Preparation and Characterization of Tubelike g-C3N4/Ag3PO4 Heterojunction with Enhanced Visible-Light Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Tubelike g-C3N4
2.2. Synthesis of Tubelike g-C3N4/Ag3PO4 Heterojunction
2.3. Characterization
2.4. Photocatalytic Activity Evaluation
3. Results and Discussion
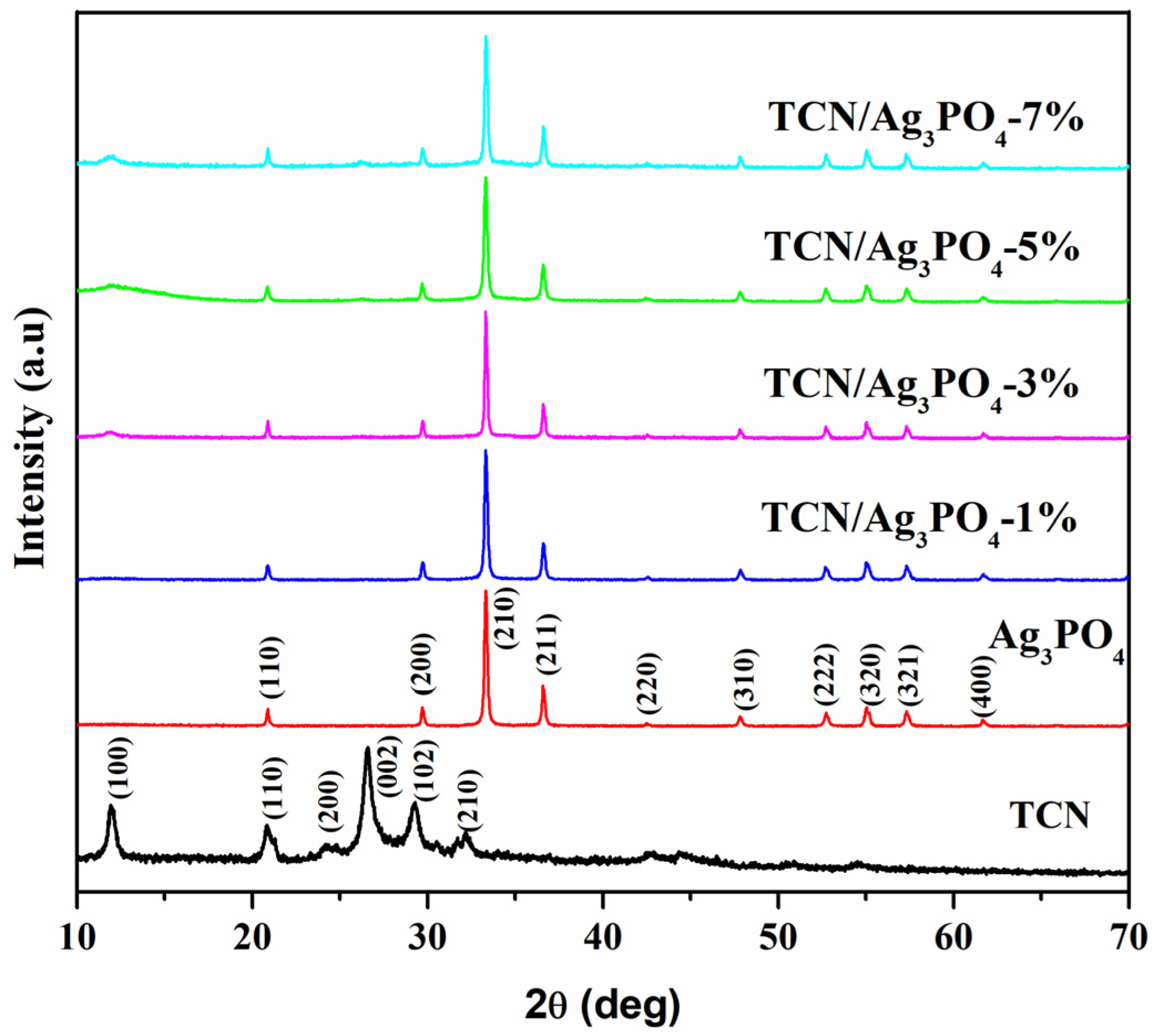
3.1. XRD and XPS Analysis
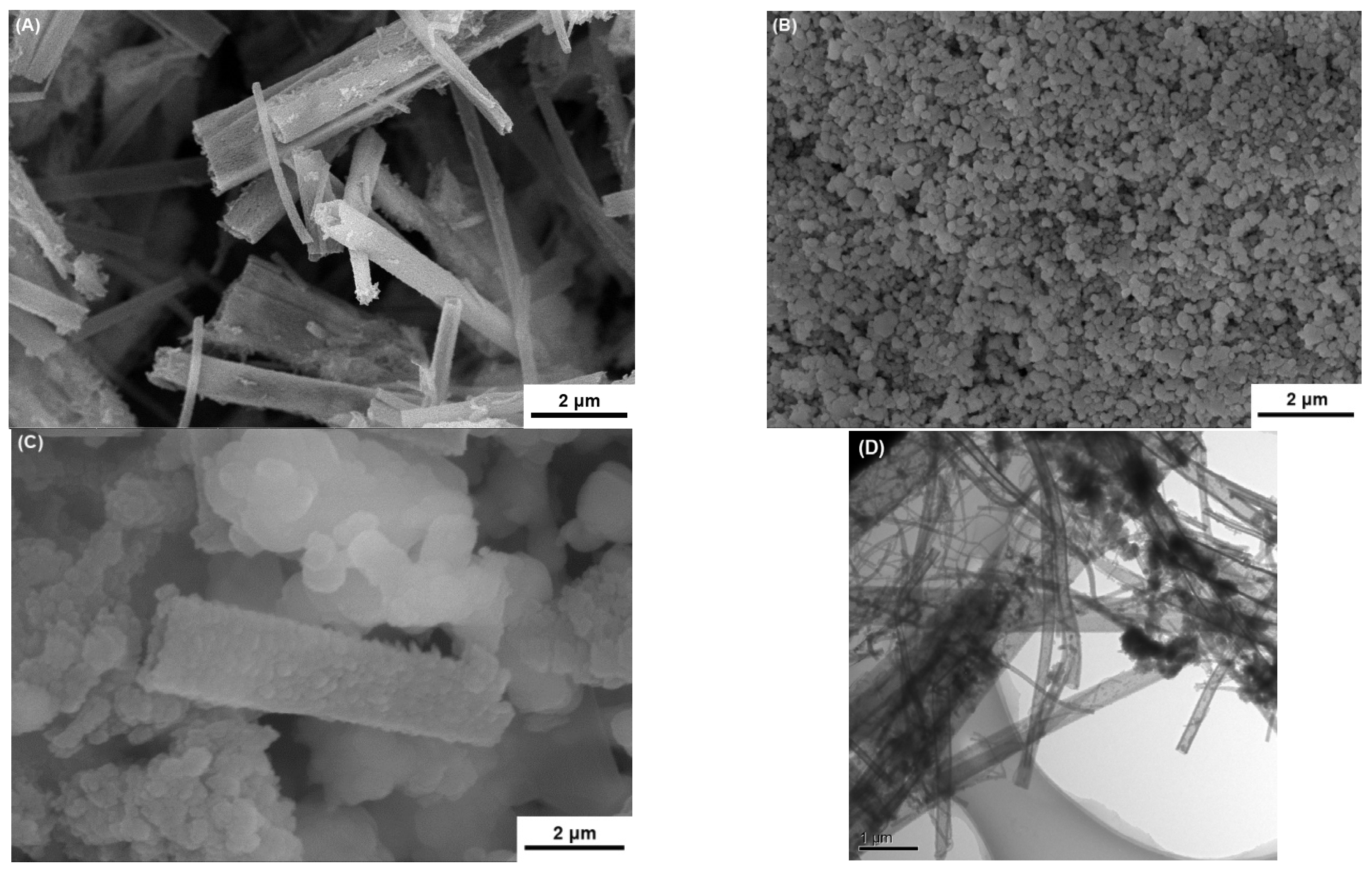
3.2. SEM Analysis
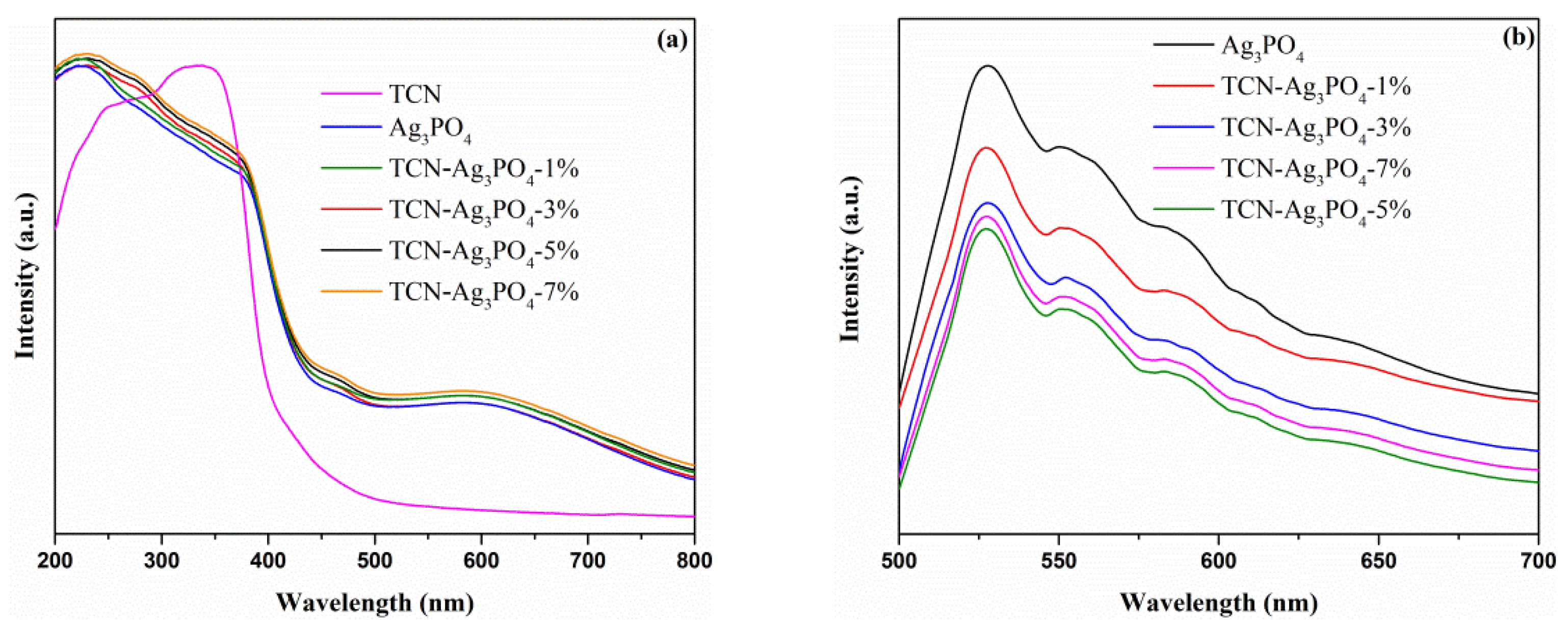
3.3. UV–vis Diffuse Reflectance Spectra and Photoluminescence Spectra Analysis
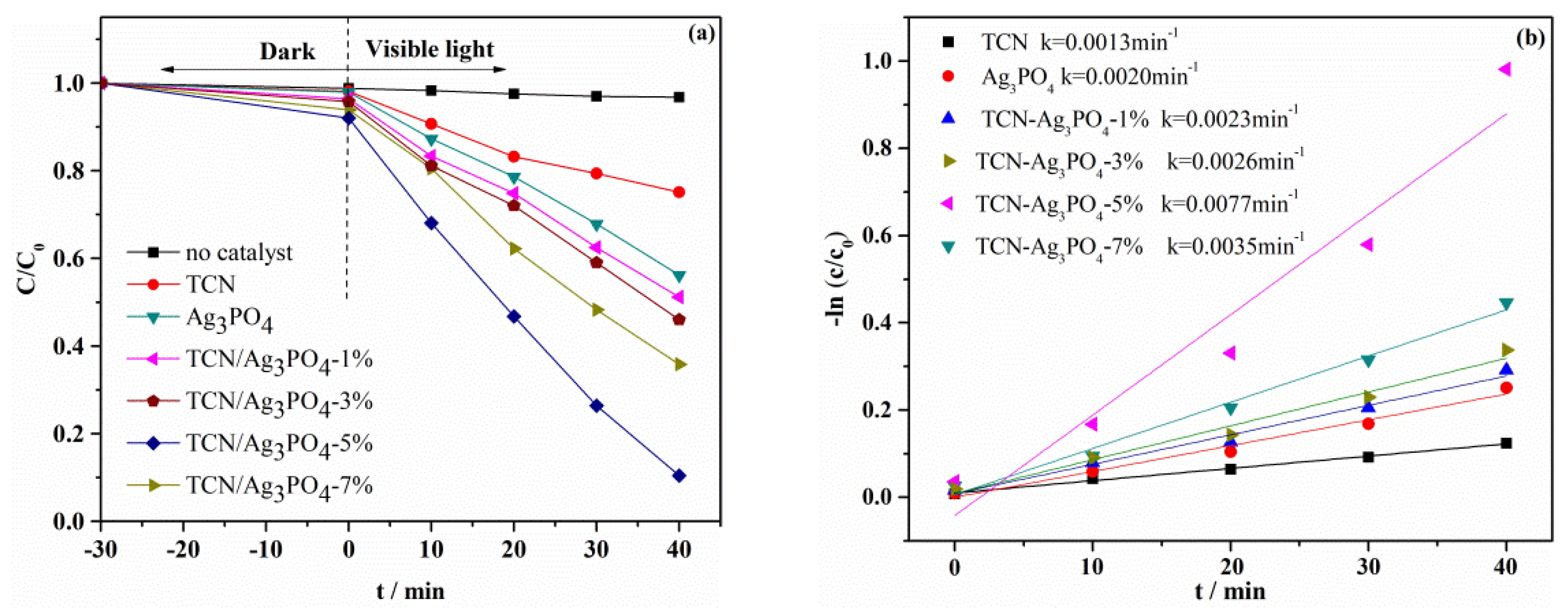
3.4. Photocatalytic Activities Analyses
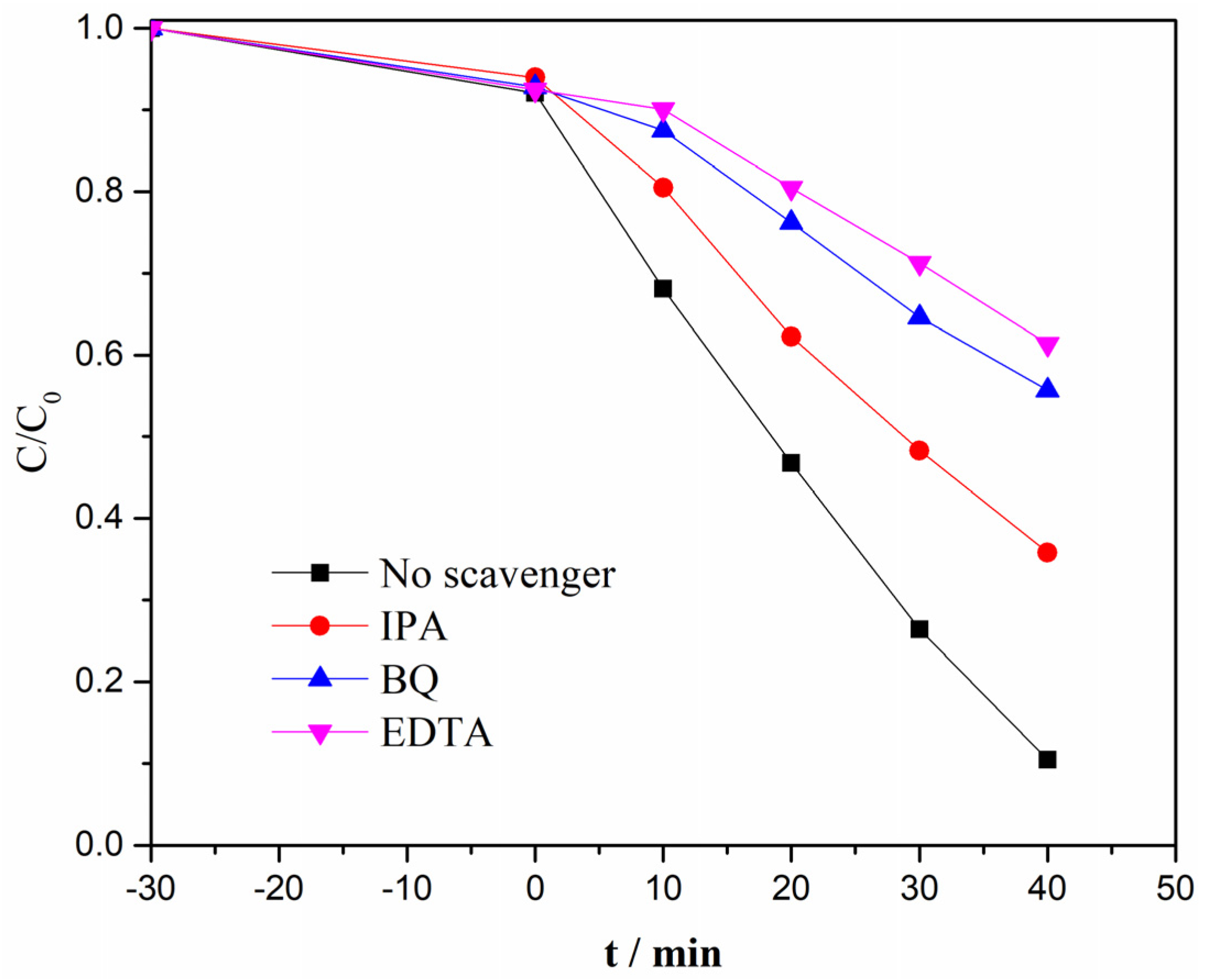
3.5. Photocatalytic Mechanism of TCN/Ag3PO4 Heterojunction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Gong, W.; Xie, C.; Yang, D.; Ling, X.; Yuan, X.; Chen, S.; Liu, X. Removal of neutral red from aqueous solution by adsorption on spent cottonseed hull substrate. J. Hazard. Mater. 2011, 185, 502–506. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Marquez, J.J.; Levchuk, I.; Ibañez, P.F.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2021, 78, 1–29. [Google Scholar] [CrossRef]
- Hoffman, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water. Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Lu, D.Z.; Zhao, B.; Fang, P.F.; Zhai, S.B.; Li, D.L.; Chen, Z.Q.; Wu, W.H.; Chai, W.Q.; Wu, Y.C.; Qi, N. Facile one-pot fabrication and high photocatalytic performance of vanadium doped TiO2-based nanosheets for visible-light-driven degradation of RhB or Cr(VI). Appl. Surf. Sci. 2015, 359, 435–448. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Zhao, J.C.; Wang, X.M.; Mai, B.X.; Sheng, G.Y.; Peng, P.A.; Fu, J.M. Preparation, characterization and photocatalytic activity of nano-sized ZnO/SnO2 coupled photocatalysts. Appl. Catal. B Environ. 2002, 39, 269–279. [Google Scholar]
- Wang, C.; Li, Y.; Huang, L.; Yang, L.; Wang, H.; Liu, J.; Liu, J.; Song, Z.; Huang, L. Enhanced photocatalytic antibacterial and degradation performance by n-p type 0D/2D SnO2−x/BiOI photocatalyst under LED Light. Chem. Eng. J. 2021, 411, 128505. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Guan, X.; Shi, J.; Guo, L. Ag3PO4 photocatalyst: Hydrothermal preparation and enhanced O2 evolution under visible-light irradiation. Inter. J. Hydrogen. Energy. 2013, 38, 11870–11877. [Google Scholar] [CrossRef]
- Petala, A.; Spyrou, D.; Frontistis, Z.; Mantzavinos, D.; Kondarides, D.I. Immobilized Ag3PO4 photocatalyst for micro-pollutants removal in a continuous flow annular photoreactor. Catal. Today. 2019, 328, 223–229. [Google Scholar] [CrossRef]
- Osman, N.S.; Sulaiman, S.N.; Muhamad, E.N.; Mukhair, H.; Tan, S.T.; Abdullah, A.H. Synthesis of an Ag3PO4/Nb2O5 photocatalyst for the degradation of dye. Catalysts 2021, 11, 458. [Google Scholar] [CrossRef]
- Li, D.; Wang, W.; Shang, X.; Tang, H.; Zulfiqar, S. Solar-driven photocatalytic water oxidation of Ag3PO4/CNTs@MoSe2 ternary composite photocatalyst. Appl. Surf. Sci. 2019, 505, 144613. [Google Scholar] [CrossRef]
- Teng, F.; Liu, Z.; Zhang, A.; Min, L. Photocatalytic performances of Ag3PO4 polypods for degradation of dye pollutant under natural indoor weak light irradiation. Environ. Sci. Technol. 2015, 49, 9489–9494. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Jin, Y.; Gao, K.; Cai, H.; Ou, G. The enhancement mechanism of ultra-active Ag3PO4 modified by tungsten and the effective degradation towards phenolic pollutants. Chemosphere 2021, 285, 131440. [Google Scholar] [CrossRef]
- Najafabadi, M.N.; Ghanbari, H.; Naghizadeh, R. Graphene/silver-based composites and coating on dead coral for degradation of organic pollution using the z-scheme mechanism. RSC Adv. 2021, 11, 19890–19901. [Google Scholar] [CrossRef]
- Muthukumar, P.; Alex, V.; Pannipara, M.; Al-Sehemi, A.G.; Anthony, S.P. Fabricating highly efficient Ag3PO4-Fe3O4-GO ternary nanocomposite photocatalyst: Effect of Fe3O4-GO preparation methods on photocatalytic activity. Mater. Res. Bull. 2021, 141, 111337. [Google Scholar] [CrossRef]
- Yan, X.; Hui, X.-Y.; Gao, Q.; Yu, G.-J.; Mo, Y.-C.; Ye, Z.-M.; Li, J.-C.; Ma, Z.-Y.; Sun, G.-D. Synthesis and visible light photocatalytic performance of Ag3PO4/MoS2 nanosheets composite photocatalyst. Chinese J. Inorg. Chem. 2017, 33, 1782–1788. [Google Scholar]
- Li, N.; Chen, J.; Chen, X.; Lai, Y.; Yu, C.; Yao, L.; Liang, Y. Novel visible-light-driven SrCoO3/Ag3PO4 heterojunction with enhanced photocatalytic performance for tetracycline degradation. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.-H.; Wei, F.; Zhang, T.; Luo, L.; Pan, Y.; Yang, X.; Yu, H.; Zhou, S. Different antibacterial effect of Ag3PO4/TiO2 heterojunctions and the TiO2 polymorphs. J. Alloy. Compd. 2021, 876, 160016. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Huang, H.; Liu, Y.; Kang, Z. Ag3PO4/SnO2 semiconductor nanocomposites with enhanced photocatalytic activity and stability. New J. Chem. 2012, 36, 1541–1544. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Xie, F.-Z.; Zhu, S.-F.; Liu, J.; Zhang, J.; Mei, S.-F.; Zhao, W. A novel photofunctional g-C3N4/Ag3PO4 bulk heterojunction for decolorization of RhB. Chem. Eng. J. 2013, 228, 435–441. [Google Scholar] [CrossRef]
- Guan, X.; Guo, L. Cocatalytic effect of SrTiO3 on Ag3PO4 toward enhanced photocatalytic water oxidation. ACS Catal. 2014, 4, 3020–3026. [Google Scholar] [CrossRef]
- He, Y.M.; Zhang, L.H.; Teng, B.T.; Fan, M.H. New application of Z-Scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015, 49, 649–656. [Google Scholar] [CrossRef]
- Cui, X.; Tian, L.; Xian, X.; Tang, H.; Yang, X. Solar photocatalytic water oxidation over Ag3PO4/g-C3N4 composite materials mediated by metallic Ag and graphene. Appl. Surf. Sci. 2018, 430, 108–115. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Wang, J.K.; Li, S.; Zhang, H.J.; Zhang, S.W. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Tuffy, B.; Mesch, M.B.; Wirnhier, C.; Martineau, F.; Taulelle, W.; Lotsch, B.V. Triazine-based carbon nitrides for visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 2013, 125, 2495–2499. [Google Scholar] [CrossRef]
- Yan, X.; Ning, G.; Zhao, P. Enhanced visible light photocatalytic reduction of Cr(VI) over a novel square nanotube poly(triazine imide)/TiO2 heterojunction. Catalysts 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Song, J.; Tan, S.; Yang, L.; Yu, G.; Chen, H.; Zhou, L.; Zhang, Z.; Zhang, Y.; Su, Y. Facile synthesis of z-scheme ZnO/Ag/Ag3PO4 composite photocatalysts with enhanced performance for the degradation of ciprofloxacin. Mater. Chem. Phys. 2021, 260, 124136. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Shi, Q.; Zhou, Q. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light. Appl. Catal. B Environ. 2016, 186, 77–87. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Z.; Zhang, Z.; Zhang, X.; Wen, R.; Liu, Y.; Fang, M.; Wu, X. Controlled synthesis and photocatalytic properties of rhombic dodecahedral Ag3PO4 with high surface energy. Appl. Surf. Sci. 2016, 389, 56–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Gu, F.L.; Wu, H.; Guo, Q. Significant visible-light photocatalytic enhancement in Rhodamine B degradation of silver orthophosphate via the hybridization of N-doped graphene and poly(3-hexylthiophene). J. Hazard. Mater. 2016, 315, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yue, X.; Ji, Z.; Shen, X.; Zhou, H.; Liu, M.; Xu, K.; Zhu, J.; Zhu, G.; Kong, L. Nitrogen-doped carbon dots decorated on g-C3N4/Ag3PO4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2018, 227, 459–469. [Google Scholar] [CrossRef]
- Liu, J.J.; Fu, X.; Chen, S.F.; Zhu, Y.F. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett. 2011, 99, 191903. [Google Scholar] [CrossRef]
- Yan, X.; Qin, J.; Ning, G.; Li, J.; Ai, T.; Su, X.; Wang, Z. A novel poly(triazine imide) hollow tube/ZnO heterojunction for tetracycline hydrochloride degradation under visible light irradiation. Adv. Powder. Technol. 2019, 30, 359–365. [Google Scholar] [CrossRef]
- Chen, Z.; Bing, F.; Liu, Q.; Zhang, Z.G.; Fang, X.M. Novel z-scheme visible-light-driven Ag3PO4/Ag/SiC photocatalysts with enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 4652–4658. [Google Scholar] [CrossRef]
- Yan, X.; Li, J.; Zhou, H. Molten salts synthesis and visible light photocatalytic activity of crystalline poly(triazine imide) with different morphologies. J. Mater. Sci. Mater. Electron. 2019, 30, 11706–11713. [Google Scholar] [CrossRef]
- Li, J.; Jin, L.; Liu, F.; Liu, X. Synthesis of cocoon-like Ag3PO4 and its high-performance in photocatalytic degradation of ciprofloxacin. Mater. Lett. 2019, 242, 139–142. [Google Scholar] [CrossRef]
- Ding, M.; Zhou, J.; Yang, H.; Cao, R.; Zhang, S.; Shao, M.; Xu, X. Synthesis of Z-scheme g-C3N4 nanosheets/Ag3PO4 photocatalysts with enhanced visible-light photocatalytic performance for the degradation of tetracycline and dye. Chinese Chem. Lett. 2020, 31, 71–76. [Google Scholar] [CrossRef]
- Yan, X.; Gao, Q.; Hui, X.; Yan, C.; Ai, T.; Wang, Z.; Sun, G.; Su, X.; Zhao, P. Fabrication of g-C3N4/MoS2 nanosheet heterojunction by facile ball milling method and its visible light photocatalytic performance. Rare. Metal. Mat. Eng. 2018, 47, 3015–3020. [Google Scholar]
- Sun, M.; Zeng, Q.; Zhao, X.; Shao, Y.; Ji, P.; Wang, C.; Yan, T.; Du, B. Fabrication of novel g-C3N4 nanocrystals decorated Ag3PO4 hybrids: Enhanced charge separation and excellent visible-light driven photocatalytic activity. J. Hazard. Mater. 2017, 339, 9–21. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Wang, Y.; Kang, B.; Li, Z.; Niu, Y. Preparation and Characterization of Tubelike g-C3N4/Ag3PO4 Heterojunction with Enhanced Visible-Light Photocatalytic Activity. Crystals 2021, 11, 1373. https://doi.org/10.3390/cryst11111373
Yan X, Wang Y, Kang B, Li Z, Niu Y. Preparation and Characterization of Tubelike g-C3N4/Ag3PO4 Heterojunction with Enhanced Visible-Light Photocatalytic Activity. Crystals. 2021; 11(11):1373. https://doi.org/10.3390/cryst11111373
Chicago/Turabian StyleYan, Xin, Yuanyuan Wang, Bingbing Kang, Zhuo Li, and Yanhui Niu. 2021. "Preparation and Characterization of Tubelike g-C3N4/Ag3PO4 Heterojunction with Enhanced Visible-Light Photocatalytic Activity" Crystals 11, no. 11: 1373. https://doi.org/10.3390/cryst11111373




