Morphological: Optical, and Mechanical Characterizations of Non-Activated and Activated Nanocomposites of SG and MWCNTs
Abstract
:1. Introduction
2. Chemicals and Methodology
2.1. Materials: Silica Gel (SG) and Multiwalled Carbon Nanotubes (MWCNTs)
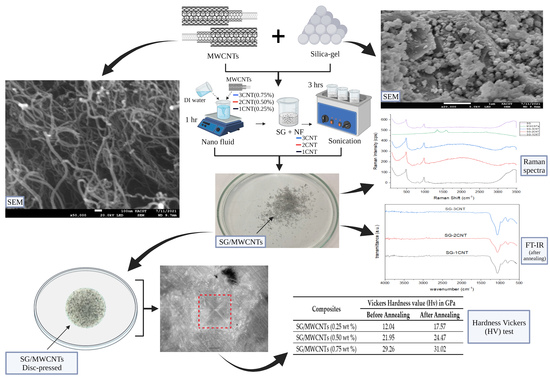
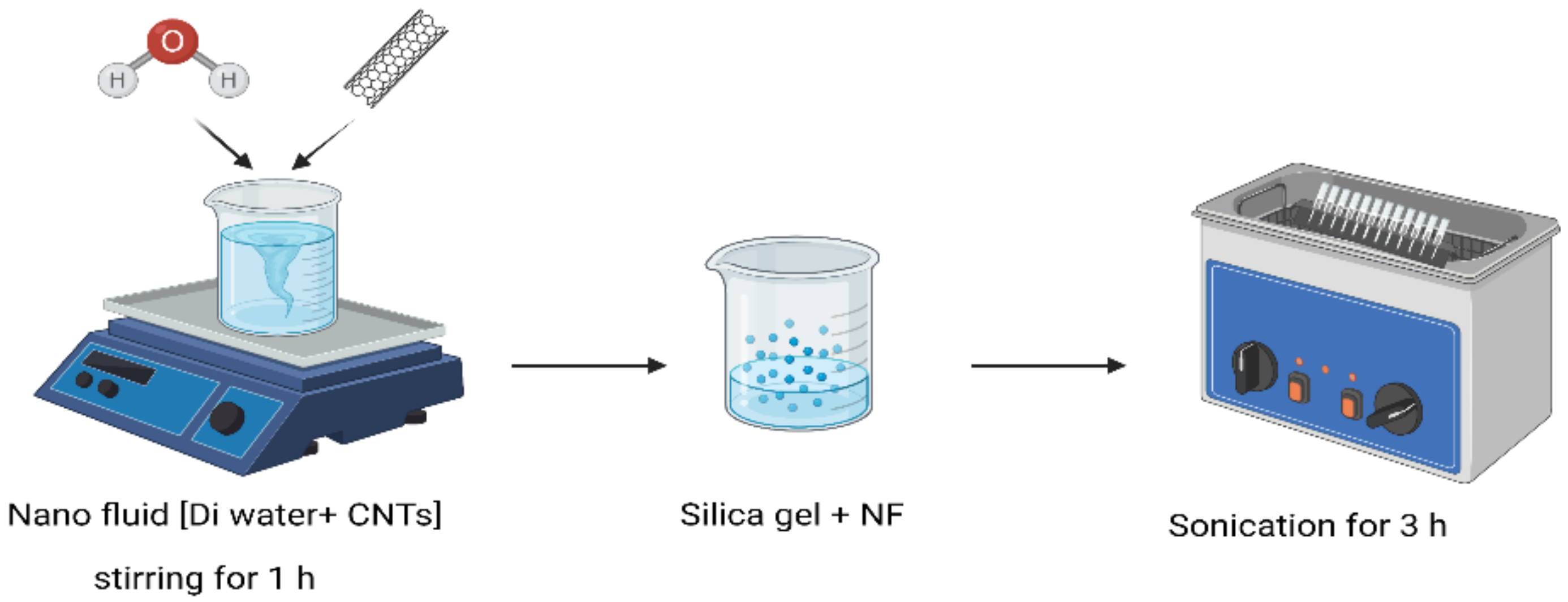
2.2. Preparation of SG with MWCNTs Using Mechanical Dispersion
3. Characterization Tools, Results, and Discussion
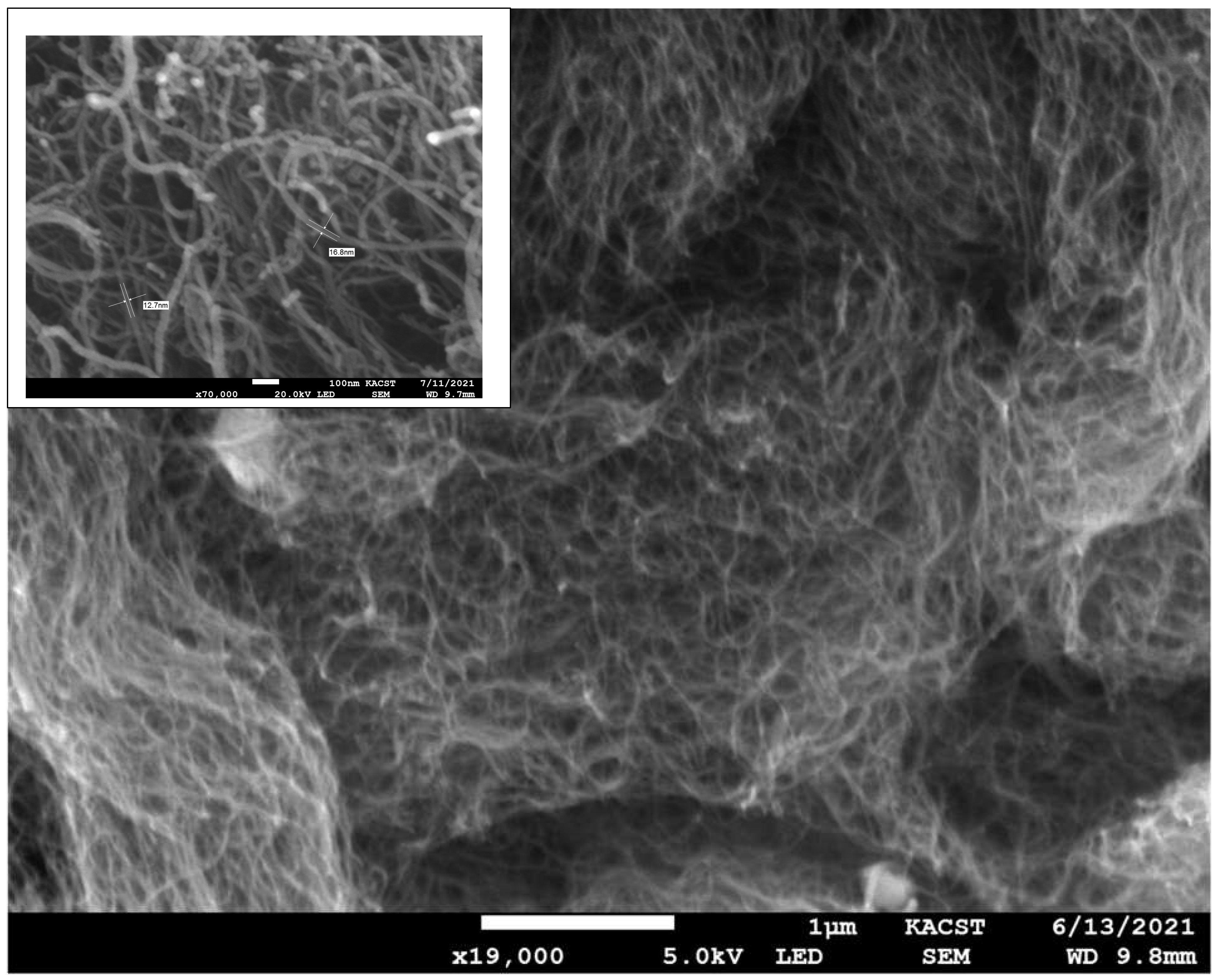
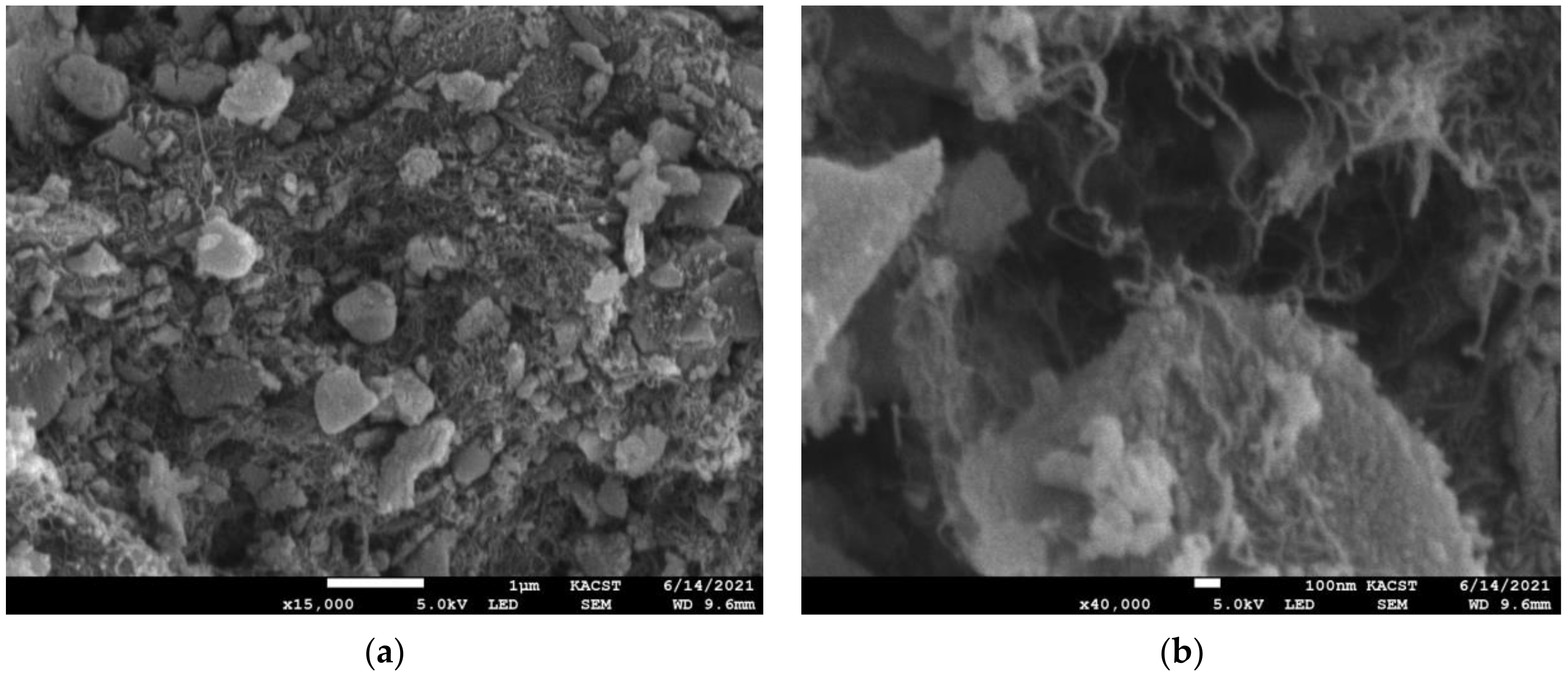
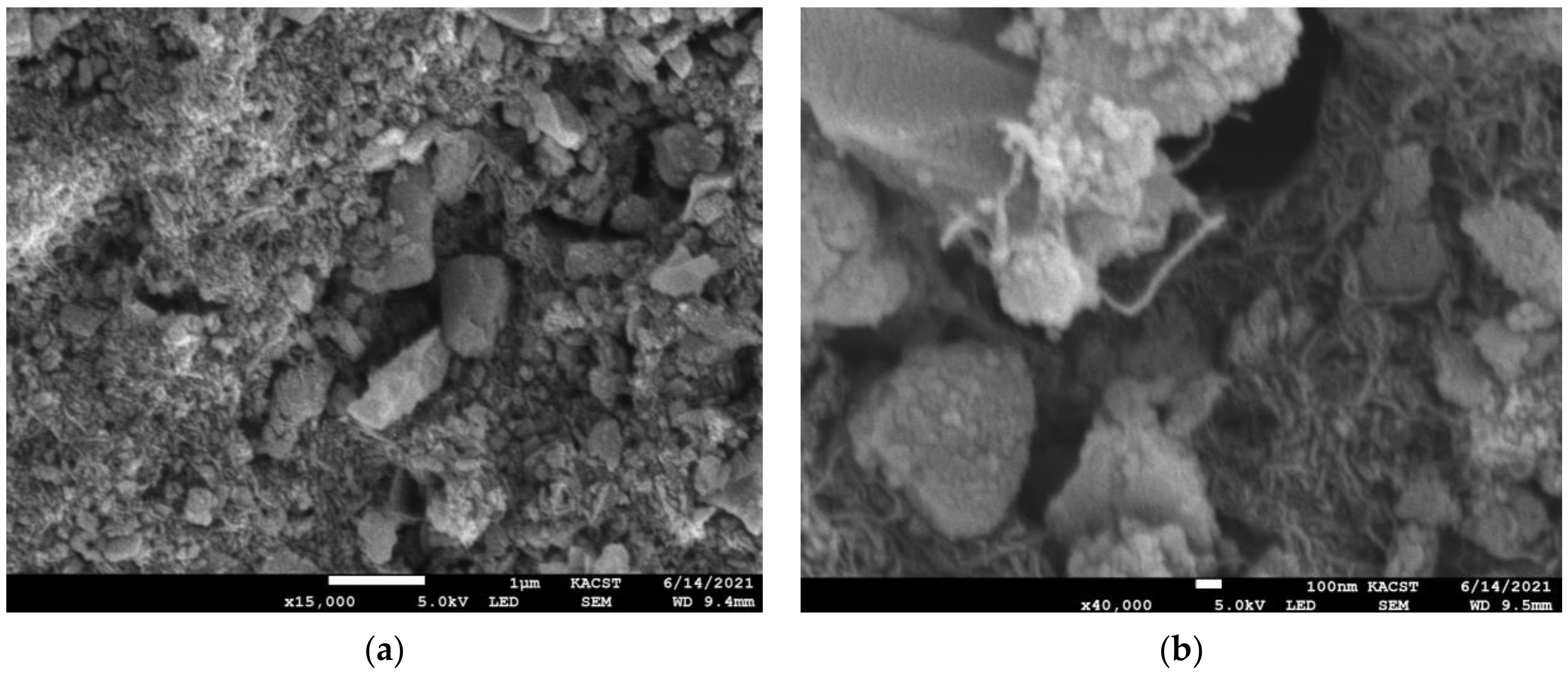
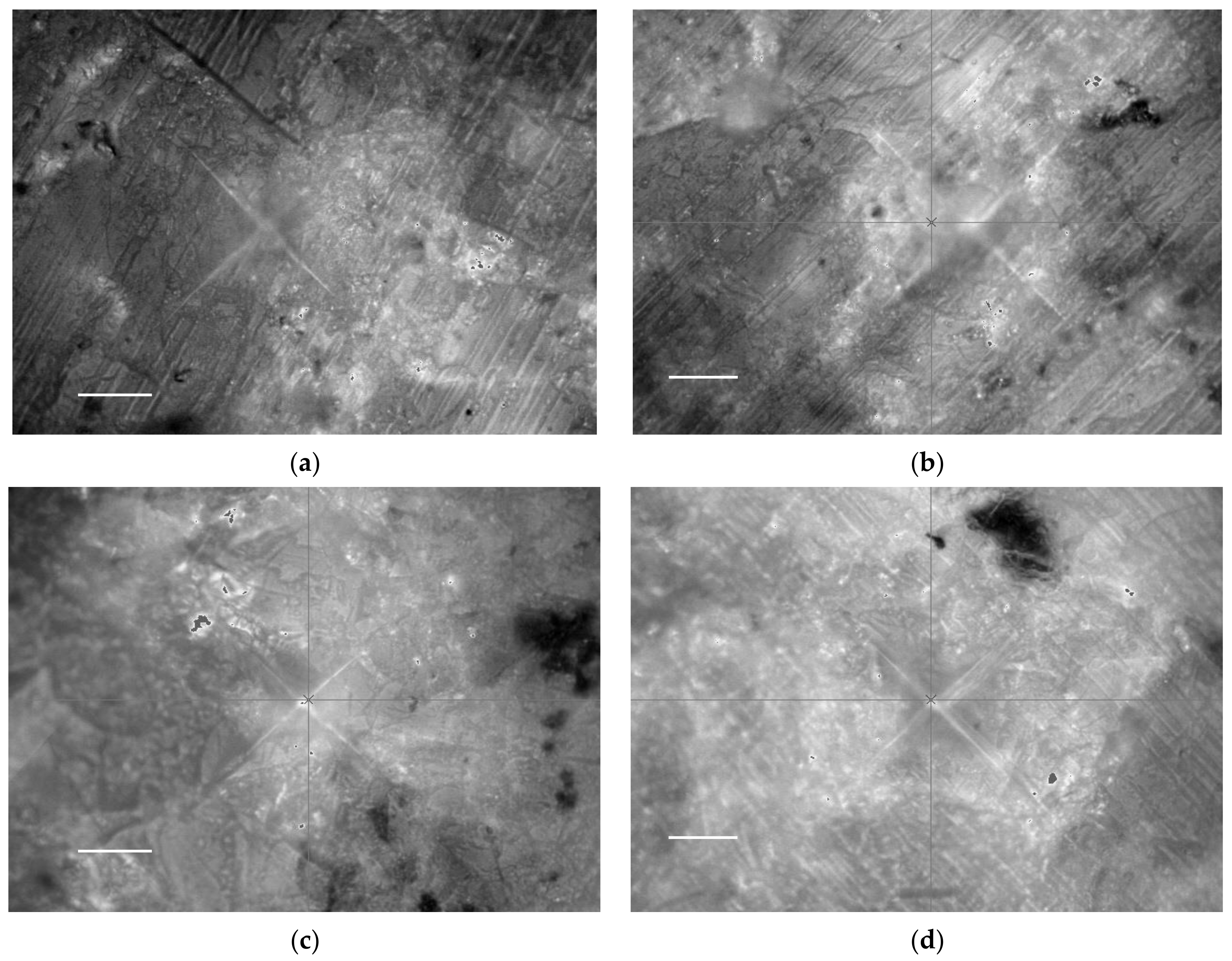
3.1. Scanning Electron Microscopy (SEM)
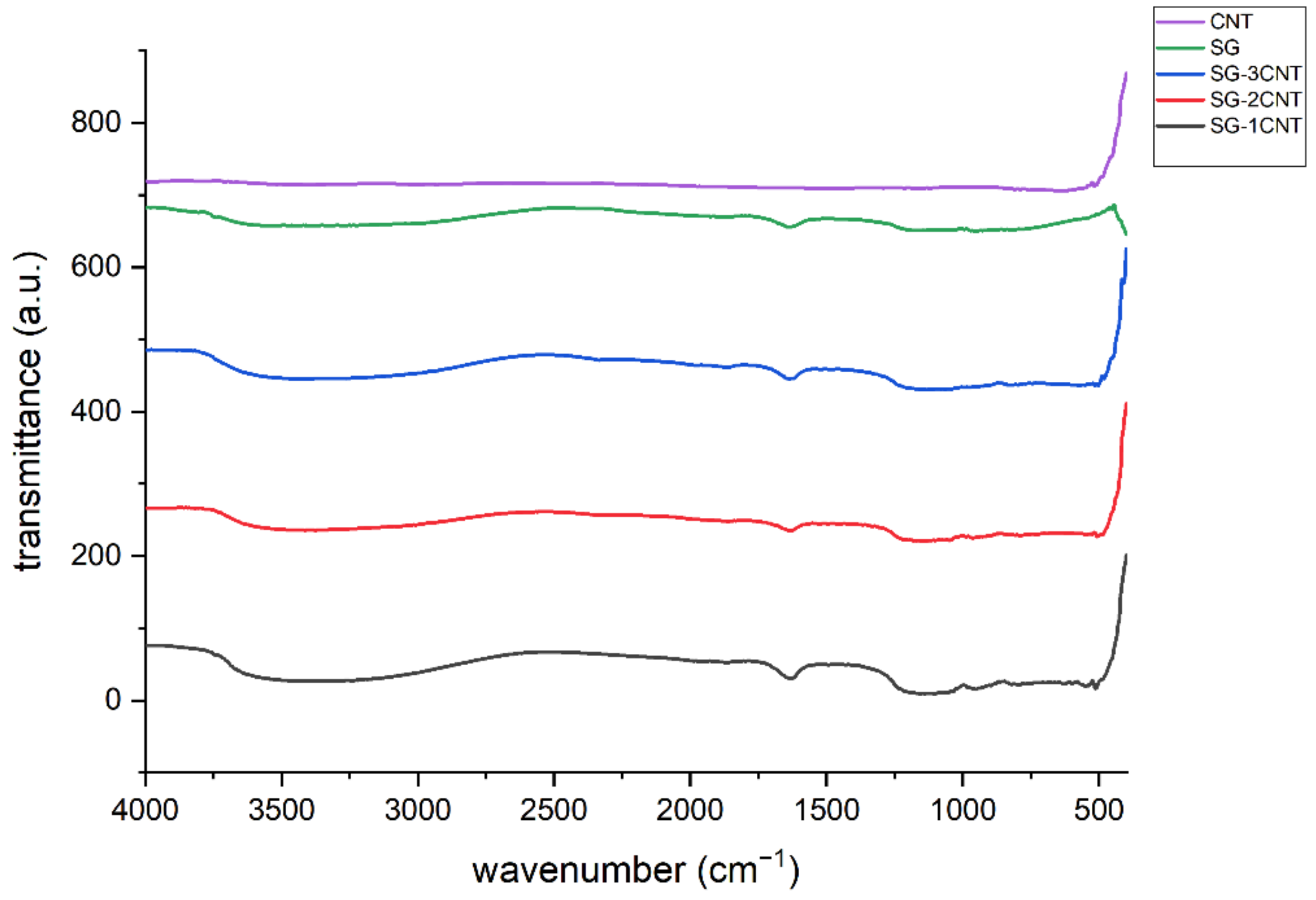
3.2. FTIR Measurements
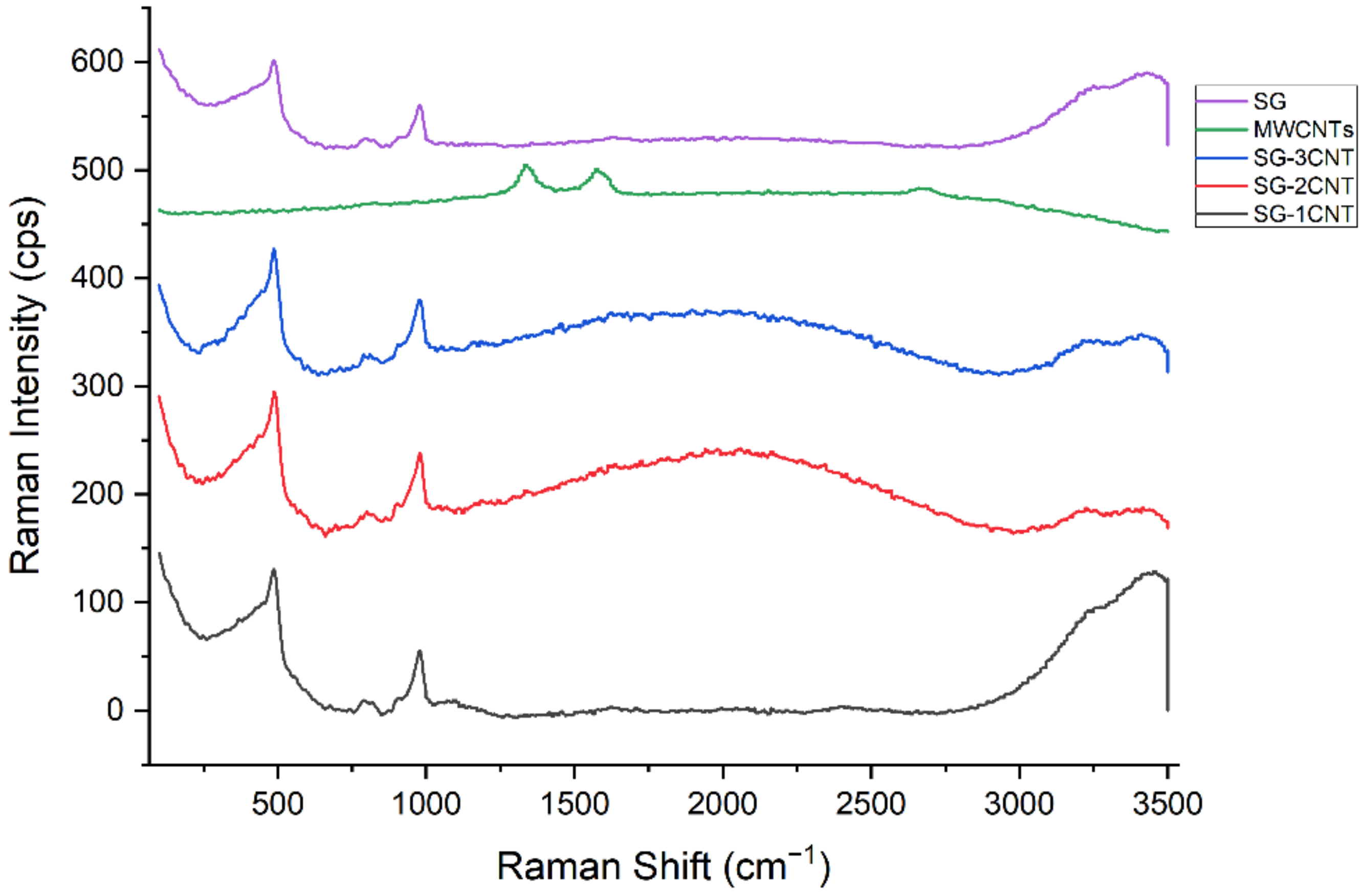
3.3. Raman Spectroscopy
3.4. Particle Size Distribution
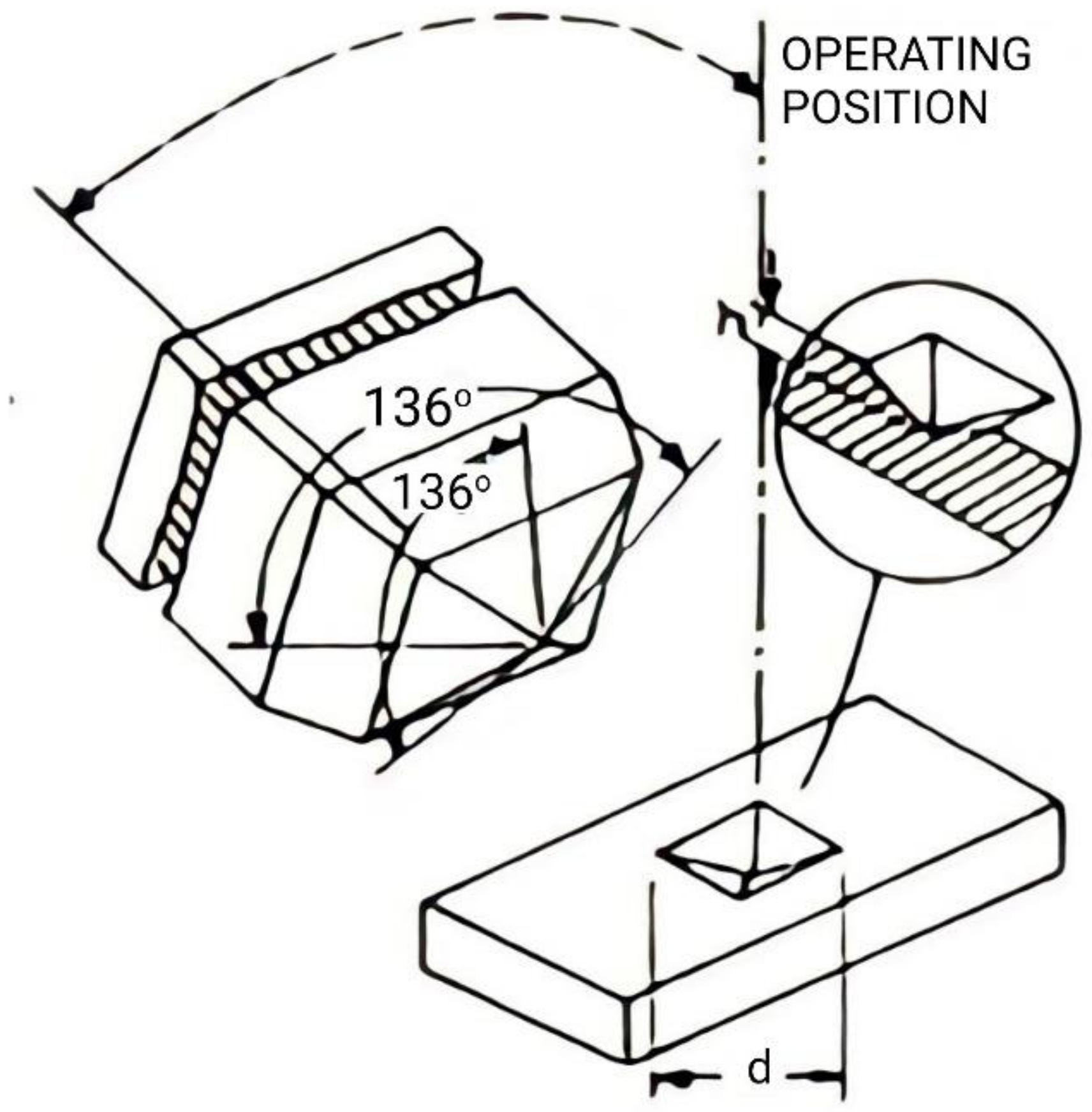
3.5. Vickers Hardness Test
4. Conclusions and Future Work
- (1)
- SEM micrographs and EDX showed that silica gel and MWCNT composites were mixed, with the lengthy MWCNTs tangled and the silica gel particulates immersed between the nanotubes. Low concentrations of MWCNTs are sufficient to produce balanced composites.
- (2)
- Annealing at 400 °C confirmed the functionalization of the composites, as shown by the FTIR measurements.
- (3)
- Raman spectra were mainly dominated by the base materials, due to the presence of MWCNTs in very low concentrations.
- (4)
- The Nanosizer showed that the best distribution of MWCNTs in the composite matrix occurred at 0.25 wt%, with high reproducibility rates.
- (5)
- As the percent weight of MWCNTs increased within the matrix of silica gel composites, the Vickers hardness value increased. Annealing further improved the strength of the composites.
- (6)
- Further studies are required to investigate the pore structure of silica gel within the matrix of MWCNTs, as the enhancement of cooling and water purification is very much associated with these micro- and nanosized particulates.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Radushkevich, L.V.; Lukyanovich, V.M. On the structure of carbon produced at thermal decomposition of carbon monoxide on an iron contact. J. Phys. Chem. 1952, 26, 88–95. [Google Scholar]
- Oberlin, A.; Endo, M. Filamentous Growth of Carbon Through Benzene Decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Singer, G.; Siedlaczek, P.; Sinn, G.; Rennhofer, H.; Mičušík, M.; Omastová, M.; Unterlass, M.M.; Wendrinsky, J.; Milotti, V.; Fedi, F.; et al. Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP. Nanomaterials 2018, 8, 912. [Google Scholar] [CrossRef] [Green Version]
- Singer, G.; Sinn, G.; Rennhofer, H.; Schuller, R.; Grünewald, T.A.; Unterlass, M.M.; Windberger, U.; Lichtenegger, H.C. High performance functional composites by in-situ orientation of carbon nanofillers. Compos. Struct. 2019, 215, 178–184. [Google Scholar] [CrossRef]
- Freni, A.; Calabrese, L.; Malara, A.; Frontera, P.; Bonaccorsi, L. Silica Gel Microfibres by Electrospinning for Adsorption Chillers. Energy 2019, 187. [Google Scholar] [CrossRef]
- Pan, Q.W.; Wang, R.Z. Study on Boundary Conditions of Adsorption Heat Pump Systems Using Different Working Pairs for Heating Application. Energy Convers. Manag. 2017, 154, 322–335. [Google Scholar] [CrossRef]
- Qadir, N.U.; Said, S.A.M.; Mansour, R.B.; Mezghani, K.; Ul-Hamid, A. Synthesis, Characterization, and Water Adsorption Properties of a Novel Multi-Walled Carbon Nanotube/MIL-100(Fe) Composite. Dalt. Trans. 2016, 45, 15621–15633. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, L.C.; Li, L.L.; Ding, M.L.; Wu, Y.P.; Holze, R. Enhanced Electrochemical and Mechanical Properties of P(VDF-HFP)-Based Composite Polymer Electrolytes with SiO2 Nanowires. J. Memb. Sci. 2011, 379, 80–85. [Google Scholar] [CrossRef]
- Jin, F.; Feng, M.; Huang, X.; Long, C.; Jia, K.; Liu, X. Effect of SiO2 Grafted MWCNTs on the Mechanical and Dielectric Properties of PEN Composite Films. Appl. Surf. Sci. 2015, 357, 704–711. [Google Scholar] [CrossRef]
- Kocyigit, A.; Orak, I.; Karteri, İ.; Uruş, S. The Structural Analysis of MWCNT-SiO2 and Electrical Properties on Device Application. Curr. Appl. Phys. 2017, 17, 1215–1222. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Lin, S.; Yan, J.; Du, S. Effects of Nano-SiO2 Coated Multi-Walled Carbon Nanotubes on Mechanical Properties of Cement-Based Composites. Constr. Build. Mater. 2021, 281, 122577. [Google Scholar] [CrossRef]
- Sharma, N.; Alam, S.N.; Ray, B.C.; Yadav, S.; Biswas, K. Silica-Graphene Nanoplatelets and Silica-MWCNT Composites: Microstructure and Mechanical Properties. Diam. Relat. Mater. 2018, 87, 186–201. [Google Scholar] [CrossRef]
- Piao, Y.; Tondare, V.N.; Davis, C.S.; Gorham, J.M.; Petersen, E.J.; Gilman, J.W.; Scott, K.; Vladár, A.E.; Hight Walker, A.R. Comparative Study of Multiwall Carbon Nanotube Nanocomposites by Raman, SEM, and XPS Measurement Techniques. Compos. Sci. Technol. 2021, 208. [Google Scholar] [CrossRef]
- Sharma, N.; Alam, S.N. Influence of Surface Roughness on Wear Behaviour of Ceramic Nanocomposites. Mater. Today Proc. 2018, 5, 28051–28060. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, S.; Ye, L.; Li, M.; Pan, Z.; Sun, R.; Xu, J. Encapsulating Carbon Nanotubes with SiO2: A Strategy for Applying Them in Polymer Nanocomposites with High Mechanical Strength and Electrical Insulation. J. Mater. Chem. C 2015, 3, 187–195. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Zhong, F.; Chen, H. Synthesis and Characterization: Silicon Oxide-Coated Multiwalled Carbon Nanotubes and Properties of Composite Coating Research. High Perform. Polym. 2015, 27, 352–361. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, M.J.; Lima, M.D.; Bergmann, C.P.; de O Ramminger, G.; Balzaretti, N.M.; Costa, T.M.H.; Gallas, M.R. Carbon Nanotube/Silica Composites Obtained by Sol–Gel and High-Pressure Techniques. Nanotechnology 2008, 19, 265607. [Google Scholar] [CrossRef]
- Contreras-Navarrete, J.J.; Ambriz-Torres, J.M.; Gutiérrez-García, C.J.; Granados-Martínez, F.G.; Flores-Ramírez, N.; Vásquez-García, S.R.; Mondragón-Sánchez, M.L.; García-González, L.; Zamora-Peredo, L.; Domratcheva-Lvova, L. Electrical Conductivity and Vickers Hardness Enhancement by Pristine and Functionalized MWCNTs Incorporation in Polycaprolactam Matrix. J. Mater. Sci. Mater. Electron. 2018, 29, 15776–15783. [Google Scholar] [CrossRef]
- Khayyat, M. Structural Phase Transformation of Semiconductors. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2004. [Google Scholar]
- Dresselhaus, M.; Lin, Y.-M.; Rabin, O.; Black, M.; Kong, J.; Dresselhaus, G. Nanowires. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 113–160. ISBN 978-3-540-29857-1. [Google Scholar]
- Wu, Y.; Fan, R.; Yang, P. Block-by-Block Growth of Single-Crystalline Si/SiGe Superlattice Nanowires. Nano Lett. 2002, 2, 83–86. [Google Scholar] [CrossRef]
- Atchudan, R.; Pandurangan, A.; Joo, J. Effects of Nanofillers on the Thermo-Mechanical Properties and Chemical Resistivity of Epoxy Nanocomposites. J. Nanosci. Nanotechnol. 2015, 15, 4255–4267. [Google Scholar] [CrossRef]
- Wang, B.; Song, K.; Han, Y.; Zhang, T. Synthesis and Characterization of Multi-Walled Carbon Nanotube Doped Silica Aerogels. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2012, 27, 512–515. [Google Scholar] [CrossRef]
- Hanzel, O.; Sedláček, J.; Hadzimová, E.; Šajgalík, P. Thermal Properties of Alumina-MWCNTs Composites. J. Eur. Ceram. Soc. 2015, 35, 1559–1567. [Google Scholar] [CrossRef]
- Javed, M.; Abbas, S.M.; Hussain, S.; Siddiq, M.; Han, D.; Niu, L. Amino-Functionalized Silica Anchored to Multiwall Carbon Nanotubes as Hybrid Electrode Material for Supercapacitors. Mater. Sci. Energy Technol. 2018, 1, 70–76. [Google Scholar] [CrossRef]
- Kulakowska, A.; Pajdak, A.; Krzywanski, J.; Grabowska, K.; Zylka, A.; Sosnowski, M.; Wesolowska, M.; Sztekler, K.; Nowak, W. Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica-Gel-Based Adsorption Bed. Energies 2020, 16, 1391. [Google Scholar] [CrossRef] [Green Version]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R.; Somoghi, R.; Frone, A.N.; Stirbescu, R.M. Chemical Synthesis of Multi-Walled Carbon Nanotubes and Their Functionalization with Carboxylated Groups. Proceedings 2020, 57, 45. [Google Scholar] [CrossRef]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J.; Elías, A.L.; Muñoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.-C.; et al. Graphene and Graphite Nanoribbons: Morphology, Properties, Synthesis, Defects and Applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Malekfar, R.; Rajabi, M.H.; Ara, M.H.M. Structural and Optical Characteristics of Silica Nano- Tubes Using CNTs as Template. Nano-Micro Lett. 2010, 2, 268–271. [Google Scholar] [CrossRef]
- Bertoluzza, A.; Fagnano, C.; Antonietta Morelli, M.; Gottardi, V.; Guglielmi, M. Raman and Infrared Spectra on Silica Gel Evolving toward Glass. J. Non. Cryst. Solids 1982, 48, 117–128. [Google Scholar] [CrossRef]
- Matsui, K.; Satoh, H.; Kyoto, M. Raman Spectra of Silica Gel Prepared from Triethoxysilane and Tetraethoxysilane by the Sol-Gel Method. J. Ceram. Soc. Japan 1998, 106, 528–530. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.M.; Richter, E.; Smalley, R.E.; Dresselhaus, G.; Dresselhaus, M.S.; Bandow, S.; Chase, B.; Eklund, P.C.; Williams, K.; Fang, S.; et al. Diameter-Selective Raman Scattering from Vibrational Modes in Carbon Nanotubes. Science 1997, 275, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Akbar, S.; Beyou, E.; Chaumont, P.; Mazzolini, J.; Espinosa, E.; D’agosto, F.; Boisson, C. Synthesis of Polyethylene-Grafted Multiwalled Carbon Nanotubes via a Peroxide-Initiating Radical Coupling Reaction and by Using Well-Defined TEMPO and Thiol End-Functionalized Polyethylenes. J. Polym. Sci. A Polym. Chem. 2011, 49, 957–965. [Google Scholar] [CrossRef]
- Gao, W.; Qi, Q.; Dong, L.; Lv, X.; Huang, W. Experimental Evaluation of the Lubrication Performance of Milling Al7075 with Different Nano-Fluids MQL. J. Phys. Conf. Ser. 2021, 1744. [Google Scholar] [CrossRef]
- Lamnini, S.; Károly, Z.; Bódis, E.; Balázsi, K.; Balázsi, C. Influence of Structure on the Hardness and the Toughening Mechanism of the Sintered 8YSZ/MWCNTs Composites. Ceram. Int. 2019, 45, 5058–5065. [Google Scholar] [CrossRef]
- Luo, J.T.; Wen, H.C.; Chou, C.P.; Wu, W.F.; Wan, B.Z. Reinforcing Porous Silica with Carbon Nanotubes to Enhance Mechanical Performance. J. Compos. Mater. 2007, 41, 979–991. [Google Scholar] [CrossRef]
- Winarto, W.; Priadi, D.; Sofyan, N.; Wicaksono, A. Wear Resistance and Surface Hardness of Carbon Nanotube Reinforced Alumina Matrix Nanocomposite by Cold Sprayed Process. Procedia Eng. 2017, 170, 108–112. [Google Scholar] [CrossRef]
- Upadhyay, A.N.; Tiwari, R.S.; Singh, K. Annealing Effect on Thermal Conductivity and Microhardness of Carbon Nanotube Containing Se80Te16Cu4 Glassy Composites. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Nemeth, K.; Varro, N.; Reti, B.; Berki, P.; Adam, B.; Belina, K.; Hernadi, K. Synthesis and Investigation of SiO2-MgO Coated MWCNTs and Their Potential Application. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhou, Y.; Yu, C.; Xiang, M.; Huang, D.; Xu, W. Synthesis and Strengthening of Ti3AlC2 by Doping with Carbon Nanotubes. J. Alloys Compd. 2016, 654, 120–125. [Google Scholar] [CrossRef]
- Li, A.; Li, W.; Ling, Y.; Gan, W.; Brady, M.A.; Wang, C. Effects of Silica-Coated Carbon Nanotubes on the Curing Behavior and Properties of Epoxy Composites. RSC Adv. 2016, 6, 23318–23326. [Google Scholar] [CrossRef] [Green Version]



| Items | Unit | Spec | Test Method | |
|---|---|---|---|---|
| Adsorption Capacity | 20% RH | % | 10 ↑ | KS T 1084 |
| 50% RH | 25 ↑ | KS T 1084 | ||
| 90% RH | 35 ↑ | KS T 1084 | ||
| Bulk Density | g/cc | 670–750 | KS T 1084 | |
| pH Value | - | 4.0–6.0 | KS T 1084 | |
| Specific Resistance | Ω·cm | 3000 ↑ | KS T 1084 | |
| Moisture Content | % | 2 ↓ | KS T 1084 | |
| Surface Area | m2/g | 670–770 | BET | |
| Pore Volume | mL/g | 0.35–0.55 | BET | |
| Pore Volume per Gram | A | 20–28 | - | |
| Particle Size | ||||
| Size | 20 mesh over | % | 5.0 ↓ | KS T 1084 |
| 40 mesh under | % | 5.0 ↓ | KS T 1084 | |
| No | Silica Gel | MWCNTs |
|---|---|---|
| 1 | 4.9875 g (99.75 w/w %) | 0.0125 g (0.25 w/w %) |
| 2 | 4.975 g (99.5 w/w %) | 0.025 g (0.5 w/w %) |
| 3 | 4.9625 g (99.25 w/w %) | 0.0375 g (0.75 w/w %) |
| Composite | Vickers Hardness Value (Hv) in GPa | |
|---|---|---|
| Before Annealing | After Annealing | |
| SG/MWCNTs (0.25 wt%) | 12.04 | 17.57 |
| SG/MWCNTs (0.50 wt%) | 21.95 | 24.47 |
| SG/MWCNTs (0.75 wt%) | 29.26 | 31.02 |
| Material | Functionalization | Characterization Techniques | Observations | Applications and Outcomes | References |
|---|---|---|---|---|---|
| SG/MWCNTs | Annealing (physical functionalization) | (1) SEM and EDX (2) FTIR (3) Raman spectroscopy (4) Micro-hardness test | (1) MWCNTs incorporated within SG matrix at low concentrations (2) Annealing functionalizing composites (3) Dominated mainly by MWCNTs (4) Hv increased with MWCNTs and annealing | Annealing at 400 °C for 4 h was sufficient for optimum results and improved optical and mechanical properties | Current study, 2021 |
| SiO2-MgO coated MWCNTs | Chemical functionalization via sol–gel Physical functionalization, annealing in air at 400 °C for 4 h | (1) Raman spectroscopy (2) SEM | (1) Scattering peaks at 1345, 1574, and 2685 cm−1 corresponding to D, G, and G’ modes. (2) Incorporated in different types of polymer matrices, kept polymer parts together | Dispersion of MWCNTs increased in polymer matrices | Nemeth et al., 2019 [40] |
| CNTs/Se80Te16Cu4 glassy composites | CNTs incorporated into Se80Te16Cu4 glassy matrix via melt-quenched technique | (1) Vickers microhardness test (2) SEM | (1) Hardness increased with increased annealing temperature (2) Thermal annealing with successive increase in temperature could improve dispersion of CNTs | Greater mechanical strength | Upadhyay et al., 2018 [39] |
| Ti3AlC2-MWCNTs composites | Hot pressing method in Ar atmosphere at 1300 °C under a pressure of 20 MPa. | (1) Vickers Hardness (2) SEM | (1) Hardness increased with increased content of MWCNTs up to 2 wt% (2) MWCNTs enwrapped in Ti3AlC2 grains were observed in SEM micrographs | Mechanical properties of Ti3AlC2 ceramic were greatly enhanced by MWCNTs | Wang et al., 2016 [41] |
| MWCNTs/MIL-100(Fe) composite | Carboxylic acid-functionalized MWCNTs | (1) FTIR (2) SEM | (1) Chemical integrity of structure preserved upon incorporation of MWCNTs using proposed synthesis method (2) MWCNTs can also be seen implanted in surrounding MIL-100(Fe) matrix | Adsorption and cyclic stability | Qadir et al. 2016 [8] |
| SiO2/MWCNTs | SiO2/MWCNTs prepared by sol–gel method | (1) FTIR (2) SEM | (1) Absorbance peaks at 1065, 964, and 793 cm−1 observed (2) Better dispersion of MWCNTs in matrix | Optimized property needed in electronic packaging applications | Li et al., 2016 [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.S.; Almousa, N.H.; Asaker, M.A.; Alkasmoul, F.S.; Khdary, N.H.; Khayyat, M. Morphological: Optical, and Mechanical Characterizations of Non-Activated and Activated Nanocomposites of SG and MWCNTs. Crystals 2021, 11, 1280. https://doi.org/10.3390/cryst11111280
Alotaibi MS, Almousa NH, Asaker MA, Alkasmoul FS, Khdary NH, Khayyat M. Morphological: Optical, and Mechanical Characterizations of Non-Activated and Activated Nanocomposites of SG and MWCNTs. Crystals. 2021; 11(11):1280. https://doi.org/10.3390/cryst11111280
Chicago/Turabian StyleAlotaibi, Mohammed S., Norah H. Almousa, Mohammed A. Asaker, Fahad S. Alkasmoul, Nezar H. Khdary, and Maha Khayyat. 2021. "Morphological: Optical, and Mechanical Characterizations of Non-Activated and Activated Nanocomposites of SG and MWCNTs" Crystals 11, no. 11: 1280. https://doi.org/10.3390/cryst11111280
APA StyleAlotaibi, M. S., Almousa, N. H., Asaker, M. A., Alkasmoul, F. S., Khdary, N. H., & Khayyat, M. (2021). Morphological: Optical, and Mechanical Characterizations of Non-Activated and Activated Nanocomposites of SG and MWCNTs. Crystals, 11(11), 1280. https://doi.org/10.3390/cryst11111280