Effects of CF4 Plasma Treatment on Indium Gallium Oxide and Ti-doped Indium Gallium Oxide Sensing Membranes in Electrolyte–Insulator–Semiconductors
Abstract
1. Introduction
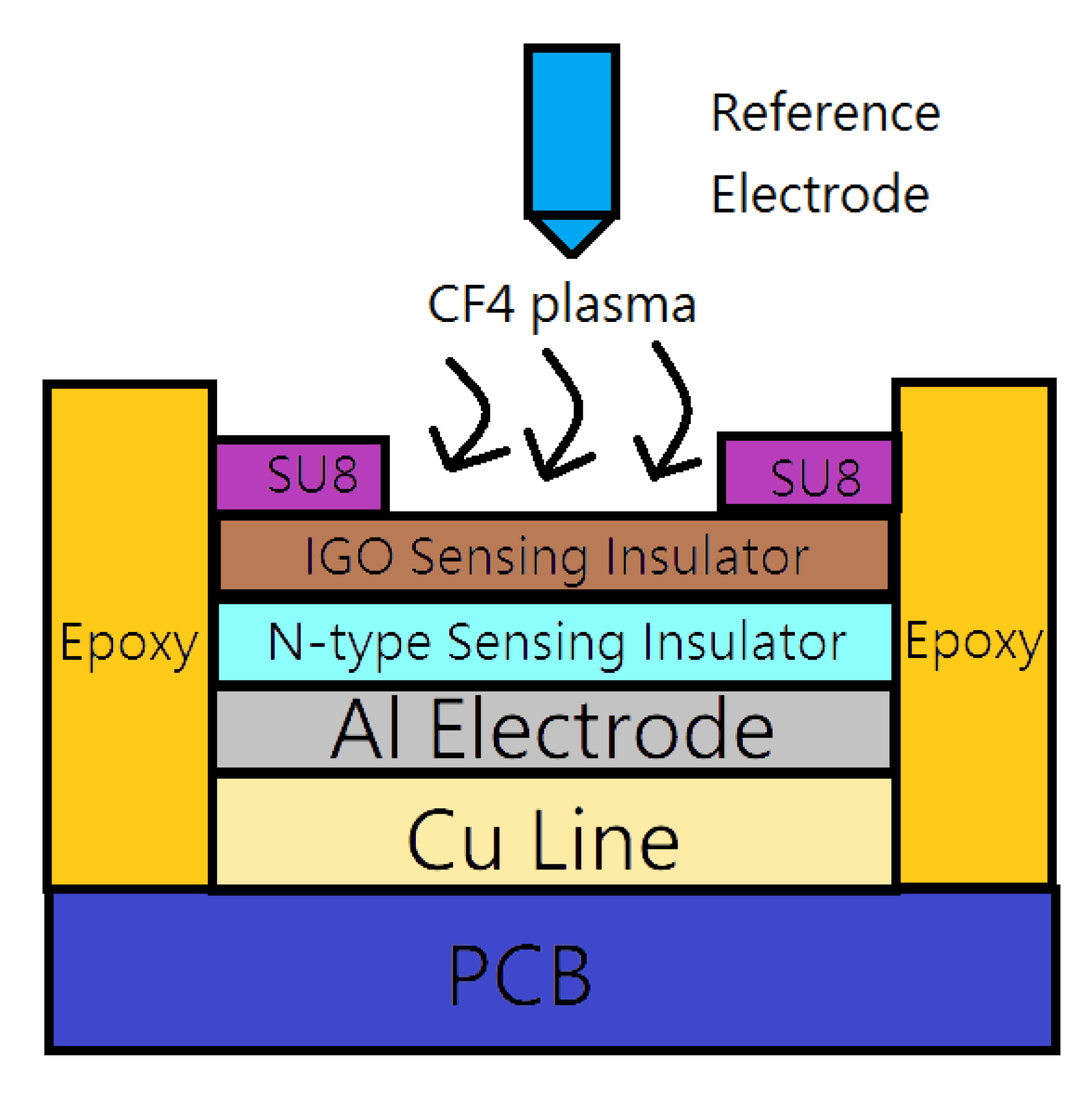
2. Materials and Methods
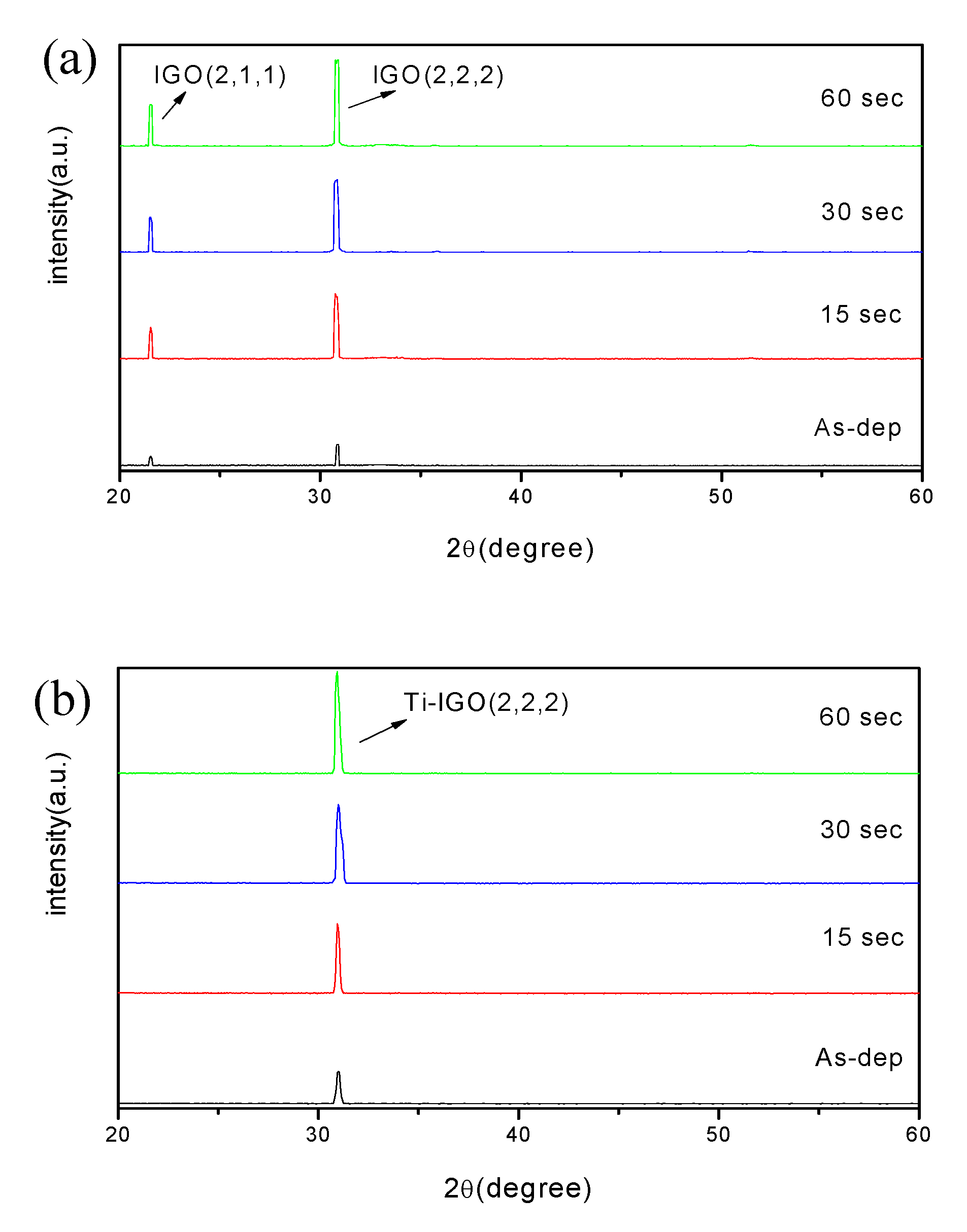
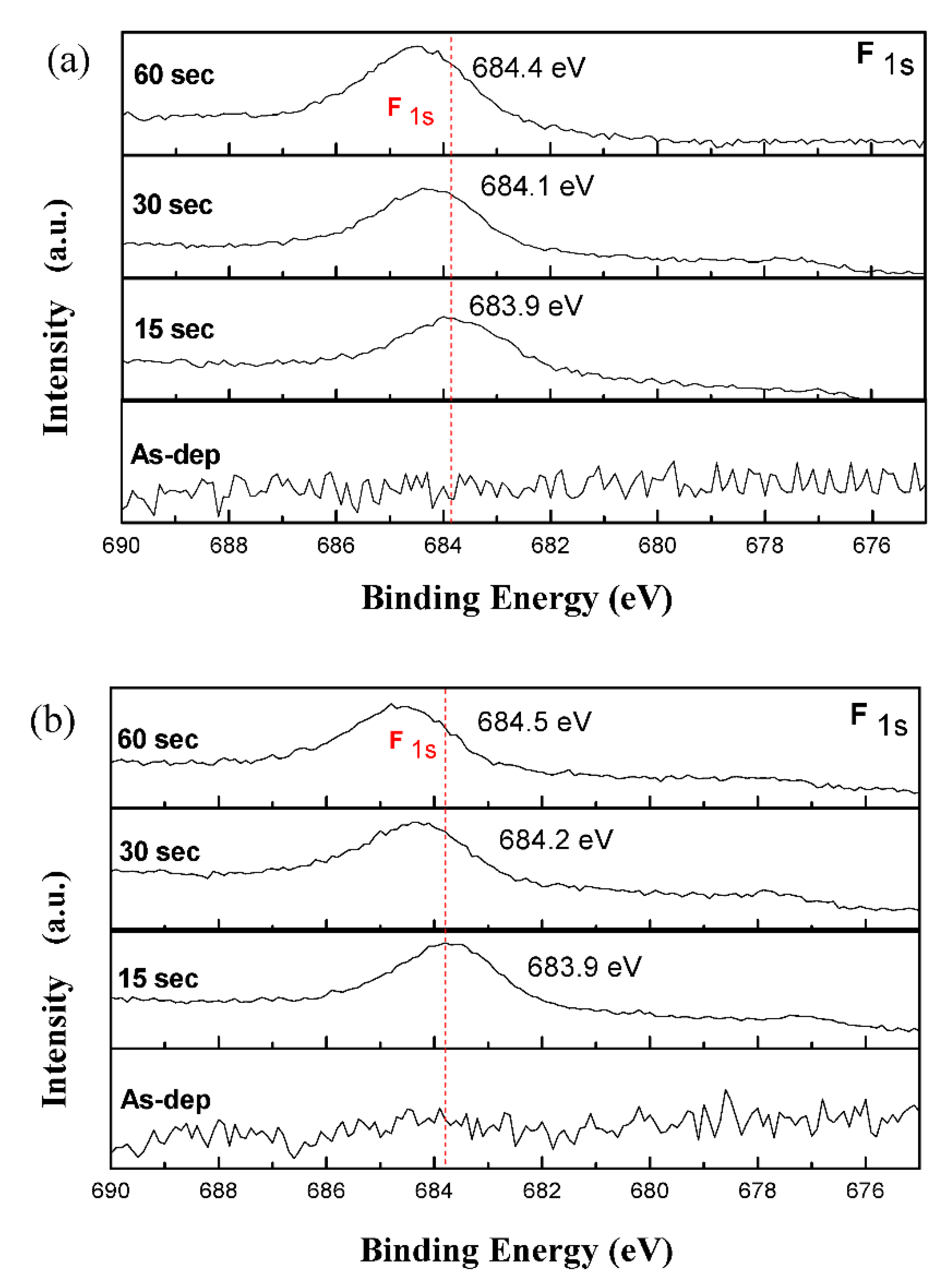
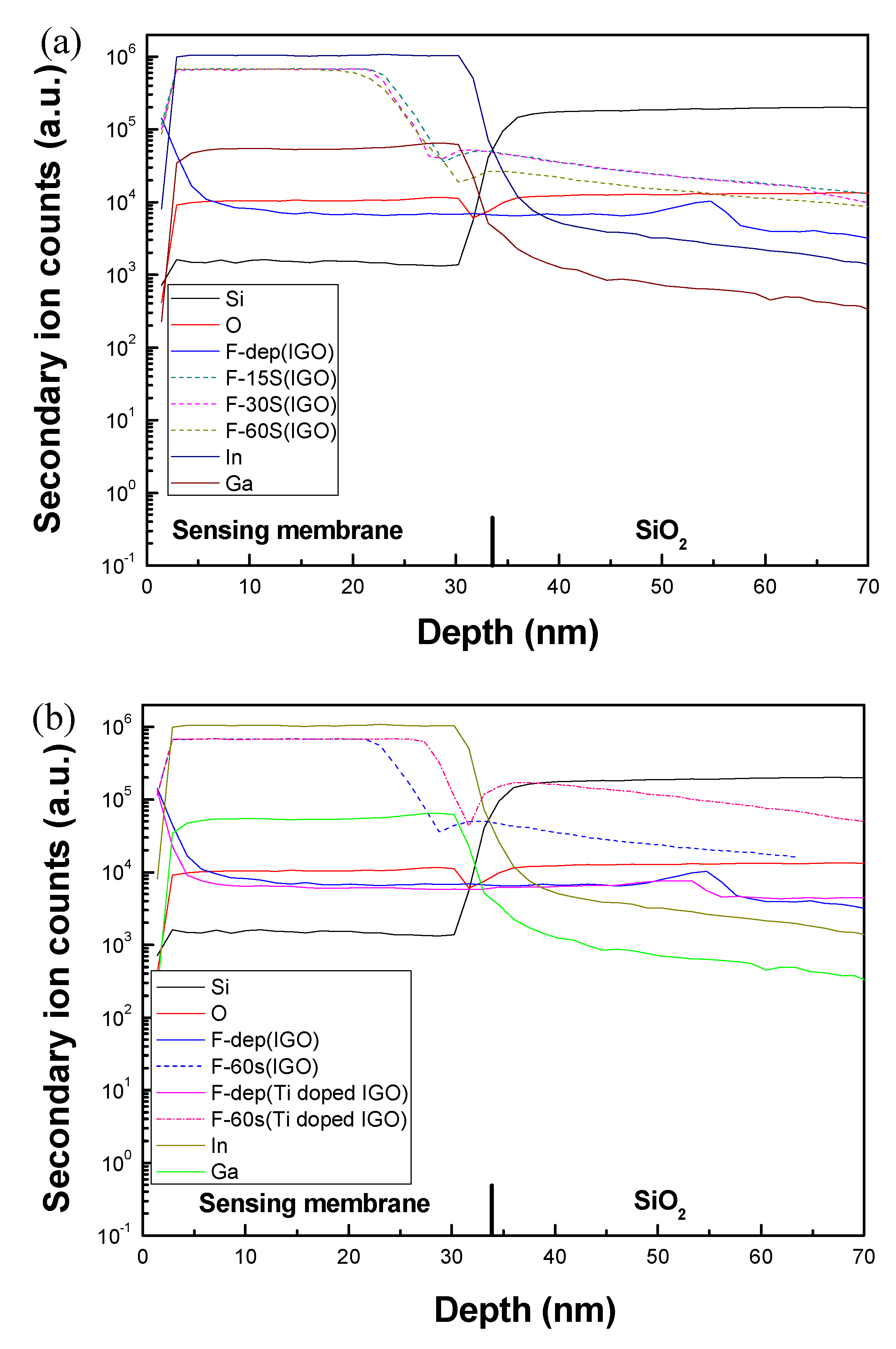
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kao, C.-H.; Liu, C.S.; Xu, C.Y.; Lin, C.F.; Chen, H. Ti-doped indium gallium oxide electrolyte–insulator–semiconductor membranes for multiple ions and solutes detectors. J. Mater. Sci. Mater. Electron. 2019, 30, 20596–20604. [Google Scholar] [CrossRef]
- Rongming, C.; Soo, S.C.; Hoi, W.M.; Fichtenbaum, N.; Brown, D.; McCarthy, L.; Keller, S.; Feng, W.; Speck, J.S.; Mishra, U.K. Impact of CF4 Plasma Treatment on GaN. IEEE Electron Device Lett. 2007, 28, 781–783. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.-M.; Gilron, J.; Kong, D.-f.; Yin, Y.; Oren, Y.; Linder, C.; He, T. CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation. J. Membr. Sci. 2014, 456, 155–161. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Moo, A.M.; Yu, T.; Xu, X.J.; Gao, X.Y.; Liu, Y.J.; Lim, C.T.; Shen, Z.X.; Ong, C.K.; Wee, A.T.S.; et al. Enhanced field emission from O2 and CF4 plasma-treated CuO nanowires. Chem. Phys. Lett. 2006, 419, 458–463. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Nitschke, M.; Janke, A.; Helbig, R.; D’Souza, F.; Donnelly, G.T.; Willemsen, P.R.; Werner, C. Fluorination of poly(dimethylsiloxane) surfaces by low pressure CF4 plasma–physicochemical and antifouling properties. Express Polym. Lett. 2009, 3, 70–83. [Google Scholar] [CrossRef]
- Sahin, H.T. RF-CF4 plasma surface modification of paper: Chemical evaluation of two sidedness with XPS/ATR-FTIR. Appl. Surf. Sci. 2007, 253, 4367–4373. [Google Scholar] [CrossRef]
- Sima, M.; Enculescu, I.; Sima, M.; Vasile, E.; Visan, T. EIS studies of electrodeposition process of manganese and copper doped ZnO wires. Surf. Interface Anal. 2008, 40, 561–565. [Google Scholar] [CrossRef]
- Valentini, L.; Puglia, D.; Armentano, I.; Kenny, J.M. Sidewall functionalization of single-walled carbon nanotubes through CF4 plasma treatment and subsequent reaction with aliphatic amines. Chem. Phys. Lett. 2005, 403, 385–389. [Google Scholar] [CrossRef]
- Chin, C.-H.; Lu, T.-F.; Wang, J.-C.; Yang, J.-H.; Lue, C.-E.; Yang, C.-M.; Li, S.-S.; Lai, C.-S. Effects of CF4 Plasma Treatment on pH and pNa Sensing Properties of Light-Addressable Potentiometric Sensor with a 2-nm-Thick Sensitive HfO2 Layer Grown by Atomic Layer Deposition. Jpn. J. Appl. Phys. 2011, 50. [Google Scholar] [CrossRef]
- Lu, T.-F.; Yang, C.-M.; Wang, J.-C.; Ho, K.-I.; Chin, C.-H.; Pijanowska, D.G.; Jaroszewicz, B.; Lai, C.-S. Characterization of K+ and Na+-Sensitive Membrane Fabricated by CF4 Plasma Treatment on Hafnium Oxide Thin Films on ISFET. J. Electrochem. Soc. 2011, 158. [Google Scholar] [CrossRef]
- Lin, C.F.; Kao, C.H.; Lin, C.Y.; Liu, C.S.; Liu, Y.W. Comparison Between Performances of In2O3 and In2TiO5-Based EIS Biosensors Using Post Plasma CF4 Treatment Applied in Glucose and Urea Sensing. Sci. Rep. 2019, 9, 3078. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.H.; Chang, C.L.; Su, W.M.; Chen, Y.T.; Lu, C.C.; Lee, Y.S.; Hong, C.H.; Lin, C.Y.; Chen, H. Magnesium Oxide (MgO) pH-sensitive Sensing Membrane in Electrolyte-Insulator-Semiconductor Structures with CF4 Plasma Treatment. Sci. Rep. 2017, 7, 7185. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.H.; Chen, H.; Hou, F.Y.S.; Chang, S.W.; Chang, C.W.; Lai, C.S.; Chen, C.P.; He, Y.Y.; Lin, S.-R.; Hsieh, K.M.; et al. Fabrication of multianalyte CeO2 nanograin electrolyte–insulator–semiconductor biosensors by using CF4 plasma treatment. Sens. Bio-Sens. Res. 2015, 5, 71–77. [Google Scholar] [CrossRef]
- Zong, X.; Zhu, R.; Guo, X. Nanostructured gold microelectrodes for SERS and EIS measurements by incorporating ZnO nanorod growth with electroplating. Sci. Rep. 2015, 5, 16454. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, A.; Romanyuk, A.; Pietzka, C.; Scharpf, J.; Kohn, E. Surface structure and surface barrier characteristics of boron-doped diamond in electrolytes after CF4 plasma treatment in RF-barrel reactor. Diam. Relat. Mater. 2010, 19, 423–427. [Google Scholar] [CrossRef]
- Kao, C.H.; Chang, C.W.; Chen, Y.T.; Su, W.M.; Lu, C.C.; Lin, C.Y.; Chen, H. Influence of NH3 plasma and Ti doping on pH-sensitive CeO2 electrolyte-insulator-semiconductor biosensors. Sci. Rep. 2017, 7, 2405. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-M.; Liu, C.-H.; Lee, V.; Lee, C.; Wang, M.-J. Highly sensitive glucose biosensor based on CF4-plasma-modified interdigital transducer array (IDA) microelectrode. Sens. Actuators B Chem. 2010, 149, 59–66. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.D. Plasma surface treatment of HDPE powders by CF4 plasma in a fluidized bed reactor. Polym. Bull. 1998, 41, 479–486. [Google Scholar] [CrossRef]
- Sahin, H.T.; Manolache, S.; Young, R.A.; Denes, F. Surface fluorination of paper in CF4-RF plasma environments. Cellulose 2002, 9, 171–181. [Google Scholar] [CrossRef]
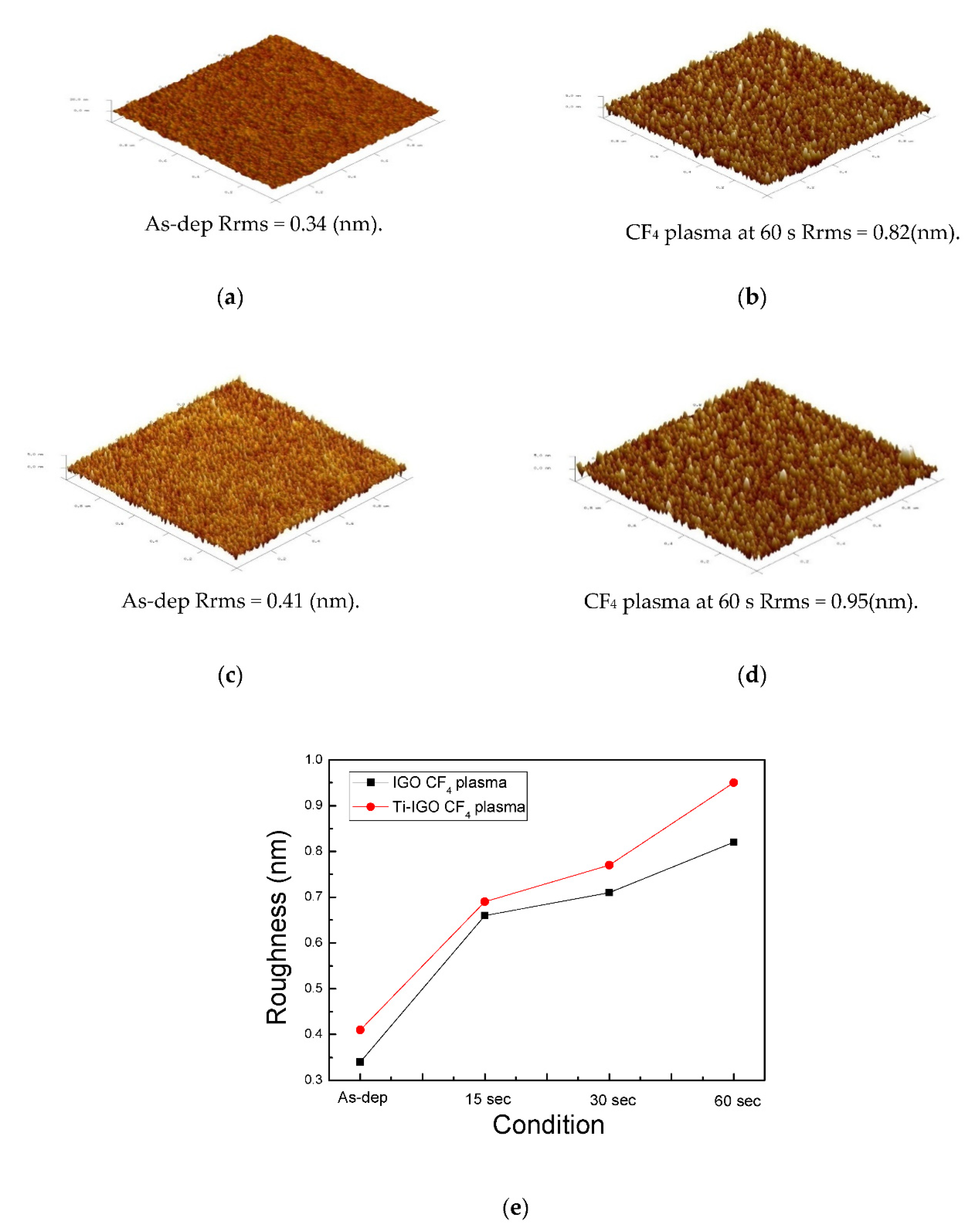
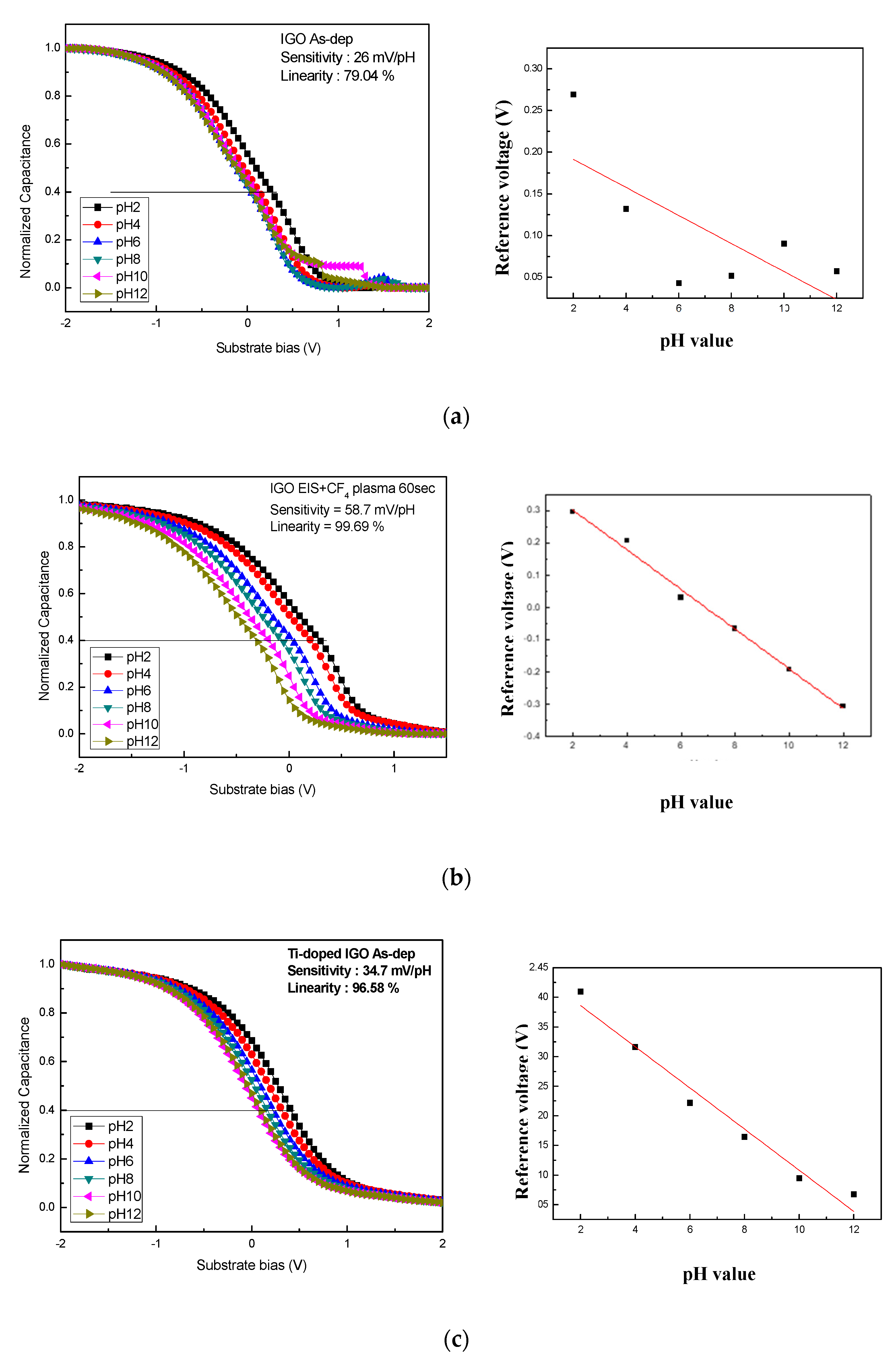
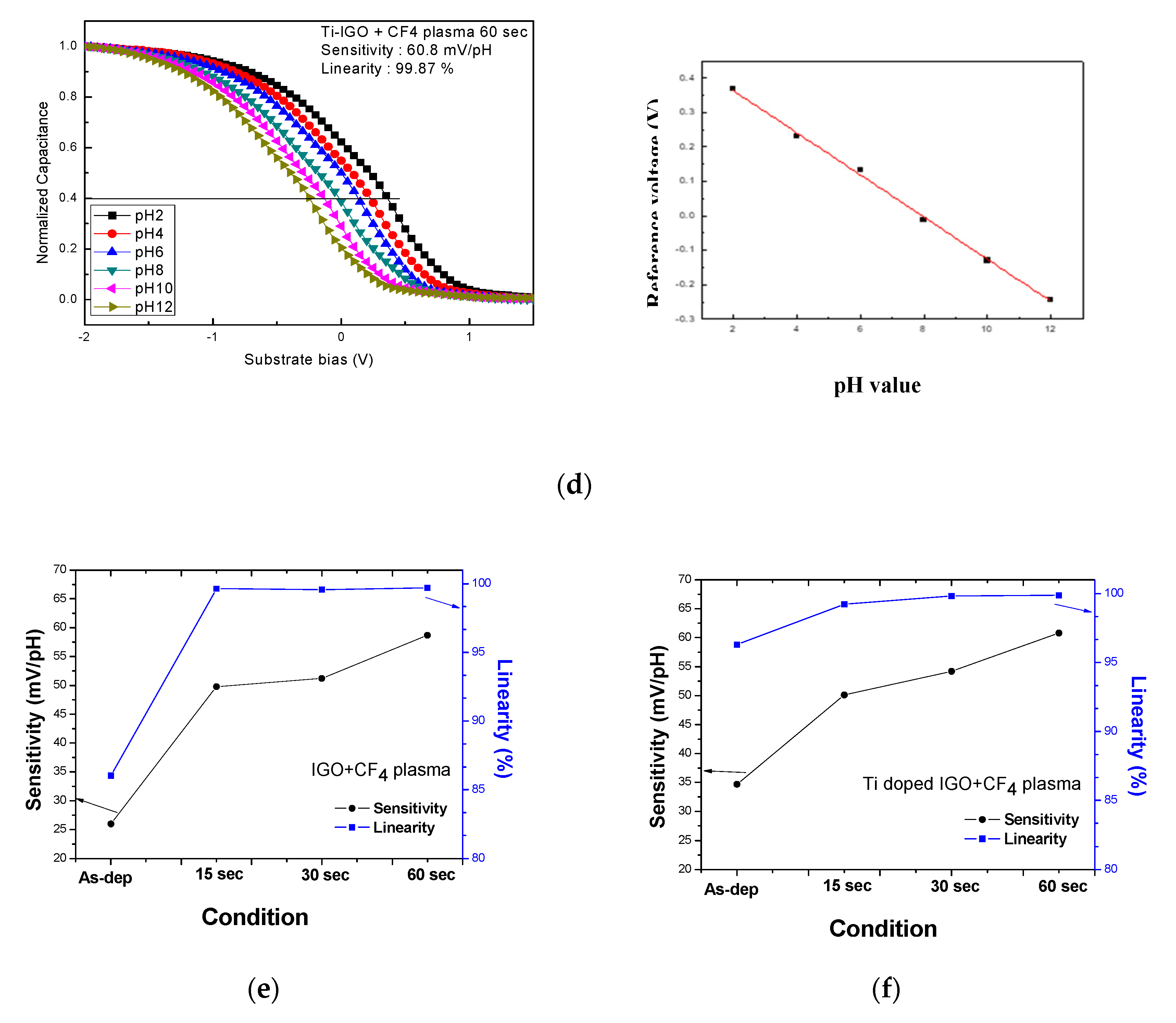

| Sensitivity (mV/pH) | Linearity (%) | Reference | |
|---|---|---|---|
| Ti-doped IGO (CF4 plasma) | 60.8 | 99.87 | This work |
| Ti-doped IGO (annealing at 600 °C) | 59.6 | 98.2 | [1] |
| IGO (CF4 plasma) | 58.7 | 99.69 | This work |
| In2TiO5 (As-deposited) | 36.34 | 94.23 | [13] |
| In2TiO5 (CF4 plasma) | 59.64 | 99.68 | [13] |
| Nb2O5 (As-deposited) | 32.76 | 93.51 | [5] |
| Nb2O5 (CF4 plasma) | 52.12 | 98.22 | [5] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-H.; Su, Y.-L.; Liao, W.-J.; Li, M.-H.; Chan, W.-L.; Tsai, S.-C.; Chen, H. Effects of CF4 Plasma Treatment on Indium Gallium Oxide and Ti-doped Indium Gallium Oxide Sensing Membranes in Electrolyte–Insulator–Semiconductors. Crystals 2020, 10, 810. https://doi.org/10.3390/cryst10090810
Kao C-H, Su Y-L, Liao W-J, Li M-H, Chan W-L, Tsai S-C, Chen H. Effects of CF4 Plasma Treatment on Indium Gallium Oxide and Ti-doped Indium Gallium Oxide Sensing Membranes in Electrolyte–Insulator–Semiconductors. Crystals. 2020; 10(9):810. https://doi.org/10.3390/cryst10090810
Chicago/Turabian StyleKao, Chyuan-Haur, Yen-Lin Su, Wei-Jen Liao, Ming-Hsien Li, Wei-Lun Chan, Shang-Che Tsai, and Hsiang Chen. 2020. "Effects of CF4 Plasma Treatment on Indium Gallium Oxide and Ti-doped Indium Gallium Oxide Sensing Membranes in Electrolyte–Insulator–Semiconductors" Crystals 10, no. 9: 810. https://doi.org/10.3390/cryst10090810
APA StyleKao, C.-H., Su, Y.-L., Liao, W.-J., Li, M.-H., Chan, W.-L., Tsai, S.-C., & Chen, H. (2020). Effects of CF4 Plasma Treatment on Indium Gallium Oxide and Ti-doped Indium Gallium Oxide Sensing Membranes in Electrolyte–Insulator–Semiconductors. Crystals, 10(9), 810. https://doi.org/10.3390/cryst10090810





