Review and Recalculation of Growth and Nucleation Kinetics for Calcite, Vaterite and Amorphous Calcium Carbonate
Abstract
:1. Introduction
2. Theory
3. Results and Discussion
3.1. Recalculating Saturation Values
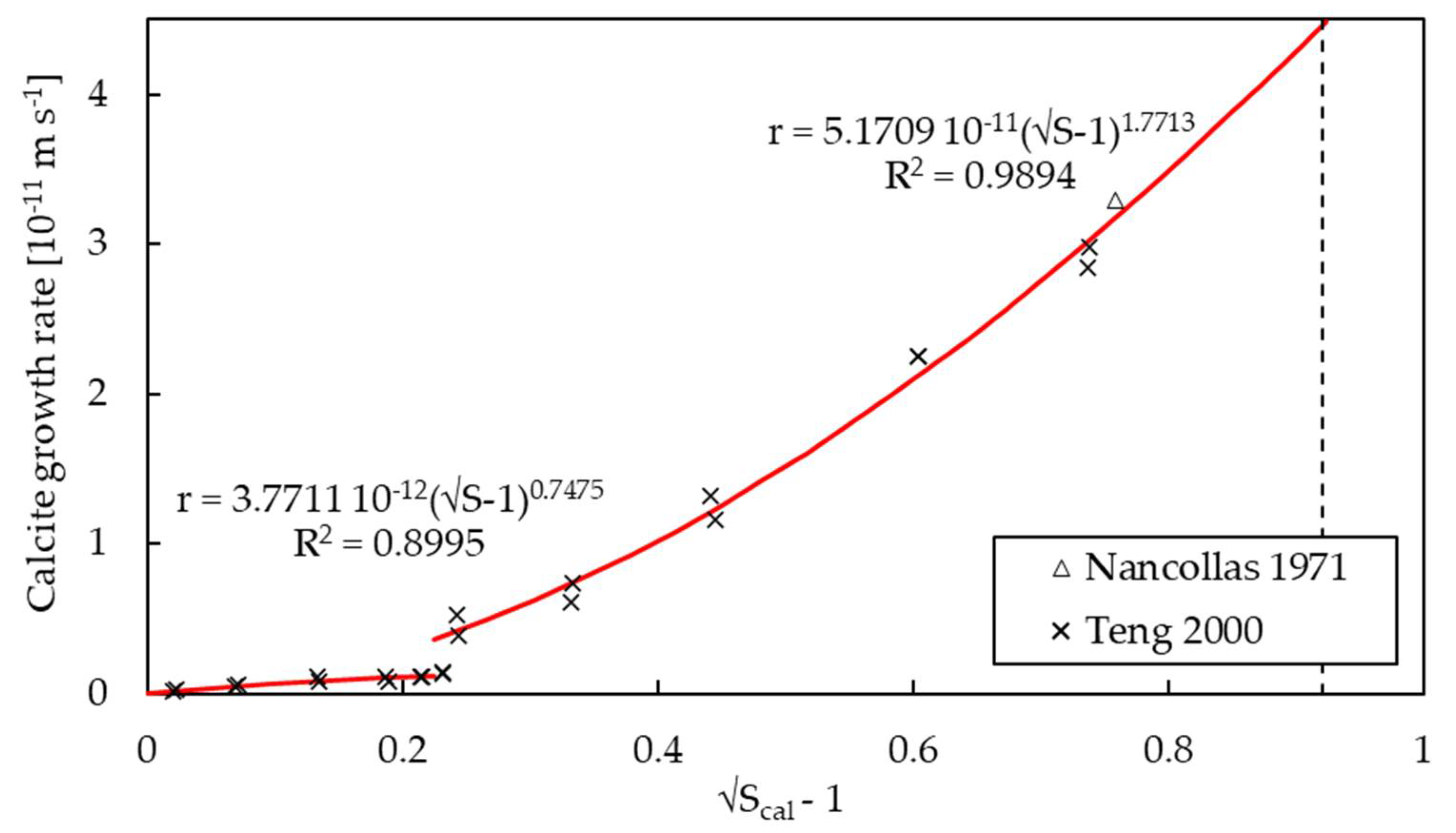
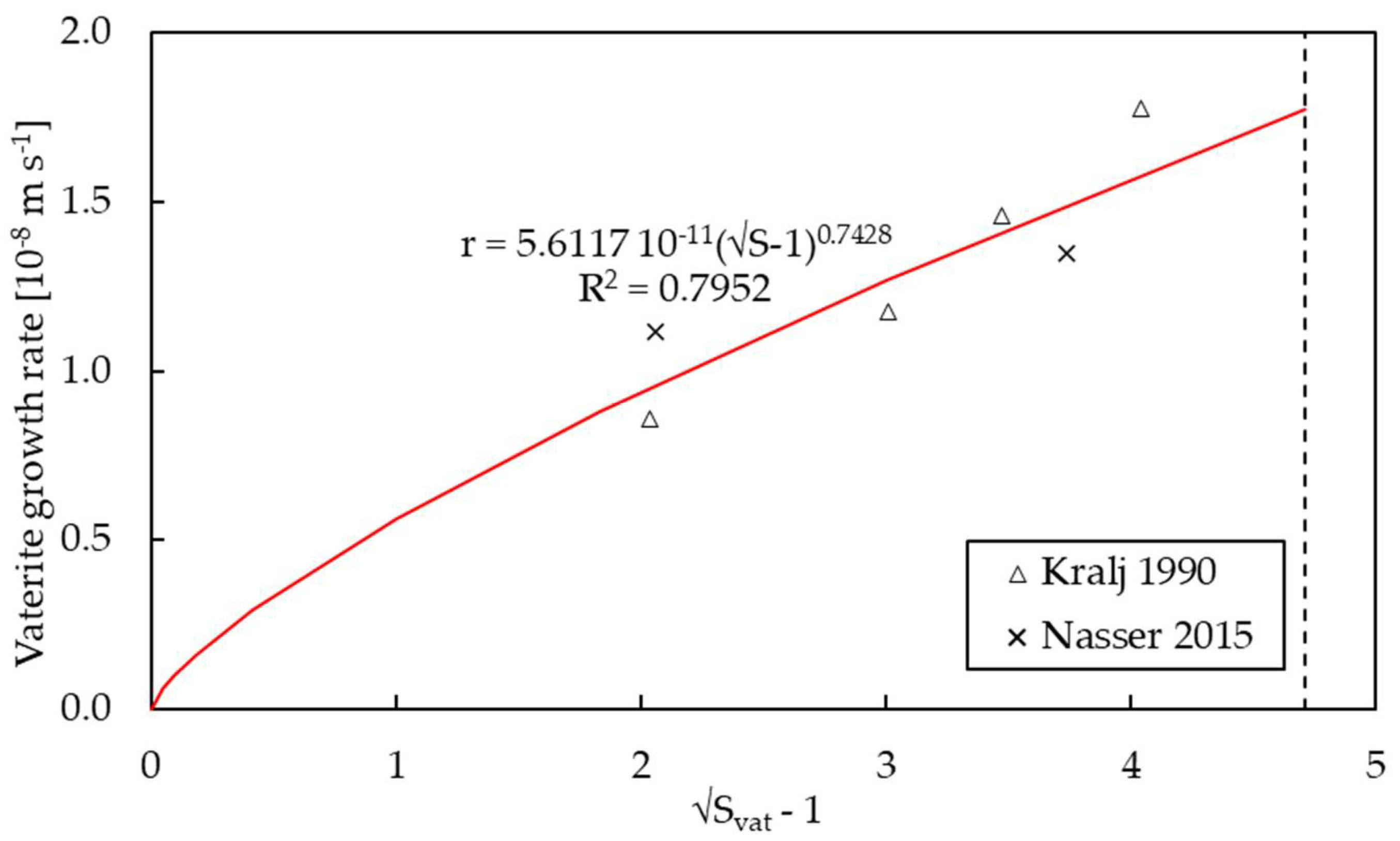
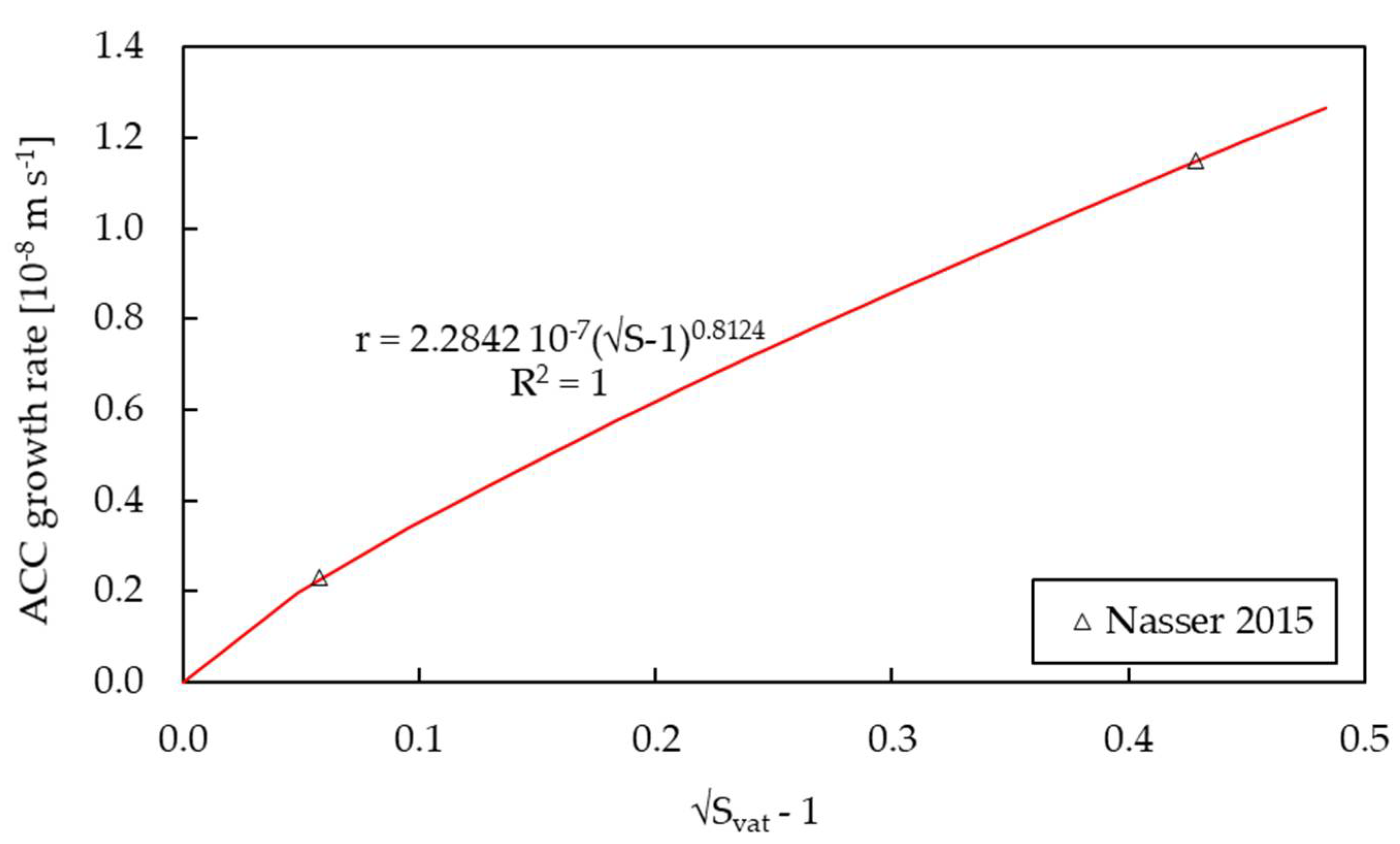
3.2. Kinetics of Growth
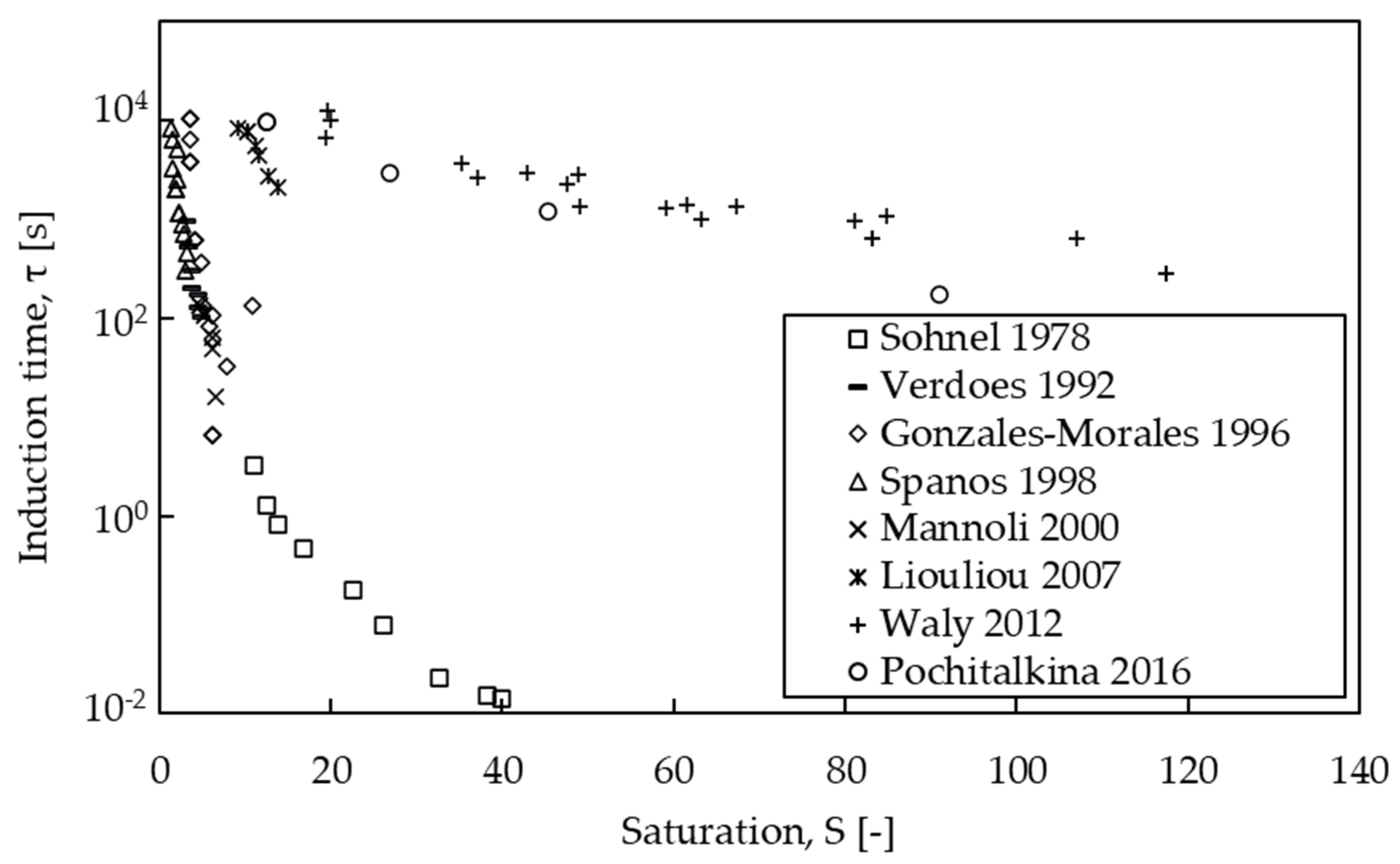
3.3. Kinetics of Nucleation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuddas, P.; Mucci, A. Kinetics of calcite precipitation from seawater: I. A classical chemical kinetics description for strong electrolyte solutions. Geochem. Cosmochem. Acta 1994, 58, 4353–4362. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chang, M.C. Growth of calcite seeds in a magnetized environment. J. Cryst. Growth 2014, 389, 5–11. [Google Scholar] [CrossRef]
- Chhim, N.; Kharbachi, C. Inhibition of calcium carbonate crystal growth by organic additives using the constant composition method in conditions of recirculating cooling circuits. J. Cryst. Growth 2017, 472, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.M.; Nancollas, G.H. The Crystallization of Calcium Carbonate 1. Isotopic Exchange and Kinetics. J. Colloid Interface Sci. 1971, 36, 166–172. [Google Scholar] [CrossRef]
- Alamdari, A.; Alamdari, A.; Mowla, D. Kinetics of calcium carbonate precipitation through CO2 absorption from flue gas into distiller waste of soda ash plant. J. Ind. Eng. Chem. 2014, 20, 3480–3486. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Kim, D.H.; Mahabadi, N.; Jang, J.; van Paassen, L.A. Assessing the kinetics and pore-scale characteristics of biological calcium carbonate precipitation in porous media using a microfluidic chip experiment. Water Resour. Res. 2021, 56, e2019WR025420. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.N. A comparative study of the dissolution and crystal growth kinetics of calcite and aragonite. Stud. Diagenesis USGS Bull. 1986, 1578, 139–168. [Google Scholar]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Kaschiev, D.; Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Parkhurst, D.L. User’s Guide to PHREEQC: A Computer Program for Speciation, Reaction-Path, Advective-Transport and Inverse Geochemical Calculations; United States Geological Survey: Lakewood, CO, USA, 1995.
- Ogino, T.; Suzuki, T.; Sawada, K. The formation and transformation mechanism of calcium carbonate in water. Geochem. Cosmochim. 1987, 51, 2757–2767. [Google Scholar] [CrossRef]
- Davies, C.W.; Jones, A.L. The precipitation of silver chloride from aqueous solutions. Trans. Faraday Soc. 1955, 51, 812–817. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Vekilov, P. Principles of crystal nucleation and growth. In Biomineralization; Reviews in Mineralogy and Geochemistry; Dove, P.M., de Yoreo, J.J., Eds.; Mineralogical Society of America: Washington, DC, USA, 2003; pp. 57–93. [Google Scholar]
- Nancollas, G.H.; Reddy, M.M. The Crystallization of Calcium Carbonate 2. Calcite Growth Mechanism. J. Colloid Interface Sci. 1971, 37, 824–830. [Google Scholar] [CrossRef]
- Mucci, A. Growth kinetics and composition of magnesian calcite overgrowths precipitated from seawater: Quantitative influence of orthophosphate ions. Geochim. Cosmochim. Acta 1986, 50, 2255–2265. [Google Scholar] [CrossRef]
- Mucci, A.; Canuel, R.; Zhong, S. The solubility of calcite and aragonite in sulfate-free seawater and the seeded growth kinetics and composition of the precipitates at 25 °C. Chem. Geol. 1989, 74, 309–320. [Google Scholar] [CrossRef]
- Dromgoole, E.L.; Walter, L.M. Inhibition of calcite growth rates by Mn2= in CaCl2 solutions at 10, 25, and 50 C. Geochim. Cosmochim. Acta 1990, 54, 2991–3000. [Google Scholar] [CrossRef]
- Gutjahr, A.; Dabringhaus, H.; Lacmann, R. Studies of the growth and dissolution kinetics of the CaCO3 polymorphs calcite and aragonite I. Growth and dissolution rates in water. J. Cryst. Growth 1996, 158, 296–309. [Google Scholar] [CrossRef]
- Gomez-Morales, J.; Torrent-Burgues, J. Precipitation of calcium carbonate from solutions with varying Ca2+/carbonate ratios. J. Cryst. Growth 1996, 166, 1020–1026. [Google Scholar] [CrossRef]
- Kralj, D.; Brecivic, L. Vaterite growth and dissolution in aqueous solution III. Kinetics of transformation. J. Cryst. Growth 1997, 177, 248–257. [Google Scholar] [CrossRef]
- Van der Weijden, R.D.; van der Heijden, A.E. The influence of total clacium and total carbonate on the growth rate of calcite. J. Cryst. Growth 1997, 171, 190–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawe, R. The kinetics of calcite precipitation. Appl. Geochem. 1998, 13, 177–184. [Google Scholar] [CrossRef]
- Zuddas, P.; Mucci, A. Kinetics of calcite precipitaiton from seawater: II. The influence of the ionic strength. Geochim. Cosmochim. Acta 1998, 62, 757–766. [Google Scholar] [CrossRef]
- Lebron, I.; Suarez, D.L. Calcite nucleation and precipitation kinetics as affected by dissolved organic matter at 25 C and pH > 7.5. Geochem. Cosmochem. 1996, 60, 2765–2776. [Google Scholar] [CrossRef]
- Nilsson, Ö.; Sternbeck, J. A mechanistic model for calcite crystal growth using surface speciation. Geochim. Cosmochim. Acta 1999, 63, 217–225. [Google Scholar] [CrossRef]
- Parsiegla, K.I.; Katz, J.L. Calcite growth inhibition by copper(II) I. Effect of supersaturation. J. Cryst. Growth 1999, 200, 213–226. [Google Scholar] [CrossRef]
- Van der Weijden, C.H.; van der Weijden, R.D. Calcite growth: Rate dependence on saturation, on ratios of dissolved calcium and (bi)carbonate and on their complexes. J. Cryst. Growth 2014, 394, 137–144. [Google Scholar] [CrossRef]
- Zhu, G.; Li, H. Crystallization behavior and kinetics of calcium carbonate in highly alkaline and supersaturated system. J. Cryst. Growth 2015, 428, 16–23. [Google Scholar] [CrossRef]
- Kralj, D.; Brecivic, L. Vaterite growth and dissolution in aqueous solution I. Kinetics of crystal growth. J. Cryst. Growth 1990, 104, 793–800. [Google Scholar] [CrossRef]
- Spanos, N.; Koutsoukos, P.G. Kinetics of Precipitation of Calcium Carbonate in Alkaline pH at Constant Supersaturation. Spontaneous and Seeded Growth. J. Phys. Chem. B 1998, 102, 6679–6684. [Google Scholar] [CrossRef]
- Olderoy, M.O.; Xie, M. Growth and Nucleation of Calcium Carbonate Vaterite Crystals in Presence of Alginate. Cryst. Growth Des. 2009, 9, 5176–5183. [Google Scholar] [CrossRef]
- Andreassen, J.P.; Hounslow, M.J. Growth and Aggregation of Vaterite in Seeded-Batch Experiments. AIChE J. 2004, 50, 2772–2782. [Google Scholar] [CrossRef]
- Wolthers, M.; Nehrke, G. Calcite growth kinetics: Modeling the effect of solution stoichiometry. Geochim. Cosmochim. Acta 2012, 77, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.P.; Dobberschütz, S. A Microkinetic Model of Calcite Step Growth. Angew. Chem. Int. Ed. 2016, 55, 11086–11090. [Google Scholar] [CrossRef]
- Sand, K.K.; Tobler, D.J. Calcite Growth Kinetics: Dependence on Saturation Index, Ca2+:CO32− Acitvity Ratio, and Surface Atomic Structure. Cryst. Growth Des. 2016, 16, 3602–3612. [Google Scholar] [CrossRef]
- Kavanagh, A.M.; Rayment, T. Inhibitor Effects on Calcite Growth at Low Supersaturations. J. Chem. Soc. Faraday Trans. 1990, 86, 965–972. [Google Scholar] [CrossRef]
- Gomez-Morales, J.; Torrent-Burgues, J. Nucleation of calcium carbonate at different initial pH conditions. J. Cryst. Growth 1996, 169, 331–338. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, J.Y. Nucleation and growth kinetics in synthesizing nanometer calcite. J. Cryst. Growth 2002, 245, 309–320. [Google Scholar] [CrossRef]
- Sugiura, Y.; Onuma, K. Growth dynamics of vaterite in relation to the physico-chemical properties of its precursor, amorphous calcium carbonate, in the Ca-CO3-PO4 system. Am. Mineral. 2016, 101, 289–296. [Google Scholar] [CrossRef]
- Lorens, R.B. Sr, Cd, Mn and Co distribution coefficients in calcite as a function of calcite precipitation rate. Geochim. Cosmochim. Acta 1981, 45, 553–561. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. The effect of sodium alginate on the crystal growth of calcium carbonate. J. Mater. Sci. Mater. Med. 2002, 13, 155–158. [Google Scholar] [CrossRef]
- Tai, C.Y.; Chang, M.C. Magnetic effects on crystal growth rate of calcite in a constant-composition environment. J. Cryst. Growth 2008, 310, 3690–3697. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Putnis, C.V. An Atomic Force Microscopy study of the growth of calcite in the presence of sodium sulfate. Chem. Geol. 2008, 253, 243–251. [Google Scholar] [CrossRef]
- Perdikouri, C.; Putnis, C.V. An Atomic Force Microscopy Study of the Growth of a Calcite Surface as a Function of Calcium/Total Carbonate Concentration Ratio in Solution at Constant Supersaturation. Cryst. Growth Des. 2009, 9, 4344–4350. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V. Specific effects of background electrolytes on the kinetics of step propagation during calcite growth. Geochim. Cosmochim. Acta 2011, 75, 3803–3814. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V. Effect of pH on calcite growth at constant aCa2+/aCO32- ratio and supersaturation. Geochim. Cosmochim. Acta 2011, 75, 284–296. [Google Scholar] [CrossRef]
- Gebrehiwet, T.A.; Reddem, G.D. The Effect of the CO32− to Ca2+ Ion activity ratio on calcite precipitation kinetics and Sr2+ partitioning. Geochem. Trans. 2012, 13, 1. [Google Scholar] [CrossRef]
- Hong, M.; Teng, H.H. Implications of solution chemistry effects: Direction-specific restraints on the step kinetics of calcite growth. Geochim. Cosmochim. Acta 2014, 141, 228–239. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Sand, K.K. Inhibition of Calcite Growth: Combined Effects of Mg2+ and SO42−. Cryst. Growth Des. 2016, 16, 6199–6207. [Google Scholar] [CrossRef]
- Teng, H.H.; Dove, P.M. Kinetics of calcite growth: Surface processes and relationships to macroscopic rate laws. Geochim. Cosmochim. Acta 2000, 64, 2255–2266. [Google Scholar] [CrossRef]
- Bracco, J.N.; Grantham, M.C. Calcite Growth Rates As a Function of Aqueous Calcium-to-Carbonate Ratio, Saturation Index, and Inhibitor Concentration: Insight into the Mechanism of Reaction and Poisoning by Strontium. Cryst. Growth Des. 2012, 12, 3540–3548. [Google Scholar] [CrossRef]
- Nasser, W.N.; Al Salhi, F.H. Kinetics determination of calcium carbonate precipitation behavior by inline techniques. Powder Technol. 2015, 270, 548–560. [Google Scholar] [CrossRef]
- Mucci, A.; Morse, J.W. The incorporation of Mg2+ and Sr2+ into calcite overgrowths: Influences of growth rate and solution composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Verdoes, D.; Kashchiev, D. Determination of nucleation and growth rate froms induction times in seeded and unseeded precipitation of calcium carbonate. J. Cryst. Growth 1992, 118, 401–413. [Google Scholar] [CrossRef]
- Waly, T.; Kennedy, M.D. On the induction time of CaCO3: Effect of ionic strength. Desalination Water Treat. 2012, 39, 55–69. [Google Scholar] [CrossRef]
- Söhnel, O.; Mullin, J.W. A method for the determination of precipitation induction periods. J. Cryst. Growth 1978, 44, 377–382. [Google Scholar] [CrossRef]
- Söhnel, O.; Mullin, J.W. Precipitation of calcium carbonate. J. Cryst. Growth 1982, 60, 239–250. [Google Scholar] [CrossRef]
- Liouliou, M.G.; Paraskeva, C.A. Heterogeneous nucleation and growth of calcium carbonate on calcite and quartz. J. Colloid Interface Sci. 2007, 308, 421–428. [Google Scholar] [CrossRef]
- Flaten, E.M.; Seiersten, M. Induction time studies of calcium carbonate in ethylene glycol and water. Chem. Eng. Res. Des. 2010, 88, 1659–1668. [Google Scholar] [CrossRef]
- He, S.; Kan, A.T. Inhibition of calcium carbonate precipitation in NaCl brines from 25 to 90 °C. Appl. Geochem. 1999, 14, 17–25. [Google Scholar] [CrossRef]
- Pochitalkina, I.A.; Kekin, P.A. Crystallization Kinetics of Calcium Carbonate at a Stoichiometric Ratio of Components. Russ. J. Phys. Chem. A 2016, 90, 2346–2351. [Google Scholar] [CrossRef]
- Brecivic, L.; Nielsen, A.E. Solubility of amorphous calcium carbonate. J. Cryst. Growth 1989, 98, 504–510. [Google Scholar] [CrossRef]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, J.H.E.; Checa, A.G. Calcium carbonate polymorphism and its role in biomineralisation: How many ACCs are there? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Bots, P.; Benning, L.G. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Saharay, M.; Yazaydin, A.O. Dehydration-Induced Amorphous Phases of Calcium Carbonate. J. Phys. Chem. B 2013, 117, 3328–3336. [Google Scholar] [CrossRef] [PubMed]

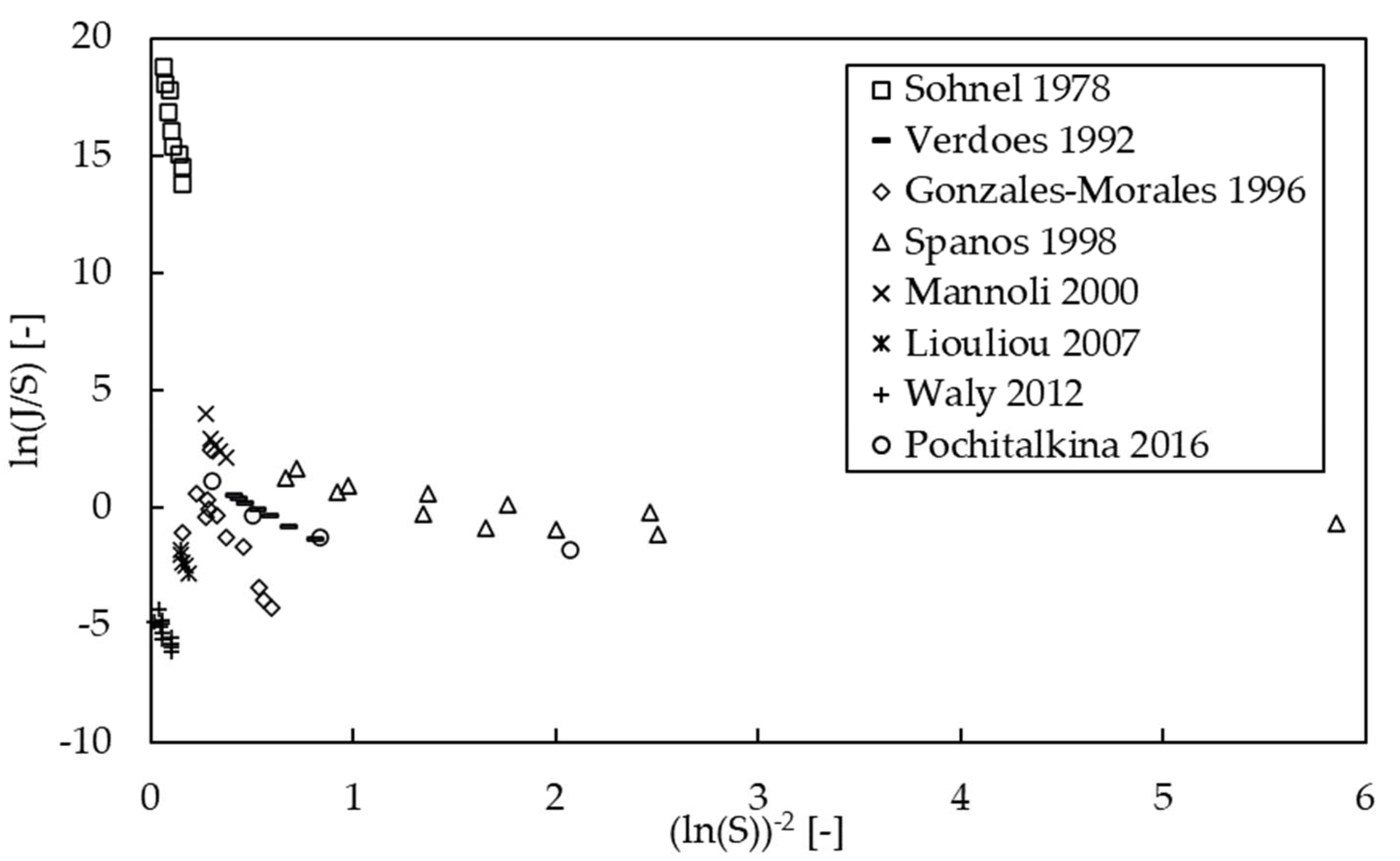
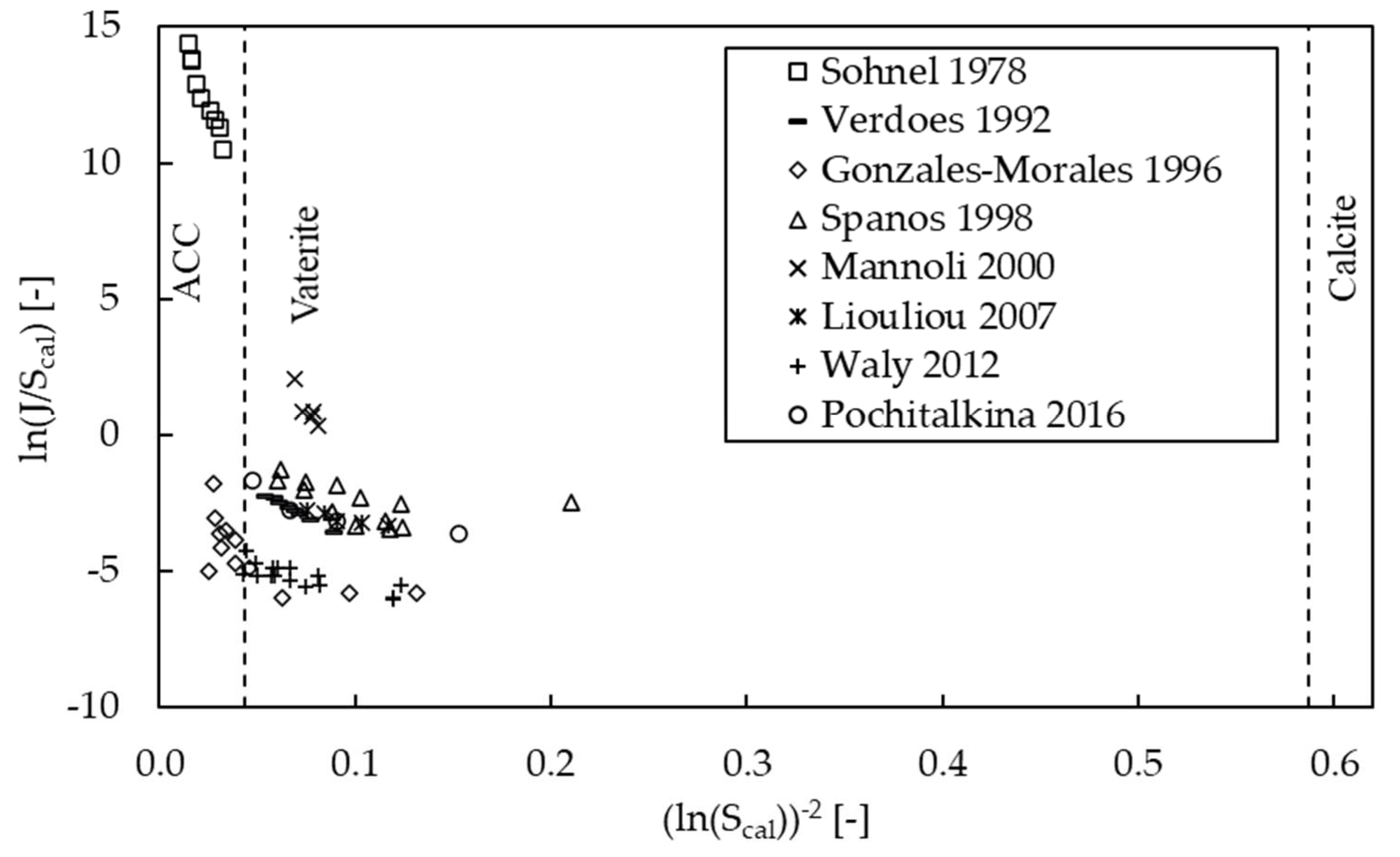

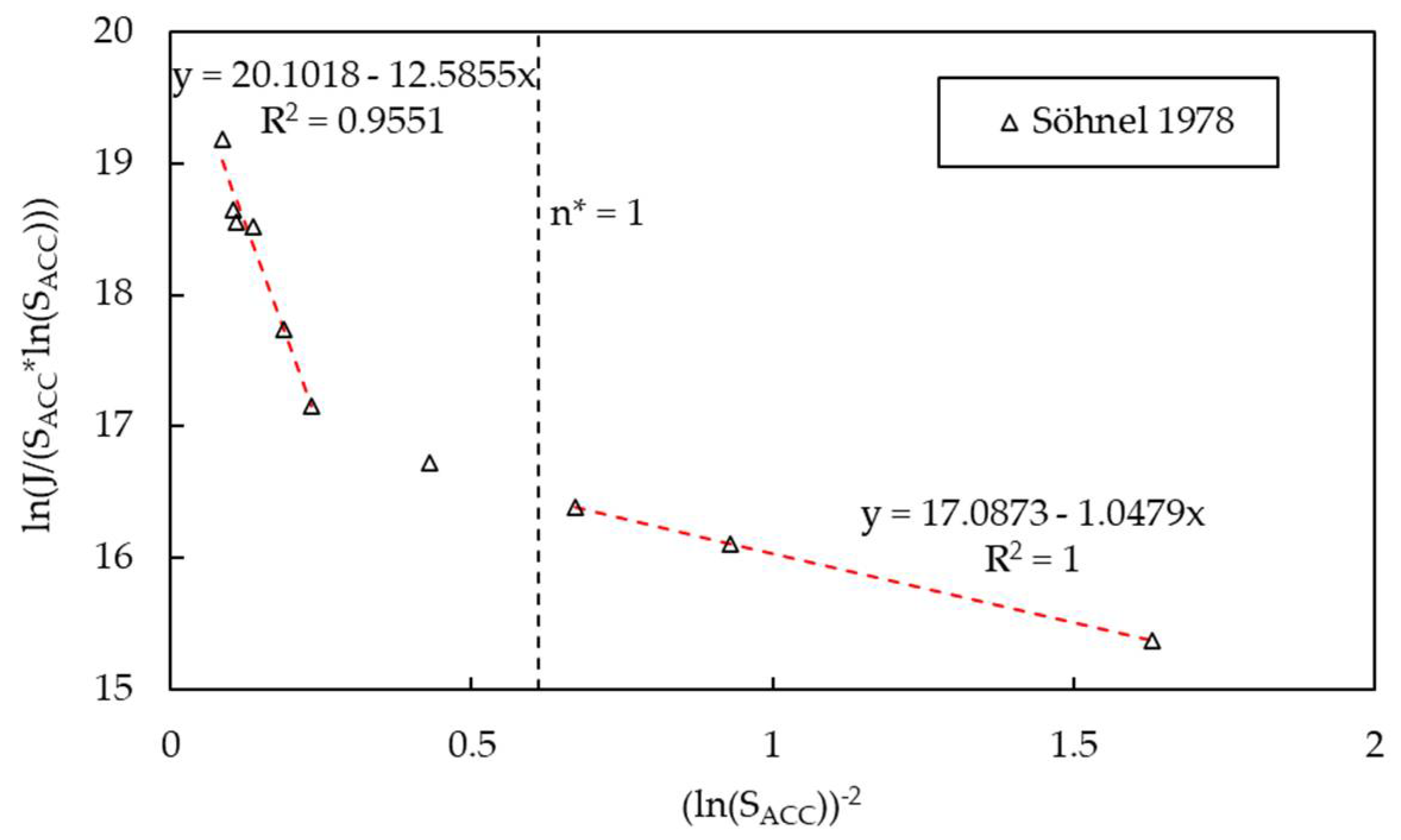
| Polymorph | Growth Rate Constant | Unit | Reference |
|---|---|---|---|
| Calcite | 4.0–8.8 | L mol−1 s−1 (gseed L−1)−1 | [4,15] |
| 6.48 × 104 | L2 mol−1 m−2 s−1 | [25] | |
| 1.43 × 10−10–1.08 × 10−9 | mol m−2 s−1 | [16,17] | |
| 5.37 × 10−7–2.34 × 10−6 | mol m−2 s−1 | [18] | |
| 2.91 × 10−9–1.39 × 10−6 | mol m−2 s−1 | [1] | |
| 1.64 × 10−7–2.81 × 10−7 | mol m−2 s−1 | [19] | |
| 2.21 × 10−8–7.48 × 10−4 | mol m−2 s−1 | [24] | |
| 3.42 × 10−7 | mol m−2 s−1 | [26] | |
| 6.64 × 10−8–7.80 × 10−7 | mol m−2 s−1 | [27] | |
| 5.8 × 10−7–3.35 × 10−6 | mol m−2 s−1 | [28] | |
| 8.56 × 10−8–2.65 × 10−7 | mol m−2 s−1 kgw−1 | [23] | |
| 4.05 × 10−11–1.98 × 10−10 | m s−1 | [20] | |
| 5.0 × 10−10 | m s−1 | [21] | |
| 1.1 × 10−10 | m s−1 | [22] | |
| 1.13 × 10−10–9.2 × 10−10 | m s−1 | [6] | |
| 8.08 × 10−26 | m3 s−1 | [29] | |
| Vaterite | 5.60 × 10−10–6.10 × 10−10 | m s−1 | [30] |
| 1.06–1.17 × 10−10 | m s−1 | [31] | |
| 1.71 × 10−9 | m s−1 | [32] | |
| 5.27 × 10−10 | m s−1 | [33] |
| Nancollas 1971 [15] | Kralj 1990 [30] | Van der Weijden 1997 [22] | Spanos 1998 [31] | Teng 2000 [51] | Bracco 2012 [52] | Van der Weijden 2014 [28] | Nasser 2015 [53] | |
|---|---|---|---|---|---|---|---|---|
| Reported morphology | Calcite | Vaterite | Calcite | Vaterite | Calcite | Calcite | Calcite | Calcite, Vaterite, ACC |
| Growth rate constant | 4.0–8.8 | 5.60–6.10 × 10−10 | 1.1 × 10−10 | 1.06–1.17 × 10−10 | - | - | 5.8 × 10−7–3.35 × 10−6 | - |
| Unit | L mol−1 s−1 (gseed L−1)−1 | m s−1 | m s−1 | m s−1 | - | - | mol m−2 s−1 | - |
| Temperature (°C) | 25 | 25 | 22 | 25 | 25 | 25 | 22 | 25 |
| pH | 8.53 | 9.57–9.85 | 7.56–8.90 | 9–10 | 8.5 | 8.25–9.35 | 7.56–8.90 | 7.62–7.84 |
| Total calcium concentration (mM) | 0.149 | 2.5 | 0.35–0.97 | 0.4–2.5 | 0.16–0.34 | 0.035–0.82 | 0.25–0.97 | 10–50 |
| Total carbonate concentration (mM) | 10.1 | 2.5 | 6.4–68 | 0.4–2.5 | 5.00–10.1 | 1.25–20.0 | 6.4–68 | 10–50 |
| Gaseous CO2 exchange | Unknown | No | Yes | No | No | Yes | Yes | No |
| Ionic strength (mM) | 15.8 | 11.2–317 | 751–856 | 1.85–11.6 | 106–112 | 3.51–22.2 | 0.75–0.86 | 38.4–184 |
| Other ions | Na, Cl | Na, Cl | K, Cl | Na, K, NO3 | Na, Cl | Na, Cl | K, Cl | Na, Cl |
| Söhnel 1978 [57] | Verdoes 1992 [55] | Gomez-Morales 1996 [38] | Spanos 1998 [31] | Mannoli 2000 [42] | Liouliou 2007 [59] | Waly 2012 [56] | Pochitalkina 2016 [62] | |
|---|---|---|---|---|---|---|---|---|
| Reported morphology | Unspecified | Vaterite | Vaterite + Calcite | Vaterite | Vaterite | Calcite | Vaterite + Calcite | Calcite |
| Working volume (L) | Variable a | 1.3 | 2 | 0.2 | 0.2 | 0.2 | 2 | 0.3 |
| pH range | 10.6–10.9 | 10 | 7.8–10 | 9-10 | 8.5 | 8.5 | 7.8–8.8 | 10 |
| Total calcium concentration (mM) | 3.2–33 | 0.2–0.5 | 10 | 0.4–3 | 4–4.75 | 3.82–5.48 | 16.9–23.7 | 1.1–3.3 |
| Total carbonate concentration (mM) | 32–33 | 28.3 | 10 | 0.4–3 | 4–4.75 | 3.82–5.48 | 3.43–4.80 | 1.1–3.3 |
| Ionic strength (mM) | 12.9–106 | 49.0–49.1 | 503–507 | 0.68–11.6 | 156–184 | 89.5–93.0 | 52–1446 | 157–166 |
| Detection method | Conducto-metric | Ca2+ electrode | pH measurement | pH measurement | pH measurement | pH measurement | pH measurement | Laser optical |
| Limitation | diffusion | unknown | kinetic | kinetic | unknown | kinetic | kinetic | kinetic |
| Other ions present | Na, Cl | Na, Cl | K, Cl | Na, NO3, K | Na, NO3, K | Na, NO3, K | Na, Cl | Na, Cl |
| Calcite | Vaterite | ACC1 | ACC2 | ||
|---|---|---|---|---|---|
| K | mol2 kgw−2 | 10−8.48 | 10−7.913 | 10−6.40 | 10−5.87–10−5.51 e |
| k | m s−1 | 3.7711 × 10−12 a 5.1709 × 10−11 a | 5.6117 × 10−9 | 2.2842 × 10−7 f | n/a |
| g | - | 0.7475 a 1.7713 a | 0.7428 | 0.8124 f | n/a |
| Ankin | m−3 s−1 | n/a | 0.282 | 2.96 × 108 bc | n/a |
| Andiff | m−3 s−1 | n/a | 0.0208 b | 2.64 × 107 c | 5.37 × 108 cd |
| Bn | - | n/a | 1.8337 | 1.0479 c | 12.59 cd |
| γ | mJ m−2 | n/a | 16.9 | 10.2 c | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergwerff, L.; van Paassen, L.A. Review and Recalculation of Growth and Nucleation Kinetics for Calcite, Vaterite and Amorphous Calcium Carbonate. Crystals 2021, 11, 1318. https://doi.org/10.3390/cryst11111318
Bergwerff L, van Paassen LA. Review and Recalculation of Growth and Nucleation Kinetics for Calcite, Vaterite and Amorphous Calcium Carbonate. Crystals. 2021; 11(11):1318. https://doi.org/10.3390/cryst11111318
Chicago/Turabian StyleBergwerff, Luke, and Leon A. van Paassen. 2021. "Review and Recalculation of Growth and Nucleation Kinetics for Calcite, Vaterite and Amorphous Calcium Carbonate" Crystals 11, no. 11: 1318. https://doi.org/10.3390/cryst11111318
APA StyleBergwerff, L., & van Paassen, L. A. (2021). Review and Recalculation of Growth and Nucleation Kinetics for Calcite, Vaterite and Amorphous Calcium Carbonate. Crystals, 11(11), 1318. https://doi.org/10.3390/cryst11111318






