Influence of Cr/Zr Ratio on Activity of Cr–Zr Oxide Catalysts in Non-Oxidative Propane Dehydrogenation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterization
2.3. Catalytic Tests
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Sattler, J.J.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X.; Wang, Q.; Wan, X.; Zhou, C.; Yang, Y. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Soc. Rev. 2021, 50, 5590–5630. [Google Scholar] [CrossRef]
- Monai, M.; Gambino, M.; Wannakao, S.; Weckhuysen, B.M. Propane to olefins tandem catalysis: A selective route towards light olefins production. Chem. Soc. Rev. 2021, 50, 11503–11529. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.; Farsad, A.; Adebayo, B.; Newport, K.; Schueddig, K.; Lowrey, E.; Polo-Garzon, F.; Rezaei, F.; Rownaghi, A.A. A Novel Method of 3D Printing High-Loaded Oxide/H-ZSM-5 Catalyst Monoliths for Carbon Dioxide Reduction in Tandem with Propane Dehydrogenation. Adv. Sustain. Syst. 2021, 5, 2000257. [Google Scholar] [CrossRef]
- De Oliveira, J.F.S.; Volanti, D.P.; Bueno, J.M.C.; Ferreira, A.P. Effect of CO2 in the oxidative dehydrogenation reaction of propane over Cr/ZrO2 catalysts. Appl. Catal. A Gen. 2018, 558, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Kharlamova, T.S.; Timofeev, K.L.; Salaev, M.A.; Svetlichnyi, V.A.; Vodyankina, O.V. Monolayer MgVOx/Al2O3 catalysts for propane oxidative dehydrogenation: Insights into a role of structural, redox, and acid-base properties in catalytic performance. Appl. Catal. A Gen. 2020, 59825, 117574. [Google Scholar] [CrossRef]
- Kharlamova, T.; Sushchenko, E.; Izaak, T.; Vodyankina, O. Phase composition, structural peculiarities and catalytic properties of supported MgO-V2O5/Al2O3 catalysts for oxidative dehydrogenation of propane: Insight into formation of surface Mg-V-O phase. Catal. Today 2016, 278, 174–184. [Google Scholar] [CrossRef]
- Jiang, X.; Sharma, L.; Fung, V.; Park, S.J.; Jones, C.W.; Sumpter, B.G.; Baltrusaitis, J.; Wu, Z. Oxidative dehydrogenation of propane to propylene with soft oxidants via heterogeneous catalysis. ACS Catal. 2021, 11, 2182–2234. [Google Scholar] [CrossRef]
- Wu, R.; Xie, P.; Cheng, Y.; Yue, Y.; Gu, S.; Yang, W.; Miao, C.; Hua, W.; Gao, Z. Hydrothermally prepared Cr2O3–ZrO2 as a novel efficient catalyst for dehydrogenation of propane with CO2. Catal. Commun. 2013, 39, 20–23. [Google Scholar] [CrossRef]
- Bugrova, T.A.; Dutov, V.V.; Svetlichnyi, V.A.; Cortés Corberán, V.; Mamontov, G.V. Oxidative dehydrogenation of ethane with CO2 over CrOx catalysts supported on Al2O3, ZrO2, CeO2 and CexZr1-xO2. Catal. Today 2019, 333, 71–80. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Mohammadkhani, B. Enhanced dispersion of Cr nanoparticles over nanostructured ZrO2-doped ZSM-5 used in CO2-oxydehydrogenation of ethane. Microp. Mesopor. Mater. 2017, 242, 34–49. [Google Scholar] [CrossRef]
- Gao, Y.; Jie, X.; Wang, C.; Jacobs, R.M.J.; Li, W.; Yao, B.; Dilworth, J.R.; Xiao, T.; Edwards, P.P. One-Pot Synthesis of Ca Oxide-Promoted Cr Catalysts for the Dehydrogenation of Propane Using CO2. Ind. Eng. Chem. Res. 2020, 59, 12645–12656. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Wang, Z.; Yuan, Z.-Y. Cr/Al2O3 catalysts with strong metal-support interactions for stable catalytic dehydrogenation of propane to propylene. Molec. Catal. 2020, 493, 111052. [Google Scholar] [CrossRef]
- Nazimov, D.A.; Klimov, O.V.; Saiko, A.V.; Trukhan, S.N.; Glazneva, T.S.; Prosvirin, I.P.; Cherepanova, S.V.; Noskov, A.S. Effect of the K loading on effective activation energy of isobutane dehydrogenation over chromia/alumina catalysts. Catal. Today 2021, 375, 401–409. [Google Scholar] [CrossRef]
- Nazimov, D.A.; Klimov, O.V.; Danilova, I.G.; Trukhan, S.N.; Saiko, A.V.; Cherepanova, S.V.; Chesalov, Y.A.; Martyanov, O.N.; Noskov, A.S. Effect of alumina polymorph on the dehydrogenation activity of supported chromia/alumina catalysts. J. Catal. 2020, 391, 35–47. [Google Scholar] [CrossRef]
- Zolotukhina, A.I.; Romanova, E.V.; Bugrova, T.A.; Knyazev, A.S.; Mamontov, G.V. Influence of impregnation conditions on the activity of CrOx/Al2O3 catalysts in dehydrogenation of isobutane in fixed bed reactor. Arab. J. Chem. 2020, 13, 9130–9138. [Google Scholar] [CrossRef]
- Salaeva, A.A.; Salaev, M.A.; Vodyankina, O.V.; Mamontov, G.V. Synergistic effect of Cu and Zn modifiers on the activity of CrOx/Al2O3 catalysts in isobutane dehydrogenation. Appl. Catal. A Gen. 2019, 581, 82–90. [Google Scholar] [CrossRef]
- Salaeva, A.A.; Salaev, M.A.; Mamontov, G.V. Effect of Cu modifier on the performance of CrOx/Al2O3 catalysts for isobutane dehydrogenation. Chem. Eng. Sci. 2020, 2156, 115462. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, R.; Yue, Y.; Yuan, P.; Bao, X.; Abou-Hamad, E.; Basset, J.-M.; Zhu, H. Bimetallic Pt-Sn nanocluster from the hydrogenolysis of a well-defined surface compound consisting of [(≡AlO–)Pt(COD)Me] and [(≡AlO–)SnPh3] fragments for propane dehydrogenation. J. Catal. 2019, 374, 391–400. [Google Scholar] [CrossRef]
- Natarajan, P.; Khan, H.A.; Yoon, S.; Jung, K.-D. One-pot synthesis of Pt–Sn bimetallic mesoporous alumina catalysts with worm-like pore structure for n-butane dehydrogenation. J. Ind. Eng. Chem. 2018, 63, 380–390. [Google Scholar] [CrossRef]
- Pham, H.N.; Sattler, J.J.H.B.; Weckhuysen, B.M.; Datye, A.K. Role of Sn in the regeneration of Pt/γ-Al2O3 light alkane dehydrogenation catalysts. ACS Catal. 2016, 6, 2257–2264. [Google Scholar] [CrossRef] [Green Version]
- Srinath, N.V.; Poelman, H.; Buelens, L.; Dendooven, J.; Reyniers, M.-F.; Marin, G.B.; Galvita, V.V. Behaviour of Platinum-Tin during CO2-assisted propane dehydrogenation: Insights from quick X-ray absorption spectroscopy. J. Catal. 2021. [Google Scholar] [CrossRef]
- Rodemerck, U.; Stoyanova, M.; Kondratenko, E.V.; Linke, D. Influence of the kind of VOx structures in VOx/MCM-41 on activity, selectivity and stability in dehydrogenation of propane and isobutane. J. Catal. 2017, 352, 256–263. [Google Scholar] [CrossRef]
- Jeon, N.; Choe, H.; Jeong, B.; Yun, Y. Propane dehydrogenation over vanadium-doped zirconium oxide catalysts. Catal. Today 2020, 352, 337–344. [Google Scholar] [CrossRef]
- Matveyeva, A.N.; Wärnå, J.; Pakhomov, N.A.; Murzin, D.Y. Kinetic modeling of isobutane dehydrogenation over Ga2O3/Al2O3 catalyst. Chem. Eng. J. 2020, 381, 122741. [Google Scholar] [CrossRef]
- Dai, Y.; Gu, J.; Tian, S.; Wu, Y.; Chen, J.; Li, F.; Du, Y.; Peng, L.; Ding, W.; Yang, Y. γ-Al2O3 sheet-stabilized isolate Co2+ for catalytic propane dehydrogenation. J. Catal. 2020, 381, 482–492. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Otroshchenko, T.; Perechodjuk, A.; Kondratenko, V.A.; Bartling, S.; Rodemerck, U.; Linke, D.; Jiao, H.; Jiang, G.; et al. Structure-Activity-Selectivity Relationships in Propane Dehydrogenation over Rh/ZrO2 catalysts. ACS Catal. 2020, 10, 6377–6388. [Google Scholar] [CrossRef]
- Natarajan, P.; Khan, H.A.; Jaleel, A.; Park, D.S.; Kang, D.-C.; Yoon, S.; Jung, K.-D. The pronounced effect of Sn on RhSn catalysts for propane dehydrogenation. J. Catal. 2020, 392, 8–20. [Google Scholar] [CrossRef]
- Perechodjuk, A.; Zhang, Y.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Bartling, S.; Kreyenschulte, C.R.; Jiang, G.; Kondratenko, E.V. The effect of supported Rh, Ru, Pt or Ir nanoparticles on activity and selectivity of ZrO2-based catalysts in non-oxidative dehydrogenation of propane. Appl. Catal. A Gen. 2020, 60225, 117731. [Google Scholar] [CrossRef]
- Wang, P.; Yao, J.; Jiang, Q.; Gao, X.; Lin, D.; Yang, H.; Wu, L.; Tang, Y.; Tan, L. Stabilizing the Isolated Pt Sites on PtGa/Al2O3 Catalyst via Silica Coating Layers for Propane Dehydrogenation at Low Temperature. Appl. Catal. B Environ. 2022, 300, 120731. [Google Scholar] [CrossRef]
- Tolek, W.; Suriye, K.; Praserthdam, P.; Panpranot, J. Effect of preparation method on the Pt-In modified Mg(Al)O catalysts over dehydrogenation of propane. Catal. Today 2020, 358, 100–108. [Google Scholar] [CrossRef]
- Rimaz, S.; Chen, L.; Monzón, A.; Kawi, S.; Borgna, A. Enhanced selectivity and stability of Pt-Ge/Al2O3 catalysts by Ca promotion in propane dehydrogenation. Chem. Eng. J. 2021, 405, 126656. [Google Scholar] [CrossRef]
- Saxena, R.; De, M. Enhanced performance of supported Pd-Pt bimetallic catalysts prepared by modified electroless deposition for butane dehydrogenation. Appl. Catal. A Gen. 2021, 610, 117933. [Google Scholar] [CrossRef]
- Han, S.; Zhao, D.; Lund, H.; Rockstroh, N.; Bartling, S.; Doronkin, D.E.; Grunwaldt, J.-D.; Gao, M.; Jiang, G.; Kondratenko, E.V. TiO2-Supported catalysts with ZnO and ZrO2 for non-oxidative dehydrogenation of propane: Mechanistic analysis and application potential. Catal. Sci. Technol. 2020, 10, 7046–7055. [Google Scholar] [CrossRef]
- Takehira, K.; Ohishi, Y.; Shishido, T.; Kawabata, T.; Takaki, K.; Zhang, Q.; Wang, Y. Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J. Catal. 2004, 224, 404–416. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Radnik, J.; Schneider, M.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Bulk binary ZrO2-based oxides as highly active alternative-type catalysts for non-oxidative isobutane dehydrogenation. Chem. Commun. 2016, 52, 8164–8167. [Google Scholar] [CrossRef]
- Han, S.; Zhao, Y.; Otroshchenko, T.; Zhang, Y.; Zhao, D.; Lund, H.; Vuong, T.H.; Rabeah, J.; Bentrup, U.; Kondratenko, V.A.; et al. Unraveling the Origins of the Synergy Effect between ZrO2 and CrOx in Supported CrZrOx for Propene Formation in Nonoxidative Propane Dehydrogenation. ACS Catal. 2020, 10, 1575–1590. [Google Scholar] [CrossRef]
- Kim, T.H.; Kang, K.H.; Baek, M.; Song, J.H.; Hong, U.G.; Park, D.S.; Choi, W.C.; Park, Y.-K.; Song, I.K. Dehydrogenation of propane to propylene with lattice oxygen over CrOy/Al2O3-ZrO2 catalysts. Molec. Catal. 2017, 433, 1–7. [Google Scholar] [CrossRef]
- Kim, T.H.; Gim, M.Y.; Song, J.H.; Choi, W.C.; Park, Y.-K.; Hong, U.G.; Park, D.S.; Song, I.K. Deactivation behavior of CrOy/Al2O3-ZrO2 catalysts in the dehydrogenation of propane to propylene by lattice oxygen. Catal. Commun. 2017, 97, 37–41. [Google Scholar] [CrossRef]
- Bugrova, T.A.; Mamontov, G.V. The Study of CrOx-Containing Catalysts Supported on ZrO2, CeO2, and CexZr(1–x)O2 in Isobutane Dehydrogenation. Kinet. Catal. 2018, 59, 143–149. [Google Scholar] [CrossRef]
- Otroshchenko, T.P.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Synergy effect between Zr and Cr active sites in binary CrZrOx or supported CrOx/LaZrOx: Consequences for catalyst activity, selectivity and durability in non-oxidative propane dehydrogenation. J. Catal. 2017, 356, 197–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Otroshchenko, T.; Han, S.; Lund, H.; Rodemerck, U.; Linke, D.; Jiao, H.; Jiang, G.; Kondratenko, E.V. The effect of phase composition and crystallite size on activity and selectivity of ZrO2 in non-oxidative propane dehydrogenation. J. Catal. 2019, 371, 313–324. [Google Scholar] [CrossRef]
- Otroshchenko, T.P.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Non-oxidative dehydrogenation of propane, n-butane, and isobutane over bulk ZrO2-based catalysts: Effect of dopant on the active site and pathways of product formation. Catal. Sci. Technol. 2017, 7, 4499–4510. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Otroshchenko, T.; Lund, H.; Pohl, M.-M.; Rodemerck, U.; Linke, D.; Jiao, H.; Jiang, G.; Kondratenko, E.V. Control of coordinatively unsaturated Zr sites in ZrO2 for efficient C–H bond activation. Nat. Commun. 2018, 9, 3794. [Google Scholar] [CrossRef]
- Han, S.; Otroshchenko, T.; Zhao, D.; Lund, H.; Rockstroh, N.; Vuong, T.H.; Rabeah, J.; Rodemerck, U.; Linke, D.; Gao, M.; et al. The effect of ZrO2 crystallinity in CrZrOx/SiO2 on non-oxidative propane dehydrogenation. Appl. Catal. A Gen. 2020, 59025, 117350. [Google Scholar] [CrossRef]
- Cutrufello, M.G.; De Rossi, S.; Ferino, I.; Monaci, R.; Rombia, E.; Solinas, V. Preparation, characterisation and activity of chromia-zirconia catalysts for propane dehydrogenation. Thermochim. Acta 2005, 434, 62–68. [Google Scholar] [CrossRef]
- Annuar, N.H.R.; Jalil, A.A.; Triwahyonoa, S.; Fatah, N.A.A.; The, L.P.; Mamat, C.R. Cumene cracking over chromium oxide zirconia: Effect of chromium(VI) oxide precursors. Appl. Catal. A Gen. 2014, 475, 487–496. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Sokolov, S.; Stoyanova, M.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. ZrO2-Based Alternatives to Conventional Propane Dehydrogenation Catalysts: Active Sites, Design, and Performance. Angew. Chem. Int. Ed. 2015, 54, 15880–15883. [Google Scholar] [CrossRef]
- Mahmoud, H.R. Highly dispersed Cr2O3–ZrO2 binary oxide nanomaterials as novel catalysts for ethanol conversion. J. Mol. Catal. A Chem. 2014, 392, 216–222. [Google Scholar] [CrossRef]
- Baronskiy, M.G.; Kostyukov, A.I.; Larina, T.V.; Snytnikov, V.N.; Zaitseva, N.A.; Zhuzhgov, A.V. Photoluminescence of surface chromium centers in the Cr/Al2O3 system that is active in isobutane dehydrogenation. Mater. Chem. Phys. 2019, 234, 403–410. [Google Scholar] [CrossRef]
- Cavani, F.; Koutyrev, M.; Trifiro, F.; Bartolini, A.; Ghisletti, D.; Iezzi, R.; Santucci, A.; Del Piero, G. Chemical and physical characterization of alumina-supported chromia-based catalysts and their activity in dehydrogenation of isobutane. J. Catal. 1996, 158, 236–250. [Google Scholar] [CrossRef]
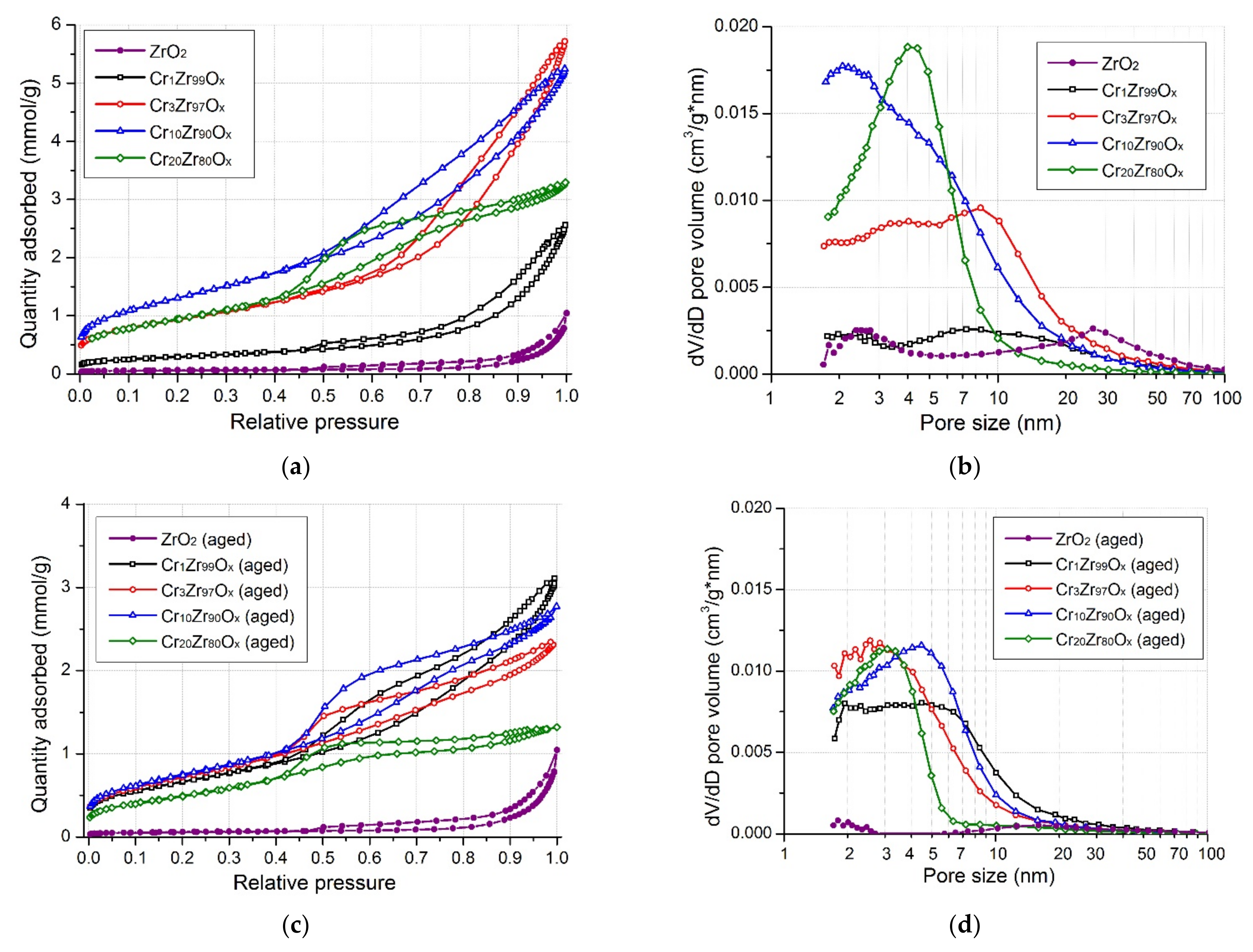
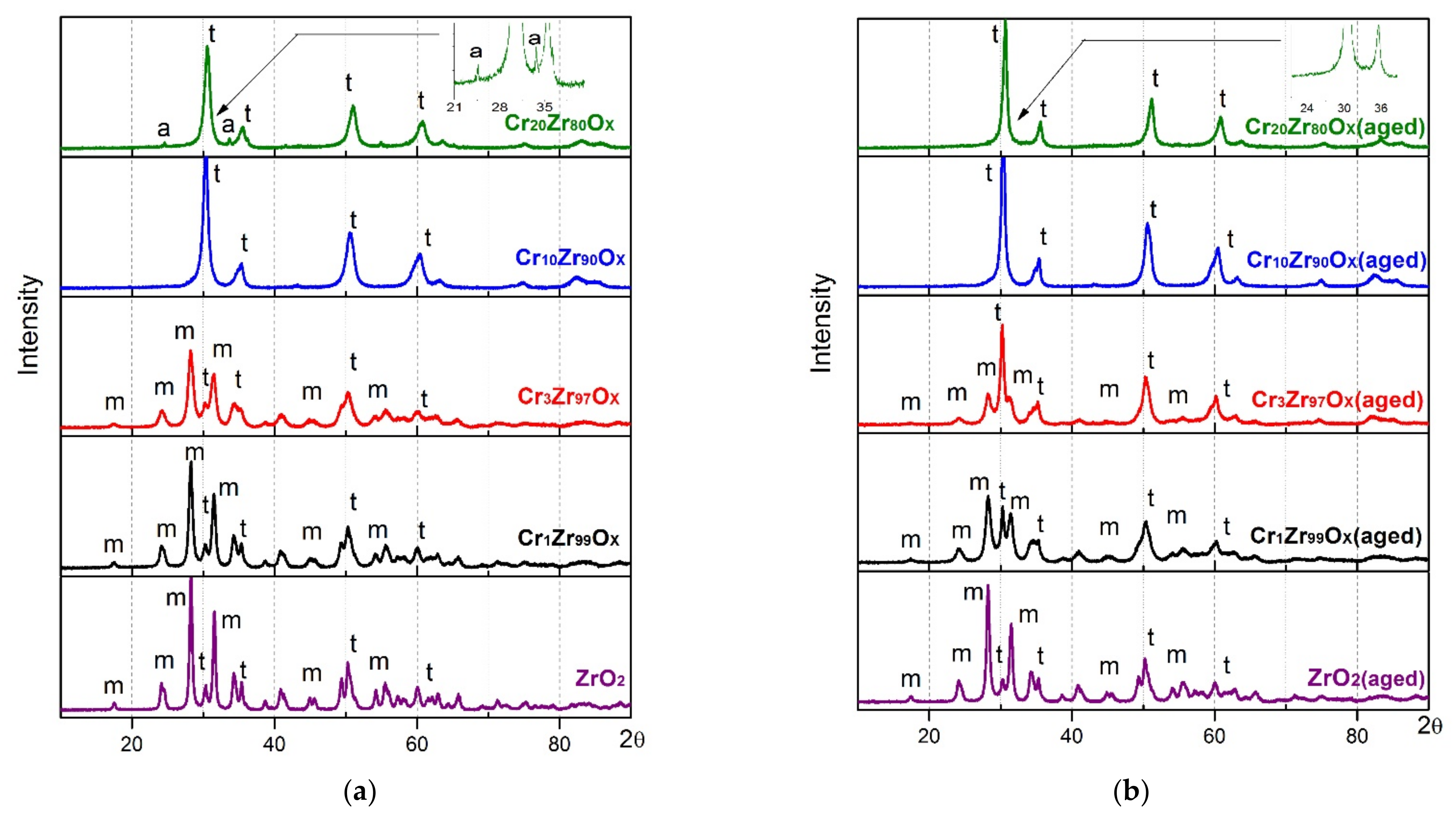
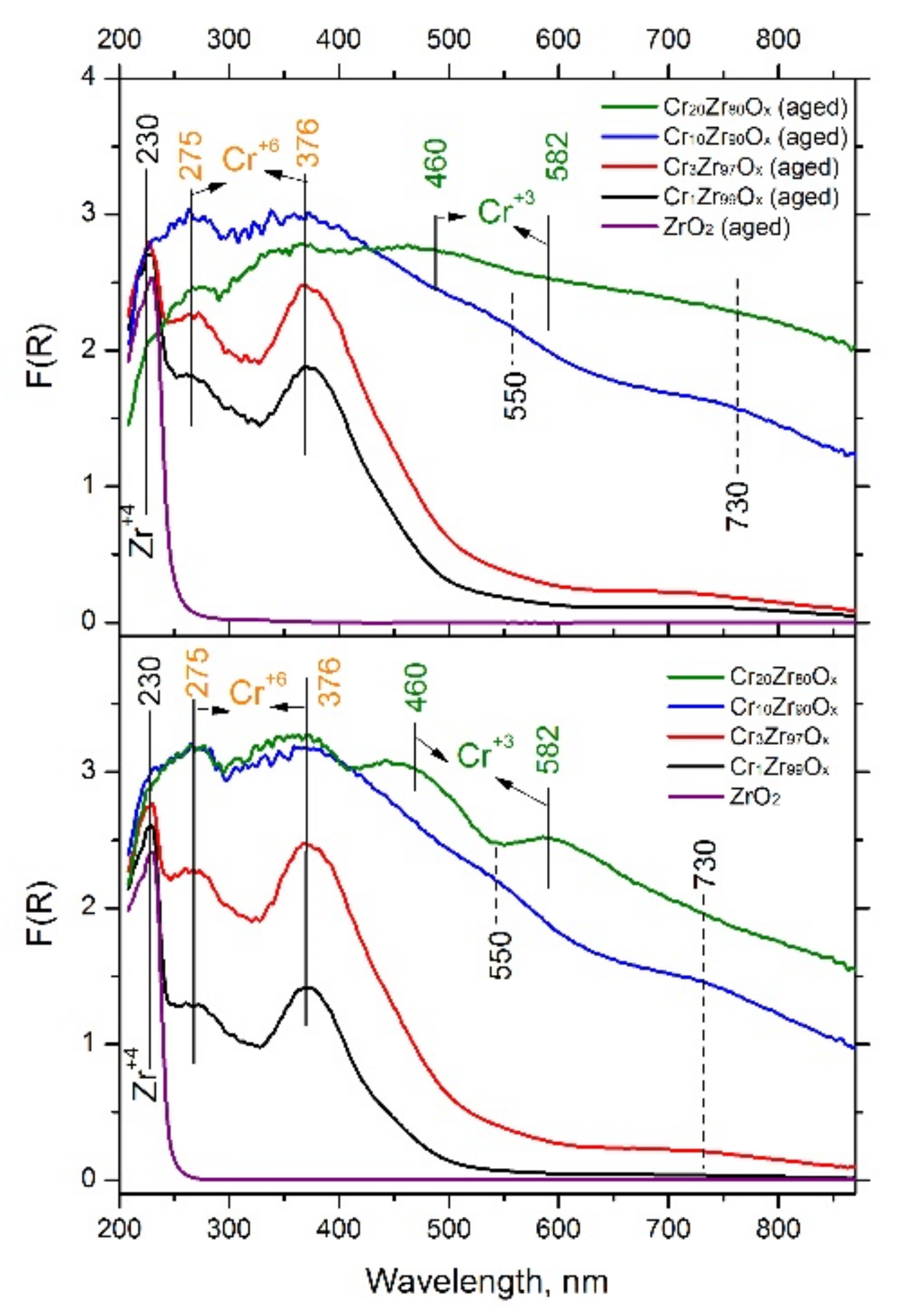
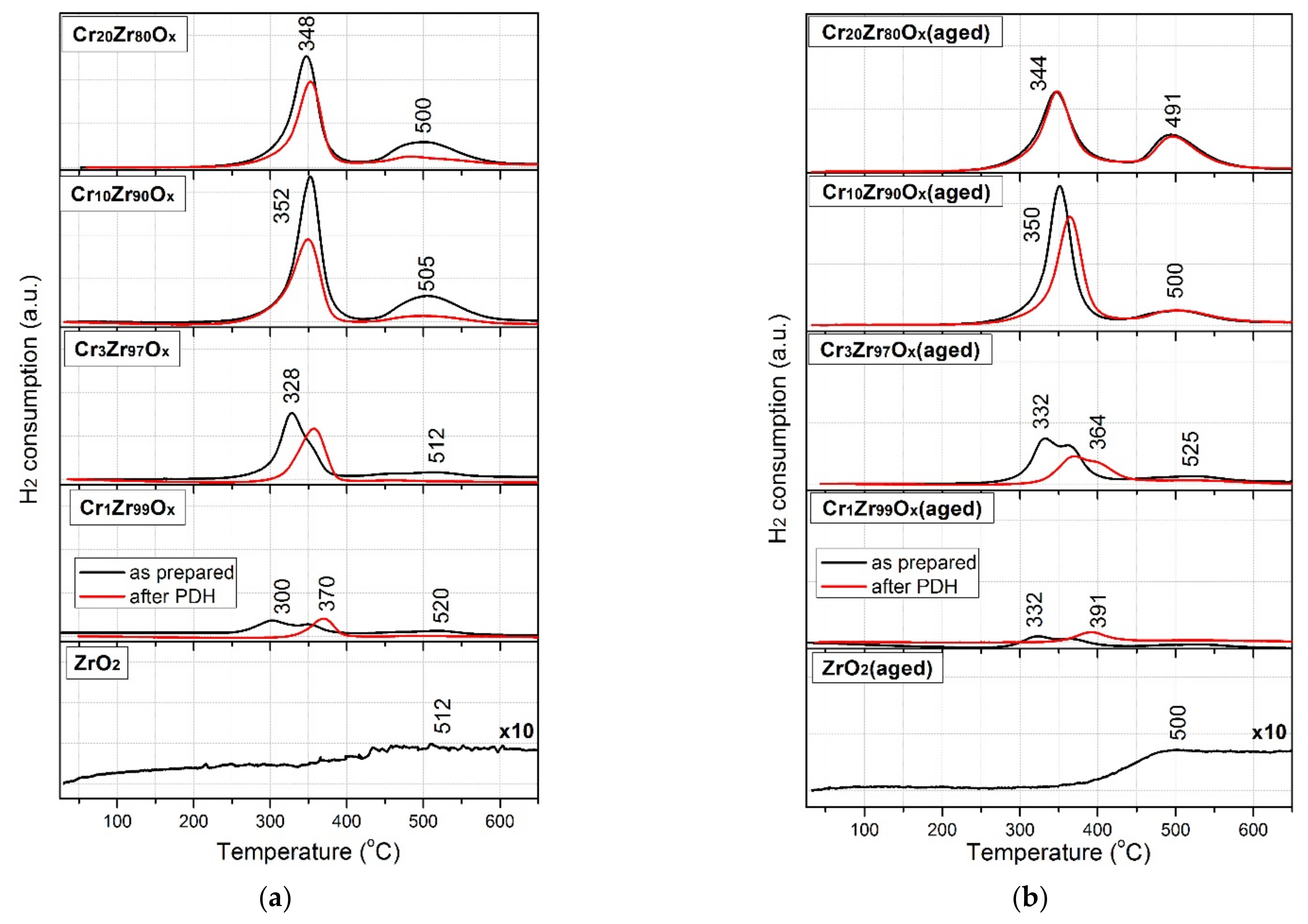

| Sample | SBET, m2/g | Vp, cm3/g | Sample | SBET, m2/g | Vp, cm3/g | ω(Cr), wt.% |
|---|---|---|---|---|---|---|
| ZrO2 | 23 | 0.14 | ZrO2(aged) | 5 | 0.04 | - |
| Cr1Zr99Ox | 24 | 0.09 | Cr1Zr99Ox(aged) | 54 | 0.11 | 0.4 |
| Cr3Zr97Ox | 76 | 0.20 | Cr3Zr97Ox(aged) | 59 | 0.09 | 1.3 |
| Cr10Zr90Ox | 107 | 0.18 | Cr10Zr90Ox(aged) | 62 | 0.10 | 4.4 |
| Cr20Zr80Ox | 77 | 0.11 | Cr20Zr80Ox(aged) | 42 | 0.05 | 9.2 |
| Sample | Phases | ϖ, wt.% | DXRD, nm | a, Å | Sample | Phases | ϖ, wt.% | DXRD, nm | a, Å |
|---|---|---|---|---|---|---|---|---|---|
| ZrO2 | m-ZrO2 | 92.32 | 20.2 | - | ZrO2 (aged) | m-ZrO2 | 90.67 | 17.3 | 5.147 |
| t-ZrO2 | 7.68 | 18.1 | - | t-ZrO2 | 9.33 | 15.4 | 3.600 | ||
| Cr1Zr99Ox | m-ZrO2 | 91.50 | 13.5 | 5.147 | Cr1Zr99Ox (aged) | m-ZrO2 | 79.45 | 8.95 | 5.157 |
| t-ZrO2 | 8.50 | 12.8 | 3.603 | t-ZrO2 | 20.55 | 19.9 | 3.597 | ||
| Cr3Zr97Ox | m-ZrO2 | 90.26 | 9.3 | 5.151 | Cr3Zr97Ox (aged) | m-ZrO2 | 48.75 | 3.33 | 5.152 |
| t-ZrO2 | 9.74 | 7.9 | 3.603 | t-ZrO2 | 51.25 | 17.3 | 3.599 | ||
| Cr10Zr90Ox | m-ZrO2 | 0 | - | - | Cr10Zr90Ox (aged) | m-ZrO2 | 0 | - | - |
| t-ZrO2 | 100 | 11.4 | 3.588 | t-ZrO2 | 100 | 17.1 | 3.587 | ||
| Cr20Zr80Ox | a-Cr2O3 | 7.22 | - | - | Cr20Zr80Ox (aged) | m-ZrO2 | 0 | - | - |
| t-ZrO2 | 92.78 | 9.6 | 3.570 | t-ZrO2 | 100 | 14.7 | 3.567 |
| Sample | before PDH | after PDH | Sample | before PDH | after PDH | H2, μmol/g (Cr6+→ Cr3+) |
|---|---|---|---|---|---|---|
| n(H2), μmol/g | n(H2), μmol/g | |||||
| ZrO2 | - | - | ZrO2(aged) | - | - | - |
| Cr1Zr99Ox | 132 | 98 | Cr1Zr99Ox(aged) | 108 | 72 | 122 |
| Cr3Zr97Ox | 345 | 218 | Cr3Zr97Ox(aged) | 318 | 207 | 369 |
| Cr10Zr90Ox | 722 | 442 | Cr10Zr90Ox(aged) | 581 | 514 | 1266 |
| Cr20Zr80Ox | 670 | 466 | Cr20Zr80Ox(aged) | 601 | 537 | 2637 |
| STY, kg/h·m3 | TOF, h−1 | |||||
|---|---|---|---|---|---|---|
| Sample | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 1 | Cycle 2 | Cycle 3 |
| ZrO2 | 8 | 8 | 5 | - | - | - |
| Cr1Zr99Ox | 857 | 238 | 155 | 259 | 78 | 57 |
| Cr3Zr97Ox | 1955 | 1274 | 922 | 196 | 127 | 94 |
| Cr10Zr90Ox | 2409 | 1572 | 1117 | 78 | 51 | 36 |
| Cr20Zr80Ox | 2344 | 1889 | 1520 | 33 | 27 | 22 |
| ZrO2(aged) | 15 | 16 | 9 | - | - | - |
| Cr1Zr99Ox(aged) | 639 | 78 | 42 | 198 | 37 | 28 |
| Cr3Zr97Ox(aged) | 1700 | 694 | 360 | 172 | 74 | 38 |
| Cr10Zr90Ox(aged) | 2209 | 1628 | 1464 | 41 | 30 | 27 |
| Cr20Zr80Ox(aged) | 1565 | 1193 | 1050 | 10 | 8 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubkov, A.; Bugrova, T.; Salaev, M.; Mamontov, G. Influence of Cr/Zr Ratio on Activity of Cr–Zr Oxide Catalysts in Non-Oxidative Propane Dehydrogenation. Crystals 2021, 11, 1435. https://doi.org/10.3390/cryst11111435
Zubkov A, Bugrova T, Salaev M, Mamontov G. Influence of Cr/Zr Ratio on Activity of Cr–Zr Oxide Catalysts in Non-Oxidative Propane Dehydrogenation. Crystals. 2021; 11(11):1435. https://doi.org/10.3390/cryst11111435
Chicago/Turabian StyleZubkov, Alexander, Tatiana Bugrova, Mikhail Salaev, and Grigory Mamontov. 2021. "Influence of Cr/Zr Ratio on Activity of Cr–Zr Oxide Catalysts in Non-Oxidative Propane Dehydrogenation" Crystals 11, no. 11: 1435. https://doi.org/10.3390/cryst11111435
APA StyleZubkov, A., Bugrova, T., Salaev, M., & Mamontov, G. (2021). Influence of Cr/Zr Ratio on Activity of Cr–Zr Oxide Catalysts in Non-Oxidative Propane Dehydrogenation. Crystals, 11(11), 1435. https://doi.org/10.3390/cryst11111435






