Abstract
CHn is the precursor unit for graphene synthesis. We have theoretically predicated a series of CHn structures with n = 1, 2, 4, 6, 8, 10, and 12 at elevated pressures (ambient pressure, 50, 100, 200, 300, 350, and 400 GPa) using evolutionary algorithms. The predicted CH and CH2 structures are graphane-type and polyethylene over the whole considered pressure range, respectively. The molecular crystalline methane is predicted for the stoichiometry of CH4. The combination of methane and H2 for CH6, CH8, CH10, and CH12 up to 300 GPa are obtained. At 400 GPa, the mixture of polymer and H2 for CH6, CH10, and CH12 comes into play. From the computed enthalpy, higher pressure and more hydrogen concentration contributed to the decomposition (to carbon and H2) of CHn systems. The total density of states for these CHn structures show that only the CH12 phase is metallic above 300 GPa. The rotational properties are traced in H2 and the CHn structures. The CH4 rotation is more sensitive to the pressure. The H2 units are nearly freely rotational. Other structures of CHn, including fcc-type and experimentally known structures, are not competitive with the structures predicted by evolutionary algorithms under high pressure region. Our results suggest that the CHn (n > 4) system is a potential candidate for hydrogen storage where H2 could be released by controlling the pressure.
1. Introduction
A significant paper by N. W Ashcroft in 2004 [1] entitled “Hydrogen Dominant Metallic Alloys: High Temperature Superconductors?” opened the door for the study of Group 14 hydrides. In the past few tens of years, a number of studies of Group 14 hydrides were performed; both experimental [2,3,4] and theoretical [5,6,7,8] studies have appeared. These studies were mainly focused on EHx with x ≥ 4 systems (E = Si, Ge, and Sn) which have a high atomic fraction of H (≥80%) and were predicted to metalize as hydrogen-dominant metallic alloys at lower pressures than pure hydrogen. These hydrogen-dominant alloys might become superconductors under high pressure with similar properties to those of pure metallic hydrogen. The strategy for the metallization of hydrogen-dominant metallic alloys is that due to the chemical precompression exerted by heavier atoms on hydrogen, the expected pressure needed for the required metallic transition as a preliminary step toward superconductivity might be within current experimental capabilities.
In Group 14, the member carbon is the basis of all known life on Earth, which is different to other members (Si, Ge, Sn, and Pb). We investigated their differences in the Group 14 elements at ambient pressure [9]. Carbon is the only element of Group 14 known to have metastable, kinetically persistent 0D (fullerenes), 1D (nanotubes), 2D (graphene, graphane), and 3D (diamond, graphite) allotropes. This kinetic persistence arises from large barriers to oligo- and polymerization and bond reorganization. Moving down the Group 14 column reveals stark changes in chemical bonding that directly affect the types of structures each element favors. At high pressure, the stable phase of C is the diamond type (up to 500 GPa), which is insulating with a wide gap. Si, Ge, and Sn become metallic at 12, 11, and 1 GPa, respectively, owing to the phase transition from diamond type to β-Sn type (six-coordination) [10].
Based on the difference between carbon and other members, the carbon hydrides (CHn) exhibit different chemical and physical properties to other Group 14 hydrides. For instance, from the thermodynamic point of view, the heat of formation is negative (exothermic) only for CH4. Methane has been detected to exist in several locations of the solar system. According to the first-principle calculation by Martinez-Canales and Bergara [11], there is no evidence for the metallization of CH4 up to 520 GPa which is quite high, well above the current experiment capabilities.
The high-pressure behavior of carbon hydrides, CHn (n ≥ 4), is just beginning to be studied. In 1996 [12], methane (CH4) was discovered to form four stoichiometric compounds (CH4(H2)2, (CH4)2H2, CH4(H2)4, and CH4H2), with hydrogen at pressures up to 30 GPa. These van der Waals compounds exhibit many interesting properties with relevance to many fields such as planetary science, hydrogen storage, energy, environment field, and so on. Experiments have demonstrated that the methane is unstable under high temperature and high pressures. At T = 2000 K and P = 20 GPa, the methane could dissociate to form diamond and hydrogen [7,13].
Following the trend of analyzing Group 14 hydrides, we studied a series of CHn structures with n = 1, 2, 4, 6, 8, 10, and 12 at elevated pressures (up to 400 GPa) by evolutionary algorithm procedures. We examined whether pressure could lead to the formation of new compounds in this series or not. The structural and electronic properties were paid attention to in the current work to check the metallization of CHn systems.
2. Materials and Methods
The calculations are based on the plane wave/pseudopotential approach using the computer program VASP (Vienna Ab-initio Simulation Package) [14,15], employing the PBE type and the projected-augmented wave (PAW) [16,17] method. The energy cutoff for plane-wave basis was set to 600 eV. The convergence criterion for electronic energy and force is 1.0 × 10−6 eV/atom and 0.01 eV/Å, respectively. The density of K-mesh was set to 0.04. In geometry optimization, we allow all structural parameters (atomic position, lattice constants, and symmetry) to relax. The evolutionary algorithm USPEX [18,19,20] was employed to find the lowest energy structures; the searching calculations were carried out at 1 atm, 50, 100, 200, 300, 350, and 400 GPa, which is consistent with our DFT calculations.
3. Results
3.1. The Tie Line of CHn Systems
We performed evolutionary prediction methodology for searching ground state CHn structures with x = 1, 2, 4, 6, 8, 10, and 12 at a series of pressures (ambient pressure, 50 GPa, 100 GPa, 200 GPa, 300 GPa, 350 GPa, and 400 GPa). The searched lowest energy CHn structures at series pressure are shown in SI (Figures S1–S8). The predicted CH and CH2 (Table S1) structures are graphane-type and polyethylene over the whole considered pressure range, respectively. The predicted structure of CH3 depends very much on the pressure: at or below 50 GPa, it forms ethane molecules; at 100 GPa, it forms butane, which can be thought of as oligomerized ethane; at 200 GPa polymerization happens, forming polyethylene (200 GPa), polyethylene and methane (300 GPa), or graphene (350 GPa). The predicated CH4 structures (Table S2) are the molecular crystalline methane. The combination of methane and H2 for CH6 (Table S3), CH8 (Table S4), CH10 (Table S5), and CH12 (Table S6) up to 300 GPa are obtained. Interestingly, at 400 GPa, the mixture of polymer and H2 for CH6, CH10, and CH12 come into play.
Figure 1 (a tie line curve) shows the static-lattice enthalpies (eV/per C atom) for the most competitive CHn structures at selected pressure. Note that the tie line energy curve has been used in our previous studies [21,22], which may be used to connect two phases, and if the enthalpy of a third falls below it, the first two will react to give the third. The enthalpy of formation of CHn is defined as ΔH (CHn) = H(CHn) − H(C) − 1/2x∙H(H2). The carbon in graphite (0–10 GPa), diamond (10–400 GPa), and solid H2 structures in the P63/m (0–100 GPa), C2/c (100–300 GPa), Cmca-12 (300–350 GPa), and Cmca (400 GPa) are taken as references.
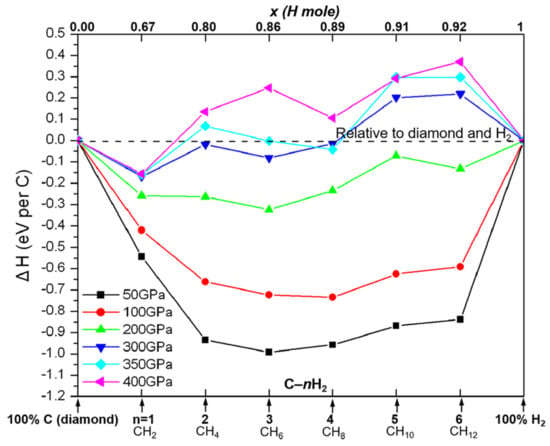
Figure 1.
Relative ground state enthalpies (with respect to carbon and H2) of the most stable CHn (n = 1, 2, 4, 6, 8, 10, and 12) solids. The abscissa x is the fraction of H in the structures: the mole fraction of H on top; the stoichiometry of CHn on bottom.
It is important to recognize that in the light-element systems, ion dynamics can significantly change the total energies [23]. On the other hand, DFT static-lattice energies, in general, reproduce experimental phase stabilities well, even for light-element high-pressure phases [24], in part owing to the cancellation of the dynamical effects in the energy differences. In the discussions that follow, we use the static-lattice enthalpies. Note that kinetic effects and metastability are not considered at this level.
From the enthalpy curve in Figure 1, one can see that (I) all of the CH and CH2 phases are stable relative to carbon and H2 over the whole given pressure range; (II) the stabilization for CH4, CH6, and CH8 are up to 300 GPa, while there is on stabilization for CH10 and CH12 above 300 GPa; (III) in the pressure region of 1 atm to 100 GPa, the “flat energy lines” for the CHn (n ≥ 4) are owing to the van der Waals forces in the combination of methane and H2; (IV) higher pressure and more hydrogen concentration contribute to the decomposition of CHn system; (V) when pressure lowers under 200 GPa, the CH4, CH6, and CH8 are more stable than other compounds, but under high pressure (p > 200 GPa), the CH4 becomes a metastable phase, which is consistent with previous studies [13]. We would like to propose that the CHn (n > 4) system is a potential candidate for hydrogen storages since such a system can release H2 under high pressure.
3.2. Compression of CHn
Figure 2b shows the calculated Wigner–Seitz radius (rs) as a function of pressure (rs-P curve) for the lowest energy structure of carbon, H2, and CHn systems. The Wigner–Seitz radius (rs) is defined by 4πrs3/3 = 1/ρ, where ρ is the average valence-electron density. In the work, we set up ρ = N/V, where N is the number of valence electrons in the unit cell; V is the volume of the unit cell. Let rs be the standard measure of the average valence electron density for a system. The volume reductions for all these structures are rapid from 0 GPa to 50 GPa due to squeezing out van der Waals space in H2 and CHn structures, and the phase transition in carbon (graphite to diamond). The computed rs for CHn structures are located between the value of H2 and carbon. The corresponding slopes reflect the compressible properties.
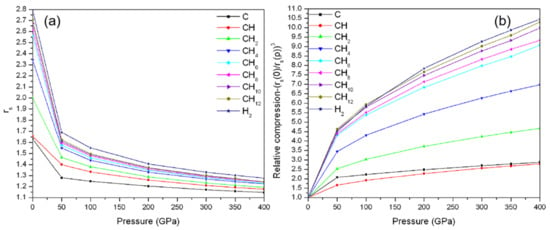
Figure 2.
(a) Wigner–Seitz radius (rs) of carbon, H2, and CHn structures (n = 1, 2, 4, 6, 8, 10, and 12) as a function of pressure. (b) Computed relative compression as a function of pressure. The relative compression is defined by , where rs(0) and rs(p) is the Wigner–Seitz radius at ambient pressure and certain high pressure, respectively.
Figure 3a shows the closest distance between C and C and the closest inter-distance of H and H (Figure 3b) in CHn structures. We can see that there are C–C chemical bonds in carbon, CH, and CH2 phase, while the carbon atoms in CHn (n ≥ 4) are separated by forming methane molecules. After 350 GPa, the complexes of polymer and H2 are formed in CH6, CH10, and CH12 phase, as shown in the jump of C–C distances. With increasing pressure (0 to 50 GPa), the distance of the closest inter H–H in CHn structures is decreased rapidly due to squeezing the van der Waals space, as shown in Figure 3b.
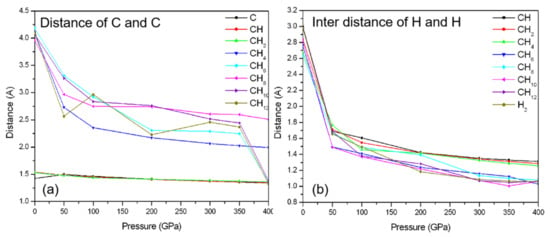
Figure 3.
(a) the closest distance of C and C in CHn structures and carbon as a function of pressure. (b) the closest inter distance of H and H in CHn structures and H2 as a function of pressure.
3.3. Electronic Properties of CHn Phase
The Goldhammer–Herzfeld criterion states that a material becomes metallic when the quantity diverges, where α is the molecular polarizability, Vm is the volume per molecule in the solid, and f is a dimensionless factor determined only by the packing of the molecules in the crystal. The divergence occurs when fα/Vm = 1. For cubic systems, f is 4π/3, and this gives . Using the definition of rs, (where Nve is number of electrons), we then have the following equation for cubic systems, , where the polarizability of a material, α for C, CH4, and H2 is 7.437, 17.53, and 5.42 bohr, respectively. One readily obtains rs = 1.23, 1.30, and 1.40 bohr for the metallization of CH4 and H2, respectively.
Figure 4 shows the computed total density of states for carbon (diamond), H2 (C2/c), and CHn structures at 300 GPa. One can see that only the CH12 phase is almost metallic above 300 GPa. The band gap for the CHn phase is decreased with the increasing concentration of H. Note that the free-electron-like shapes are also shown in Figure 4.
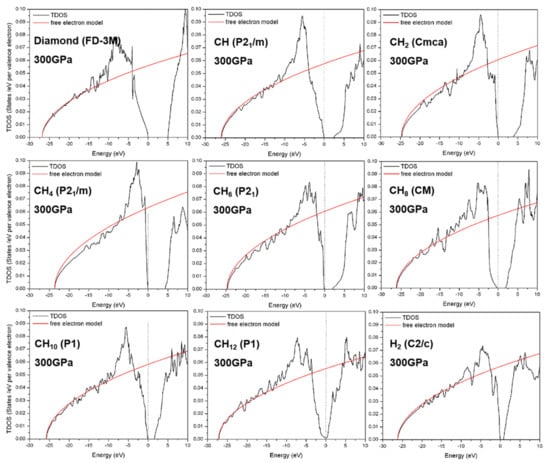
Figure 4.
Computed total density of states of diamond, H2, and CHn (n = 1, 2, 4, 6, 8, 10, and 12) phases at 300 GPa. The free electron models are shown.
3.4. The Rotational Solid for CHn
The term—the rotational solid—is mainly used to describe the properties of a molecular crystal, such as solid H2 [25], solid CH4 [26], and C60 crystal structure [27]. For molecular crystals, the rotational dynamic might be an important factor for the complicated phase transition at low temperature and pressure.
The rotational barriers in H2 and CHn (n ≥ 4) structures are examined via scanning potential energy surface (shown in Figures S9–S15). Table 1 and Table S7 show the rotational barriers of H2 unit and CH4 unit in corresponding structure at 100 GPa and 300 GPa. As expected, the rotational barriers for H2 and CH4 units are elevated with increasing pressure. At ambient pressure, these rotations are free (no energy barriers were found), owing to the dispersion forces. The energy barrier of CH4 rotation is higher than that of H2 rotation under same pressure, indicating that the CH4 rotation is more sensitive to the pressure. From the small rotational barriers for H2 units, even at 300 GPa, one can know that the these H2 units are nearly in free rotation. The rotational solid is a good term to describe the CHn phases.

Table 1.
The computed barriers of rotation in H2 and CHn (n ≥ 4) structures.
3.5. Other Possible Structures for CHn Systems
In 2009, Strobel et. al. [4] reported novel molecular compound formation from silane–hydrogen mixtures (SiH4–2H2) and proposed that the molecular compound adopts an fcc lattice. The study on SiH4–2H2 offers possible structural information for the methane–hydrogen mixtures, even though the C is different to Si, as mentioned.
The fcc primitive cell with CH4 unit (one CH4 in the cell) is shown in Figure 5. There are two tetrahedral holes (at site 2 and 3) and one octahedral hole (at site 1) along the diagonal—red dot line in the primitive cell. When only the one site is H2-occupied, the stoichiometry of the phase is CH4H2. The CH4–2H2 can be obtained via occupying one more. The sites will be all occupied in CH4–3H2. In the work, we pick up the CH4–H2 containing one occupied octahedral hole, CH4–2H2, with occupied octahedral hole and tetrahedral hole. Note that there are similar energies for other possible (H2-occupied) fcc isomers. Though these structures begin with fcc type, they will not adopt perfect fcc-type at high pressure any more (see Figure S16). This might be attributed to the properties of rational solid.
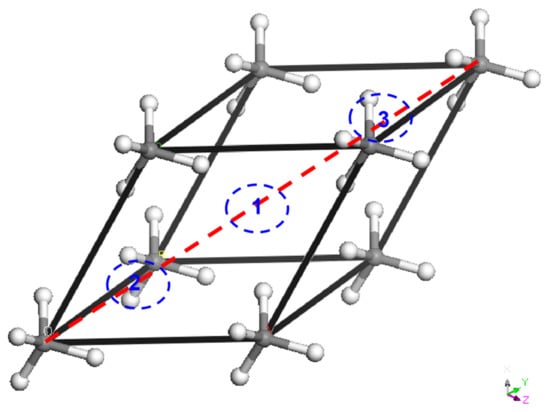
Figure 5.
Schematic fcc primitive cell with CH4 unit. 1, 2, and 3 denotes the location of atoms in fcc structures.
The calculated enthalpy (with respect to carbon and H2) of these fcc-type CHn structures as a function of pressure is shown in Figure 6. For comparison, the corresponding CHn structures by USPEX are also inserted as dot lines. Due to van der Waals interaction, it is not surprising that the relative enthalpies of these structures are close together in the low-pressure zone, while they are separated with elevating pressure. As one can see, the structures searched for by evolutionary algorithm are more stable than their corresponding fcc type structures, indicating that the fcc-type CHn structures are not competitive (Table S8). These fcc-type CHn structures at 300 GPa are still semiconductors or insulators.
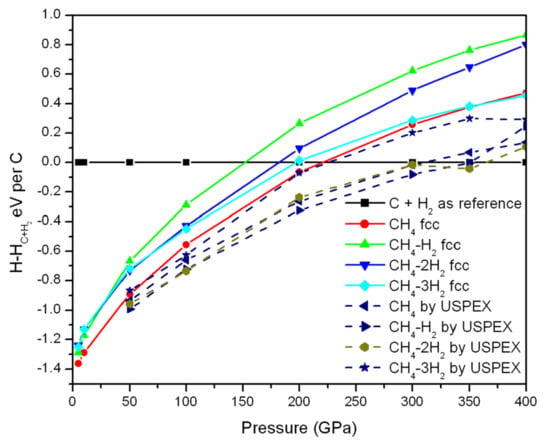
Figure 6.
Computed enthalpy (with respect to carbon and H2) as a function of pressure.
Experimentally, the observed CH4–H2 in the range of 7.9–30 GPa is consistent with the wurtzite structure. The CH4–2H2 (which is stable between 5.8 and 7 GPa) is that of a MgZn2 (Laves phase; H2 occupy the Zn sites and the CH4 is in the Mg sites) structure.
Based on the observation in experiment, the CH4–H2 wurtzite phase and CH4–2H2 MgZn2 phase are also examined, as shown in Figure 7. The enthalpy curve shows that the CH4–H2 wurtzite phase is more stable than the corresponding fcc type below 180 GPa, while the CH4–2H2 MgZn2 phase is more favorable than fcc-type CH4–2H2 phase over the whole range of pressure. Note the blue line (CH4–H2 wurtzite) undergoes a big jump, indicating that there might be a phase transition (see Figures S17 and S18).
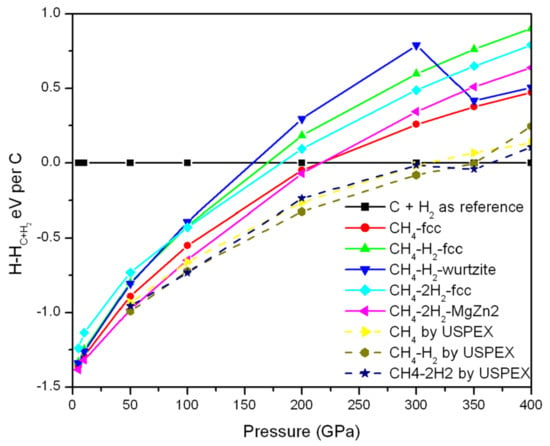
Figure 7.
Computed enthalpy of wurtzite-type structures (with respect to carbon and H2) as a function of pressure.
In a word, the considered fcc-type CHn structures and the experimental known structures are not competitive with the corresponding CHn structures obtained by evolutionary algorithms above 50 GPa. However, the relative enthalpies of these considered CHn structures are close together in the low-pressure zone. These phenomena are possibly owing to the rotational solid or liquid-like.
4. Conclusions
We have systematically investigated the CHn crystalline structures with n = 1, 2, 4, 6, 8, 10, and 12 under various pressures (ambient pressure, 50, 100, 200, 300, 350, and 400 GPa) by evolutionary algorithms procedures (USPEX). CH and CH2 are the graphane-type and polyethylene, respectively, over the whole considered pressure range. The predicted stoichiometry of CH4 is molecular crystalline methane. The combination of methane and H2 for CH6, CH8, CH10, and CH12 up to 300 GPa are obtained. The mixture of polymer and H2 for CH6, CH10, and CH12 come into play at 400 GPa. From the computed static-lattice enthalpy, the CHn (n ≥ 4) phase decomposes to carbon and H2 under high pressure (above 300 GPa). In addition, the concentration of hydrogen also contributes to the decomposition (of carbon and H2). Based on the calculations, we propose that the CHn (n > 4) system is a potential candidate for hydrogen storages since such a system can release H2 under high pressure. The calculated total density of states for the CHn structures shows that only the CH12 phase is almost metallic above 300 GPa. The rotational properties are traced in H2 and the CHn structures. The energy barrier of CH4 rotation is higher than that of H2 rotation under the same pressure, indicating that the CH4 rotation is more sensitive to the pressure. From the small rotational barriers for H2 units, even at 300 GPa, one can know that the H2 units are nearly freely rotational. Other structures of CHn, including fcc-type and experimentally known structures, are not competitive with the structures predicted by evolutionary algorithms under high pressure.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11121499/s1, Figure S1: CH2 structures under various pressure by USPEX, Table S1: Calculated volume (Å), density (g/cm3), rs of CH2 structures by USPEX. The Z and electron number in corresponding unit cell are shown here, Figure S2: CH4 structures under various pressure by USPEX, Table S2: Calculated volume (Å), density (g/cm3), rs of CH4 structures by USPEX. The Z and electron number in corresponding unit cell are shown here, Figure S3: CH4–H2 structures under various pressure by USPEX, Table S3: Calculated volume (Å), density (g/cm3), rs of CH4–H2 structures by USPEX. The Z and electron number in corresponding unit cell are shown here, Figure S4: CH4–2H2 structures under various pressure by USPEX, Table S4: Calculated volume (Å), density (g/cm3), rs of CH4–2H2 structures by USPEX. The Z and electron number in corresponding unit cell are shown here, Figure S5: CH4–3H2 structures under various pressure by USPEX, Table S5: Calculated volume (Å), density (g/cm3), rs of CH4–H2 structures by USPEX. The Z and electron number in corresponding unit cell are shown here, Figure S6: CH4–4H2 structures under various pressure by USPEX, Table S6: Calculated volume (Å), density (g/cm3), rs of CH4–4H2 structures by USPEX. The Z and electron number in corresponding unit cell are shown here, Figure S7: CHx structures at 1 atm by USPEX, Figure S8: CH2 structures by USPEX, Figure S9: Scanned potential energy surfaces of rotation in two solid H2 structures (at 100 GPa and 300 GPa, respectively), Figure S10: Scanned potential energy surfaces of rotation in two solid CH4 structures (at 100GPa and 300GPa, respectively), Figure S11: Scanned potential energy surfaces of rotation in two solid CH4–H2 fcc (at 100GPa and 300GPa, respectively) structures, Figure S12: Scanned potential energy surfaces of rotation in two solid CH4–H2 structures (at 100 GPa and 300 GPa, respectively) by USPEX, Figure S13: Scanned potential energy surfaces of rotation in two solid CH4–2H2 structures (at 100 GPa and 300 GPa, respectively) by USPEX, Figure S14: Scanned potential energy surfaces of rotation in two solid CH4–3H2 structures (at 100 GPa and 300 GPa, respectively) by USPEX, Figure S15: Scanned potential energy surfaces of rotation in two solid CH4–4H2 structures (at 100 GPa and 300 GPa, respectively) by USPEX, Table S7: The biggest barriers of rotation in H2 and CHx structures, Figure S16: Simulated XRD pattern of a perfect fcc CH4 structure and optimized “fcc” CH4 structure at 300 GPa. The Synchrotron with a wave length by 1.000 Å is used, Figure S17: Simulated XRD pattern of CH4–H2 wurtzite structure at 300 GPa and 350 GPa. Here, the radiation source is from Synchrotron with a wave length by 1.000 Å, Figure S18: Simulated XRD pattern of CH4–H2 wurtzite structure (the starting structure and optimized at 5 GPa) and CH4–H2 by USPEX at 5 GPa, Table S8: Computed the relative enthalpy of three CH4–H2 structures at 5 GPa.
Author Contributions
Conceptualization, T.Y.; methodology, T.Y.; software, T.Y.; writing—original draft preparation, T.Y. and J.L.; writing—review and editing, X.L. (Xiaotong Liu); visualization, X.L. (Xiulei Liu); supervision, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful for the financial support from Beijing Advanced Innovation Center for Materials Genome Engineering.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ashcroft, N.W. Hydrogen dominant metallic alloys: High temperature superconductors? Phys. Rev. Lett. 2004, 92, 187002. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mao, H.K.; Chen, X.J.; Mao, W.L. High pressure chemistry in the H2-SiH4 system. Proc. Natl. Acad. Sci. USA 2009, 106, 14763–14767. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Struzhkin, V.V.; Song, Y.; Goncharov, A.F.; Ahart, M.; Liu, Z.; Mao, H.K.; Hemley, R.J. Pressure-induced metallization of silane. Proc. Natl. Acad. Sci. USA 2008, 105, 20–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, T.A.; Somayazulu, M.; Hemley, R.J. Novel pressure-induced interactions in silane-hydrogen. Phys. Rev. Lett. 2009, 103, 65701. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Grochala, W.; Jaroń, T.; Hoffmann, R.; Bergara, A.; Ashcroft, N.W. Erratum: Structures and potential superconductivity in SiH4 at high pressure: En route to “metallic hydrogen” (Physical Review Letters (2006) 96 (017006)). Phys. Rev. Lett. 2006, 97, 17006. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Oganov, A.R.; Li, P.; Li, Z.; Wang, H.; Cui, T.; Ma, Y.; Bergara, A.; Lyakhov, A.O.; Iitaka, T.; et al. High-pressure crystal structures and superconductivity of Stannane (SnH4). Proc. Natl. Acad. Sci. USA 2010, 107, 1317–1320. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Oganov, A.R.; Bergara, A.; Martinez-Canales, M.; Cui, T.; Iitaka, T.; Ma, Y.; Zou, G. Superconducting high pressure phase of germane. Phys. Rev. Lett. 2008, 101, 107002. [Google Scholar] [CrossRef] [Green Version]
- Tse, J.S.; Yao, Y.; Tanaka, K. Novel superconductivity in metallic SnH4 under high pressure. Phys. Rev. Lett. 2007, 98, 117004. [Google Scholar] [CrossRef]
- Wen, X.D.; Cahill, T.J.; Hoffmann, R. Exploring group 14 structures: 1D to 2D to 3D. Chem.—A Eur. J. 2010, 16, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Mujica, A.; Rubio, A.; Muñoz, A.; Needs, R.J. High-pressure phases of group-IV, III–V, and II–VI compounds. Rev. Mod. Phys. 2003, 75, 863–912. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Canales, M.; Bergara, A. No evidence of metallic methane at high pressure. High Press. Res. 2006, 26, 369–375. [Google Scholar] [CrossRef]
- Somayazulu, M.S.; Finger, L.W.; Hemley, R.J.; Mao, H.K. High-pressure compounds in methane-hydrogen mixtures. Science 1996, 271, 1400–1402. [Google Scholar] [CrossRef]
- Liu, H.; Naumov, I.I.; Hemley, R.J. Dense Hydrocarbon Structures at Megabar Pressures. J. Phys. Chem. Lett. 2016, 7, 4218–4222. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Perdew, Burke, and Ernzerhof Reply. Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Kresse, G. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B—Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W.; Ono, S. High-pressure phases of CaCO3: Crystal structure prediction and experiment. Earth Planet. Sci. Lett. 2006, 241, 95–103. [Google Scholar] [CrossRef]
- Oganov, A.R.; Glass, C.W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, C.W.; Oganov, A.R.; Hansen, N. USPEX-Evolutionary crystal structure prediction. Comput. Phys. Commun. 2006, 175, 713–720. [Google Scholar] [CrossRef]
- Feng, J.; Hennig, R.G.; Ashcroft, N.W.; Hoffmann, R. Emergent reduction of electronic state dimensionality in dense ordered Li-Be alloys. Nature 2008, 451, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Zurek, E.; Hoffmann, R.; Ashcroft, N.W.; Oganov, A.R.; Lyakhov, A.O. A little bit of lithium does a lot for hydrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 17640–17643. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, N.W. Quantum-solid behavior and the electronic structure of the light alkali metals. Phys. Rev. B 1989, 39, 10552–10559. [Google Scholar] [CrossRef] [PubMed]
- Hanfland, M.; Syassen, K.; Christensen, N.E.; Novikov, D.L. New high-pressure phases of lithium. Nature 2000, 408, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Ashcroft, N.W. Structure and bandgap closure in dense hydrogen. Nature 2000, 403, 632–635. [Google Scholar] [CrossRef]
- Hüller, A.; Prager, M.; Press, W.; Seydel, T. Phase III of solid methane: The orientational potential and rotational tunneling. J. Chem. Phys. 2008, 128, 34503. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.D.; Yannoni, C.S.; Dorn, H.C.; Salem, J.R.; Bethune, D.S. C60 rotation in the solid state: Dynamics of a faceted spherical top. Science 1992, 255, 1235–1238. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).