Microstructure and Corrosion Resistance in Bimetal Materials of Q345 and 308 Steel Wire-Arc Additive Manufacturing
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Experimental System and Process Parameters
2.3. Microstructure Observation and Performance Test
3. Results and Discussion
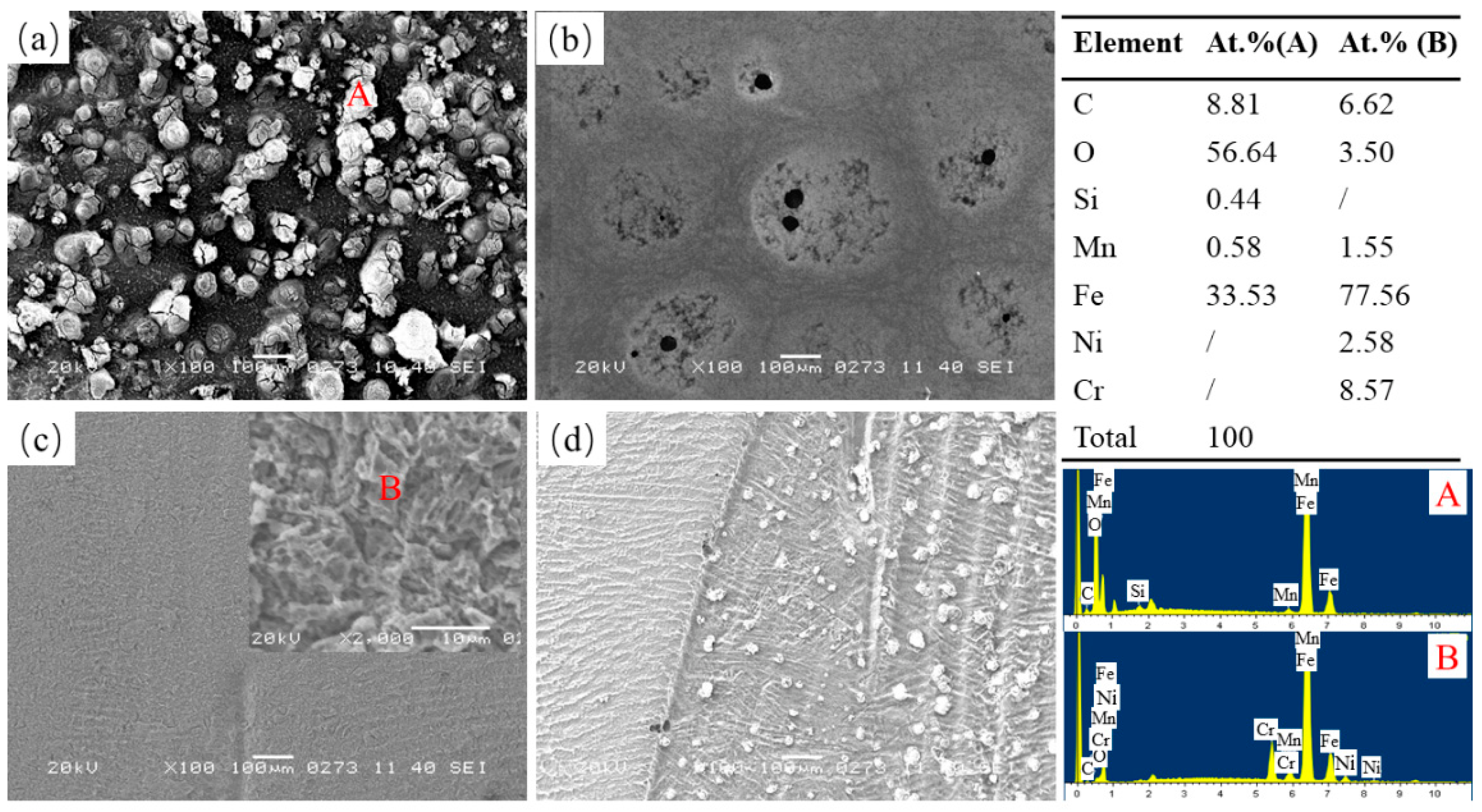
3.1. Macroscopic and Microstructure Observation
3.2. Hardness Analysis
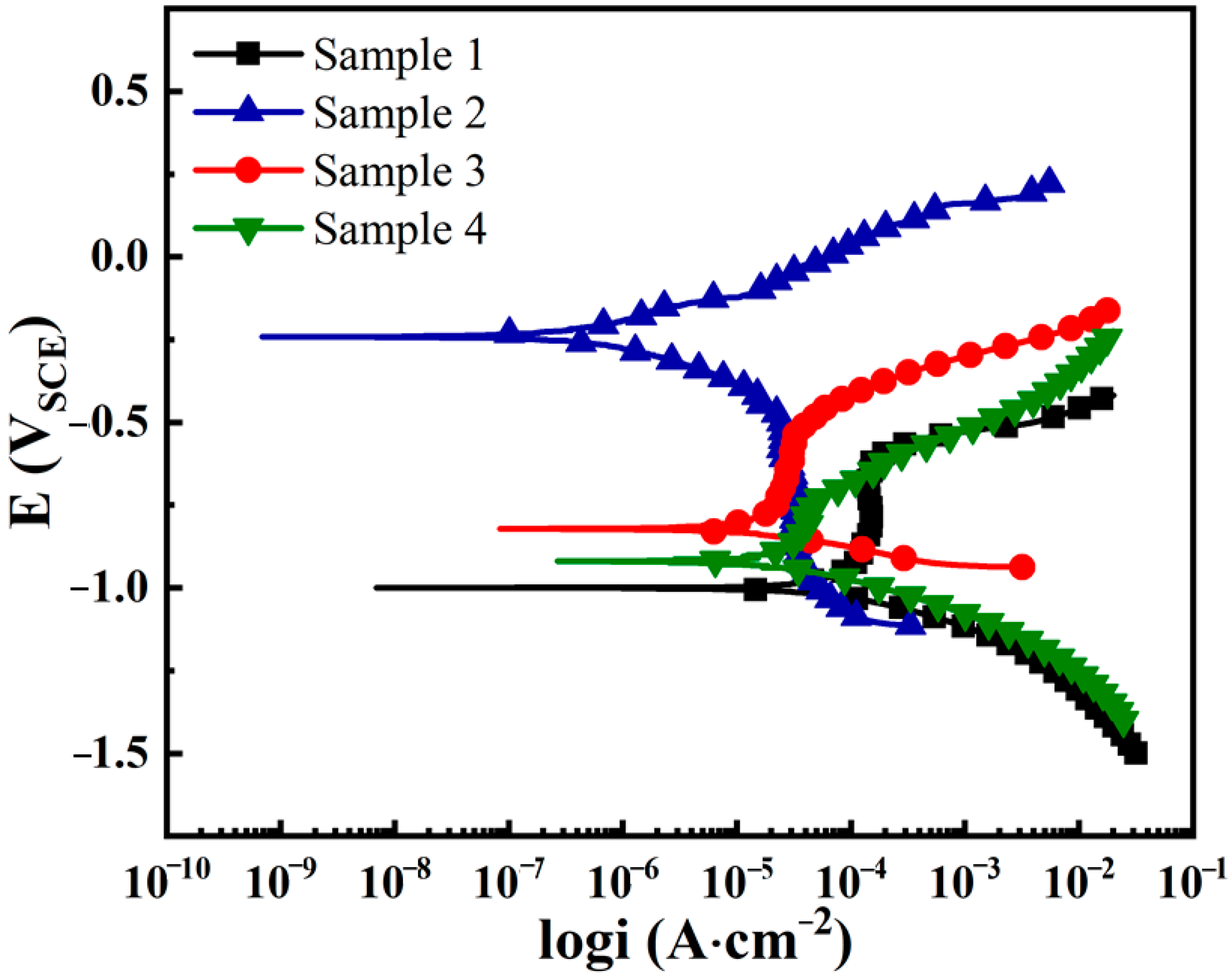
3.3. Corrosion Resistance
4. Conclusions
- (1)
- The Q345/308 bimetallic material parts were fabricated by wire-arc additive manufacturing. Its microstructure depended on the process. When the feeding wire mode was horizontal parallel wire feeding, its microstructure was fine and had many sub-crystal boundaries, and there was no apparent interface between Q345 and 308. When the feeding wire mode was upper and lower interval wire feeding, it had an obvious interface. The average hardness of bimetal wire additive was higher than that of single wire-arc additive manufacturing.
- (2)
- The corrosion resistance of bimetal wire Q345/308 wire-arc additive manufacturing was better than that of Q345. Pitting corrosion had been improved with Q345 and 308 bimetallic additive manufacturing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loh, G.H.; Pei, E.; Harrison, D.; Monzon, M.D. An overview of functionally graded additive manufacturing. Addit. Manuf. 2018, 23, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Monkova, K.; Zetkova, I.; Kučerova, L.; Zetek, M.; Monka, P.; Dana, M. Study of 3D printing direction and effects of heat treatment on mechanical properties of MS1 maraging steel. Arch. Appl. Mech. 2019, 89, 791–804. [Google Scholar] [CrossRef]
- Monkova, K.; Monka, P.P.; Tkac, J.; Vanca, J. A bending test of the additively produced porous sample. In Proceedings of the 5th International Conference on the Industry 4.0 Model for Advanced Manufacturing, Belgrade, Serbia, 1–4 June 2020; Lecture Notes in Mechanical Engineering. Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Unocic, R.; DuPont, J. Process efficiency measurements in the laser engineered net shaping process. Metall. Mater. Trans. B 2004, 35, 143–152. [Google Scholar] [CrossRef]
- Rannar, L.E.; Glad, A.; Gustafson, C.G. Efficient cooling with tool inserts manufactured by electron beam melting. Rapid Prototyp. J. 2007, 13, 128–135. [Google Scholar] [CrossRef]
- DuPont, J.; Marder, A. Thermal efficiency of arc welding processes. Weld. J. Incl. Weld. Res. Suppl. 1995, 74, 406. [Google Scholar]
- Stenbacka, N. On arc efficiency in gas tungsten arc welding. Soldag. Inspeção 2013, 18, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; Na, S.J.; Zhang, L.J.; Wang, X.; Long, J.; Cho, W.I. Improving thermal efficiency and stability of laser welding process for magnesium alloy by combining power modulation and subatmospheric pressure environment. J. Magnes. Alloy 2021, in press. [Google Scholar] [CrossRef]
- Hu, Q.X.; Miao, J.Y.; Wang, X.L.; Li, C.T.; Fang, K.W. Microstructure and Properties of ER50-6 Steel Fabricated by Wire Arc Additive Manufacturing. Scanning 2021, 2021, 7846116. [Google Scholar] [CrossRef]
- Ma, Y.; Cuiuri, D.; Li, H.; Pan, Z.; Shen, C. The effect of postproduction heat treatment on γ-TiAl alloys produced by the GTAW-based additive manufacturing process. Mater. Sci. Eng. A 2016, 657, 86–95. [Google Scholar] [CrossRef]
- Martina, F.; Mehnen, J.; Williams, S.W.; Colegrove, P.; Wang, F. Investigation of the benefits of plasma deposition for the additive layer manufacture of Ti-6Al-4V. J. Mater. Process. Technol. 2012, 212, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Ozsoy, A.; Tureyen, E.B.; Baskan, M.; Yasa, E. Microstructure and mechanical properties of hybrid additive manufactured dissimilar 17-4 ph and 316l stainless steels. Mater. Today Commun. 2021, 28, 102561. [Google Scholar]
- Chen, N.N.; Khan, H.A.; Wan, Z.X.; Lipper, J.; Sun, H.; Shang, S.L.; Liu, Z.K.; Li, J.J. Microstructural characteristics and crack formation in additively manufactured bimetal material of 316l stainless steel and Inconel 625. Addit. Manuf. 2020, 32, 101037. [Google Scholar] [CrossRef]
- Ytab, C.; Jsab, C.; Sha, B.; Jian, G.; Yan, C. Effects of cold metal transfer mode on the reaction layer of wire and arc additive-manufactured Ti-6Al-4V/Al-6.25Cu dissimilar alloys—Sciencedirect. J. Mater. Sci. Technol. 2020, 74, 35–45. [Google Scholar]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-feed additive manufacturing of metal components: Technologies, developments and future interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481. [Google Scholar]
- Hao, X.H.; Dong, H.G.; Yu, F.Y.; Li, P.; Yang, Z.L. Arc welding of titanium alloy to stainless steel with cu foil as interlayer and ni-based alloy as filler metal. J. Mater. Res. Technol. 2021, 13, 48–60. [Google Scholar] [CrossRef]
- Shamanian, M.; Eghlimi, A.; Eskandarian, M.; Szpunar, J.A. Interface microstructure across cladding of super duplex stainless steel with austenitic stainless steel buffer layer. Surf. Coat. Technol. 2014, 259, 532–542. [Google Scholar] [CrossRef]
- Tarasov, S.Y.; Filippov, A.V.; Shamarin, N.N.; Fortuna, S.V.; Maier, G.G.; Kolubae, E.A. Microstructural evolution and chemical corrosion of electron beamwire-feed additively manufactured AISI 308 stainless steel. J. Alloys Compd. 2019, 803, 364–370. [Google Scholar] [CrossRef]
- Kura, W.; Fisher, D.J. Fundamentals of Solidification, 3rd ed.; Trans Tech Publications: Aedermansdorf, Switzerland, 1992. [Google Scholar]
- Saboori, A.; Aversa, A.; Bosio, B.; Bassini, E.; Librera, E.; De Chirico, M.; Biamino, S.; Ugues, D.; Fino, P.; Lombardi, M. An investigation on the effect of powder recycling on the microstructure and mechanical properties of AISI 316L produced by Directed Energy Deposition. Mater. Sci. Eng. A 2019, 766, 138360. [Google Scholar] [CrossRef]
- Zhang, C.H.; Zhang, H.; Wu, C.L.; Zhang, S.; Sun, Z.L.; Dong, S.Y. Multilayer functional graded stainless steel fabricated by laser melting deposition. Vaccum 2017, 141, 181–187. [Google Scholar] [CrossRef]
- Ares, A.E.; Gassa, L.M. Corrosion susceptibility of Zn-Al alloys with different grains and dendritic microstructures in NaCl solutions. Corros. Sci. 2012, 59, 290–306. [Google Scholar]
- Nady, H.; EL-Rabiei, M.M.; Samy, M. Corrosion behavior and electrochemical properties of carbon steel, commercial pure titanium, copper and copper-aluminum-nickel alloy in 35% sodium chloride containing sulfide ions. Egypt. J. Petrol. 2017, 26, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Hamadou, L.; Kadri, A.; Benbrahi, M.N. Characterisation of passive film formed on low carbon steel in borate buffer solution by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Chen, S.S.; Dou, Y.P.; Han, S.K.; Wang, L.W.; Man, C.; Wang, X.; Chen, S.G.; Cheng, Y.F.; Li, X.G. Passivation behavior and surface chemistry of 2507 super duplex stainless steel in artifificial seawater: Influence of dissolved oxygen and pH. Corros. Sci. 2019, 150, 218–234. [Google Scholar]
- Chen, C.; Liu, N.; Zhang, J.; Cao, J.; Wang, L.J.; Xiang, H.F. Microstructure stability and oxidation behavior of (FeCoNiMo)90(Al/Cr)10 high entropy alloys. Mater. Sci. Tech. 2019, 35, 1883–1890. [Google Scholar] [CrossRef]
- Liu, N.; Ding, W.; Wang, X.J.; Zhang, J.; Zhou, P.J.; Mu, C. Phase, microstructures and properties of multi-component FeCoNi-based alloys. Mater. Sci. Tech. 2020, 36, 654–660. [Google Scholar]
- Liu, N.; Xu, M.; Qian, Y.Y.; Zhou, P.J. Microstructure, Phase stability, and oxidation resistance of (FeCoNi)60Al15Cr25-xTix High entropy alloys. J. Alloys Compd. 2021, 870, 159320. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Balbo, A.; Monticelli, C.; Zucchi, F. Stree corrosion cracking of LDX 2101 duplex stainless steel in chloride solutions in the presence of thiosulphate. Corros. Sci. 2014, 80, 205–212. [Google Scholar] [CrossRef]
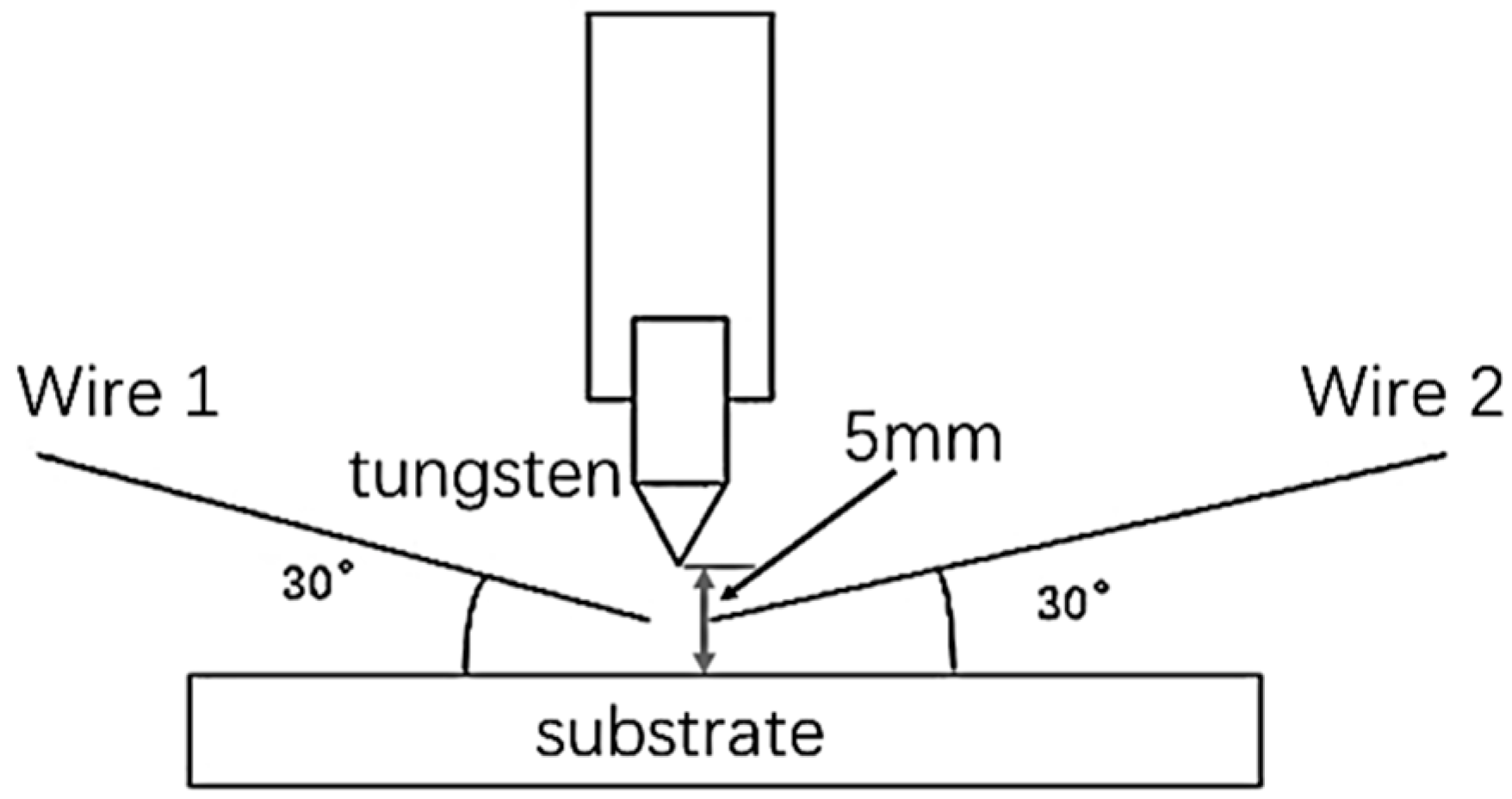
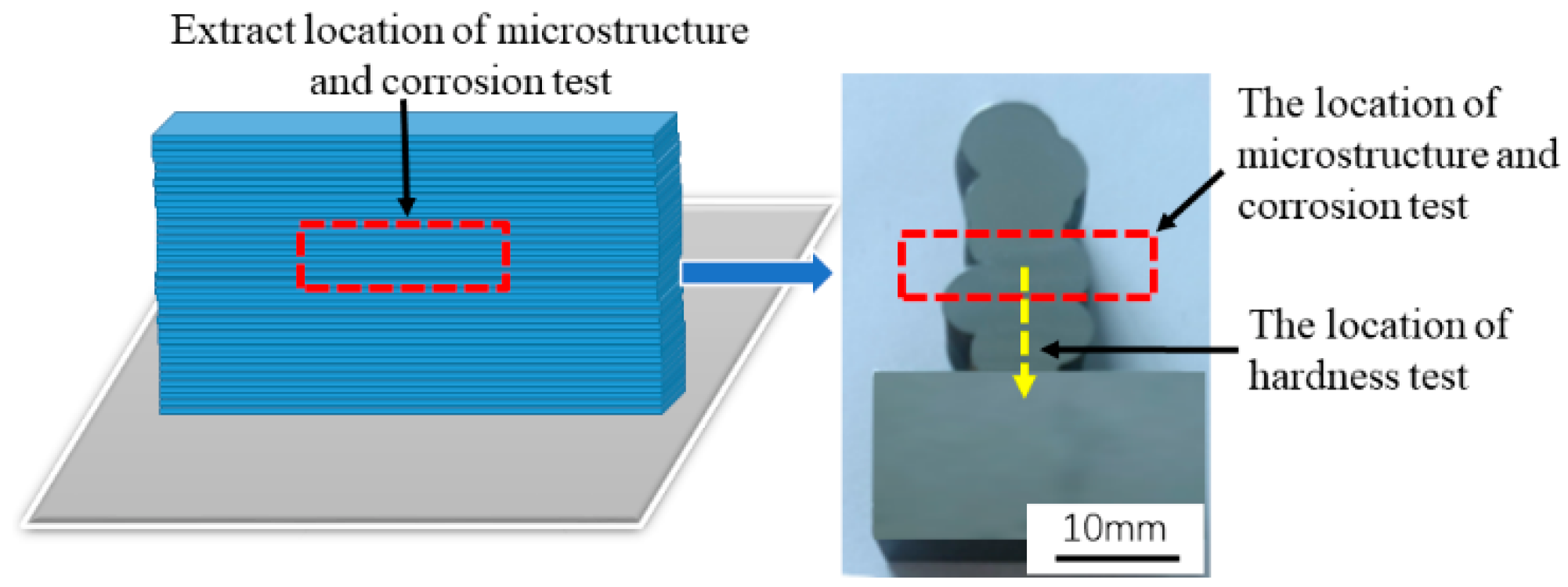
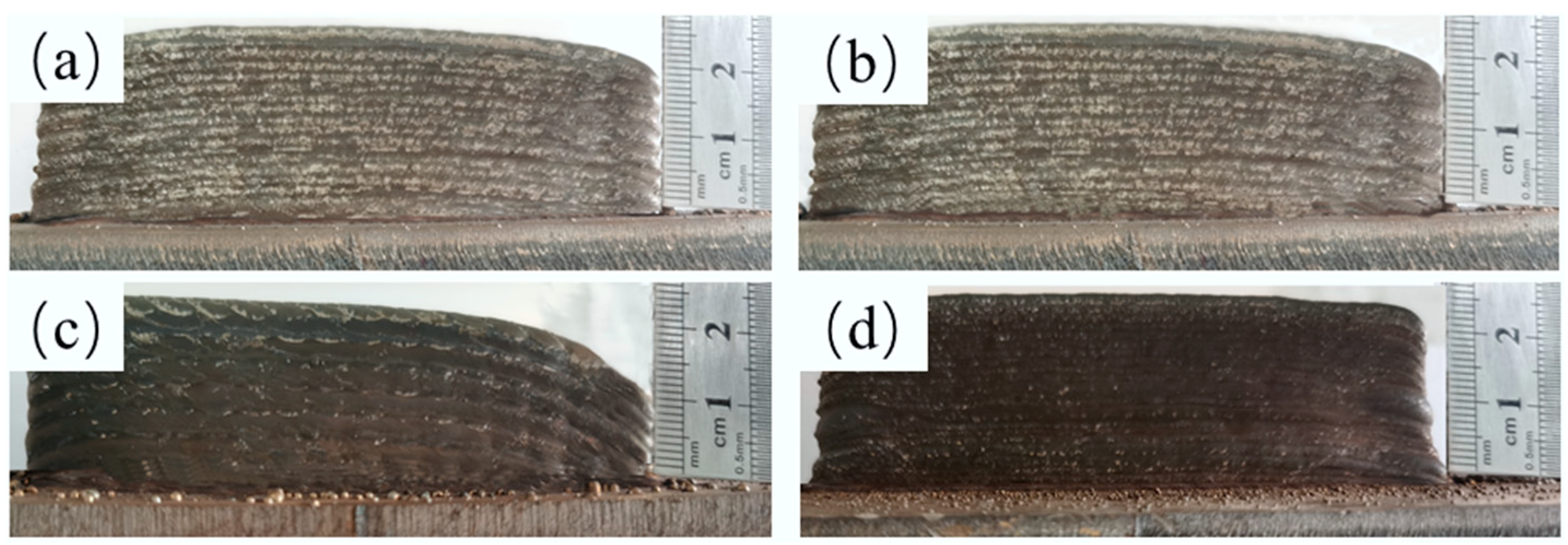

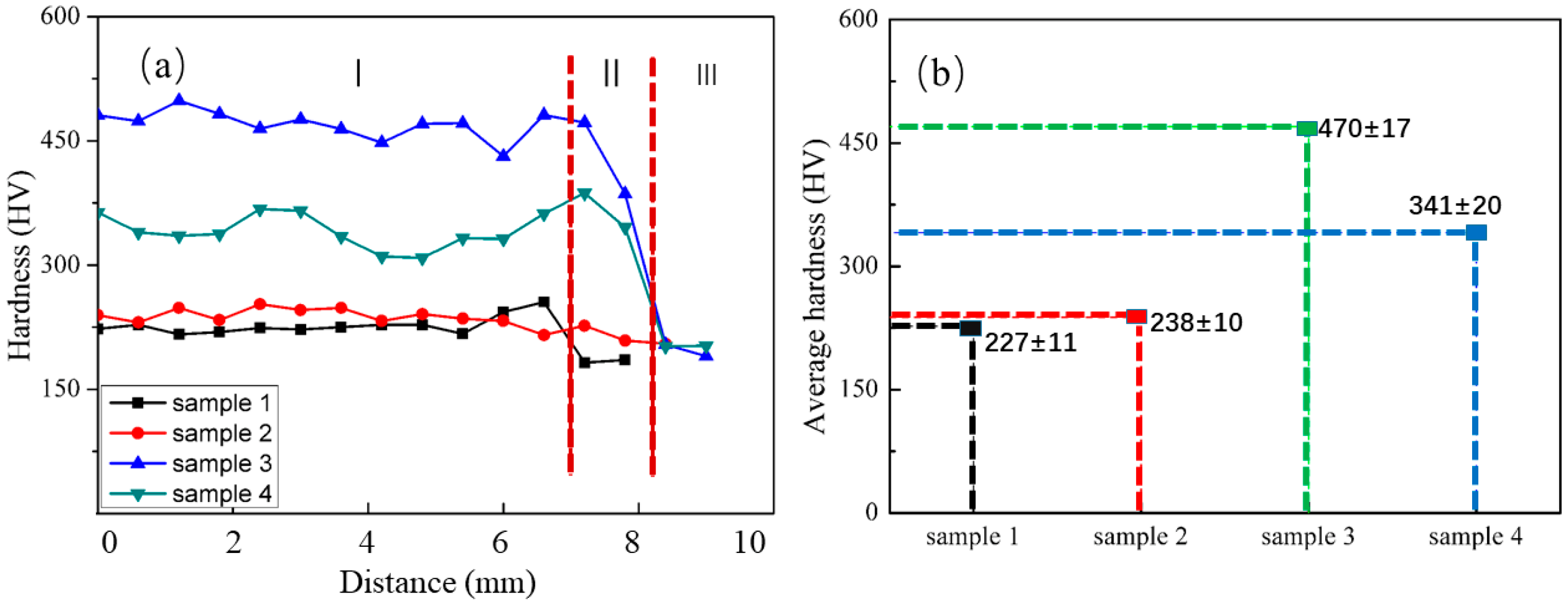




| Wire | C | Mn | Si | Cr | Ni | S | P | Fe |
|---|---|---|---|---|---|---|---|---|
| Q345 | 0.2 | 1.0–1.6 | ≤0.5 | ≤0.3 | ≤0.5 | 0.035 | 0.035 | Bal. |
| 308 | 0.05 | 1.63 | 0.51 | 18.52 | 8.64 | 0.012 | 0.024 | Bal. |
| Sample | Current (A) | Q345/308 Wire Feeding Speed (cm/min) | Wire Feeding Mode |
|---|---|---|---|
| 1 (Q345) | 150 | 100 | / |
| 2 (308) | 150 | 100 | / |
| 3 (Q345 + 308) | 150 | 80 (Q345) + 100 (308) | Horizontal parallel wire feeding |
| 4 (Q345 + 308) | 150 | 100 (Q345) + 100 (308) | Upper and lower interval wire feeding |
| Sample | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Ecorr (VSCE) | −1.00 | −0.24 | −0.82 | −0.92 |
| icorr (A·cm−2) | 1.14 × 10−4 | 3.92 × 10−7 | 1.81 × 10−5 | 5.17 × 10−5 |
| Sample | Rs | Qdl/Cdl | n | Qa | n | Rct | Ra | L |
|---|---|---|---|---|---|---|---|---|
| 1# | 4.01 | 1.08 × 10−3 | 0.78 | — | — | 55.91 | 22.71 | 5.59 |
| 2# | 4.57 | 3.17 × 10−5 | 0.57 | 8.87 × 10−5 | 0.91 | 1.21 | 2.57 × 104 | — |
| 3# | 5.44 | 4.56 × 10−5 | — | — | — | 4.27 | 5.78 | 0.2 |
| 4# | 4.11 | 6.60 × 10−5 | — | — | — | 4.87 | 0.71 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Wang, X.; Shen, X.; Tan, Z. Microstructure and Corrosion Resistance in Bimetal Materials of Q345 and 308 Steel Wire-Arc Additive Manufacturing. Crystals 2021, 11, 1401. https://doi.org/10.3390/cryst11111401
Hu Q, Wang X, Shen X, Tan Z. Microstructure and Corrosion Resistance in Bimetal Materials of Q345 and 308 Steel Wire-Arc Additive Manufacturing. Crystals. 2021; 11(11):1401. https://doi.org/10.3390/cryst11111401
Chicago/Turabian StyleHu, Qingxian, Xiaoli Wang, Xinwang Shen, and Zemin Tan. 2021. "Microstructure and Corrosion Resistance in Bimetal Materials of Q345 and 308 Steel Wire-Arc Additive Manufacturing" Crystals 11, no. 11: 1401. https://doi.org/10.3390/cryst11111401
APA StyleHu, Q., Wang, X., Shen, X., & Tan, Z. (2021). Microstructure and Corrosion Resistance in Bimetal Materials of Q345 and 308 Steel Wire-Arc Additive Manufacturing. Crystals, 11(11), 1401. https://doi.org/10.3390/cryst11111401






