Natural Fluorite from Órgiva Deposit (Spain). A Study of Its Pozzolanic and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Sample
2.2.2. Calcination Test
2.2.3. X-ray Diffraction Analysis (XRD)
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Raman Spectroscopy (RS)
2.2.6. Chemical Pozzolanicity Test (CPT)
- −
- [OH−]: is the concentration in hydroxyl ions (mmol/L).
- −
- V3: is the volume of the hydrochloric acid solution (0.1 mol/L).
- −
- f2: is the factor of the hydrochloric acid solution (0.1 mol/L).
- −
- [CaO]: is the concentration in calcium oxide (mmol/L).
- −
- V4: is the volume of EDTA solution used in the titration.
- −
- f1: is the factor of the EDTA solution.
2.2.7. Chemical Quality Analysis (CQA)
2.2.8. Mechanical Strength Test (MST)
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
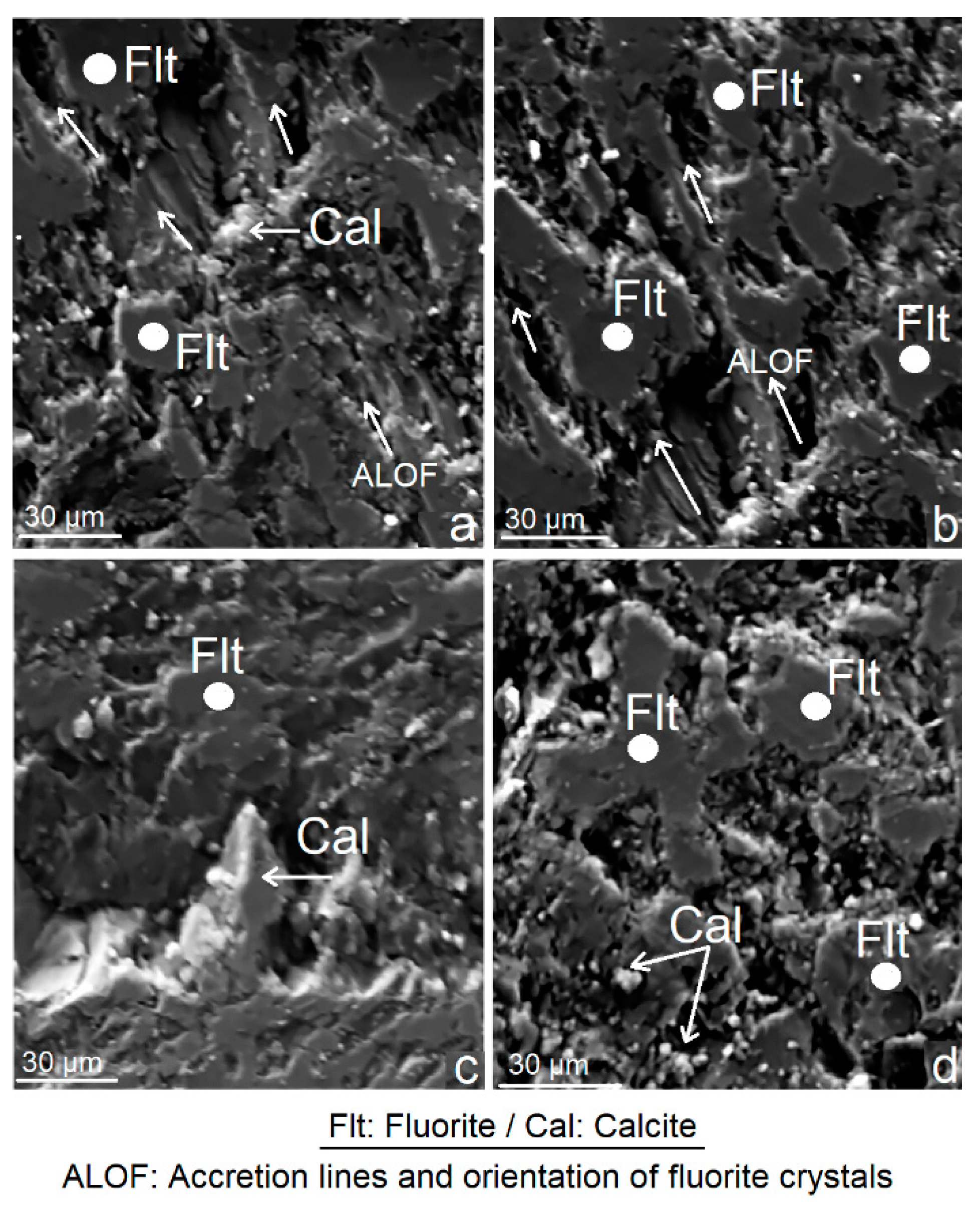
3.2. Scanning Electron Microscopy (SEM)
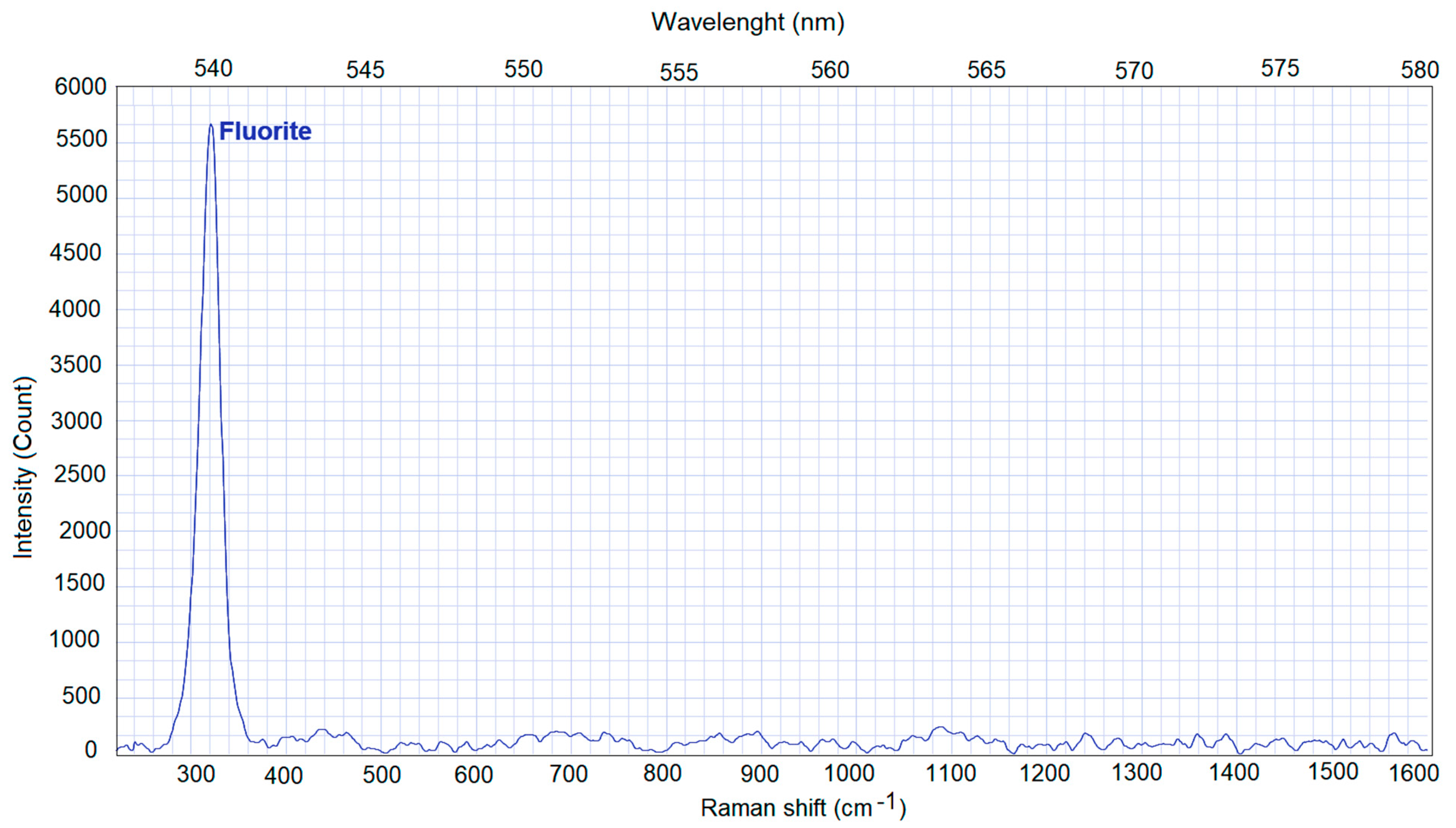
3.3. Raman Spectroscopy (RS)
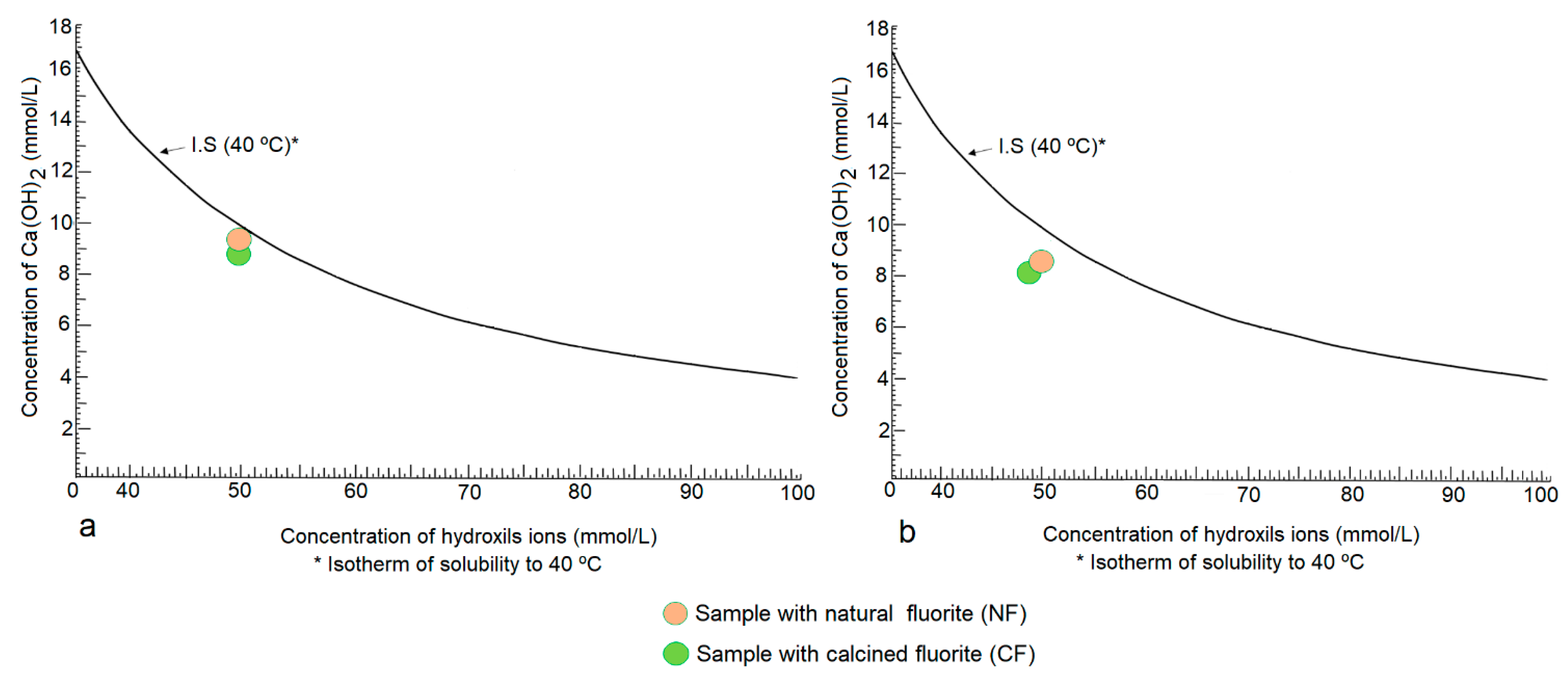
3.4. Pozzolanicity Test (CPT) at 8 and 15 Days
3.5. Chemical Quality Analysis (CQA)
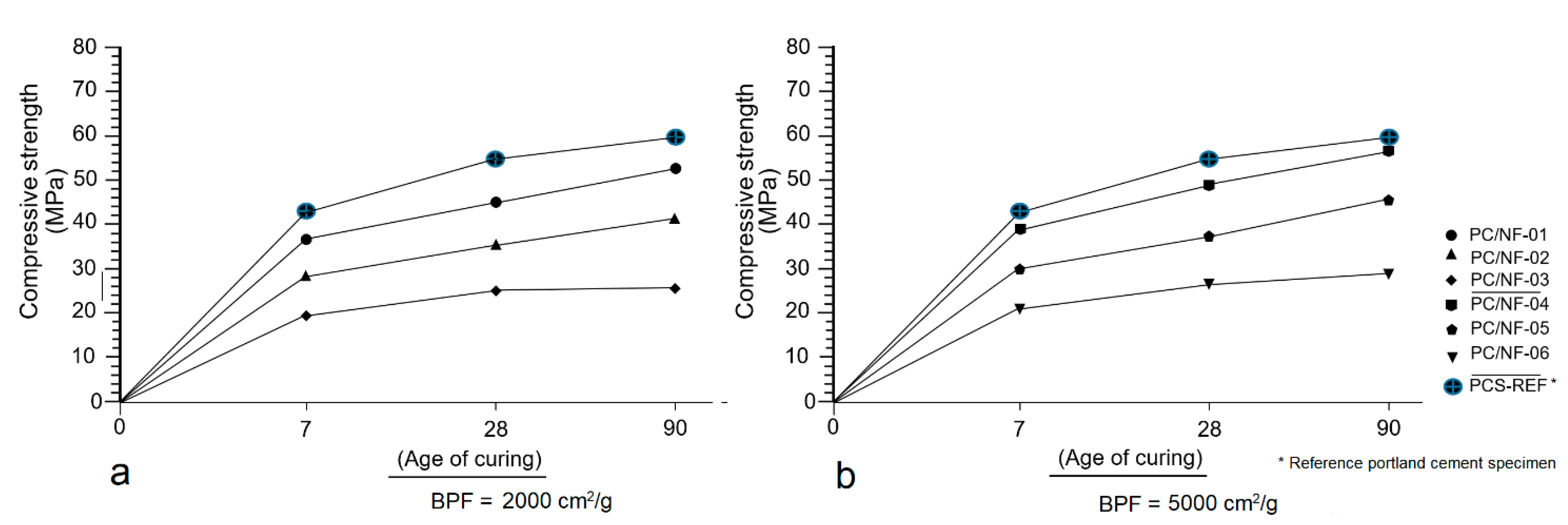
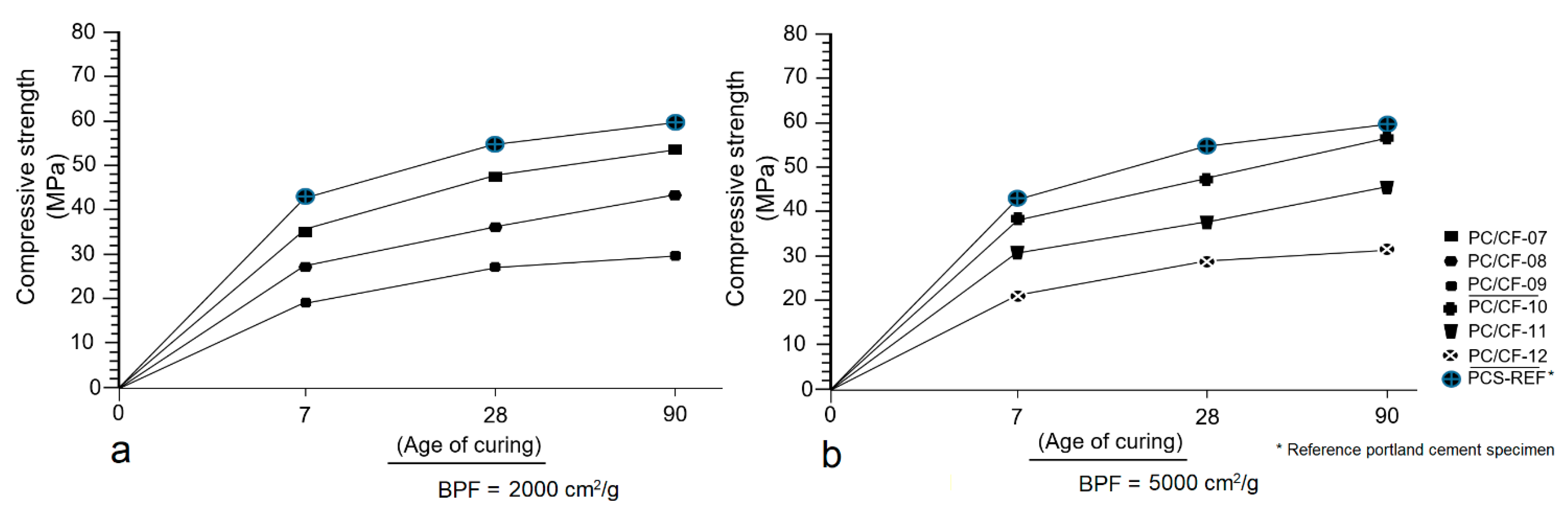
3.6. Mechanical Strength Test (MST)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilvonio, R.; Domínguez, F. Ahorro de energía en el proceso de fabricación de clinker de cemento empleando mineral fluorita, (CaF2). Rev. Soc. Quím. Perú 2009, 75, 303–309. [Google Scholar]
- Dahhou, M.; El Hamidi, A.; El Moussaouiti, M.; Azeem Arshadb, M. Synthesis and characterization of belite clinker by sustainable utilization of alumina sludge and natural fluorite (CaF2). Materialia 2021, 20, 101204. [Google Scholar] [CrossRef]
- Najafi Kani, E.; Allahverdi, A. Fast set and high early strength cement from limestone, natural pozzolan, and fluorite. Int. J. Civ. Eng. Technol. 2010, 8, 362–369. Available online: http://ijce.iust.ac.ir/article-1-306-en.html (accessed on 20 October 2021).
- Dominguez, O.; Torres-Castillo, A.; Flores-Veleza, L.M.; Torres, R. Characterization using thermomechanical and differential thermal analysis of the sinterization of portland clinker doped with CaF2. Mater. Charact. 2010, 61, 459–466. [Google Scholar] [CrossRef]
- Endzhievskaya, I.G.; Demina, A.V.; Lavorenko, A.A. Synthesis of a mineralizing agent for portland cement from aluminum production waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 945. [Google Scholar] [CrossRef]
- Fridrichová, M.; Gazdič, D.; Dvoøák, K.; Magrla, R. Optimizing the reactivity of a raw-material mixture for portland clinker firing. Mater. Technol. 2017, 51, 219–223. [Google Scholar] [CrossRef]
- Zea-Garcia, J.D.; Santacruz, I.; Aranda, M.A.G.; De la Torre, A.G. Alite-belite-ye’elimite cements: Effect of dopants on the clinker phase composition and properties. Cem. Concr. Res. 2019, 115, 192–202. [Google Scholar] [CrossRef]
- Güleç, A.; Oğuzhanoğlu, M. Fluorite mineral waste as natural aggregate replacement in concrete. J. Build. Rehabil. 2021, 6, 26. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L.Y.; Hong, D.; Qian, G.R.; Zhou, M. A study of making synthetic oxy-fluoride construction material using waste serpentine and kaolin mining tailings. Int. J. Miner. Process. 2012, 104–105, 31–36. [Google Scholar] [CrossRef]
- Achmania, J.; Chraibi, I.; Blaise, T.; Barbarand, J.; Brigaud, B.; Bounajma, H. Virtues of fluorite-case of Bou-Izourane fluorite. Mater. Today Proc. 2020, 31, S114–S121. [Google Scholar] [CrossRef]
- Makin, S.A.; Simand, G.J.; Marshall, D. Fluorite and its potential as an indicator mineral for carbonatite-related rare earth element deposits. Geol. Fieldwork 2013, 2014-1, 207–212. [Google Scholar]
- UNE-EN. Cemento. Parte 1: Composición, Especificaciones y Criterios de Conformidad de Los Cementos Comunes; Standard UNE-EN 197-1:2011; AENOR: Madrid, Spain, 2011. [Google Scholar]
- UNE-EN. Métodos de Ensayo de Cementos. Parte 5: Ensayo de Puzolanicidad Para Cementos Puzolánicos; Standard UNE-EN 196-5:2006; AENOR: Madrid, Spain, 2006. [Google Scholar]
- Mielenz, R.C.; Greene, K.T.; Schieltz, N.C. Natural pozzolans for concrete. Econ. Geol. 1951, 46, 311–328. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Aggelakopoulou, E. Evaluation of pozzolanic activity of natural and artificial pozzolans by thermal analysis. Thermochim. Acta 2004, 420, 135–140. [Google Scholar] [CrossRef]
- UNE-EN. Métodos de Ensayo de Cementos. Parte 2: Análisis Químico de Cementos; Standard UNE-EN 196-2:2014; AENOR: Madrid, Spain, 2014. [Google Scholar]
- UNE-EN. Métodos de Ensayo de Cementos. Parte 1: Determinación de Resistencias Mecánicas; Standard UNE-EN 196-1:2005; AENOR: Madrid, Spain, 2005. [Google Scholar]
- Magotra, R.; Namga, S.; Singh, P.; Arora, N.; Srivastava, P.K. A new classification scheme of fluorite deposits. Int. J. Geosci. 2017, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhu, Q.; Yang, X. Electrical Conductivity of fluorite and fluorine conduction. Minerals 2019, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Costafreda, J.L.; Martín, D.A.; Presa, L.; Parra, J.L. Altered volcanic tuffs from Los Frailes Caldera. A study of their pozzolanic properties. Molecules 2021, 26, 5348. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Lam, M.; Villar-Cociña, E.; Frías, M. Study on the pozzolanic properties of a natural Cuban zeolitic rock by conductometric method: Kinetic parameters. Constr. Build. Mater. 2011, 25, 644–650. [Google Scholar] [CrossRef]
- Pei, F.; Zhu, G.; Li, P.; Guo, H.; Yang, P. Effects of CaF2 on the sintering and crystallisation of CaO–MgO–Al2O3–SiO2 glass-ceramic. Ceram. Int. 2020, 46, 17825–17835. [Google Scholar] [CrossRef]
- Hou, P.; Qian, J.; Cheng, X.; Shahd, S.P. Effects of the pozzolanic reactivity of nanoSiO2 on cement-based materials. Cem. Concr. Compos. 2015, 55, 250–258. [Google Scholar] [CrossRef]
- Grist, E.R.; Paine, K.A.; Heath, A.; Norman, J.; Pinde, H. Compressive strength development of binary and ternary lime–pozzolan mortars. Mater. Des. 2013, 52, 514–523. [Google Scholar] [CrossRef]


| Sample | Proportion (Ratios) | Temperature of Calcination (°C) | Blaine Particle Fineness (BPF) for NF and CF (cm2/g) | |||
|---|---|---|---|---|---|---|
| PC 1:NF 2 (%) | PC:CF 3 (%) | NS 4 (g) | DW 5 (g) | |||
| PC/NF-01 PC/NF-02 PC/NF-03 PC/NF-04 PC/NF-05 PC/NF-06 | 90:10 75:25 60:40 90:10 75:25 60:40 | - | 1350 | 225 | - | 2000 |
| 5000 | ||||||
| PC/CF-07 PC/CF-08 PC/CF-09 PC/CF-10 PC/CF-11 PC/CF-12 | - | 90:10 75:25 60:40 90:10 75:25 60:40 | 1350 | 225 | 900 | 2000 |
| 5000 | ||||||
| Compounds | Samples | |
|---|---|---|
| Natural Fluorite (NF) (%) | Calcined Fluorite (CF) (%) | |
| Total SiO2 | 53.28 | 56.70 |
| Reactive SiO2 | 51.36 | 55.75 |
| MgO | 0.02 | 0.06 |
| Total CaO | 30.34 | 28.66 |
| Reactive CaO | 28.58 | 28.40 |
| Fe2O3 | 0.10 | 0.15 |
| Al2O3 | 0.23 | 0.15 |
| I.R. * | 3.85 | 2.84 |
| SiO2/(CaO + MgO) | 1.86 | 1.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, D.A.; Costafreda, J.L.; Estévez, E.; Presa, L.; Calvo, A.; Castedo, R.; Sanjuán, M.Á.; Parra, J.L.; Navarro, R. Natural Fluorite from Órgiva Deposit (Spain). A Study of Its Pozzolanic and Mechanical Properties. Crystals 2021, 11, 1367. https://doi.org/10.3390/cryst11111367
Martín DA, Costafreda JL, Estévez E, Presa L, Calvo A, Castedo R, Sanjuán MÁ, Parra JL, Navarro R. Natural Fluorite from Órgiva Deposit (Spain). A Study of Its Pozzolanic and Mechanical Properties. Crystals. 2021; 11(11):1367. https://doi.org/10.3390/cryst11111367
Chicago/Turabian StyleMartín, Domingo A., Jorge Luis Costafreda, Esteban Estévez, Leticia Presa, Alicia Calvo, Ricardo Castedo, Miguel Ángel Sanjuán, José Luis Parra, and Rafael Navarro. 2021. "Natural Fluorite from Órgiva Deposit (Spain). A Study of Its Pozzolanic and Mechanical Properties" Crystals 11, no. 11: 1367. https://doi.org/10.3390/cryst11111367
APA StyleMartín, D. A., Costafreda, J. L., Estévez, E., Presa, L., Calvo, A., Castedo, R., Sanjuán, M. Á., Parra, J. L., & Navarro, R. (2021). Natural Fluorite from Órgiva Deposit (Spain). A Study of Its Pozzolanic and Mechanical Properties. Crystals, 11(11), 1367. https://doi.org/10.3390/cryst11111367









