1. Introduction
In recent years, ABO
4 compounds have attracted a great deal of interest due to their applications. The wolframite and scheelite structures are common structure types for ABO
4 compounds [
1]. Tungstate materials like BaWO
4, PbWO
4, CaWO
4, or SrWO
4 are very interesting because of their applications as laser host material or scintillators. Among the tungstate materials barium tungstate (BaWO
4, BWO) is a very attractive inorganic optical material due to its emission properties and its potential as a material for stimulated Raman scattering for application in Raman shifters [
2] of laser radiation or as scintillators and X-ray phosphor [
3].
Doped barium tungstate single crystals with alkali earth metals (Me) or rare-earth ions (Re) display the greater possibility of further applications as luminescence and solid-state laser materials. Doping with trivalent ions to make them applicable and requires charge compensation which may be provided, for example, by structural defects or proper codoping with alkaline metal ions. Voronina et al. have obtained BaWO
4: Nd
3+ single crystals by the Czochralski method using compensating dopants: Nb
5+, Na
+ [
4]. Liao et al. [
5] performed chemical analyses of the BaWO
4: Pr
3+ microcrystalline samples by energy-dispersive X-ray spectroscopy (EDS). Na
+, Pr
3+, Ba
2+, W and O were detected for all samples. Moreover, the Pr
3+ content was nearly equal to the Na
+ content. They supposed that the charge compensation mechanism of BaWO
4: Pr
3+ phosphors may be such that two Ba
2+ ions are replaced by one Pr
3+ ion and one Na
+ ion, 2Ba
2+ = Pr
3+ + Na
+. The charge compensation pattern has been discussed in detail in the case of Eu
3+ and Na
+ codoped CaWO
4 phosphors [
6]. The hypothesis of Codoping of Eu
3+ and Na
+ at Ca
2+ sites were verified, too [
6]. Kaczmarek et al. published another paper about Pr
3+ and Na
+ sites in BaWO
4 single crystals doped with Pr and codoped with Na [
7].
The doping process with different RE ions leads to different optical or magnetic properties of the doped hosts. The application of different methods may lead to different structural defects in grown crystals and changed optical properties. We decided to codope BaWO
4: Ce single crystals with Na ions using the Czochralski growth method with inductive heating [
8,
9]. A deeper understanding of the influence of structural properties of BWO on their luminescent behavior and electro-optical properties is crucial for their applications [
10,
11]. The understanding is particularly important in the case of doped BaWO
4 single crystal. Doped barium tungstate single crystals with alkali earth metals (Me) or rare-earth ions (Re) greatly increases the further possibility of application as luminescence and solid-state laser materials. Currently, many papers are focused on the investigation of BWO thin films or the synthetization of nanoscale rods, cylinders made of BaWO
4 [
12,
13]. However, there are a few papers that deal with doping, absorption bands or defect study of barium tungstate (BaWO
4) crystal [
14,
15,
16].
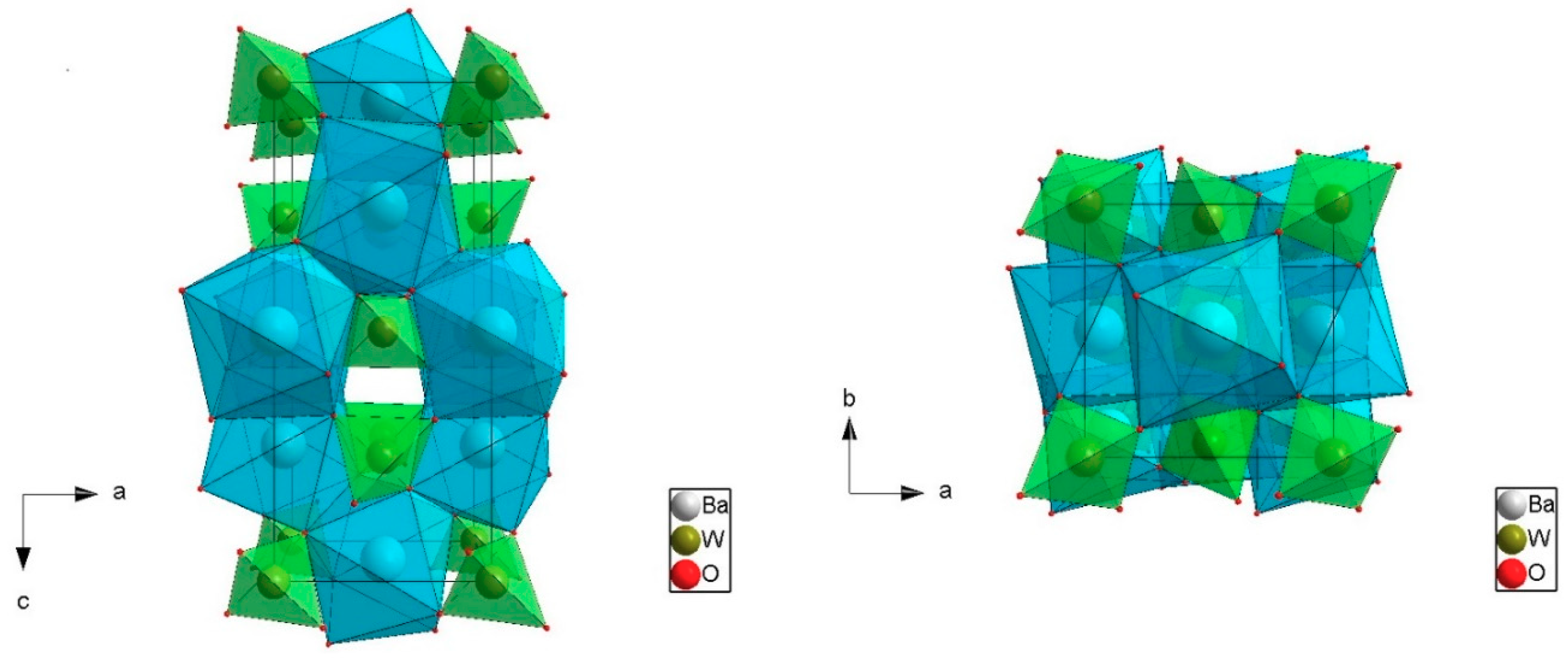
BWO crystal with scheelite structure crystallized in the tetragonal space group with C
64h (I4
1/a) [
17]. Lattice parameters of BWO are: a = b = 5.6148 Å, c = 12.721 Å [
1,
17] (see
Figure 1). Both Ba
2+ and W
6+ sites have S
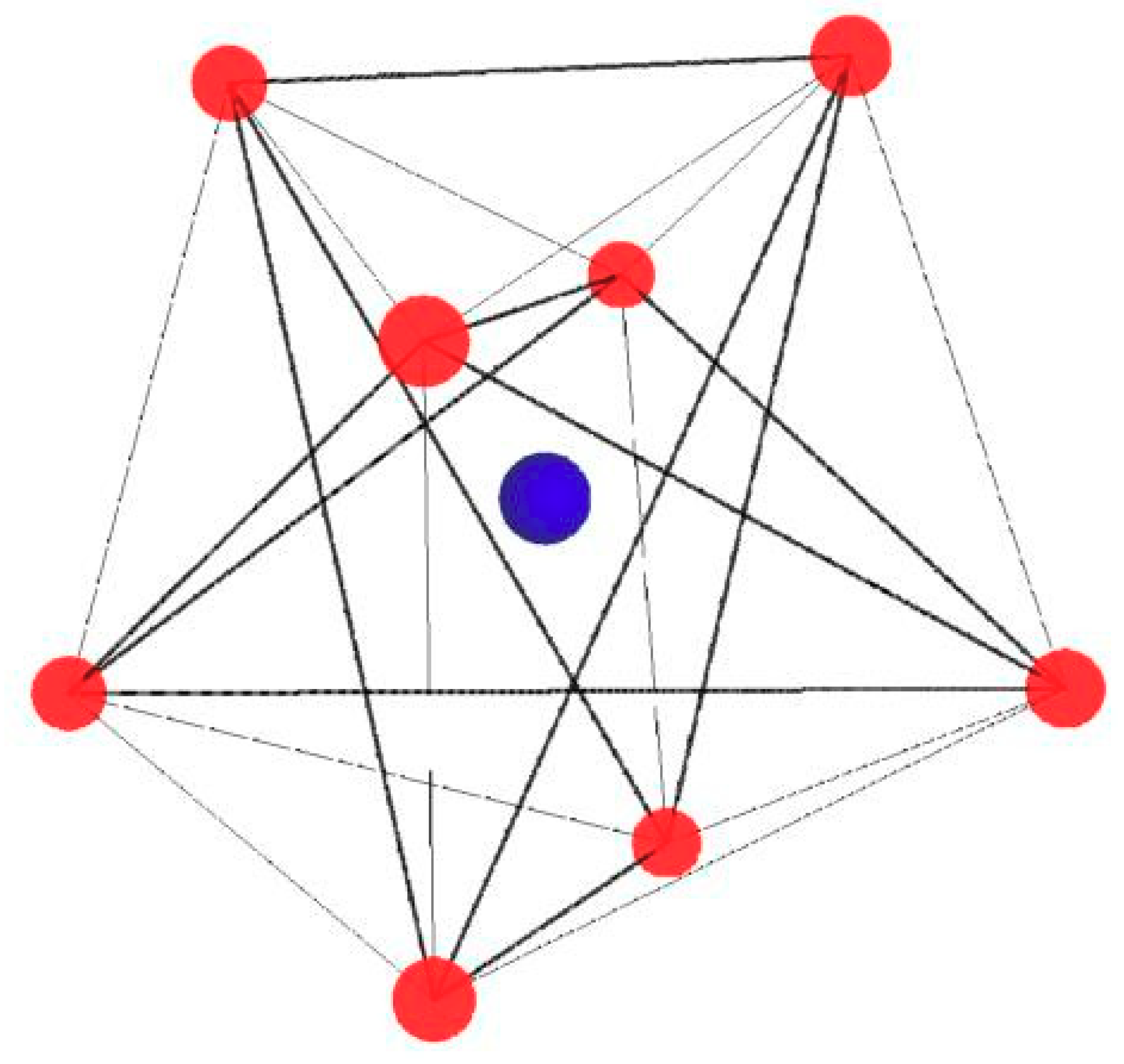
4 point symmetry. In the tungstate compound, Ba
2+ is coordinated by eight O
2− ions in the form of a dodecahedron [BaO
8] made of two rotated, interpenetrated tetrahedra (see
Figure 2). The distance Ba–O is 2.7857 Å and 2.8310 Å, respectively, for two tetrahedra forming a dodecahedron [BaO
8]. The [WO
4] tetrahedron has a nearby regular shape only slightly distorted along the
c (S
4) axis. In the tetrahedron [WO
4], the W–O bond length is 1.8230 Å [
1]. All data for pure, undoped lattice structure. In the
c (
z) directions, [BaO
8] dodecahedra are connected by its edges. Barium dodecahedra [BaO
8] and tungsten tetrahedra [WO
4] are connected by O atoms. Therefore, each O atoms links with two Ba atoms and one W atom. It is worth mentioning that the volume of the cell BaWO
4 is the greatest one among ABO
4 molybdates and tungstate with the structure of wolframite and scheelite, according to Sleight (V = 401.0 Å
3) [
1].
For a cerium ion (Ce
3+, f
1 electronic configuration) in tetragonal symmetry, its
2F
5/2 ground state would be split into tree Kramers doublets. Only the lowest doublet is populated. Therefore, the Ce
3+ ion has an effective spin S = ½. This article continues our previous papers related to the investigation of BaWO
4 single crystals doped with Ce and codoped with Na [
8,
9]. Barium tungstate doped and codoped with cerium and sodium (BaWO
4: Ce, Na) with different amounts of impurities were investigated by Electron Paramagnetic Resonance (EPR). New paramagnetic centers with axial or lower symmetry were detected and spin Hamiltonian parameters were established [
8,
9]. A few articles about investigations of BWO doped and codoped with different elements were found. Kunti et al. published an article about BaWO
4 doped with Dy
3+ and codoped with K
+ (Ba
1-x-yDy
xK
yWO
4 (x = 0.10; y = 0.05)) [
14]. However, they focused on optical and XRD measurements with detailed structural analysis, without EPR data and analysis [
14].
The EPR measurements is a sensitive method to detect a local surrounding of the impurity ions. An impurity ion serves as a probe to study local surroundings. However, the extraction of more detailed structural information from EPR spectra such that one about lattice distortion around an impurity ion is not an easy task. Generally, there are two methods of obtaining this kind of information from spin—Hamiltonian parameters calculated on the basis of ERP measurements: (a) superposition model (SPM) [
18] and/or (b) perturbation methods (PM) up to second-order (or higher) [
19]. However, only g-parameters were calculated for BaWO
4: Ce, Na single crystals. There are
for axial symmetry centers, and
for lower symmetry centers [
8,
9]. Therefore, the use of the SPM model is impossible. Nevertheless, a simplified method proposed by Newman can be used [
20]. Newman suggested a method to extract structural information from the g-shift parameters [
18,
20]. In this paper, his method will be applied to results already published in previous articles [
8,
9,
21]. The goal is to find out where the dopant ions are located in the host crystal and the changes in their surroundings. Those results will be analysed on the basis of crystallographic data and other publications.
2. Theoretical Background
The spin Hamiltonian (SH) may be written as [
22,
23]:
where
is a spin operator,
is crystal field parameters, where
,
J is an angular momentum, assuming the absence of the hyperfine structure. Equation (1) is applied both to transition metal ions (TM) and rare-earth ions (RE) except for the replacement of
J by
S for transition metal (TM) ions [
22]. The Zeeman term in case the axial symmetry is given by [
22]:
In SPM model,
factors are the sum of contributions from surrounding ligands [
18,
20]:
where
is a g-shift.
parameters are determined by the angular coordination of ligands
i.
are parameters depend only on distance from ligand. The transformation properties of
gives the expressions for
in terms of angular position of ligands in spherical polar coordinates
. Coordination factors
are given in
Appendix A. Coordination factors
can be obtained from the relation [
20]:
The parameter
is just a function of polar angle
. In the SPM model, g-shift depends on the ratio (
) [
19]. However, Newman proposed the following formulae defining the normalizing g-shift as a function of
angle [
20]:
where
g0 = 2.0023 is the free-ion value,
mean value and
n is a number of ligands [
20]. In Equations (4) and (5), only the polar angle (
) is present. One polar angle suffices for a partial description of an ion–host system and its deformations.
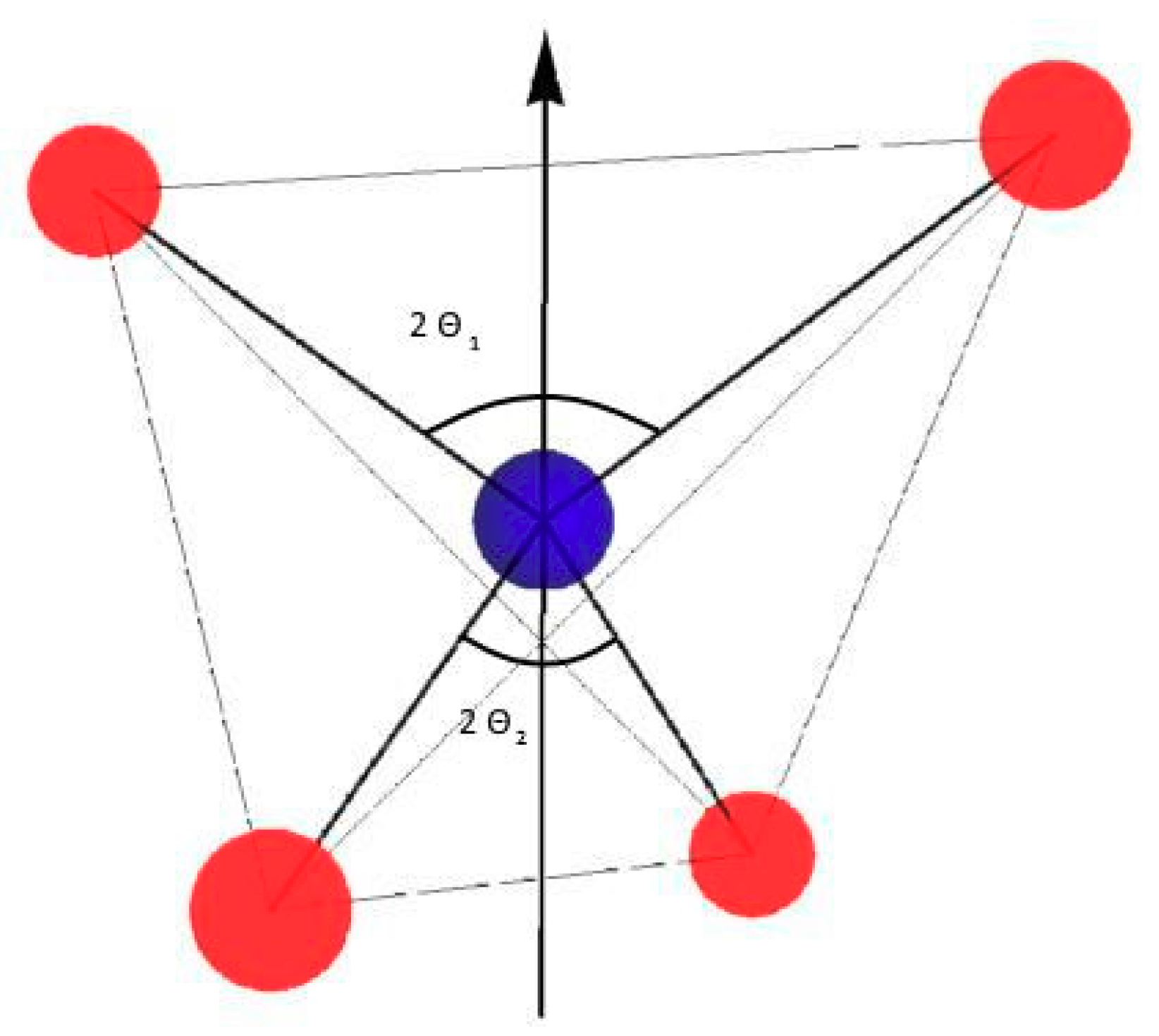
Figure 3 shows the WO
4 tetrahedron taken from the BWO unit cell. The two marked angles O-W-O (upper and lower) are equal and their value is
The axis symmetry (
c-axis) is marked too.
If , the tungstate (W) ion is placed in the middle of the tetrahedron [WO4] and the system has cubic symmetry. Other data suggest that the tetrahedron [WO4] is slightly distorted, and . If the tungsten ion is shifted along the axis symmetry these angles will become different () the system will display an axial symmetry. If tungsten ion moves out of the c-axis, the WO4 system will have lower symmetry than axial. Finally, one parameter (e.g., the angle to the positive axis symmetry) describes the position of the impurity ion on the axis symmetry in the oxygen tetrahedron or dodecahedron with axial symmetry. The value of this angle describes the position and the displacement of the ion from the midway plane. Detailed crystallographic analysis is also necessary because any mathematical model can give results without physical sense. Of course, the polar angle includes only a part of the structural information about axial symmetry systems. We cannot conclude from it anything about azimuthally angles () or the rotation angle of the lower oxygens.
3. Results and Discussion
The following BaWO
4 single crystals were investigated by EPR in previous papers: (a) BaWO4: 0.5% at. Ce, (b) BaWO4: 1.0% at. Ce, (c) BaWO4: 0.5% at. Ce, 1.0% at. Na and (d) BaWO4: 1.0% at. Ce, 2.0% at. Na [
8,
9]. Two crystals were doped with cerium, two were doped with cerium and codoped with sodium. One axial symmetry (S
4 or D
2d) paramagnetic center was found present in all four single crystals, and ten lower symmetry centers (C
2) were identified [
8,
9]. Some centers clearly differ in the values of g parameters (
). However, some other centers have similar values (
)
Table 1 collects original data for those, selected paramagnetic centers with similar values of (
) taken from [
8,
9].
The paramagnetic center with axial symmetry (Number 1 is strong and present in all four BWO single crystals. Centers 2–5 with lower symmetry are present only in selected BaWO
4 single crystals. In previous papers only Zeeman term was taken into account in spin—Hamiltonian without crystal field terms which additionally may have increased the error of calculated values of g-parameters [
8,
9]. The values of g
x are equal to g
y (within the error limit) or very close for all five centers (see
Table 1). Therefore, one can assume that all centers from
Table 1 have axial symmetry (
).
Table 2 gathers g-parameters for Ce
3+ centers with axial symmetry. It was assumed that the g perpendicular
and g parallel is on average
.
We can now use Equation (5) to determine the position of the impurity ion (Ce
3+) and its displacement. In the literature, it is generally accepted that trivalent rare-earth dopant ions (e.g., Ce
3+, or Er
3+) are located on the barium ion (Ba
2+) site and preserve the tetragonal site symmetry (S
4) [
8,
9,
14,
24]. Of course, the charge compensation is necessary. The same is applied to F type color centers, or color centers in BWO [
15,
16]. Rare-earth ions like cerium (Ce
3+) or erbium (Er
3+) cannot located on the W
6+ site because of the charge compensation and ionic radii. The ionic radii data are collected in
Table 3, according to Shannon [
25]. Radii Dy
3+ and K
+ ions were added for comparison to the paper by Kunti et al. [
14].
The polar angles (
) were calculated for Ce
3+ centers in BWO single crystals based on the structural parameters. The polar angles (
) were calculated using Equation (5). The results have been composed together in
Table 4. The host polar angles
were collected in
Table 4, too (first row,
Table 4). Calculations were made for: (a) dodecahedra [CeO
8], assuming that Ce
3+ ion substitutes the Ba
2+ site, n = 8 and (b) tetrahedra [CeO
4] assuming that Ce
3+ ion substitutes W
6+ site, n = 4, where n is number of ligands. The polar angles
are angles between Ba-O (W-O) and the
c-axes, respectively (see
Figure 3).
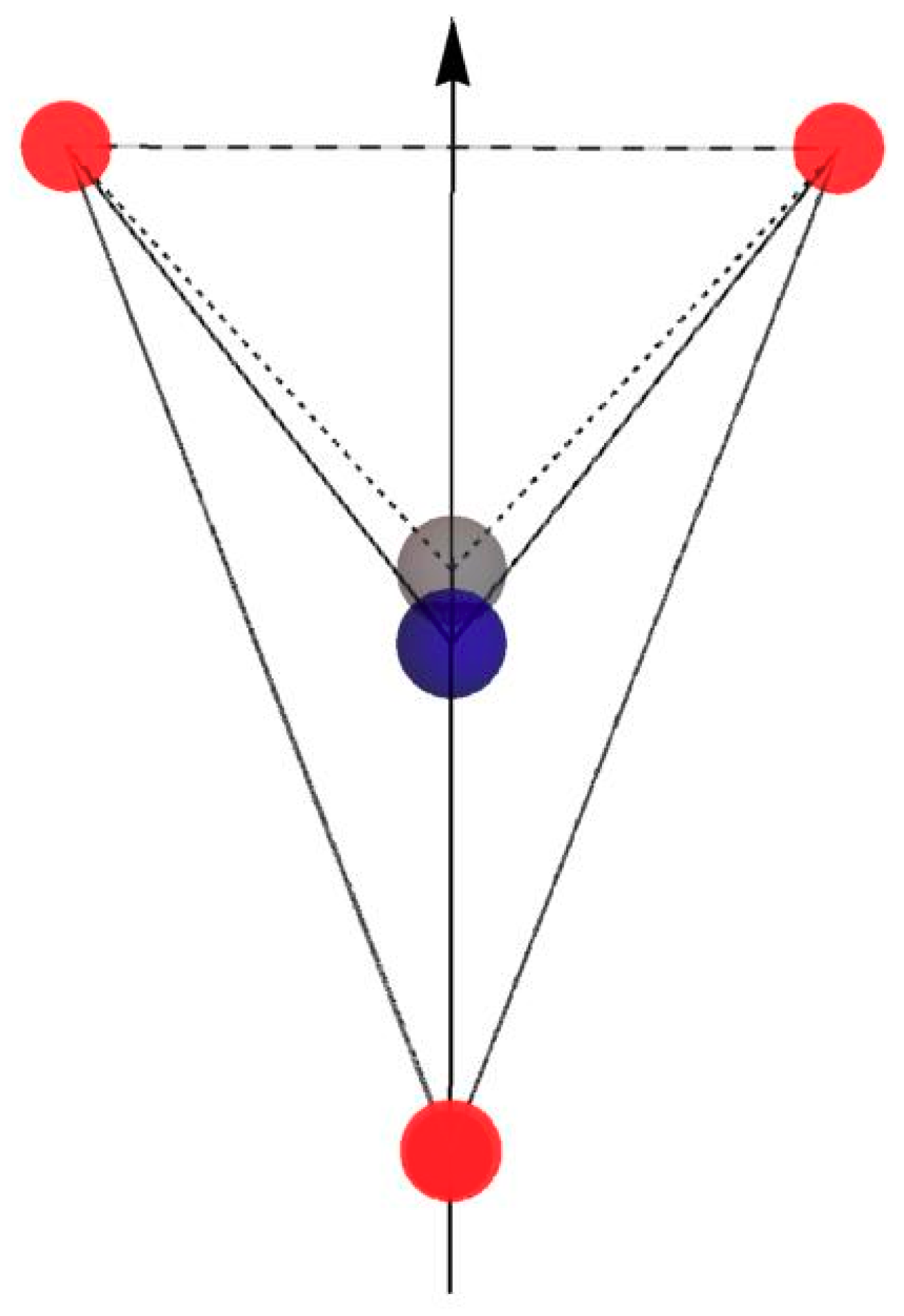
Figure 4 shows [BaO
4] and [CeO
4] tetrahedra with marked angles O-Ba-O, O-Ce-O and
c-axes. The dodecahedron [BaO
8] is made of two rotated, interpenetrated tetrahedra (see
Figure 2). The
Figure 4 presents the displacement of the dopant ion (Ce
3+, gray color) with respect to the Ba ion (blue color) in one of the [BaO
4] tetrahedrons for axial center presents in all, four measured samples (number 1, white background,
Table 4).
Let us examine the results of the calculations in
Table 4, which contains two groups of results, the first of which for when it was assumed that the cerium ion (Ce
3+) substitutes the Ba
2+ site in the dodecahedron [BaO
8] for all four BaWO
4 single crystals with different amounts of impurities. The second group is for when the cerium ion (Ce
3+) substitutes the W
6+ site in the tetrahedron [WO
4] (
Table 3, lines, gray background). The last column shows the difference between the calculated angle and the structure angle, respectively, for the barium dodecahedron and tungsten tetrahedron. The angular distortion for dodecahedra [CeO
8] is within a few degrees, from 1.6° to 4.4° for BaWO
4: 0.5% at. Ce, 1.0% and BaWO
4: 0.5% at. Ce, Na, respectively. One can see that the Ce
3+ ion only slightly shifts along the
c-axes (see
Figure 4). The dodecahedron [BaO
8] is made of two rotated, interpenetrated tetrahedra (
Figure 2). We get only one polar angle. However, the results qualitatively describe the distortion of the [CeO
8] dodecahedron.
Shao-Yi and Hui-Ning published an article about theoretical investigations of the ERP g factor and the local structure for Er
3+ ions in BaWO
4 [
24]. They used the full SPM model for calculation and estimated the angular distortion
for the erbium ion (Er
3+) in BaWO
4 [
24]. They also assumed that Er
3+ ions substitute Ba
2+ in the dodecahedron [BaO
8]. We used a simplified model based on g-shift parameters for different rare-earth ions. Nevertheless, we can say that our results are quantitatively consistent. The angular distortion data obtained from the g-shift parameters’ model confirm that Ce
3+ ion substitutes the Ba
2+ site in the dodecahedron [BaO
8] for all BaWO
4 single crystals and shows only a small displacement along
c-axes (see
Table 4).
The second data group, when the cerium ion (Ce
3+) substitutes the W
6+ site in the tetrahedron [WO
4] looks quite different (
Table 4, lines with a gray background). The angular distortions have a negative sign and quite large values, approximately
. It means that the tetrahedron [CeO
4] would be strongly distorted. Ce
3+ would be shifted approximately to the upper (or lower) limit of the tetrahedron (see
Figure 3). Therefore, the tetrahedron [CeO
4] should be discarded. However, there is one exception. The angular distortion is
for BaWO
4: 1.0% at. Ce (see
Table 4). This result looks quite reasonable. It suggests a slight shift of the Ce
3+ ion along
c-axes in the tetrahedron [CeO
4] comparable to shifts in the dodecahedron [CeO
8]. This result should not be a priori rejected but should be subjected to a deeper analysis.
Table 5 presents summary data based on g-shift parameter calculations. Positive sign (+) means that Ce
3+ion can substitute Ba
2+or W
6+ ions. The negative sign (−) means that Ce
3+ ion cannot substitute Ba
2+, or W
6+ sites in the dodecahedron [BaO
8] or tetrahedron [WO
4], respectively.
Obtained results confirm that impurity ion (i.e., Ce
3+) can substitute the Ba
2+ site, and generally cannot substitute the W
6+ site in BWO crystal [
8,
9,
14,
15,
16,
24]. However, relatively small angular distortion for BaWO
4: 1.0% at. Ce may suggest the existence of such a possibility. Why is the Re
3+ ion (i.e., Ce
3+) unable to substitute the W
6+ site in the BWO cell? There are two problems with such substitutions: ionic radii and charge compensations. The W
6+ ion has the smallest ionic radii. Barium ion (Ba
2+) is approximately three times greater: 0.42 Å (4 coordinate) and 0.6 Å (8 coordinate) respectively (
Table 3). The distance Ba–O is equal
, which is 156% greater than W–O (
Å). Therefore, there is much more space for a dopant ion in the dodecahedron [BaO
8] than in the tetrahedron [WO
4]. The charge compensation is achieved by vacancies on the barium site (V
Ba) [
14,
15,
16,
24]. However, there are oxygens ions sharing among the dodecahedron [BaO
8] and tetrahedron [WO
4]. Each oxygen O ion links with two Ba and one W ions. In the tetrahedron [WO
4], each oxygen O ion links to two dodecahedra [BaO
8] connected by the edge. In the case of vacancies on the barium site (V
Ba), the bond angle and bond length of the B-O and W-O changes. It causes distortion in the dodecahedron [BaO
8] and tetrahedral structure of [WO
4] [
14]. In the case of two pairs of barium vacancies (V
Ba) (each pair [BaO
8] linked to two upper (or lower) oxygen ions in the tetrahedron [WO
4] (see
Figure 3) the displacement of these two O ions can make enough space to substitute tungsten by impurity ions (Ce
W). Additionally, this new paramagnetic center would show an axial symmetry. The biggest volume of unit cell among ABO
4 molybdates and tungstates [
1] from which comes the relative flexibility of the unit cell structure BaWO
4 can also be helpful. Ions of second dopant (i.e., Na
+) substitute also Ba
2+ site (Na
Ba). Barium vacancies (V
Ba) and codopant sites (Na
Ba) guarantee compensation mechanism and new cerium (Ce
W) centers are strongly forbidden. Therefore, the new possible paramagnetic center can be present in the case of BaWO
4 with a significant amount of impurities, BaWO
4: 1.0% at. Ce. The Ce
W paramagnetic centers can be observed only in EPR measurements, not in optics or XRD measurements. Of course, the is only a theoretical explanation of one computational result therefore more evidence is needed. However, the dopant ion in the tungsten tetrahedron [WO
4] may be one of the reasons for the increase in the lattice volume of the unit cell reported by Kunti et al. [
14]. Each larger dopant ion (Ce
3+ or Dy
3+) will push out oxygens influencing via oxygens on the surrounding dodecahedra. The volume of the unit cell increases as a result. The influence of the dopant and codopant in the case of a significant amount of impurities, such as those reported by Kunti et al. [
14], should be subject to further investigations.
4. Conclusions
The values of g-parameters were published previously for two doped with rare-earth cerium (Ce
3+) ions and two additionally codoped with sodium ions (Na): (a) BaWO4: 0.5% at. Ce, (b) BaWO4: 1.0% at. Ce, (c) BaWO4: 0.5% at. Ce, 1.0% at. Na and (d) BaWO4: 1.0% at. Ce, 2.0% at. Na [
8,
9]. One axial symmetry paramagnetic center was found, and ten with lower symmetry centers were identified previously. Five new, paramagnetic centers (Ce
3+) with axial symmetry were suggested after a deeper analysis (
Table 2). A method to extract structural information from g-shift, proposed by Newman, was used [
20]. The following assumptions were made: (a) cerium ion (Ce
3+) substitutes the Ba
2+ ion in the dodecahedron [BaO
8], (b) ion (Ce
3+) substitutes W
6+ ion in the dodecahedron tetrahedron [WO
4]. On the basis g-shift model for all paramagnetic centers with axial symmetry the polar angles of the oxygen ions making dodecahedron [BaO
8] and tetrahedron [WO
4], respectively, were calculated.
Results of calculations display that the angular distortions for dodecahedra [CeO
8] are within a few degrees, in the range from 1.6° to 4.4° for BaWO
4: 0.5% at. Ce, 1.0% at. Na and BaWO
4: 0.5% at. Ce, respectively. There is a quantitative consistency of our results with other results already present in the literature which were obtained by applications of the superposition model (SPM) for erbium ions (Er
3+) in BaWO
4 [
24]. However, we used a simplified g-shift model. The angular distortion data obtained from the g-shift model confirm that Ce
3+ ion substitutes the Ba
2+ site in the dodecahedron [BaO
8]. In the case of cerium ion (Ce
3+) substitutes the W
6+ site in the tetrahedron [WO
4] the angular distortions have a negative sign and quite large values, approximately
. Therefore, the tetrahedron [CeO
4] should be discarded. However one exception was observed. The angular distortion is equal
for BaWO
4: 1.0% at. Ce (see
Table 3). It is generally accepted that impurity ions, like rare-earth ions, cannot substitute tungsten (W) in the tetrahedron [WO
4] in BaWO
4 crystal. Nevertheless, after structural analysis, we conclude that in certain circumstances this possibility is worth considering.
Our calculations show a close relationship between g-shift and the local structure of the paramagnetic center with axial symmetry. Simple g-shift model, proposed by D J. Newman, can give important information about positions or displacements of paramagnetic ions in the case of paramagnetic centers with axial symmetry.