Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthetic Procedures
3. Results and Discussion
3.1. Synthesis and Crystal Structure Description
3.2. Thermal Properties
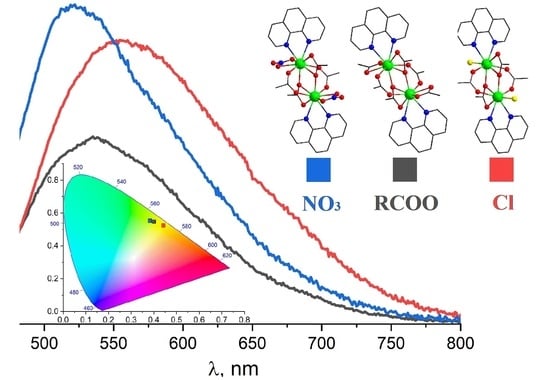
3.3. Luminescence Spectrocopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Crystal Structure of a New Complex [Gd(DMF)2(phen)]Cl3 (5)

Appendix B. The Crystallographic Data for 1–5
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Chemical formula | C44H48Gd2N4O13 | C49.5H49.5Gd2N4.50O12.5 | C46H50Gd2N8O16 | C47.7H51.7Cl2Gd2N4.3O11.7 | C18H22Cl3GdN4O2 |
| Mr, g/mol | 1155.36 | 1221.93 | 1285.44 | 1257.83 | 589.99 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/n | P21/n | P21/n | P21/n | P¯1 |
| Temperature, K | 100 | 140 | 140 | 140 | 140 |
| a, Å | 10.422(2) | 10.4479(4) | 12.3986(6) | 13.2219(5) | 9.2772(4) |
| b, Å | 17.551(4) | 17.9554(7) | 16.8287(6) | 13.3512(7) | 9.6641(4) |
| c, Å | 12.294(3) | 12.8866(7) | 12.7182(5) | 15.5831(7) | 12.2954(5) |
| α, ° | 90 | 90 | 90 | 90 | 102.460(4) |
| b, ° | 103.90(3) | 99.104(4) | 112.863(5) | 109.100(5) | 94.032(3) |
| γ, ° | 90 | 90 | 90 | 90 | 92.587(3) |
| V, Å3 | 2183.0(8) | 2387.02(19) | 2445.20(19) | 2599.4(2) | 1071.67(8) |
| Z | 2 | 2 | 2 | 2 | 2 |
| F(000) | 1144 | 1212 | 1276 | 1247 | 578 |
| D(calc.), g/cm3 | 1.758 | 1.700 | 1.746 | 1.607 | 1.828 |
| m, mm−1 | 3.462 | 2.82 | 2.77 | 2.69 | 3.49 |
| Crystal size, mm | 0.20 × 0.04 × 0.04 | 0.30 × 0.14 × 0.10 | 0.30 × 0.21 × 0.11 | 0.25 × 0.17 × 0.14 | 0.65 × 0.25 × 0.20 |
| θ range for data collection, ° | 2.42 ≤ θ ≤ 26.68 | 2.3 ≤ θ ≤ 25.4 | 2.0 ≤ θ ≤ 25.4 | 2.1 ≤ θ ≤ 25.4 | 2.2 ≤ θ ≤ 25.3 |
| No. of reflections: measured/independent/observed [I > 2σ(I)] | 14,396/3987/3615 | 10,535/4383/3520 | 11,222/4487/3921 | 11,529/4755/3827 | 9676/3919/3637 |
| Rint | 0.0356 | 0.0249 | 0.0251 | 0.0231 | 0.0368 |
| Index ranges | −12 ≤ h ≤ 12 −21 ≤ k ≤ 20 −14 ≤ l ≤ 14 | −12 ≤ h ≤ 12 −21 ≤ k ≤ 19 −15 ≤ l ≤ 15 | −14 ≤ h ≤ 12 −15 ≤ k ≤ 20 −12 ≤ l ≤ 15 | −11 ≤ h ≤ 15 −16 ≤ k ≤ 14 −18 ≤ l ≤ 18 | −11 ≤ h ≤ 9 −11 ≤ k ≤ 11 −14 ≤ l ≤ 14 |
| Final R indices [I > 2σ(I)] | R1 = 0.0205 wR2 = 0.0482 | R1 = 0.0286 wR2 = 0.0630 | R1 = 0.0227 wR2 = 0.0480 | R1 = 0.0475 wR2 = 0.1365 | R1 = 0.0231 wR2 = 0.0459 |
| R indices (all data) | R1 = 0.0240 wR2 = 0.0496 | R1 = 0.0437 wR2 = 0.0673 | R1 = 0.0296 wR2 = 0.0502 | R1 = 0.0572 wR2 = 0.1443 | R1 = 0.0261 wR2 = 0.0470 |
| Goodness-of-fit on F2 | 1.026 | 1.043 | 1.061 | 1.070 | 1.049 |
| Largest diff. peak/hole, e/Å3 | 0.525, −0.601 | 0.73, −0.80 | 0.66, −0.78 | 2.36, −0.86 | 0.63, −0.68 |
References
- Nonat, A.M.; Charbonnière, L.J. Upconversion of light with molecular and supramolecular lanthanide complexes. Coord. Chem. Rev. 2020, 409, 213192. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate com-pounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.-S.; Kang, X.-M.; Zhao, B. Lanthanide-based metal–organic frameworks as luminescent probes. Dalton Trans. 2016, 45, 18003–18017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Chen, S.; Zhou, Y.; Zhang, Y.; Xu, M. Recent Progress in Metal–Organic Framework (MOF) Based Luminescent Che-modosimeters. Nanomaterials 2019, 9, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Z.; Jia, Y.; Li, D.; Zhang, X.; Hu, M. A water-stable terbium-MOF sensor for the selective, sensitive, and recyclable detection of Al3+ and CO32− ions. Dalton Trans. 2019, 48, 15255–15262. [Google Scholar] [CrossRef]
- Saraci, F.; Quezada-Novoa, V.; Donnarumma, P.R.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, Y.M.; Gayfulin, Y.M.; Samsonenko, D.G.; Dorovatovskiy, P.V.; Lazarenko, V.A.; Brylev, K.A.; Mironov, Y.V. Coordination polymers based on rhenium octahedral chalcocyanide cluster [Re6Se8(CN)6]4– and lanthanide ions solvated with dimethylformamide. Inorg. Chim. Acta 2021, 528, 120597. [Google Scholar] [CrossRef]
- Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers 2020, 12, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caravan, P. Protein-Targeted Gadolinium-Based Magnetic Resonance Imaging (MRI) Contrast Agents: Design and Mechanism of Action. Accounts Chem. Res. 2009, 42, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Farhood, B.; Haghi-Aminjan, H.; Mortezazadeh, T.; Aliasgharzadeh, A.; Mohseni, M.; Najafi, M.; Abbasi, H. Gadolinium nanoparticles as diagnostic and therapeutic agents: Their delivery systems in magnetic resonance imaging and neutron capture therapy. J. Drug Deliv. Sci. Technol. 2018, 44, 457–466. [Google Scholar] [CrossRef]
- Clough, T.J.; Jiang, L.; Wong, K.-L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zairov, R.; Pizzanelli, S.; Dovzhenko, A.P.; Nizameev, I.; Orekhov, A.S.; Arkharova, N.; Podyachev, S.N.; Sudakova, S.; Mustafina, A.R.; Calucci, L. Paramagnetic Relaxation Enhancement in Hydrophilic Colloids Based on Gd(III) Complexes with Tetrathia- and Calix[4]arenes. J. Phys. Chem. C 2020, 124, 4320–4329. [Google Scholar] [CrossRef]
- Parker, D.; Suturina, E.; Kuprov, I.; Chilton, N.F. How the Ligand Field in Lanthanide Coordination Complexes Determines Magnetic Susceptibility Anisotropy, Paramagnetic NMR Shift, and Relaxation Behavior. Accounts Chem. Res. 2020, 53, 1520–1534. [Google Scholar] [CrossRef]
- Tan, Q.-H.; Wang, Y.-Q.; Guo, X.-Y.; Liu, H.-T.; Liu, Z.-L. A gadolinium MOF acting as a multi-responsive and highly selective luminescent sensor for detecting o-, m-, and p-nitrophenol and Fe3+ ions in the aqueous phase. RSC Adv. 2016, 6, 61725–61731. [Google Scholar] [CrossRef]
- Casanovas, B.; Speed, S.; Maury, O.; El Fallah, M.S.; Font-Bardí, M.; Vicente, R. Dinuclear LnIII Complexes with 9-Anthracenecarboxylate Showing Field-Induced SMM and Visible/NIR Luminescence. Eur. J. Inorg. Chem. 2018, 34, 3859–3867. [Google Scholar] [CrossRef]
- Casanovas, B.; Speed, S.; Maury, O.; Font-Bardia, M.; Vicente, R. Homodinuclear lanthanide 9-anthracenecarboxylate complexes: Field induced SMM and NIR-luminescence. Polyhedron 2019, 169, 187–194. [Google Scholar] [CrossRef]
- Casanovas, B.; Speed, S.; Vicente, R.; Font-Bardia, M. Sensitization of visible and NIR emitting lanthanide(III) ions in a series of dinuclear complexes of formula [Ln2(μ-2-FBz)2(2-FBz)4(terpy)2]·2(2-HFBz)·2(H2O). Polyhedron 2019, 173, 114113. [Google Scholar] [CrossRef]
- Taydakov, I.V.; Belousov, Y.A.; Lyssenko, K.A.; Varaksina, E.; Drozdov, A.A.; Marchetti, F.; Pettinari, R.; Pettinari, C. Synthesis, phosphorescence and luminescence properties of novel europium and gadolinium tris-acylpyrazolonate complexes. Inorg. Chim. Acta 2019, 502, 119279. [Google Scholar] [CrossRef]
- Bryleva, Y.A.; Artem’Ev, A.V.; Glinskaya, L.A.; Komarov, V.Y.; Bogomyakov, A.S.; Rakhmanova, M.I.; Larionov, S.V. A series of bis(2-phenethyl)dithiophosphinate-based Ln(III) complexes: Synthesis, magnetic and photoluminescent properties. Inorg. Chim. Acta 2021, 516, 120097. [Google Scholar] [CrossRef]
- Kim, J.H.; Lepnev, L.S.; Utochnikova, V.V. Dual vis-NIR emissive bimetallic naphthoates of Eu–Yb–Gd: A new approach toward Yb luminescence intensity increase through Eu → Yb energy transfer. Phys. Chem. Chem. Phys. 2021, 23, 7213–7219. [Google Scholar] [CrossRef]
- Gontcharenko, V.; Kiskin, M.; Dolzhenko, V.; Korshunov, V.; Taydakov, I.; Belousov, Y. Mono- and Mixed Metal Complexes of Eu3+, Gd3+, and Tb3+ with a Diketone, Bearing Pyrazole Moiety and CHF2-Group: Structure, Color Tuning, and Kinetics of Energy Transfer between Lanthanide Ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef] [PubMed]
- Utochnikova, V.V.; Aslandukov, A.N.; Vashchenko, A.A.; Goloveshkin, A.S.; Alexandrov, A.A.; Grzibovskis, R.; Bunzli, J.-C.G. Identifying lifetime as one of the key parameters responsible for the low brightness of lanthanide-based OLEDs. Dalton Trans. 2021, 50, 12806–12813. [Google Scholar] [CrossRef] [PubMed]
- Llabres-Campaner, P.J.; Pitarch-Jarque, J.; Ballesteros-Garrido, R.; Abarca, B.; Ballesteros, R.; García-España, E. Bicy-clo[2.2.2]octane-1,4-dicarboxylic acid: Towards transparent metal-organic frameworks. Dalton Trans. 2017, 46, 7397–7402. [Google Scholar] [CrossRef]
- Demakov, P.A.; Sapchenko, S.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Coordination polymers based on zinc(ii) and manganese(ii) with 1,4-cyclohexanedicarboxylic acid. Russ. Chem. Bull. 2018, 67, 490–496. [Google Scholar] [CrossRef]
- Yin, J.; Yang, H.; Fei, H. Robust, Cationic Lead Halide Layered Materials with Efficient Broadband White-Light Emission. Chem. Mater. 2019, 31, 3909–3916. [Google Scholar] [CrossRef]
- Demakov, P.A.; Poryvaev, A.S.; Kovalenko, K.A.; Samsonenko, D.G.; Fedin, M.V.; Fedin, V.P.; Dybtsev, D.N. Structural Dy-namics and Adsorption Properties of the Breathing Microporous Aliphatic Metal–Organic Framework. Inorg. Chem. 2020, 59, 15724–15732. [Google Scholar] [CrossRef]
- Svetogorov, R.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-Throughput Small-Molecule Crystallography at the ‘Belok’ Beamline of the Kurchatov Synchrotron Radiation Source: Transition Metal Complexes with Azomethine Ligands as a Case Study. Crystals. 2017, 7, 325. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W. XDS. Acta Crystallogr. 2010, D66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- CrysAlisPro, Version: 1.171.38.46. Rigaku Oxford Diffraction; Rigaku Americas Holding Company, Inc. : The Woodlands, TX, USA, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Ryadun, A.A.; Dorovatovskii, P.V.; Lazarenko, V.A.; Samsonenko, D.G.; Brylev, K.A.; Fedin, V.P.; Dybtsev, D.N. Intense multi-colored luminescence in a series of rare-earth metal–organic frameworks with aliphatic linkers. Dalton Trans. 2021, 50, 11899–11908. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Jiang, X.-M.; Zhu, S.-D.; Du, Z.-Y.; Liu, C.-M.; Xie, Y.-R.; Liu, L.-X. Anion Effects on Lanthanide(III) Tetrazole-1-acetate Dinuclear Complexes Showing Slow Magnetic Relaxation and Photofluorescent Emission. Inorg. Chem. 2016, 55, 3738–3749. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Barsukova, M.; Samsonenko, D.G.; Fedin, V.P. Crystal structure of metal-organic frameworks based on terbium and 1,4-naphthalenedicarboxylic acid. J. Struct. Chem. 2020, 61, 1090–1096. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Structure and luminescent properties of europium(III) coordination polymers with thiophene ligands. J. Struct. Chem. 2020, 61, 1965–1974. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Cherezova, S.V.; Sapianik, A.A.; Lundovskaya, O.V.; Samsonenko, D.G.; Fedin, V.P. Lanthanide contraction effect and white-emitting luminescence in a series of metal–organic frameworks based on 2,5-pyrazinedicarboxylic acid. RSC Adv. 2020, 10, 38252–38259. [Google Scholar] [CrossRef]
- Cherezova, S.V.; Barsukova, M.O.; Samsonenko, D.G.; Fedin, V.P. Crystal structure of dense metal-organic frameworks based on sc(III) and two types of ligands. J. Struct. Chem. 2021, 62, 897–904. [Google Scholar] [CrossRef]





| Bond | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Gd−N, Å | 2.566(2), 2.600(3) | 2.499(19)−2.59(3) | 2.553(2), 2.591(3) | 2.564(7), 2.579(5) |
| Gd−O(COOchelate), Å | 2.3482(19)–2.514(2) | 2.334(3)–2.539(3) | 2.330(2)–2.573(2) | 2.294(5)–2.513(5) |
| Gd–O(COOnon-chelate), Å or Gd–O(NO3,non-chelate), Å | 2.429(2)–2.4800(19) | 2.463(3)–2.465(3) | - | - |
| - | - | 2.458(2)–2.529(2) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demakov, P.A.; Vasileva, A.A.; Lazarenko, V.A.; Ryadun, A.A.; Fedin, V.P. Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks. Crystals 2021, 11, 1375. https://doi.org/10.3390/cryst11111375
Demakov PA, Vasileva AA, Lazarenko VA, Ryadun AA, Fedin VP. Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks. Crystals. 2021; 11(11):1375. https://doi.org/10.3390/cryst11111375
Chicago/Turabian StyleDemakov, Pavel A., Alena A. Vasileva, Vladimir A. Lazarenko, Alexey A. Ryadun, and Vladimir P. Fedin. 2021. "Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks" Crystals 11, no. 11: 1375. https://doi.org/10.3390/cryst11111375
APA StyleDemakov, P. A., Vasileva, A. A., Lazarenko, V. A., Ryadun, A. A., & Fedin, V. P. (2021). Crystal Structures, Thermal and Luminescent Properties of Gadolinium(III) Trans-1,4-cyclohexanedicarboxylate Metal-Organic Frameworks. Crystals, 11(11), 1375. https://doi.org/10.3390/cryst11111375