Critical Review of Solidification of Sandy Soil by Microbially Induced Carbonate Precipitation (MICP)
Abstract
:1. Introduction
2. Methods of Solidifying Sand
2.1. Microbial Sand Consolidation
2.2. Other Sand Fixation Methods
3. Microbial Sources of Solidified Sand
3.1. External Bacteria Solidifying the Sandy Soil
3.2. Solidification of Sandy Soil by Indigenous Bacteria
4. Models for Predicting the Curing Process of MICP in the Field
5. Factors Affecting Microbial Solidification of Sandy Soil
5.1. Concentration of Cementation Solution
5.2. Bacterial Concentration
5.3. Temperature
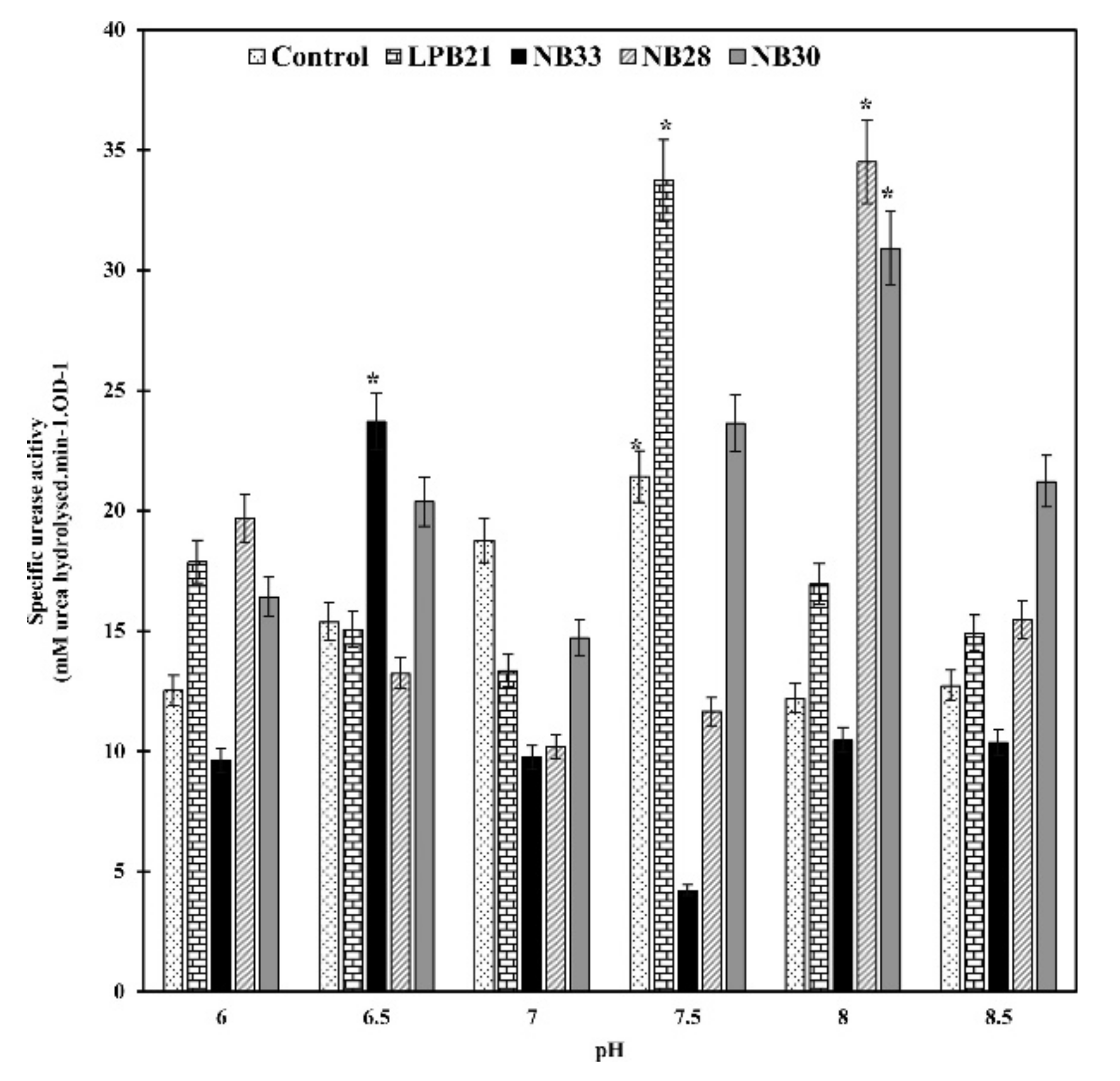
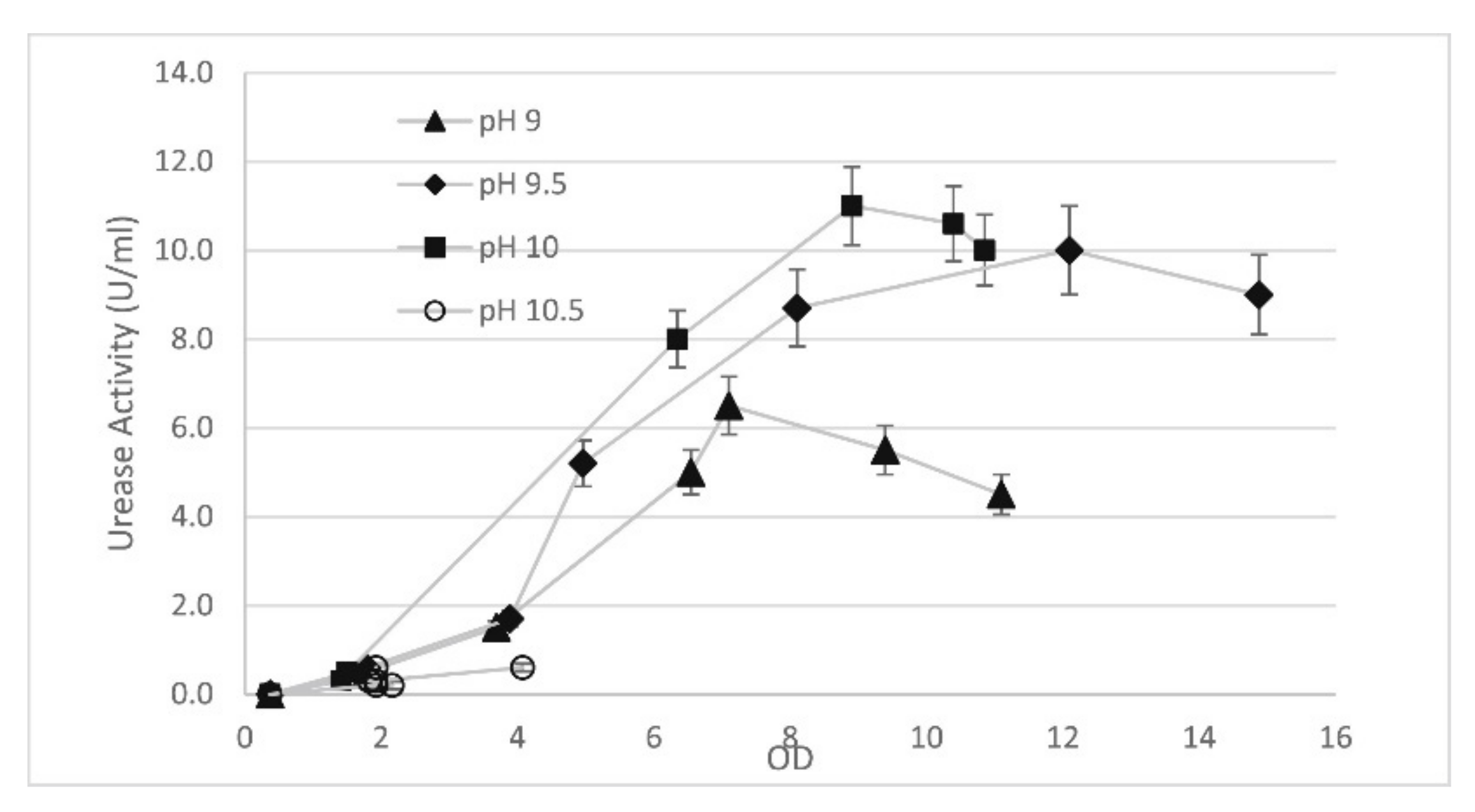
5.4. pH Value
5.5. Sources of Calcium
6. Conclusions and Suggestions for Future Research
- (1)
- At present, micro-organism solidification faces some problems. For example, when microbe grouting enters sand and other media, CaCO3 deposit is not uniformly distributed, and induced CaCO3 deposit is deposited near the injection port. This may be due to an imbalance between the rate of hydrolysis of urea and the rate of transport of the cementation solution to the sand or the rapid precipitation rate of calcium carbonate generated at the injection port [159,160]. It needs to add the cementation solution several times in batches, and the cost is relatively high. In addition, the sand body after grouting needs to be heated and dried, and the operation is complicated. Wang et al. [161] added anion admixture based on the concept of ionic lattice energy. The introduction of anion admixtures can combine Ca2+ in the cementation solution to form ionic crystals, which can effectively reduce the number of grout times and avoid heating and drying this step after grout. However, the strength of sand consolidation is lower than that of other studies, so more efficient admixtures should be explored next.
- (2)
- Urease-producing bacteria will produce the by-product of NH4+ in the process of catalyzing urea hydrolysis. A high concentration of NH4+ will harm the environment. At present, the research on ammonia removal mainly includes using natural zeolite to remove ammonium ions, the use of struvite to reduce ammonium by two steps, and reducing the pH in the reaction process to reduce ammonium ions [57,58,59]. More research on ammonium ion removal methods is needed in the future.
- (3)
- There are also problems in solidifying sandy soil using the urease bacteria contained in the soil. It is troublesome to screen urease-producing bacteria in sandy soil, because of the need to carry out primary screening, re-screening, strain identification, and the urease activity of the selected strain may not be sufficient. However, the introduction of external bacteria could compete with bacteria inside the sand. In future, if measures can be taken to allow internal urea-producing bacteria and exogenous bacteria at the same time to solidify sand, this may be a promising research direction for the optimization of microbial sand consolidation.
- (4)
- Although there are successful cases of microbial sand consolidation technology in field practice, there have been few large-scale experiments. Up until now, very few experiments on microbial sand consolidation technology have been conducted in deserts. Meng et al. [26] conducted field experiments with MICP in the Ulan Buh Desert and found that MICP can improve the resistance of sand to wind erosion. The high temperature of the desert made it easy for the solution to evaporate and posed a significant challenge to the survival of the species. Therefore, the treatment of sand by MICP is conducted after sunset. The effect of field sand consolidation experiments can be measured by the calcium carbonate content, the bearing capacity, and erosion depth of sand under wind conditions. When determining the optimal curing experimental conditions in the field, field environment and operation feasibility should be considered. The influences of temperature, pH, bacterial liquid concentration, cementation solution concentration, and calcium source on MICP should be studied. MICP-treated sand has poor durability in harsh environments, such as wet–dry cycles, freeze–thaw cycles, and acid rain conditions [8]. There are few studies on the durability of MICP-treated sand under acid rain conditions, which can be studied more in the future. In addition, future research can overcome the adverse conditions of high temperatures by mutating genetic strains, thus achieving large-scale sand consolidation in deserts.
- (5)
- Sand fixation methods include sand fixation with the sand barrier, chemical sand consolidation, and microbial sand consolidation. Each method has its advantages and disadvantages. Currently, most researchers use one curing method to solidify sandy soil, but no researchers to date have combined multiple curing methods to solidify sandy soil. This can be regarded as a future research direction in the field of solidifying sandy soil.
- (6)
- Some experimental results have shown that the combined actions of non-urease bacteria and urease-producing bacteria enhance the microbial solidification of sandy soil. However, there have been few studies of the simultaneous use of non-urease bacteria and urease-producing bacteria to date. Gat et al. [162] found that the precipitation rate of calcium carbonate generated by non-urease bacteria B. subtilis and S. pasteurii was faster than that generated by S. pasteurii alone. They speculated that non-urease bacteria provided additional nucleation sites for MICP, which promoted the precipitation of calcium carbonate and accelerated the MICP process. This should be considered as a factor in the subsequent optimization of solidification experiments.
- (7)
- The accuracy of the prediction results of the model will be affected by some factors. Kim et al. [163] found that the results predicted by the numerical model deviated from the actual value because the predicted results of the model were affected by factors such as the assumed shape of calcium carbonate particles and local pore blockage. Therefore, it was difficult to directly and accurately predict the pore-scale characteristics of MICP solidified sand through the model. Existing numerical models should be continuously optimized, and new numerical models should be proposed.
- (8)
- Biological consolidation techniques mainly include the MICP method and another promising method, enzyme-induced carbonate precipitation (EICP) [164,165]. MICP produces urease using urease-producing bacteria in the culture environment, but the cultural environment of urease-producing bacteria is complicated, and urease activity is difficult to control [80]. EICP is derived from the direct use of free urease, and the enzymes can be derived from microbes, fungi, and agricultural sources. Microbially induced carbonate precipitation cannot treat sandy soils with pores smaller than 0.5 μm because bacteria are 0.5 to 3 μm in size. EICP can treat finer clays because the enzyme particle size is about 12 nm [80]. Compared with MICP, EICP has the advantage of not involving biosafety issues, while the calcium carbonate production efficiency of EICP is lower than that of MICP [166].
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Portugal, C.R.M.E.; Fonyo, C.; Machado, C.C.; Meganck, R.; Jarvis, T. Microbiologically Induced Calcite Precipitation bio-cementation, green alternative for roads–is this the breakthrough? A critical review. J. Clean. Prod. 2020, 262, 121372. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Hasi, E.; Li, J. Desertification in China: An assessment. Earth-Sci. Rev. 2008, 88, 188–206. [Google Scholar] [CrossRef]
- Kimura, R.; Moriyama, M. Determination by MODIS satellite-based methods of recent global trends in land surface aridity and degradation. J. Agric. Meteorol. 2019, 75, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhang, G.; Zhang, Y.; Guan, X.; Wei, Y.; Guo, R. Global desertification vulnerability to climate change and human activities. Land Degrad. Dev. 2020, 31, 1380–1391. [Google Scholar] [CrossRef]
- Mariina; Fahriani, F.; Apriyanti, Y. Utilization of palm kernel shell ash as stabilization materials for clay to settlement con-solidation. In IOP Conference Series. Earth and Environmental Science; IOP Publishing Ltd.: Bangka, Indonesia, 2020; p. 599. [Google Scholar]
- Yang, J.; Cheng, Y.; Chen, W. Experimental Study on Diffusion Law of Post-Grouting Slurry in Sandy Soil. Adv. Civ. Eng. 2019, 2019, 3493942. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Ran, F.; Feng, E.; Dong, Z.; Lei, Z. Effectiveness of an Eco-friendly Polymer Composite Sand-Fixing Agent on Sand Fixation. Water Air Soil Pollut. 2015, 226, 221. [Google Scholar] [CrossRef]
- Liu, S.; Wen, K.; Armwood, C.; Bu, C.; Li, C.; Amini, F.; Li, L. Enhancement of MICP-Treated Sandy Soils against Environmental Deterioration. J. Mater. Civ. Eng. 2019, 31, 04019294. [Google Scholar] [CrossRef]
- Qu, J.; Zu, R.; Zhang, K.; Fang, H. Field observations on the protective effect of semi-buried checkerboard sand barriers. Geomorphology 2007, 88, 193–200. [Google Scholar] [CrossRef]
- Pan, X.; Chu, J.; Yang, Y.; Cheng, L. A new biogrouting method for fine to coarse sand. Acta Geotech. 2019, 15, 1–16. [Google Scholar] [CrossRef]
- Peng, S.; Di, H.; Fan, L.; Fan, W.; Qin, L. Factors Affecting Permeability Reduction of MICP for Fractured Rock. Front. Earth Sci. 2020, 8. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Zhao, X.; Zhao, L.; Xu, S.; Gu, S.; Zhang, F.; Yu, G. Seasonal variations and mechanism for environmental con-trol of NEE of CO2 concerning the Potentilla fruticosa in alpine shrub meadow of Qinghai-Tibet Plateau. Sci. China Ser. D Earth Sci. 2006, 49, 174–185. [Google Scholar] [CrossRef]
- Sharaky, A.M.; Mohamed, N.; Elmashad, M.E.; Shredah, N.M. Application of microbial biocementation to improve the physico-mechanical properties of sandy soil. Constr. Build. Mater. 2018, 190, 861–869. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, C.; Gao, Y. Improving the Erosion Resistance Performance of Pisha Sandstone Weathered Soil Using MICP Technology. Crystals 2021, 11, 1112. [Google Scholar] [CrossRef]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.K.; Santamarina, J.C. Biological Considerations in Geotechnical Engineering. J. Geotech. Geoenviron. Eng. 2005, 131, 1222–1233. [Google Scholar] [CrossRef] [Green Version]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2016, 34, 524–537. [Google Scholar] [CrossRef]
- Han, Z.; Cheng, X.; Ma, Q. An experimental study on dynamic response for MICP strengthening liquefiable sands. Earthq. Eng. Eng. Vib. 2016, 15, 673–679. [Google Scholar] [CrossRef]
- Eryürük, K.; Yang, S.; Suzuki, D.; Sakaguchi, I.; Akatsuka, T.; Tsuchiya, T.; Katayama, A. Reducing hydraulic conductivity of porous media using CaCO3 precipitation induced by Sporosarcina pasteurii. J. Biosci. Bioeng. 2015, 119, 331–336. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuwano, R. Undrained cyclic triaxial testing on sand with non-plastic fines content cemented with microbially induced CaCO3. Soils Found. 2016, 56, 485–495. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Tang, C.-S.; Xie, Y.-H.; Yin, L.-Y.; Cheng, Q.; Shi, B. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 2019, 264, 105389. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. Effects of different clay’s percentages on improvement of Sand-Clay mixtures with microbially in-duced calcite precipitation. Geomicrobiol. J. 2019, 36, 810–818. [Google Scholar] [CrossRef]
- Nassar, M.K.; Gurung, D.; Bastani, M.; Ginn, T.R.; Shafei, B.; Gomez, M.G.; Graddy, C.M.R.; Nelson, D.C.; Dejong, J.T. Large-scale experiments in microbially induced calcite precipitation (MICP): Reactive transport model development and predic-tion. Water Resour. Res. 2018, 54, 480–500. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.T.; Jabbour, H.M.; Brown, D.G. Bio-grouting to enhance axial pull-out response of pervious concrete ground improvement piles. Can. Geotech. J. 2018, 55, 119–130. [Google Scholar] [CrossRef]
- Liu, S.; Du, K.; Huang, W.; Wen, K.; Amini, F.; Li, L. Improvement of erosion-resistance of bio-bricks through fiber and mul-tiple MICP treatments. Constr. Build. Mater. 2021, 271, 121573. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2020, 383, 114723. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Class, H.; Ebigbo, A.; Gerlach, R.; Phillips, A.; Hommel, J. Field-scale modeling of microbially induced calcite precipitation. Comput. Geosci. 2018, 23, 399–414. [Google Scholar] [CrossRef] [Green Version]
- Kadhim, F.J.; Zheng, J. Review of the factors that influence on the microbial induced calcite precipitation. J. Civ. Environ. Res. 2016, 8, 69–76. [Google Scholar]
- Fang, C.; Achal, V. Biostimulation of calcite precipitation process by bacterial community in improving cement stabilized rammed earth as sustainable material. Appl. Microbiol. Biotechnol. 2019, 103, 7719–7727. [Google Scholar] [CrossRef]
- Montoya, B.; DeJong, J.T. Stress-Strain Behavior of Sands Cemented by Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2015, 141, 04015019. [Google Scholar] [CrossRef]
- Wangjie, L.; Chunxiang, Q.; Ruixing, W. Study on soil solidification based on microbiological precipitation of CaCO3. Sci. China Technol. Sci. 2010, 53, 2372–2377. [Google Scholar]
- Sun, X.; Miao, L.; Chen, R.; Wang, H.; Xia, J. Surface rainfall erosion resistance and freeze-thaw durability of bio-cemented and polymer-modified loess slopes. J. Environ. Manag. 2021, 301, 113883. [Google Scholar] [CrossRef]
- Gebru, K.A.; Kidanemariam, T.G.; Gebretinsae, H.K. Bio-cement production using microbially induced calcite precipitation (MICP) method: A review. Chem. Eng. Sci. 2021, 238, 116610. [Google Scholar] [CrossRef]
- Li, G. The countermeasures people should take facing desertification problem. J. Environ. Manag. Coll. China 2004, 14, 65–67. [Google Scholar]
- Sarlak, M.; Ferretti, L.V.; Biasi, R. The productive landscape in the desert margin for the sustainable development of rural settlements: An innovative greenbelt for maranjab desert in iran. Sustainability 2021, 13, 2077. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.T.; Brown, D.G.; Kavazanjian, E., Jr. Mechanical behavior of sands treated by microbially induced car-bonate precipitation. J. Geotech. Geoenviron. Eng. 2016, 142, 4015066. [Google Scholar] [CrossRef]
- Van Paassen, L.A.; Ghose, R.; Van der Linden, T.J.M.; Van der Star, W.R.L.; Van Loosdrecht, M.C.M. Quantifying biomediated ground improvement by ureolysis: Large-Scale biogrout experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Venuleo, S.; Laloui, L.; Terzis, D.; Hueckel, T.; Hassan, M. Microbially induced calcite precipitation effect on soil thermal conductivity. Géotechnique Lett. 2016, 6, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-M.; Huang, Y.-H.; Nimje, V.R.; Lee, W.-C.; Chen, H.-J.; Kuo, Y.-H.; Huang, C.-H.; Chen, C.-C. Comparative Study on the Sand Bioconsolidation through Calcium Carbonate Precipitation by Sporosarcina pasteurii and Bacillus subtilis. Crystals 2018, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J. Ind. Microbiol. Biotechnol. 2009, 36, 981–988. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Improvements in engineering properties of soils through microbial-induced calcite precipitation. KSCE J. Civ. Eng. 2013, 17, 718–728. [Google Scholar] [CrossRef]
- Amarakoon, G.; Kawasaki, S. Factors Affecting Sand Solidification Using MICP with Pararhodobacter sp. Mater. Trans. 2018, 59, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Youn, H. Microbially Induced Calcite Precipitation Employing Environmental Isolates. Materials 2016, 9, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarda, D.; Choonia, H.S.; Sarode, D.D.; Lele, S.S. Biocalcification by Bacillus pasteurii urease: A novel application. J. Ind. Microbiol. Biotechnol. 2009, 36, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Sarıçiçek, Y.E.; Gurbanov, R.; Pekcan, O.; Gozen, A.G. Comparison of microbially induced calcium carbonate precipitation eligibility using sporosarcina pasteurii and bacillus licheniformis on two different sands. Geomicrobiol. J. 2018, 36, 42–52. [Google Scholar] [CrossRef]
- Ma, L.; Pang, A.-P.; Luo, Y.; Lu, X.; Lin, F. Beneficial factors for biomineralization by ureolytic bacterium Sporosarcina pasteurii. Microb. Cell Factories 2020, 19, 12. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-cementation of sandy soil using microbial-induced carbonate precipitation (MICP) for marine environments. Géotechnique 2014, 64, 1010–1013. [Google Scholar] [CrossRef]
- Kumari, D.; Pan, X.; Lee, D.-J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Dikshit, R.; Jain, A.; Dey, A.; Kumar, A. Microbially induced calcite precipitation using Bacillus velezensis with guar gum. PLoS ONE 2020, 15, e0236745. [Google Scholar] [CrossRef]
- Rajasekar, A.; Moy, C.K.S.; Wilkinson, S.; Sekar, R. Microbially induced calcite precipitation performance of multiple land-fill indigenous bacteria compared to a commercially available bacteria in porous media. PLoS ONE 2021, 16, e254676. [Google Scholar] [CrossRef]
- Gowthaman, S.; Iki, T.; Nakashima, K.; Ebina, K.; Kawasaki, S. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 2019, 1, 1480. [Google Scholar] [CrossRef] [Green Version]
- Gowthaman, S. Microbial induced slope surface stabilization using industrial-grade chemicals: A preliminary laboratory study. Int. J. Geomate 2019, 17. [Google Scholar] [CrossRef]
- Torres-Aravena, Á.; Duarte-Nass, C.; Azócar, L.; Mella-Herrera, R.; Rivas, M.; Jeison, D. Can microbially induced calcite pre-cipitation (MICP) through a ureolytic pathway be successfully applied for removing heavy metals from wastewaters? Crystals 2018, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Gomez, M.G.; San Pablo, A.C.M.; Kolbus, C.M.; Graddy, C.M.R.; Dejong, J.T.; Nelson, D.C. Investigating ammonium by-product removal for ureolytic bio-cementation using meter-scale experiments. Sci. Rep. 2019, 9, 18313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keykha, H.A.; Mohamadzadeh, H.; Asadi, A.; Kawasaki, S. Ammonium-Free Carbonate-Producing bacteria as an ecofriendly soil biostabilizer. Geotech. Test. J. 2019, 42, 19–29. [Google Scholar] [CrossRef]
- Gowthaman, S.; Mohsenzadeh, A.; Nakashima, K.; Kawasaki, S. Removal of ammonium by-products from the effluent of bio-cementation system through struvite precipitation. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Gowthaman, S.; Yamamoto, M.; Nakashima, K.; Ivanov, V.; Kawasaki, S. Calcium phosphate biocement using bone meal and acid urease: An eco-friendly approach for soil improvement. J. Clean. Prod. 2021, 128782. [Google Scholar] [CrossRef]
- Liu, D.; Shao, A.; Li, H.; Jin, C.; Li, Y. A study on the enhancement of the mechanical properties of weak structural planes based on microbiologically induced calcium carbonate precipitation. Bull. Int. Assoc. Eng. Geol. 2020, 79, 4349–4362. [Google Scholar] [CrossRef]
- Tiwari, N.; Satyam, N.; Sharma, M. Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method. Sci. Rep. 2021, 11, 0324. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Sharma, M.; Satyam, N.; Reddy, K.R. Effect of freeze-thaw cycles on engineering properties of biocemented sand under dif-ferent treatment conditions. Eng. Geol. 2021, 284, 106022. [Google Scholar] [CrossRef]
- Sharma, M.; Satyam, N. Strength and durability of biocemented sands: Wetting-drying cycles, ageing effects, and liquefaction resistance. Geoderma 2021, 402, 115359. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Durability analysis of bio-cemented slope soil under the exposure of acid rain. J. Soils Sediments 2021, 21, 2831–2844. [Google Scholar] [CrossRef]
- Tobler, D.J.; Cuthbert, M.O.; Greswell, R.B.; Riley, M.S.; Renshaw, J.C.; Handley-Sidhu, S.; Phoenix, V.R. Comparison of rates of ureolysis between Sporosarcina pasteurii and an indigenous groundwater community under conditions required to precip-itate large volumes of calcite. Geochim. Cosmochim. Acta 2011, 75, 3290–3301. [Google Scholar] [CrossRef]
- Feng, C.; Cui, B.; Ge, H.; Huang, Y.; Zhang, W.; Zhu, J. Reinforcement of recycled aggregate by Microbial-Induced minerali-zation and deposition of calcium carbonate—Influencing factors, mechanism and effect of reinforcement. Crystals 2021, 11, 887. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Jiang, G.; Fang, C.; Sun, J.; Zheng, S.; Liu, H.; Leusheva, E.; Morenov, V.; Nikolaev, N. Field application of mi-crobial Self-Healing cement slurry in chunguang 17-14 well. Energies 2021, 14, 1544. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Effect of calcifying bacteria on permeation properties of concrete structures. J. Ind. Microbiol. Biotechnol. 2010, 38, 1229–1234. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose mother liquor as an alternative nutrient source for microbial con-crete production by Sporosarcina pasteurii. J. Ind. Microbiol. Biotechnol. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ngu, L.H.; Ong, D.E.L.; Nissom, P.M. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotechnol. 2019, 17, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Cuzman, O.A.; Richter, K.; Wittig, L.; Tiano, P. Alternative nutrient sources for biotechnological use of Sporosarcina pasteurii. World J. Microbiol. Biotechnol. 2015, 31, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, S.; Rojas, H.; Caicedo, G.; Romanelli, G.; Pineda, A.; Luque, R.; Martínez, J. Whey as an Alternative Nutrient Medium for Growth of Sporosarcina pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation. Materials 2021, 14, 2470. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Bundur, Z.B. Use of corn-steep liquor as an alternative carbon source for biomineralization in cement-based materials and its impact on performance. Constr. Build. Mater. 2018, 165, 655–662. [Google Scholar] [CrossRef]
- Yoosathaporn, S.; Tiangburanatham, P.; Bovonsombut, S.; Chaipanich, A.; Pathom-Aree, W. A cost effective cultivation medium for biocalcification of Bacillus pasteurii KCTC 3558 and its effect on cement cubes properties. Microbiol. Res. 2016, 186, 132–138. [Google Scholar] [CrossRef]
- Gowthaman, S.; Mitsuyama, S.; Nakashima, K.; Komatsu, M.; Kawasaki, S. Biogeotechnical approach for slope soil stabiliza-tion using locally isolated bacteria and inexpensive low-grade chemicals: A feasibility study on Hokkaido expressway soil, Japan. Soils Found. 2019, 59, 484–499. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.; Gao, Y. Effects of compound sand barrier for habitat restoration on sediment grain-size distribution in ulan buh desert. Sci. Rep. 2020, 10, 2566. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineer-ing application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Huang, N.; Xia, X.; Tong, D. Numerical simulation of wind sand movement in straw checkerboard barriers. Eur. Phys. J. E 2013, 36, 99. [Google Scholar] [CrossRef]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-Art review of the applicability and challenges of Mi-crobial-Induced calcite precipitation (MICP) and Enzyme-Induced calcite precipitation (EICP) techniques for geotechnical and geoenvironmental applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Imran, M.A.; Nakashima, K.; Kawasaki, S. Bio-Mediated soil improvement using plant derived enzyme in addition to mag-nesium ion. Crystals 2021, 11, 516. [Google Scholar] [CrossRef]
- Gomez, M.G.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C. Biogeochemical Changes During Bio-cementation Mediated by Stimulated and Augmented Ureolytic Microorganisms. Sci. Rep. 2019, 9, 11517. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, D.; DeJong, J.; Montoya, B.; Martinez, B. Bio-bricks: Biologically cemented sandstone bricks. Constr. Build. Mater. 2014, 55, 462–469. [Google Scholar] [CrossRef]
- Nafisi, A.; Safavizadeh, S.; Montoya, B.M. Influence of Microbe and Enzyme-Induced Treatments on Cemented Sand Shear Response. J. Geotech. Geoenviron. Eng. 2019, 145, 06019008. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Teasdale, P.R. A comparison of mechanical responses for microbial- and en-zyme-induced cemented sand. Géotechnique Lett. 2020, 10, 559–567. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zheng, H.; Wang, J.; Ding, X.; Chen, P. Laboratory method of microbial induced solidification/stabilization for mu-nicipal solid waste incineration fly ash. MethodsX 2019, 6, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, N.; Ding, J.; Lu, C.; Jin, Y. Experimental Study on Wind Erosion Resistance and Strength of Sands Treated with Microbial-Induced Calcium Carbonate Precipitation. Adv. Mater. Sci. Eng. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martín-Sánchez, I.; González-Muñoz, M.T.; Rodriguez-Navarro, C. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 2017, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Surface Percolation for Soil Improvement by Biocementation Utilizing In Situ Enriched Indigenous Aerobic and Anaerobic Ureolytic Soil Microorganisms. Geomicrobiol. J. 2016, 34, 546–556. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Williams, B.C.; Crawford, R.L. Urease Activity of Ureolytic Bacteria Isolated from Six Soils in which Calcite was Precipitated by Indigenous Bacteria. Geomicrobiol. J. 2012, 29, 389–395. [Google Scholar] [CrossRef]
- Burbank, M.; Weaver, T.; Lewis, R.; Williams, T.; Williams, B.; Crawford, R. Geotechnical tests of sands following bioinduced calcite precipitation catalyzed by indigenous bacteria. J. Geotech. Geoenviron. Eng. 2013, 139, 928–936. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R. Permeation properties of concrete made with fly ash and silica fume: Influence of ureolytic bacteria. Constr. Build. Mater. 2013, 49, 161–174. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Han, X.-L.; Jiang, N.-J.; Wang, J.; Feng, J. The effect of enrichment media on the stimulation of native ureolytic bacteria in calcareous sand. Int. J. Environ. Sci. Technol. 2019, 17, 1795–1808. [Google Scholar] [CrossRef]
- Khan, M.N.H.; Amarakoon, G.G.N.N.; Shimazaki, S.; Kawasaki, S. Coral sand solidification test based on microbially induced carbonate precipitation using ureolytic bacteria. Mater. Trans. 2015, 56, 1725–1732. [Google Scholar] [CrossRef] [Green Version]
- Oualha, M.; Bibi, S.; Sulaiman, M.; Zouari, N. Microbially induced calcite precipitation in calcareous soils by endogenous Bacillus cereus, at high pH and harsh weather. J. Environ. Manag. 2019, 257, 109965. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yang, Y.; Qi, R.; Li, J.; Pan, X. Suppression of coal dust by microbially induced carbonate precipitation usingStaphylococcus succinus. Environ. Sci. Pollut. Res. 2019, 26, 35968–35977. [Google Scholar] [CrossRef]
- Imran, M.A.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Factors affecting the urease activity of native ureolytic bacteria isolated from coastal areas. Geomech. Eng. 2019, 17, 421–427. [Google Scholar]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Phillips, A.J.; Lauchnor, E.; Eldring, J.; Esposito, R.; Mitchell, A.C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.H. Potential CO2 Leakage Reduction through Biofilm-Induced Calcium Carbonate Precipitation. Environ. Sci. Technol. 2012, 47, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Cord-Ruwisch, R. Upscaling Effects of Soil Improvement by Microbially Induced Calcite Precipitation by Surface Percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, M.O.; McMillan, L.A.; Handley-Sidhu, S.; Riley, M.S.; Tobler, D.J.; Phoenix, V.R. A Field and Modeling Study of Fractured Rock Permeability Reduction Using Microbially Induced Calcite Precipitation. Environ. Sci. Technol. 2013, 47, 13637–13643. [Google Scholar] [CrossRef]
- van Paassen, L.A. Bio-Mediated Ground Improvement: From Laboratory Experiment to Pilot Applications. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; ASCE: Reston, VA, USA, 2011. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Ohan, J.A.; Saneiyan, S.; Lee, J.; Bartlow, A.W.; Ntarlagiannis, D.; Burns, S.E.; Colwell, F.S. Microbial and geochemical dy-namics of an aquifer stimulated for microbial induced calcite precipitation (MICP). Front. Microbiol. 2020, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Montoya, B.; Evans, T. Discrete element method simulations of bio-cemented sands. Comput. Geotech. 2017, 85, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Fauriel, S.; Laloui, L. A bio-chemo-hydro-mechanical model for microbially induced calcite precipitation in soils. Comput. Geotech. 2012, 46, 104–120. [Google Scholar] [CrossRef]
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Gai, X.; Sánchez, M. An elastoplastic mechanical constitutive model for microbially mediated cemented soils. Acta Geotech. 2018, 14, 709–726. [Google Scholar] [CrossRef]
- Wang, X.; Nackenhorst, U. A coupled bio-chemo-hydraulic model to predict porosity and permeability reduction during microbially induced calcite precipitation. Adv. Water Resour. 2020, 140, 103563. [Google Scholar] [CrossRef]
- Martinez, B.C.; Dejong, J.T.; Ginn, T.R. Bio-geochemical reactive transport modeling of microbial induced calcite precipitation to predict the treatment of sand in one-dimensional flow. Comput. Geotech. 2014, 58, 1–13. [Google Scholar] [CrossRef]
- Colwell, F.S.; Smith, R.W.; Ferris, F.G.; Reysenbach, A.; Fujita, Y.; Tyler, T.L.; Taylor, J.L.; Banta, A.; Delwiche, M.E.; Mcling, T.L.; et al. Microbially mediated subsurface calcite precipitation for removal of hazardous divalent cations: Microbial activity, molecular biology, and modeling. Subsurf. Contam. Remediat. 2005, 904, 117–137. [Google Scholar]
- Yang, P.; Kavazanjian, E.; Neithalath, N. Particle-Scale Mechanisms in Undrained Triaxial Compression of Biocemented Sands: Insights from 3D DEM Simulations with Flexible Boundary. Int. J. Géoméch. 2019, 19, 04019009. [Google Scholar] [CrossRef]
- Tobler, D.J.; Cuthbert, M.O.; Phoenix, V.R. Transport of Sporosarcina pasteurii in sandstone and its significance for subsurface engineering technologies. Appl. Geochem. 2014, 42, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Hassanizadeh, S.M.; Ebigbo, A. Pore-scale network modeling of microbially induced calcium carbonate precipita-tion: Insight into scale dependence of biogeochemical reaction rates. Water Resour. Res. 2016, 52, 8794–8810. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Ding, J.; Li, Q.; Xu, J. Thermal conductivity of sands treated with microbially induced calcite precipita-tion (MICP) and model prediction. Int. J. Heat Mass Tran. 2020, 147, 118899. [Google Scholar] [CrossRef]
- DeJong, J.; Martinez, B.C.; Ginn, T.R.; Hunt, C.; Major, D.; Tanyu, B. Development of a Scaled Repeated Five-Spot Treatment Model for Examining Microbial Induced Calcite Precipitation Feasibility in Field Applications. Geotech. Test. J. 2014, 37. [Google Scholar] [CrossRef]
- Minto, J.M.; Lunn, R.J.; El Mountassir, G. Development of a Reactive Transport Model for Field-Scale Simulation of Microbially Induced Carbonate Precipitation. Water Resour. Res. 2019, 55, 7229–7245. [Google Scholar] [CrossRef]
- Ebigbo, A.; Phillips, A.; Gerlach, R.; Helmig, R.; Cunningham, A.B.; Class, H.; Spangler, L.H. Darcy-scale modeling of mi-crobially induced carbonate mineral precipitation in sand columns. Water Resour. Res. 2012, 48, 1–17. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors Affecting Efficiency of Microbially Induced Calcite Precipitation. J. Geotech. Geoenvironmental Eng. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 04014006. [Google Scholar] [CrossRef]
- Sotoudehfar, A.R.; Sadeghi, M.M.; Mokhtari, E.; Shafiei, F. Assessment of the Parameters Influencing Microbial Calcite Precipitation in Injection Experiments Using Taguchi Methodology. Geomicrobiol. J. 2015, 33, 163–172. [Google Scholar] [CrossRef]
- Keykhahamed, A.; Asadi, A.; Huatbujang, B.K.; Kawasaki, S. Microbial induced calcite precipitation by Sporosarcina pasteurii and Sporosarcina aquimarina. Environ. Geotech. 2018, 6, 562–566. [Google Scholar]
- Xu, J.; Tang, Y.; Wang, X. A correlation study on optimum conditions of microbial precipitation and prerequisites for self-healing concrete. Process. Biochem. 2020, 94, 266–272. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Palombo, E.A.; Ong, D.E.L.; Nissom, P.M. Biocementation of sand by Sporosarcina pasteurii strain and tech-nical-grade cementation reagents through surface percolation treatment method. Constr. Build. Mater. 2019, 228, 116828. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, J.; Xie, X.; Xu, C.; Zheng, L. Experimental study on the strength of silt soil solidified by MICP. Low Temp. Archit. Technol. 2020, 42, 17–20. [Google Scholar]
- Duo, L.; Kan-Liang, T.; Hui-Li, Z.; Yu-Yao, W.; Kang-Yi, N.; Shi-Can, Z. Experimental investigation of solidifying desert aeolian sand using microbially induced calcite precipitation. Constr. Build. Mater. 2018, 172, 251–262. [Google Scholar] [CrossRef]
- Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Mujah, D.; Cheng, L.; Shahin, M. Microstructural and Geomechanical Study on Biocemented Sand for Optimization of MICP Process. J. Mater. Civ. Eng. 2019, 31, 04019025. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, J.M.; Vanapalli, S.; Fortin, D. Improving the strength of sandy soils via ureolytic CaCO3 solidification by Sporo-sarcina ureae. Biogeosciences 2018, 15, 4367–4380. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Deng, C.; Song, W.; Zhang, D.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M.; Pan, X. Biostabilization of Desert Sands Using Bacterially Induced Calcite Precipitation. Geomicrobiol. J. 2016, 33, 243–249. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Tang, C.-S.; Yin, L.-Y.; Xie, Y.-H.; Shi, B. Applicability of Microbial Calcification Method for Sandy-Slope Surface Erosion Control. J. Mater. Civ. Eng. 2019, 31, 04019250. [Google Scholar] [CrossRef]
- Chahal, N.; Siddique, R.; Rajor, A. Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of concrete incorporating silica fume. Constr. Build. Mater. 2012, 37, 645–651. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Amini, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2020, 15, 17–27. [Google Scholar] [CrossRef]
- Andalib, R.; Majid, M.Z.A.; Hussin, M.W.; Ponraj, M.; Keyvanfar, A.; Mirza, J.; Lee, H.-S. Optimum concentration of Bacillus megaterium for strengthening structural concrete. Constr. Build. Mater. 2016, 118, 180–193. [Google Scholar] [CrossRef]
- Omoregie, A.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Nakashima, K.; Achal, V.; Kawasaki, S. Whole-cell evaluation of urease activity of Pararhodobacter sp. isolated from peripheral beachrock. Biochem. Eng. J. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Youn, H. Effect of Temperature, pH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. [Google Scholar] [CrossRef] [Green Version]
- Imran, A.; Shinmura, M.; Nakashima, K.; Kawasaki, S. Effects of Various Factors on Carbonate Particle Growth Using Ureolytic Bacteria. Mater. Trans. 2018, 59, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Wang, Y. Investigating the Factors Affecting the Properties of Coral Sand Treated with Microbially Induced Calcite Precipitation. Adv. Civ. Eng. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of Key Environmental Conditions on Microbially Induced Cementation for Soil Stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chu, J.; Cao, B.; Liu, H.; Cheng, L. Biocementation of soil using non-sterile enriched urease-producing bacteria from activated sludge. J. Clean. Prod. 2020, 262, 121315. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Zhang, Q.; Xue, D.; Zhao, Y. Growth environment optimization for inducing bacterial mineralization and its application in concrete healing. Constr. Build. Mater. 2019, 209, 631–643. [Google Scholar] [CrossRef]
- Alonso, M.J.C.; Ortiz, C.E.L.; Perez, S.O.G.; Narayanasamy, R.; Miguel, G.D.J.F.S.; Hernández, H.H.; Balagurusamy, N. Improved strength and durability of concrete through metabolic activity of ureolytic bacteria. Environ. Sci. Pollut. Res. 2017, 25, 21451–21458. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, J.; Yuan, Z.; Wang, T.; Zhang, Y.; Wang, F. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation. Environ. Sci. Pollut. Res. 2016, 24, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chu, J.; Cheng, L.; Wu, S. Biogrouting of aggregates using premixed injection method with or without pH adjustment. J. Mater. Civil Eng. 2019, 31, 06019008. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.; Kim, S.; Park, J.; Kang, C.; Jeong, J.; So, J. Effects of different calcium salts on calcium carbonate crystal formation by Sporosarcina pasteurii KCTC 3558. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, Z.; Guo, N.; Liu, T. One-Step Removal of Calcium, Magnesium, and Nickel in Desalination by Alcaligenes aquatilis via Biomineralization. Crystals 2019, 9, 633. [Google Scholar] [CrossRef] [Green Version]
- Røyne, A.; Phua, Y.J.; Balzer Le, S.; Eikjeland, I.G.; Josefsen, K.D.; Markussen, S.; Myhr, A.; Throne-Holst, H.; Sikorski, P.; Wentzel, A. Towards a low CO2 emission building material employing bacterial metabolism (1/2): The bacterial system and prototype production. PLoS ONE 2019, 14, e212990. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Chu, J.; Brown, R.C.; Wang, K.; Wen, Z. Sustainable biocement production via microbially induced calcium car-bonate precipitation: Use of limestone and acetic acid derived from pyrolysis of lignocellulosic biomass. ACS Sustain. Chem. Eng. 2017, 5, 5183–5190. [Google Scholar] [CrossRef]
- Choi, S.; Wu, S.; Chu, J. Biocementation for sand using an eggshell as calcium source. J. Geotech. Geoenviron. Eng. 2016, 142, 06016010. [Google Scholar] [CrossRef]
- Liang, S.; Chen, J.; Niu, J.; Gong, X.; Feng, D. Using recycled calcium sources to solidify sandy soil through microbial in-duced carbonate precipitation. Mar. Georesour. Geotec. 2020, 38, 393–399. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Xiao, Y.; Chu, J.; Xiao, P.; Wang, Y. Biocementation of calcareous sand using soluble calcium derived from calcareous sand. Bull. Int. Assoc. Eng. Geol. 2017, 77, 1781–1791. [Google Scholar] [CrossRef]
- Pan, L.; Li, Q.; Zhou, Y.; Song, N.; Yu, L.; Wang, X.; Xiong, K.; Yap, L.; Huo, J. Effects of different calcium sources on the min-eralization and sand curing of CaCO3 by carbonic anhydrase-producing bacteria. RSC Adv. 2019, 9, 40827–40834. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, H.; Cheng, X. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.; Ali, A.; Talkhan, F.N.; Abdel-Gawwad, H. Utilization of microbial induced calcite precipitation for sand consolidation and mortar crack remediation. HBRC J. 2012, 8, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Whiffin, V.S.; van Paassen, L.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Wu, C.; Chu, J.; Wu, S.; Cheng, L.; van Paassen, L. Microbially induced calcite precipitation along a circular flow channel under a constant flow condition. Acta Geotech. 2018, 14, 673–683. [Google Scholar] [CrossRef]
- Wang, L.; Li, K.; Fu, P.; Li, N.; Zhao, W. Preliminary laboratory study on microbial slurry admixture for sand reinforcement. J. China Inst. Water Resour. Hydropower Res. 2020, 18, 130–135. [Google Scholar]
- Gat, D.; Tsesarsky, M.; Shamir, D.; Ronen, Z. Accelerated microbial-induced CaCO3 precipitation in a defined coculture of ureolytic and non-ureolytic bacteria. Biogeosciences 2014, 11, 2561–2569. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Mahabadi, N.; Jang, J.; van Paassen, L.A. Assessing the Kinetics and Pore-Scale Characteristics of Biological Calcium Carbonate Precipitation in Porous Media using a Microfluidic Chip Experiment. Water Resour. Res. 2020, 56. [Google Scholar] [CrossRef]
- Arab, M.G.; Omar, M.; Almajed, A.; Elbaz, Y.; Ahmed, A.H. Hybrid technique to produce bio-bricks using enzyme-induced carbonate precipitation (EICP) and sodium alginate biopolymer. Constr. Build. Mater. 2021, 284, 122846. [Google Scholar] [CrossRef]
- Almajed, A.; Abbas, H.; Arab, M.; Alsabhan, A.; Hamid, W.; Al-Salloum, Y. Enzyme-Induced Carbonate Precipitation (EICP)-Based methods for ecofriendly stabilization of different types of natural sands. J. Clean. Prod. 2020, 274, 122627. [Google Scholar] [CrossRef]
- Cui, M.; Lai, H.; Hoang, T.; Chu, J. One-phase-low-pH enzyme induced carbonate precipitation (EICP) method for soil im-provement. Acta Geotech. 2021, 16, 481–489. [Google Scholar] [CrossRef]






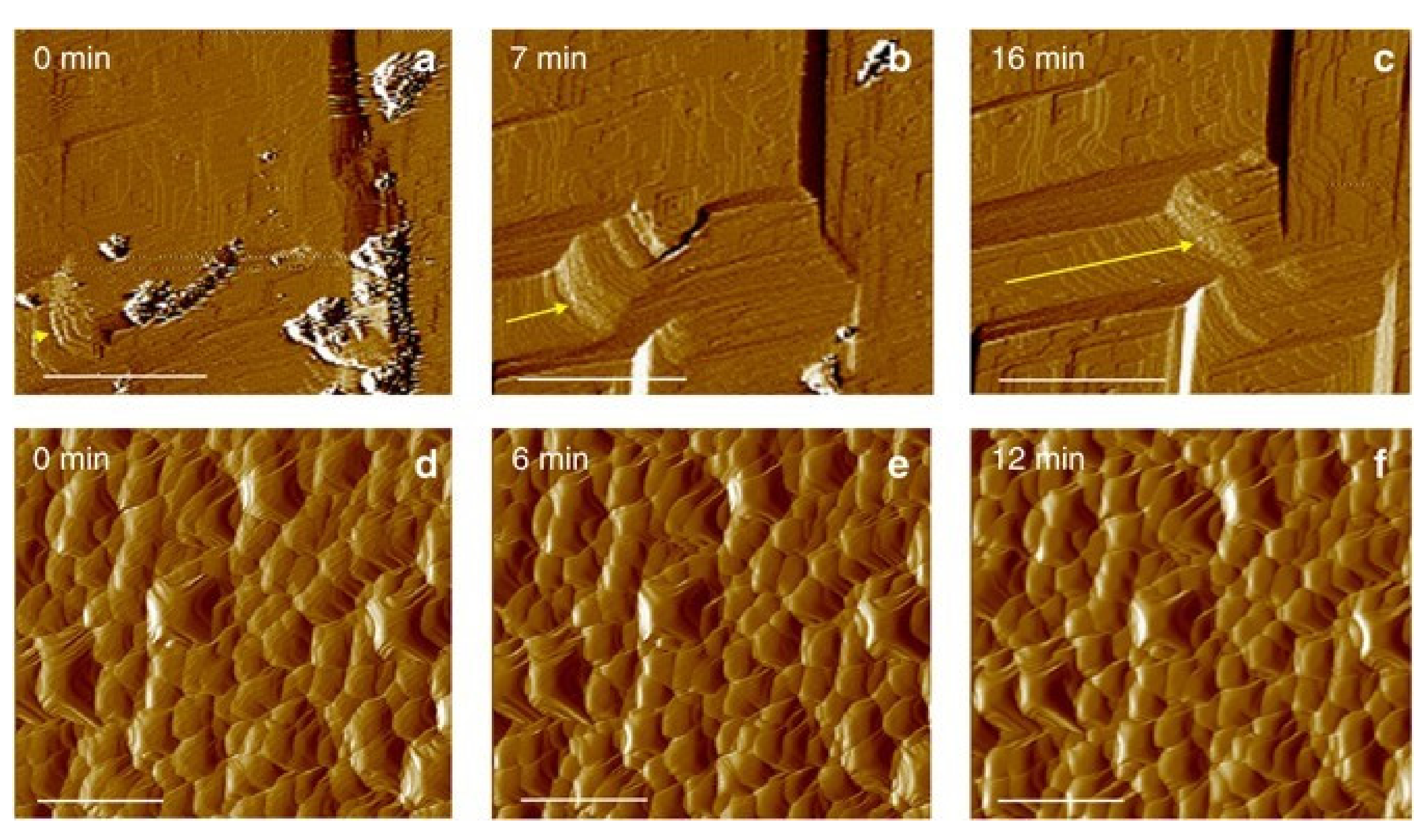


| The Name of the Bacteria | The Sources of the Bacteria | References, Year |
|---|---|---|
| Sporosarcina pasteurii MTCC 1761 | The Institute of Microbial Technology, Chandigarh, India | [42], 2009 |
| B. megaterium ATCC 14581 | American Type Culture Collection | [43], 2013 |
| Pararhodobacter sp. | The soil near beach rock in Sumuide, Nago Okinawa, Japan | [44], 2018 |
| Staphylococcus saprophyticus subsp. saprophyticus Sporosarcina globispora Bacillus lentus strain NCIMB8773 Sporosarcina sp. | Calcareous sand and limestone cave soils | [45], 2016 |
| Bacillus pasteurii NCIM 2477 Brevibacterium ammoniagenes ATCC 6871 Bacillus lentus 2466-NCIB 8773 | The National Collection of Industrial Microorganisms (NCIM) | [46], 2009 |
| B. licheniformis ATCC 14580 S. pasteurii ATCC 11859 | American Type Culture Collection | [47], 2019 |
| S. pasteurii BNCC 337394 | BeNa Culture Collection | [48], 2020 |
| Bacillus sp. DSM 23526 | Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) | [49], 2014 |
| E. undae YR10 | Isolated from Yangtze River near Chongming County, Shanghai, China | [50], 2014 |
| Bacillus velezensis | Isolated from native Indian soil | [51], 2020 |
| Pseudomonas nitroreducens szh_asesj15 | Isolated from landfill groundwater | [52], 2021 |
| Bacillus sp. xjlu_herc15 | Isolated from leachate | |
| Bacillus licheniformis adseedstjo15 | Isolated from leachate | |
| Lysinibacillus xylanilyticus | Isolated from Hokkaido, Japan | [53], 2019 |
| Psychrobacillus sp | [54], 2019 |
| The Name of the Bacteria | Economical Alternatives | Substitution of Nutrients in the Medium | References, Year |
|---|---|---|---|
| Sporosarcina pasteurii | Corn-steep liquor | Protein | [69], 2011 |
| Sporosarcina pasteurii NCIM 2477 | Lactose mother liquor | Protein | [70], 2009 |
| Sporosarcina pasteurii NB28 | Food-grade yeast | Nitrogen source | [71], 2019 |
| Sporosarcina pasteurii | By-products coming from the dairy and brewery industries | Protein | [72], 2015 |
| Fertilizer urea | Urea | ||
| Sporosarcina pasteurii isolated from agricultural soils of Sotaquirá and Nobsa | Whey | Protein | [73], 2021 |
| S. pasteurii DSMZ 33 | Corn-steep liquor | Carbon source | [74], 2018 |
| Bacillus pasteurii KCTC 3558 | Effluent from chicken manure bio-gas plant | Protein | [75], 2016 |
| Psychrobacillus sp. | Beer Yeast | Nitrogen source | [76], 2019 |
| Sand Consolidation Method | Figure | References | Advantages | Disadvantages |
|---|---|---|---|---|
| Sand fixation with sand barrier |  | [9] | Good curing effect, use of plants for environmental pollution, simple operation and cost is not high. | Cannot improve sandy soil after desertification, but can play a role in curbing desertification. |
| Chemical sand fixation |  | [7] | High economic benefits and good curing effects. | Complex operation and most chemical hardeners pollute the environment. |
| Solidification of sand by enzyme-induced carbonate precipitation (EICP) technology |  | [81] | Can treat fine soil; easier operation. | High cost; the precipitated calcium carbonate may not bind to soil particles due to the lack of nucleation sites. |
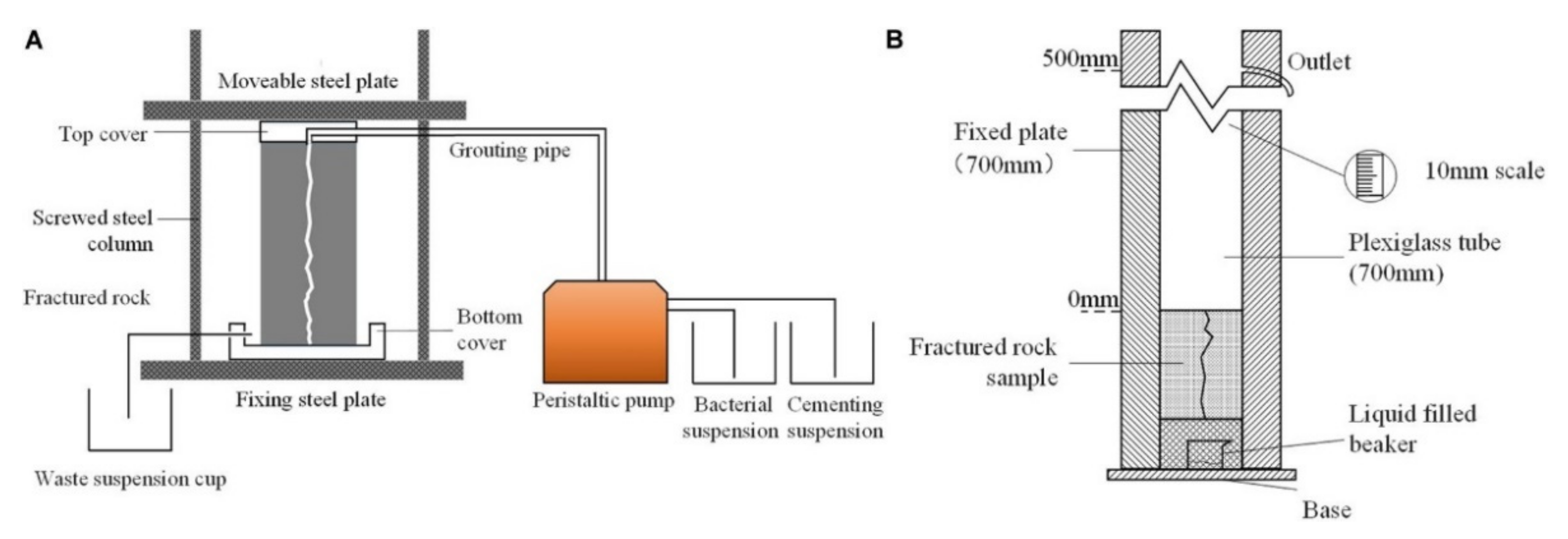
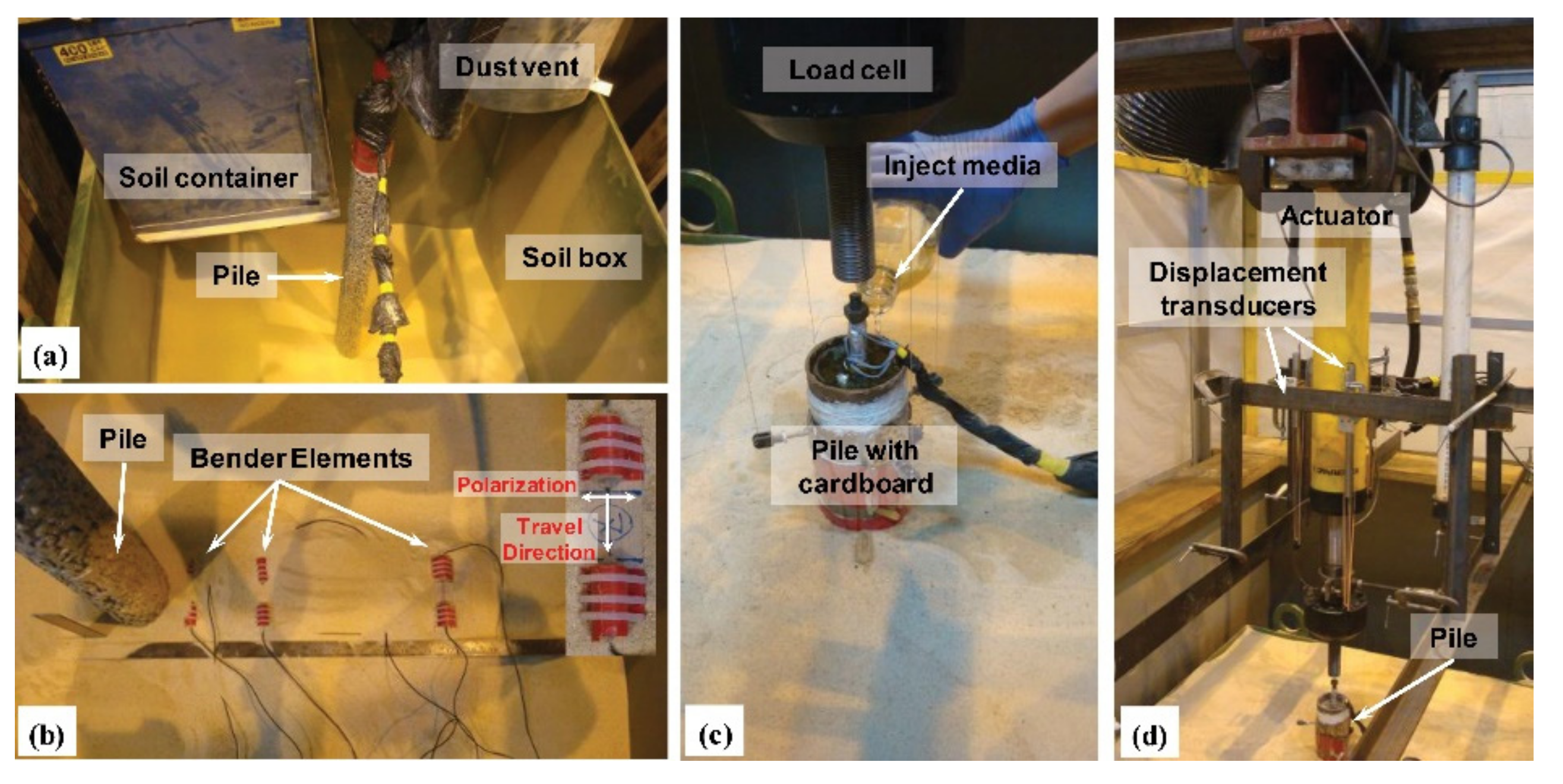
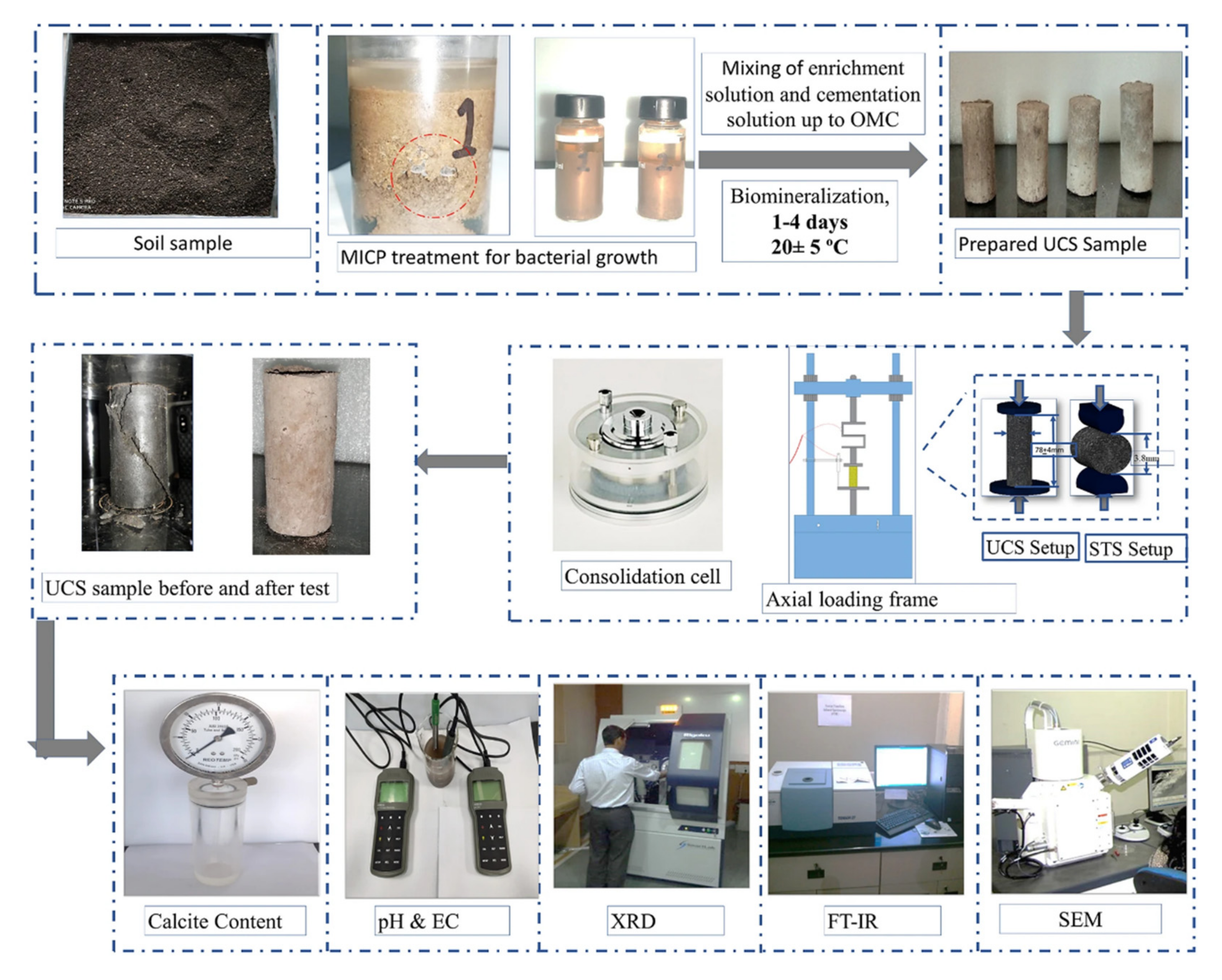
| MICP sand fixation |  (a) Full contact flexible mold; (b) samples before the MICP reactions; (c) samples after the MICP reactions; and (d) sample after cutting mold. | [8] | A good sand consolidation effect; well improves the properties of sandy soil; environmental friendly. | Complex operation and high cost. |
| Model Names | The Role of Models | References, Year |
|---|---|---|
| Aquifer conceptual model | Finding that the sedimentation rate of calcite is closely related to the hydrolysis rate of urea | [113], 2005 |
| A three-dimensional (3D) discrete element method (DEM)-based numerical model | Simulating the macroscopic mechanical properties of CaCO3 sediment-solidified sandy soil induced by micro-organisms under the condition of no triaxial compression of the drainage system | [114], 2019 |
| A loose sandstone numerical model based on a one-dimensional advective dispersion model | Predicting the movement of micro-organisms in soil and rock | [115], 2014 |
| A pore-scale network model | Simulating the CaCO3 precipitation process and the influence of different operations on CaCO3 precipitation | [116], 2016 |
| Thermal conductivity predictive models | Predicting the thermal conductivities of MICP-treated sands | [117], 2020 |
| A small repeated five-point treatment model | Predicting solidification treatment in large-scale field experiments | [118], 2014 |
| The biogrouting foam model | Simulating key solidification processes such as on-site bacterial solution perfusion and adhesion and urea hydrolysis | [119], 2019 |
| Microbe Name | Optimum Curing Condition | Reference, Year | |||
|---|---|---|---|---|---|
| Microbial Concentration | The Concentration of Cementation Solution | Temperature | Cure Time | ||
| Sporosarcina pasteurii | 1 × 107 cells/mL | 0.5 M urea-CaCl2 | 20 °C | 16 days | [121], 2012 |
| B. megaterium | 1 × 108 cfu/mL | 0.5 M urea-CaCl2 | 22–27 °C | 48 h | [122], 2014 |
| Parahodobacter sp. | 109 cfu/mL | 0.5 M urea-CaCl2 | 30 °C | 21 days | [96], 2015 |
| Sporosarcina pasteurii | OD600 = 4 | 3.0 M urea, 1.5 M CaCl2 | 30 °C | 21 days | [123], 2016 |
| Staphylococcus succinu | OD600 = 0.7 | 40 mmol Ca2+, 6%(w/w) urea | 30 °C | 35 days | [98], 2019 |
| S. aquimarina | 12.8 × 109 cells/ml | 0·25 M urea, 2 M CaCl2 | — | — | [124], 2018 |
| S. pasteurii | 9 × 109 cells/ml | 1 M urea, 2 M CaCl2 | — | — | |
| Pararhodobacter sp. | — | 0.5 M CaCl2 | 25 °C | 14 days | [44], 2018 |
| Sporosarcina pasteurii | 1 × 108 cells/mL | 50 mM Ca2+ | — | — | [125], 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Song, Y.; Huang, J.; Lai, C.; Jiao, H.; Fang, H.; Zhu, J.; Song, X. Critical Review of Solidification of Sandy Soil by Microbially Induced Carbonate Precipitation (MICP). Crystals 2021, 11, 1439. https://doi.org/10.3390/cryst11121439
Chen L, Song Y, Huang J, Lai C, Jiao H, Fang H, Zhu J, Song X. Critical Review of Solidification of Sandy Soil by Microbially Induced Carbonate Precipitation (MICP). Crystals. 2021; 11(12):1439. https://doi.org/10.3390/cryst11121439
Chicago/Turabian StyleChen, Liuxia, Yuqi Song, Jicheng Huang, Chenhuan Lai, Hui Jiao, Hao Fang, Junjun Zhu, and Xiangyang Song. 2021. "Critical Review of Solidification of Sandy Soil by Microbially Induced Carbonate Precipitation (MICP)" Crystals 11, no. 12: 1439. https://doi.org/10.3390/cryst11121439
APA StyleChen, L., Song, Y., Huang, J., Lai, C., Jiao, H., Fang, H., Zhu, J., & Song, X. (2021). Critical Review of Solidification of Sandy Soil by Microbially Induced Carbonate Precipitation (MICP). Crystals, 11(12), 1439. https://doi.org/10.3390/cryst11121439








