. [Google Scholar] [CrossRef]

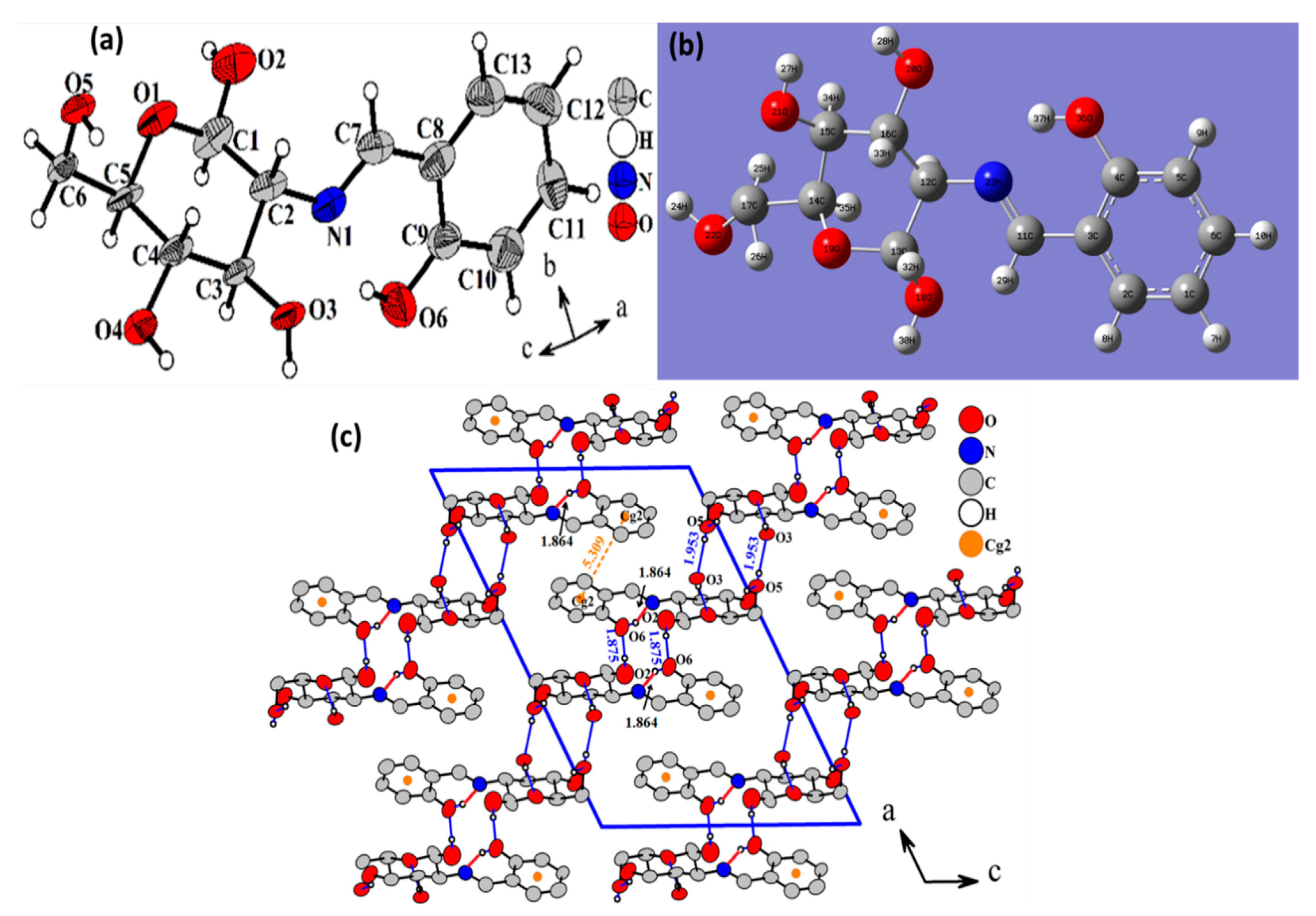
| Chemical Formula | C13H17NO6 |
|---|---|
| CCDC | 2054297 |
| Mr | 283.3 |
| Flack parameter | 0.4(9) |
| Crystal system, space group | Monoclinic, C2 |
| Temperature (K) | 150 |
| a, b, c (Å) | 18.438 (3), 6.0431 (11), 13.133 (2) |
| β (°) | 117.486 (7) |
| V (Å3) | 1298.1 (4) |
| Z | 4 |
| Radiation type | Cu Kα |
| µ (mm−1) | 0.98 |
| Crystal size (mm) | 0.26 × 0.06 × 0.03 |
| Data collection | |
| Diffractometer | Bruker D8 VENTURE |
| Absorption correction | Multi-scan SADABS |
| Tmin, Tmax | 0.884, 1 |
| No. of measured, independent and observed [I > 4σ(I)] reflections | 4820, 1667, 674 |
| Rint | 0.104 |
| (sin θ/λ)max (Å−1) | 0.597 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.068, 0.228, 1.01 |
| No. of reflections | 1667 |
| No. of parameters | 196 |
| No. of restraints | 10 |
| Δρmax, Δρmin (e Å−3) | 0.39, −0.25 |
| Absolute structure | 486 of Friedel pairs used in the refinement |
| No. | Bond | XRD | DFT | No. | Angle | XRD | DFT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | O1 | C1 | 1.41(1) | 1.417 | 18 | C1 | O1 | C5 | 113.9(6) | 112.5 |
| 2 | O1 | C5 | 1.44(1) | 1.434 | 19 | C2 | N1 | C7 | 120.6(7) | 121.83 |
| 3 | O2 | C1 | 1.28(1) | 1.302 | 20 | O1 | C1 | O2 | 108.6(7) | 109.08 |
| 4 | O3 | C3 | 1.419(9) | 1.422 | 21 | O1 | C1 | C2 | 107.9(7) | 108.67 |
| 5 | O4 | C4 | 1.41(1) | 1.405 | 22 | O2 | C1 | C2 | 113.9(8) | 113.68 |
| 6 | O5 | C6 | 1.413(8) | 1.405 | 23 | N1 | C2 | C1 | 109.9(7) | 108.38 |
| 7 | O6 | C9 | 1.32(1) | 1.342 | 24 | N1 | C2 | C3 | 111.2(7) | 112.94 |
| 8 | N1 | C2 | 1.48(1) | 1.502 | 25 | C1 | C2 | C3 | 109.6(7) | 108.38 |
| 9 | N1 | C7 | 1.27(1) | 1.254 | 26 | O3 | C3 | C2 | 110.3(6) | 109.5 |
| 10 | C1 | C2 | 1.55(1) | 1.561 | 27 | O3 | C3 | C4 | 111.0(6) | 111.85 |
| 11 | C2 | C3 | 1.52(1) | 1.521 | 28 | C2 | C3 | C4 | 109.6(7) | 110.83 |
| 12 | C3 | C4 | 1.50(1) | 1.498 | 29 | O4 | C4 | C3 | 113.5(7) | 113.68 |
| 13 | C4 | C5 | 1.53(1) | 1.515 | 30 | O4 | C4 | C5 | 105.3(6) | 107.61 |
| 14 | C5 | C6 | 1.51(1) | 1.521 | 31 | C3 | C4 | C5 | 111.2(6) | 113.68 |
| 15 | C7 | C8 | 1.48(1) | 1.476 | 32 | O1 | C5 | C4 | 109.2(6) | 111.33 |
| 16 | C8 | C9 | 1.39(1) | 1.372 | 33 | O1 | C5 | C6 | 107.1(6) | 109.26 |
| 17 | C8 | C13 | 1.36(2) | 1.367 | 34 | C4 | C5 | C6 | 114.3(6) | 111.96 |
| Donor | Hydrogen | Acceptor | D-H Distance | H...A Distance | D-A Distance | D-H...A Angle |
|---|---|---|---|---|---|---|
| O2 | H1O2 | O6 | 0.83(6) | 1.87(7) | 2.684(10) | 168(8) |
| O3 | H1O3 | O1 | 0.821(16) | 2.09(3) | 2.885(7) | 164(5) |
| O4 | H1O4 | O5 | 0.82(6) | 2.01(5) | 2.727(7) | 146(9) |
| O5 | H1O5 | O3 | 0.819(19) | 1.95(2) | 2.725(6) | 157.2(16) |
| O6 | H1O6 | N1 | 0.82(6) | 1.86(6) | 2.596(9) | 148(9) |
| No. | Atom | MAC | NBA | No. | Atom | MAC | NBA |
|---|---|---|---|---|---|---|---|
| 1 | C | −0.0986 | −0.19484 | 19 | O | −0.39559 | −0.75684 |
| 2 | C | −0.06331 | −0.18638 | 20 | O | −0.36058 | −0.60764 |
| 3 | C | −0.14837 | −0.0848 | 21 | O | −0.38535 | −0.72255 |
| 4 | C | −0.02168 | −0.1673 | 22 | O | −0.35858 | −0.71719 |
| 5 | C | −0.09702 | −0.1905 | 23 | O | −0.36984 | −0.6475 |
| 6 | C | −0.08268 | −0.18756 | 24 | N | −0.30484 | −0.46408 |
| 7 | H | 0.095839 | 0.19838 | 25 | H | 0.249019 | 0.44549 |
| 8 | H | 0.087757 | 0.19803 | 26 | H | 0.13298 | 0.26307 |
| 9 | H | 0.109898 | 0.2228 | 27 | H | 0.213206 | 0.37366 |
| 10 | H | 0.099062 | 0.19882 | 28 | H | 0.210967 | 0.43698 |
| 11 | H | 0.097445 | 0.19754 | 29 | H | 0.217824 | 0.44051 |
| 12 | C | 0.097101 | 0.12806 | 30 | H | 0.118774 | 0.18125 |
| 13 | C | −0.10795 | −0.09328 | 31 | H | 0.253056 | 0.4681 |
| 14 | C | 0.205948 | 0.4415 | 32 | H | 0.148058 | 0.20596 |
| 15 | C | −0.06965 | 0.19739 | 33 | H | 0.144472 | 0.16871 |
| 16 | C | 0.117445 | 0.13276 | 34 | H | 0.122554 | 0.17625 |
| 17 | C | 0.103363 | 0.12963 | 35 | H | 0.115282 | 0.16932 |
| 18 | C | −0.2357 | −0.50734 | 36 | H | 0.159658 | 0.1536 |
| No. | λ(nm) | Osc. Str. (f) | Major Contribution | Minor Contribution |
|---|---|---|---|---|
| 1 | 545.9 | 0.0116 | HOMO->L+1(100%) | |
| 2 | 317.1 | 0.0015 | H-2->LUMO(76%), H-1->LUMO(18%) | H-4->LUMO(2%) |
| 3 | 309.1 | 0.0205 | H-2->LUMO(20%), H-1->LUMO(73%) | H-4->LUMO(5%) |
| 4 | 307.3 | 0.0165 | H-6->LUMO(13%), H-5->LUMO(18%), H-4->LUMO(57%) | H-7->LUMO(3%), H-1->LUMO(6%) |
| 5 | 294.2 | 0.0028 | H-2->L+1(93%) | H-2->L+3(2%), H-1->L+1(3%) |
| 6 | 276.9 | 0.0073 | H-6->LUMO(73%), H-5->LUMO(20%) | H-7->LUMO(4%) |
| 7 | 270.1 | 0.0035 | HOMO->L+3(86%), HOMO->L+4(14%) | |
| 8 | 267.6 | 0.0303 | HOMO->L+3(13%), HOMO->L+4(84%) | |
| 9 | 255.8 | 0.0121 | H-3->L+1(78%), H-1->L+2 (19%) | H-1->L+1 (3%) |
| 10 | 247.5 | 0.5811 | H-1->L+1(97%) | H-2->L+1 (3%) |
| 11 | 241.1 | 0.0104 | H-7->LUMO(86%) | H-9->LUMO(3%), H-5->LUMO(9%) |
| 12 | 230.9 | 0.0007 | H-4->L+1(95%) | HOMO->L+6(2%) |
| 13 | 230.1 | 0.0062 | HOMO->L+6(94%) | H-4->L+1(2%) |
| 14 | 224.9 | 0.0126 | H-9->LUMO(14%), H-8->LUMO(76%) | HOMO->L+5(8%) |
| 15 | 223.4 | 0.0047 | HOMO->L+5 (91%) | H-9->LUMO(3%), H-8->LUMO(5%) |
| 16 | 218.7 | 0.0380 | H-9->LUMO(67%), H-8->LUMO(16%) | H-10->LUMO(9%), H-7->LUMO(3%) |
| 17 | 215 | 0.0027 | H-5->L+1(88%) | H-6->L+1(8%) |
| 18 | 207.3 | 0.0064 | HOMO->L+7(14%), HOMO->L+8(15%), HOMO->L+9(68%) | |
| 19 | 204.8 | 0.0217 | HOMO->L+7(12%), HOMO->L+8(52%), HOMO->L+9(26%) | H-1->L+2(4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijji, Y.; Rajan, R.; Ben Yahia, H.; Mansour, S.; Zarrouk, A.; Warad, I. One-Pot Microwave-Assisted Synthesis of Water-Soluble Pyran-2,4,5-triol Glucose Amine Schiff Base Derivative: XRD/HSA Interactions, Crystal Structure, Spectral, Thermal and a DFT/TD-DFT. Crystals 2021, 11, 117. https://doi.org/10.3390/cryst11020117
Hijji Y, Rajan R, Ben Yahia H, Mansour S, Zarrouk A, Warad I. One-Pot Microwave-Assisted Synthesis of Water-Soluble Pyran-2,4,5-triol Glucose Amine Schiff Base Derivative: XRD/HSA Interactions, Crystal Structure, Spectral, Thermal and a DFT/TD-DFT. Crystals. 2021; 11(2):117. https://doi.org/10.3390/cryst11020117
Chicago/Turabian StyleHijji, Yousef, Rajeesha Rajan, Hamdi Ben Yahia, Said Mansour, Abdelkader Zarrouk, and Ismail Warad. 2021. "One-Pot Microwave-Assisted Synthesis of Water-Soluble Pyran-2,4,5-triol Glucose Amine Schiff Base Derivative: XRD/HSA Interactions, Crystal Structure, Spectral, Thermal and a DFT/TD-DFT" Crystals 11, no. 2: 117. https://doi.org/10.3390/cryst11020117
APA StyleHijji, Y., Rajan, R., Ben Yahia, H., Mansour, S., Zarrouk, A., & Warad, I. (2021). One-Pot Microwave-Assisted Synthesis of Water-Soluble Pyran-2,4,5-triol Glucose Amine Schiff Base Derivative: XRD/HSA Interactions, Crystal Structure, Spectral, Thermal and a DFT/TD-DFT. Crystals, 11(2), 117. https://doi.org/10.3390/cryst11020117







