First-Principles Electronic-Structure Study of Graphene Decorated with 4d-Transition Atoms
Abstract
1. Introduction
2. Calculation Methodology
3. Results and Discussion
3.1. Adsorption Energy and Configuration
3.2. Adsorption Bonding Characteristics
3.3. Band Structure and Magnetism
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castro Neto, A.H.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties andintrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Z.; Yu, G.; Chen, W.; Chen, Z. CO catalytic oxidation on iron-embedded graphene: Computational quest for low-cost nanocatalysts. J. Phys. Chem. C 2010, 114, 6250–6254. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energ. Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- Kaukonen, M.; Krasheninnikov, A.V.; Kauppinen, E.; Nieminen, R.M. Doped graphene as a material for oxygen reduction reaction in hydrogen fuel cells: A computational study. ACS Catal. 2013, 3, 159–165. [Google Scholar] [CrossRef]
- Liao, L.; Lin, Y.C.; Bao, M.; Cheng, R.; Bai, J.; Liu, Y.; Qu, Y.; Wang, K.L.; Huang, Y.; Duan, X. High-speed graphene transistors with a self-aligned nanowire gate. Nature 2010, 467, 305–308. [Google Scholar] [CrossRef]
- Lin, Y.M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.Y.; Grill, A.; Avouris, P. 100-GHz Transistors from wafer-scale epitaxial graphene. Science 2010, 327, 662. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Katsnelson, M.I. Magnetic correlations at graphene edges: Basis for novel spintronics devices. Phys. Rev. Lett. 2008, 100, 047209. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Ding, F. Strain-induced orientation-selective cutting of graphene into graphene nanoribbons on oxidation. Angew. Chem. Int. Ed. 2012, 51, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, J.; Ding, F. Recent progress and challenges in graphene nanoribbon synthesis. Chem. Phys. Chem 2013, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Xiu, S.L.; Zheng, M.M.; Zhao, P.; Zhang, Y.; Liu, H.Y.; Li, S.J.; Chen, G.; Kawazoe, Y. An effective method of tuning conducting properties: First-principles studies on electronic structures of graphene nanomeshes. Carbon 2014, 79, 646–653. [Google Scholar] [CrossRef]
- Santos, E.J.; Sánchez-Portal, D.; Ayuela, A. Magnetism of substitutional Co impurities in graphene: Realization of single π vacancies. Phys. Rev. B 2010, 81, 125433. [Google Scholar] [CrossRef]
- López-Corral, I.; Germán, E.; Juan, A.; Volpe, M.A.; Brizuela, G.P. DFT study of hydrogen adsorption on palladium decorated graphene. J. Phys. Chem. C 2011, 115, 4315–4323. [Google Scholar] [CrossRef]
- Valencia, H.; Gil, A.; Frapper, G. Trends in the adsorption of 3d transition metalatoms onto graphene and nanotube surfaces: A DFT study and molecular orbital analysis. J. Phys. Chem. C 2010, 114, 14141–14153. [Google Scholar] [CrossRef]
- Yagi, Y.; Briere, T.M.; Sluiter, M.H.; Kumar, V.; Farajian, A.A.; Kawazoe, Y. Stable geometries and magnetic properties of single-walled carbon nanotubes doped with 3d transition metals: A first-principles study. Phys. Rev. B 2004, 69, 075414. [Google Scholar] [CrossRef]
- Chan, K.T.; Neaton, J.; Cohen, M.L. First-principles study of metal adatom adsorption on graphene. Phys. Rev. B 2008, 77, 235430. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhou, M.; Lu, M.; Fa, W.; Chen, Y. First-principles study of the IVA group atoms adsorption on graphene. J. Appl. Phys. 2010, 107, 114311. [Google Scholar] [CrossRef]
- Sun, M.; Tang, W.; Ren, Q.; Wang, S.; Yu, J.; Du, Y.; Zhang, Y. First-principles study of the alkali earth metal atoms adsorption on graphene. Appl. Surf. Sci. 2015, 356, 668–673. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.Z.; Hupalo, M.; Lin, H.Q.; Ho, K.M.; Tringides, M. Metals on graphene: Interactions, growth morphology, and thermal stability. Crystals 2013, 3, 79–111. [Google Scholar] [CrossRef]
- Ataca, C.; Aktürk, E.; Ciraci, S. Hydrogen storage of calcium atoms adsorbed ongraphene: First-principles plane wave calculations. Phys. Rev. B 2009, 79, 041406. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, Y.; Zhang, C.; Feng, Y.P. Strain effects on hydrogen storage capability of metal-decorated graphene: A first-principles study. Appl. Phys.Lett. 2010, 97, 103109. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural defects in graphene. ACS Nano 2010, 5, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Holmström, E.; Toikka, L.; Krasheninnikov, A.V.; Nordlund, K. Response of mechanically strained nanomaterials to irradiation: Insight from atomistic simulations. Phys. Rev. B 2010, 82, 045420. [Google Scholar] [CrossRef]
- Lehtinen, O.; Kotakoski, J.; Krasheninnikov, A.V.; Tolvanen, A.; Nordlund, K.; Keinonen, J. Effects of ion bombardment on a two-dimensional target: Atomistic simulations of graphene irradiation. Phys. Rev. B 2010, 81, 153401. [Google Scholar] [CrossRef]
- Alonso-Lanza, T.; Ayuela, A.; Aguilera-Granja, F. Substitutional 4d and 5d impurities in graphene. Phys. Chem. Chem. Phys. 2016, 18, 21913–21920. [Google Scholar] [CrossRef]
- Sun, M.; Ren, Q.; Zhao, Y.; Chou, J.P.; Yu, J.; Tang, W. Electronic and magnetic properties of 4d series transition metal substituted graphene: A first-principles study. Carbon 2017, 120, 265–273. [Google Scholar] [CrossRef]
- Wu, Z.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Milman, V.; Lee, M.H.; Payne, M.C. Ground-state properties of CoSi2 determined by a total-energy pseudopotential method. Phys. Rev. B 1994, 49, 16300. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations—A reply. Phys. Rev. B 1977, 16, 1748. [Google Scholar]
- Pfrommer, B.G.; Cote, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-newton method. J. Comput. Phys. 1997, 131, 133–140. [Google Scholar] [CrossRef]
- Choi, S.M.; Jhi, S.H. Self-assembled metal atom chains on graphene nanoribbons. Phys. Rev. Lett. 2008, 101, 266105. [Google Scholar] [CrossRef]
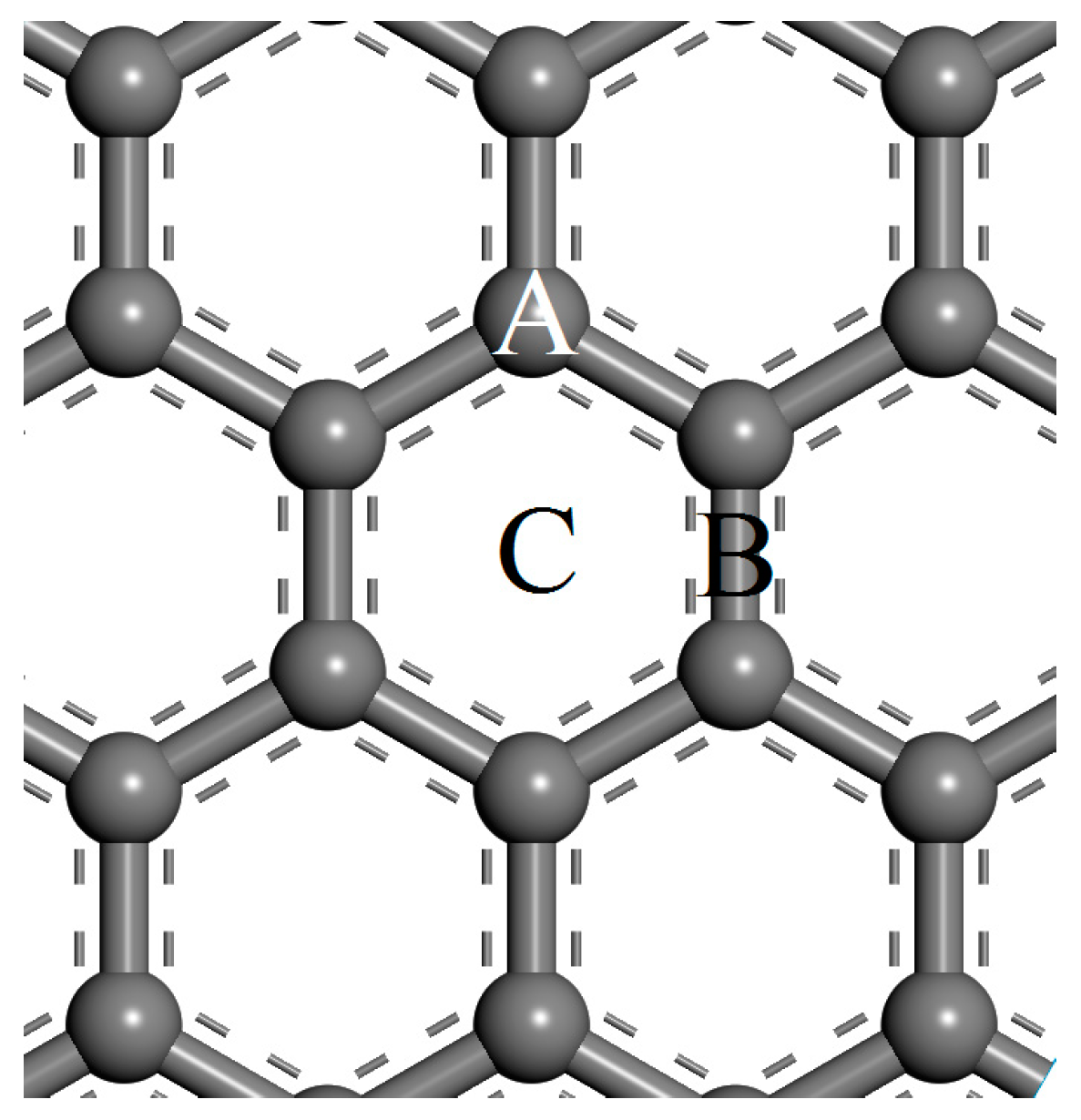
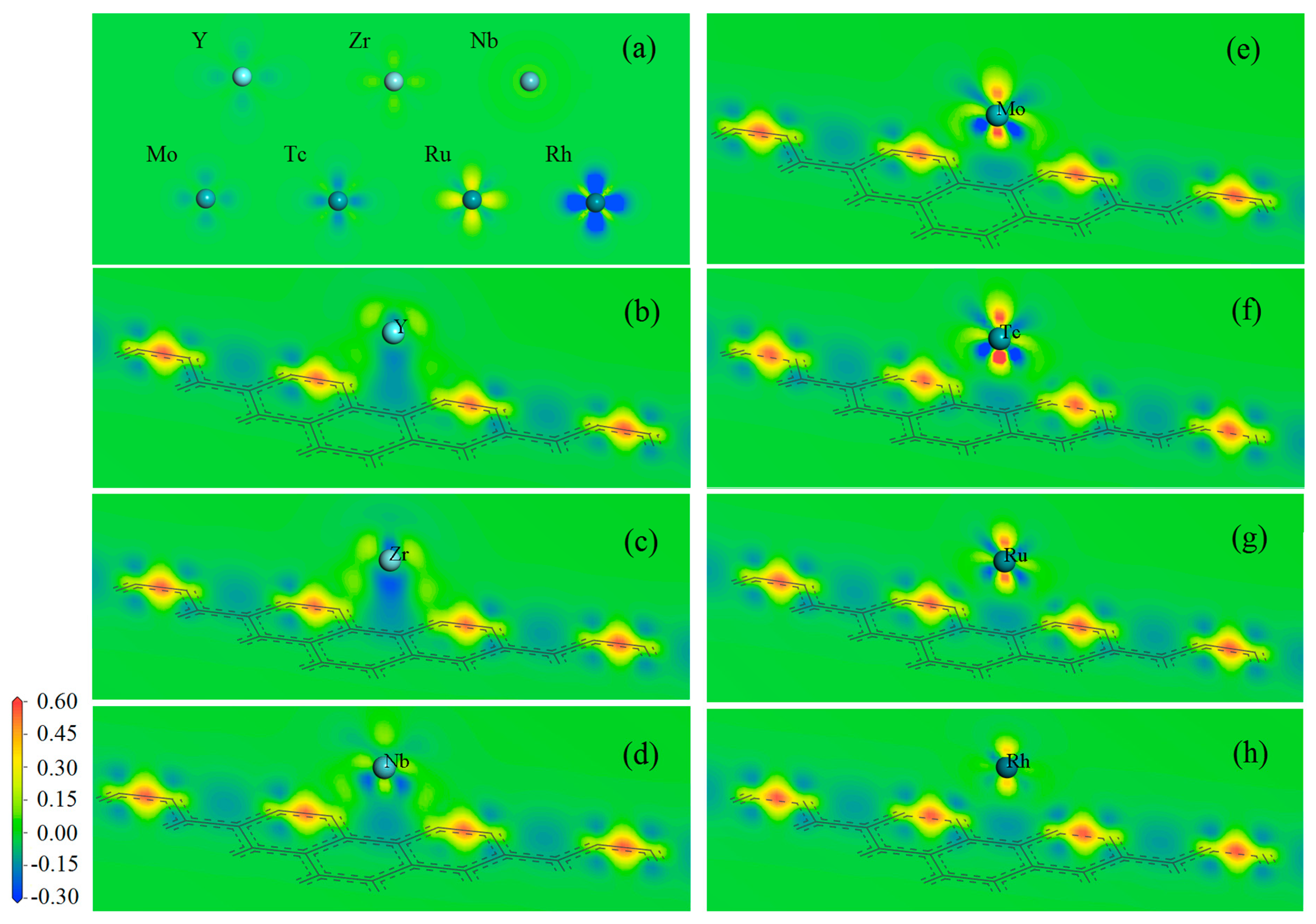
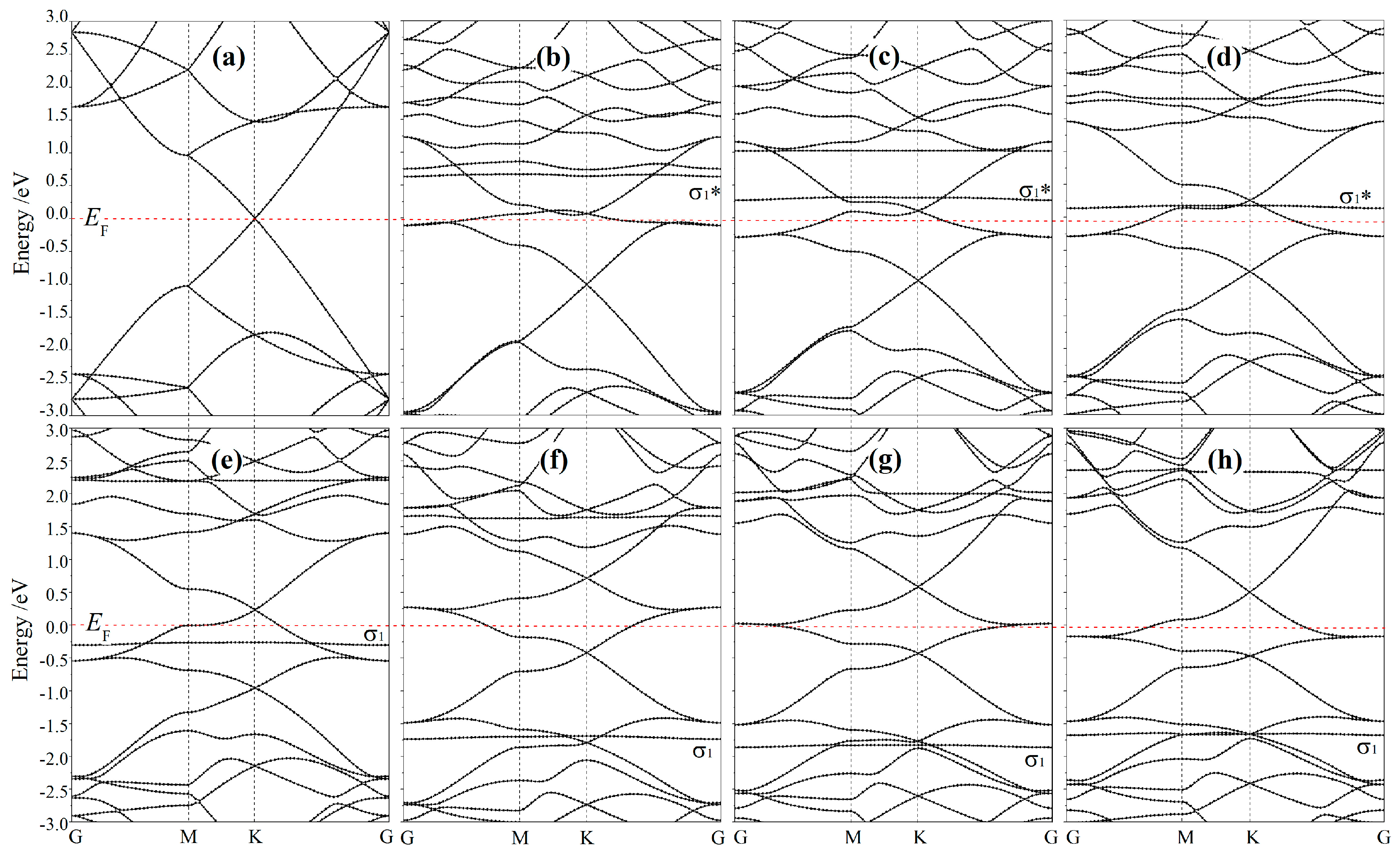

| Site | Ead/eV | dad/Å | ∆c/Å | qM/e | sn/(ћ/2) | Site | Ead/eV | dad/Å | ∆c/Å | qM/e | sn/(ћ/2) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | A | 2.763 | 1.687 | −0.555 | 1.32 | 0 | Ru | A | 3.213 | 1.652 | −0.320 | 0.65 | 0 |
| B | 1.735 | 2.055 | −0.212 | 0.76 | 0 | B | 2.095 | 2.063 | −0.032 | 0.41 | 2.00 | ||
| C | 3.028 | 1.727 | −0.214 | 1.42 | 0 | C | 3.817 | 1.542 | −0.126 | 0.58 | 0 | ||
| Zr | A | 4.313 | 1.496 | −0.473 | 1.25 | 1.23 | Rh | A | 2.169 | 1.863 | −0.110 | 0.47 | 0 |
| B | 2.026 | 2.088 | −0.148 | 0.60 | 3.87 | B | 1.663 | 2.091 | −0.031 | 0.34 | 1.00 | ||
| C | 4.770 | 1.586 | −0.205 | 1.37 | 0 | C | 2.249 | 1.666 | −0.068 | 0.46 | 0 | ||
| Nb | A | 2.670 | 1.652 | −0.480 | 0.99 | 1.87 | Pd | A | 1.956 | 2.202 | −0.070 | 0.34 | 0 |
| B | 1.830 | 2.184 | −0.018 | 0.48 | 4.89 | B | 1.452 | 2.151 | −0.051 | 0.25 | 0 | ||
| C | 3.363 | 1.520 | −0.224 | 1.06 | 0 | C | 1.570 | 2.019 | −0.026 | 0.34 | 0 | ||
| Mo | A | 1.840 | 1.770 | −0.298 | 0.74 | 2.58 | Ag | A | 0.729 | 2.605 | −0.067 | 0.27 | 0 |
| B | 1.793 | 2.290 | −0.029 | 0.35 | 5.18 | B | 0.242 | 3.083 | −0.004 | 0.05 | 1.00 | ||
| C | 2.148 | 1.469 | −0.210 | 0.69 | 0 | C | 0.484 | 2.501 | −0.030 | 0.24 | 0 | ||
| Tc | A | 2.095 | 1.541 | −0.477 | 0.55 | 0 | Cd | A | 0.505 | 3.526 | −0.044 | 0.01 | 0 |
| B | 1.936 | 2.144 | −0.038 | 0.39 | 3.01 | B | 0.207 | 3.569 | −0.009 | 0.07 | 0 | ||
| C | 3.147 | 1.470 | −0.176 | 0.56 | 0 | C | 0.508 | 3.541 | −0.031 | 0.01 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Zhang, W.-C.; Sun, W.-F. First-Principles Electronic-Structure Study of Graphene Decorated with 4d-Transition Atoms. Crystals 2021, 11, 29. https://doi.org/10.3390/cryst11010029
Hu R, Zhang W-C, Sun W-F. First-Principles Electronic-Structure Study of Graphene Decorated with 4d-Transition Atoms. Crystals. 2021; 11(1):29. https://doi.org/10.3390/cryst11010029
Chicago/Turabian StyleHu, Ran, Wei-Chao Zhang, and Wei-Feng Sun. 2021. "First-Principles Electronic-Structure Study of Graphene Decorated with 4d-Transition Atoms" Crystals 11, no. 1: 29. https://doi.org/10.3390/cryst11010029
APA StyleHu, R., Zhang, W.-C., & Sun, W.-F. (2021). First-Principles Electronic-Structure Study of Graphene Decorated with 4d-Transition Atoms. Crystals, 11(1), 29. https://doi.org/10.3390/cryst11010029






