Abstract
Bi-doped compounds recently became the subject of an extensive research due to their possible applications as scintillator and phosphor materials. The oxides co-doped with Bi3+ and trivalent rare-earth ions were proposed as prospective phosphors for white light-emitting diodes and quantum cutting down-converting materials applicable for enhancement of silicon solar cells. Luminescence characteristics of different Bi3+-doped materials were found to be strongly different and ascribed to electronic transitions from the excited levels of a Bi3+ ion to its ground state, charge-transfer transitions, Bi3+ dimers or clusters, radiative decay of Bi3+-related localized or trapped excitons, etc. In this review, we compare the characteristics of the Bi3+-related luminescence in various compounds; discuss the possible origin of the corresponding luminescence centers as well as the processes resulting in their luminescence; consider the phenomenological models proposed to describe the excited-state dynamics of the Bi3+-related centers and determine the structure and parameters of their relaxed excited states; address an influence of different interactions (e.g., spin-orbit, electron-phonon, hyperfine) as well as the Bi3+ ion charge and volume compensating defects on the luminescence characteristics. The Bi-related luminescence arising from lower charge states (namely, Bi2+, Bi+, Bi0) is also reviewed.
1. Introduction
Luminescence of various Bi3+-doped materials (alkali halides; alkaline-earth oxides, sulfates and phosphates; tungstates; garnets; perovskites; silicates; borates; vanadates; niobates, etc.) was systematically investigated starting from the 1960s (see, e.g., review papers [1,2,3,4,5,6,7,8,9,10,11] and references therein). Bi3+-doped complex oxides, where a trivalent Bi3+ ion substitutes for a trivalent rare-earth ion, became the subject of special interest and extensive research due to their possible applications as scintillator and phosphor materials. For instance, Bi3+-doped garnets [12,13,14,15,16,17,18,19], oxyorthosilicates [20,21], perovskites [22], borates [23] and phosphates [24] were considered as prospective scintillator materials and the materials for X-ray screens due to the presence of an intense and fast Bi3+-related luminescence at room temperature. The materials co-doped with Bi3+ and trivalent rare-earth ions (Ln3+: Dy3+, Er3+, Yb3+, Eu3+, Sm3+, Ho3+, Nd3+) were found to be potentially applicable as spectral converters for solar cells and solid state light sources of a new generation, so called white light-emitting diodes, owing to the presence of broad and intense absorption bands in the ultraviolet region, the intense broad visible Bi3+-related emission band, and an effective energy transfer from the Bi3+-related excited state to Ln3+ ions, giving rise to an intense visible emission from Ln3+ ions (see, e.g., [11,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]). Due to an effective Bi3+ → Ln3+ energy transfer, the needed luminescence color of the Bi3+, Ln3+ co-doped compounds can easily be obtained by varying the concentration ratio of Bi3+ and Ln3+ ions in the host material (see, e.g., [26,39,40,43]). The oxides co-doped with Bi3+ and Yb3+ are of interest as quantum cutting down-converting materials possibly applicable for enhancement of silicon solar cells (see, e.g., [46,47,48] and references therein). Some Bi3+-doped materials, e.g., ZnGa2O4:Bi [49], CaGa2O4:Bi [50], SrGa2O4:Bi [51], Ca0.9Sr0.1S:Bi [52], KGaGeO4:Bi [53], CaWO4:Bi [54], CdSiO3:Bi [55], Lu2CaGeO6:Bi [56], CaZnGe2O6:Bi [57], Sr3Ga4O9:Bi [58], NaLuGeO4:Bi,Eu [59], CdSiO3:Bi,Gd [60], CdSiO3:Bi, Dy [61], etc. were found to be long persistent phosphors which may have potential applications in photo-catalysis, anti-counterfeiting, water disinfecting, photochemistry, and dosimetry (see also review [62]).
The drastically increasing interest in the investigation of various bismuth based materials, observed since 2004 and reflecting the increasing importance of these materials in modern technological applications, is clearly illustrated in Figure 1 of Ref. [11]. The quick growth of the number of publications on the bismuth luminescence can be explained by the fact that, owing to a large number of possible valence states, bismuth containing materials exhibit a rich variety of luminescence properties, showing emission from the ultraviolet to infrared. This allows the application of these materials in many areas such as telecommunication, biomedicine, white light illumination, and lasers. An excellent review on different types of bismuth-activated photonic materials, their synthesis, characterization, future research trends and prospective applications in broadband optical amplifiers, fiber lasers, bioimaging, and phosphors for white light-emitting diodes is given in Ref. [8].
In recent years, the interest in Bi3+-doped materials of various types increased drastically not only due to their possible new applications (see, e.g., [8,11,63] and references therein), but also owing to very interesting phenomena appearing under photoexcitation in the Bi3+-related absorption bands. For example, an unusually strong dependence of luminescence characteristics on the host material found many years ago was recently connected with the position of Bi3+ energy levels with respect to the bottom of the host conduction band (see, e.g., [7,10,64,65]). To understand the mechanisms of observed features and to clarify the origin of the emission bands in different materials, a detailed spectroscopic study and comparison of various Bi3+-doped compounds are needed.
Absorption spectra of Bi3+ centers in different compounds are caused by the transitions between the electronic configurations of the ground (6s2) and the excited (6s)(6p) states of Bi3+. The ground state of a free Bi3+ ion is 1S0, and the two lowest-energy excited states are triplet 3P and singlet 1P. The triplet state is split due to the spin-orbit interaction into the 3P0, 3P1, and 3P2 states. According to [66], the energies of electronic transitions from the ground 1S0 level to the excited 3P1, 3P2, and 1P1 levels of a free Bi3+ ion (Efree) are 9.41 eV, 11.96 eV, and 14.21 eV, respectively. Only the dipole 1S0 → 1P1 transitions are allowed. The 1S0 → 3P1 transitions are partly allowed due to the mixing of the wave functions of the singlet 1P1 and triplet 3P1 states by the spin-orbit interaction. In the crystals, the forbidden 1S0 → 3P2 transitions also become partly allowed due to vibronic mixing of the 3P2-related state with the triplet or singlet states by non-totally symmetric lattice vibrations. The 1S0 → 3P0 transitions are strongly forbidden. Thus, in the crystal, the absorption (excitation) bands labeled as A, B, and C and corresponding to the electronic transitions from the ground 1S0 level of a free Bi3+ ion to the excited 3P1, 3P2, and 1P1 levels, respectively, can be observed.
In many cases, only the lowest-energy A band, corresponding to the 1S0 → 3P1 transition of a free Bi3+ ion, clearly appears in the absorption (excitation) spectra. From the comparison of the A band energy in a Bi3+-doped compound with the 1S0 → 3P1 transition energy of the free Bi3+ ion (9.41 eV), the approximate positions of the other absorption (excitation) bands of the same compound can be estimated. For example, in Lu3Al5O12:Bi, the A band is located around 4.63 eV, i.e., the transition energy in the crystal (Ecrys) is about twice as small as in a free Bi3+ ion. Taking into account the fact that the Efree/Ecrys ratio increases with the increasing Efree according to the approximate equation Efree/Ecrys = 1 + kEfree experimentally found in [67] and confirmed to be valid for all the ns2-ion-doped alkali halides, it was suggested that the 5.95 eV absorption band of Lu3Al5O12:Bi could arise from the 1S0 → 1P1 transitions, and the weak B band should be located at about 5.2 eV.
In this paper, mainly luminescence characteristics of Bi3+-doped compounds (collected in Table 1 and Table 2) are addressed. As it was noticed many years ago [1,68,69], two types of Bi3+-related emission bands with strongly different characteristics exist in these materials (see, e.g., review papers [4,7,9,10,11] and references therein). The emission of both types was found to arise from the triplet relaxed excited state (RES) of a luminescence center. For some compounds, not only the characteristics of the triplet luminescence but also the structure and parameters of the corresponding triplet RES were studied (see, e.g., [3,9,13,16,17,18,20,21,22,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]). Due to the spin-orbit interaction, the triplet RES is split and consists of the upper emitting level and the lower metastable level. The energy distance (D) between these levels depends on the spin-orbit interaction energy in the triplet state. The probability of the radiative transitions from the emitting level (k2) is much higher as compared to that from the metastable level (k1). Due to that, the radiative decay of the emitting and metastable levels can result in the appearance of two different, the fast (FC) and slow (SC), luminescence decay components with characteristic temperature dependences of their decay times and light sums, strongly depending on the D value.

Table 1.
Emission peak positions (Eem), full widths at half maxima (FWHM), the Stokes shifts (S), positions of the lowest-energy excitation band (Eexc), and decay times (τSC) of the slow decay component obtained at 4.2 K for the triplet emission of Bi3+ centers (denoted in this work the UV emission). The parameters of the triplet relaxed excited state (RES): the spin-orbit splitting energy (D), the probabilities of the radiative decay of the metastable (k1) and emitting (k2) levels of the triplet RES, and the zero-temperature nonradiative transition rate (K) between the metastable and emitting levels.

Table 2.
Emission peak positions (Eem), full widths at half maxima (FWHM), the Stokes shifts (S), positions of the lowest-energy excitation band (Eexc) and decay times (τSC) of the slow decay component obtained at LHeT for the lower-energy triplet emission of Bi3+-doped compounds (denoted in this work the VIS emission). The parameters of the triplet RES: the spin-orbit splitting energy (D), the probabilities of the radiative decay of the metastable (k1) and emitting (k2) levels of the triplet RES, and the zero-temperature nonradiative transition rate (K) between the metastable and emitting levels.
The main difference between the emissions of the two types is in the Stokes shift (S) and full width at half maximum (FWHM) of the emission band and in the temperature dependence of the luminescence decay kinetics. The higher-energy emission band of Bi3+-related centers, located usually in the ultraviolet (UV) spectral region, further on called the UV emission, is characterized by relatively small values of FWHM and S. The slow component decay time of this emission is temperature-independent up to rather high temperatures (40–100 K) indicating a high spin-orbit splitting energy D of the triplet RES of the luminescence center (see Table 1). The FWHM and S values of the lower-energy emission band are much larger, the slow component decay time is temperature-independent only up to 1–5 K, and the D values are 1–2 orders of magnitude smaller as compared to the UV emission (see Table 2). As these bands are usually located in the visible (VIS) spectral range, we denote them the VIS emission.
A clear relation between the energy distance D and the values of the Stokes shift S was noticed for some Bi3+-containing compounds many years ago (see, e.g., [5,68,100,101] and references therein). It was also discussed in the recent paper [7], but not explained yet. In [68], the authors concluded that the S value depends on the coordination number of a Bi3+ ion and the ionic radius of the crystal lattice ion which Bi3+ is substituted for. However, later studies revealed completely different origin of the UV and VIS emissions of Bi3+-doped materials.
As evident from Table 1 and Table 2, in many Bi3+-doped compounds, e.g., in the garnets Y3Al5O12:Bi and Lu3Al5O12:Bi, silicate Lu2SiO5:Bi, borate LaBO3:Bi, both types of emission bands were found to co-exist (see, e.g., [9,15,16,17,18,20,21,78]). Only the VIS emission was observed, e.g., in LiLaP4O12:Bi [72,102], Sr3Ga4O9:Bi [58], Li2BaP2O7:Bi [103], GdBr3O6:Bi [104], YOCl:Bi [99], GdOCl:Bi [99], Gd3Ga5O12:Bi [12,74,75], PbWO4:Bi [73], rare-earth vanadates [76], and niobates [77], while only the UV emission, in alkali-earth fluorides, oxides, and sulfides [79,84,85,86,87,88,89,90,92], YAlO3:Bi [22], Y4Al2O9:Bi [70], Y2SiO5:Bi [20], Y2O3:Bi [71], etc.
Luminescence characteristics of different Bi3+-doped materials were connected in the literature with the single Bi3+ ions and with dimer {Bi3+ - Bi3+} centers or Bi3+ clusters. The UV emission was usually concluded to arise from the electronic transitions from the excited levels of a single Bi3+ ion to its ground state, corresponding to the 3P1,0 → 1S0 transitions of a free Bi3+ ion. The VIS emission was ascribed in the literature to the same 3P1,0 → 1S0 transitions; to Bi3+ pairs or clusters of Bi3+ ions; to charge-transfer (CT) transitions (in particular, to metal-to-metal charge transfer—MMCT, the Bi3+ → Bi3+ charge transfer inside a {Bi3+ - Bi3+} dimer called the intervalence charge transfer—IVCT, and O− → Bi3+ charge transfer); to radiative decay of impurity-trapped exciton; to D-state emission; to radiative decay of an exciton localized around a Bi3+-related center (e.g., a Bi3+ ion, dimer {Bi3+ - Bi3+} center, {Bi3+ - defect} center).
In addition to rich literature dealing with the Bi3+-based luminescence in many kinds of crystalline solids mentioned above, there is an increasing interest in the study of bismuth centers with the valence lower than 3+ for their specific emission characteristics given by radiative transitions within the 6p shell. Namely, the Bi2+ center was ascribed to red luminescence in SrB4O7 host in 1994 [105] and other examples can be found in the review paper of Sun et al. in 2014 [8]. More recently, several studies appeared ascribing the near infrared luminescence to Bi0 center, e.g., in Ba2P2O7 host [106]. Even the Bi+ center was ascribed to new luminescence bands in the visible-near infrared spectral region in Ba2B5O9Cl:Bi. These new bands appear among those associated with the Bi2+ and Bi0 centers during the annealing cycles in air and the reduction atmosphere [107].
In this review, we compare the characteristics of the Bi3+-related luminescence in different compounds; discuss the possible origin of the corresponding luminescence centers; consider theoretical models of their RES and phenomenological models allowing to describe the excited-state dynamics of the Bi3+-related centers of different types and determine the structure and parameters of their RES; address an influence of different interactions (e.g., spin-orbit, electron-phonon, hyperfine) as well as the Bi3+ ion charge and volume compensating defects on the luminescence characteristics.
In Section 6 we review the state-of-art regarding emission characteristics of bismuth centers with the valence lower than 3+.
The small Stokes shifts of the UV emission are reported also for NaGdO2:Bi (S = 0.6 eV), LiScO2:Bi (S = 0.9 eV), NaScO2:Bi, YAl3B4O12:Bi, and Cs2NaYCl6:Bi (S < 0.5 eV) (see [68] and references therein).
2. Ultraviolet Luminescence of Single Bi3+ Centers in Bi3+-Doped Compounds
2.1. Characteristics of the Ultraviolet Luminescence
The characteristics of the higher-energy (UV) emission in various Bi3+-doped materials are similar (Table 1). Let us demonstrate them at an example of the Lu3Al5O12:Bi, Y3Al5O12:Bi, and Lu2SiO5:Bi single crystalline films investigated in [16,17,18,21].
In Figure 1, the emission spectra of Lu3Al5O12:Bi and Y3Al5O12:Bi are shown. The absorption and excitation spectra of Lu3Al5O12:Bi are presented in Figure 2. At low temperatures (T < 100 K in Lu3Al5O12:Bi and Y3Al5O12:Bi), the UV emission arises from the radiative decay of the lowest-energy metastable level corresponding to the 3P0 level of a free Bi3+ ion. Temperature dependences of the maximum position and FWHM of the UV emission of Y3Al5O12:Bi and Lu2SiO5:Bi are displayed in Figure 3. As the temperature increases, the UV emission spectrum is shifting to higher energies and becomes broader (see the insets to Figure 1). This effect is caused by the thermally stimulated population of the higher 3P1 excited level from the lower 3P0 level. Further increase of the temperature results in thermal equilibrium between the 3P0 and 3P1 levels. As the temperature increases further, a gradual lower-energy shift of the emission band takes place. These processes also appear in the decay kinetics of the UV emission.
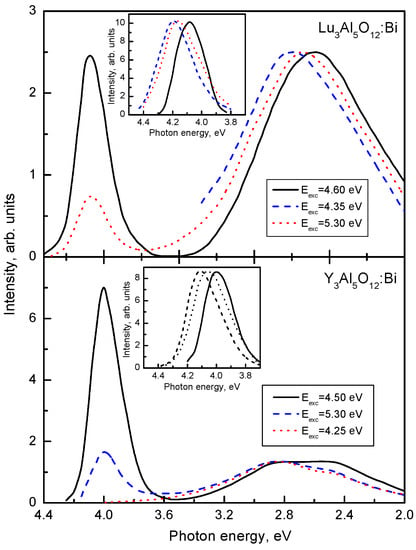
Figure 1.
Emission spectra (normalized) of Lu3Al5O12:Bi and Y3Al5O12:Bi measured at 80 K under different excitations shown in the legends. In the insets, the ultraviolet emission spectra of Bi3+ centers (normalized) measured at 80 K (solid line), 150 K (dashed line), and 300 K (dotted line). Based on the data reported in [17,18], presented with the publisher’s permission.
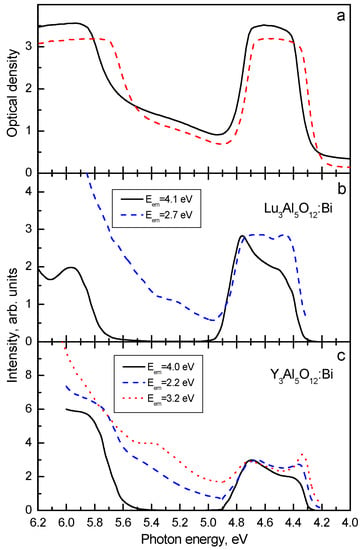
Figure 2.
(a) Absorption spectra of Lu3Al5O12:Bi (solid line) and Y3Al5O12:Bi (dashed line) at 295 K. Excitation spectra (normalized) of (b) Lu3Al5O12:Bi and (c) Y3Al5O12:Bi measured at 80 K for different emission spectra regions shown in the legends. Based on the data reported in [16,17,18], presented with the publisher’s permission.
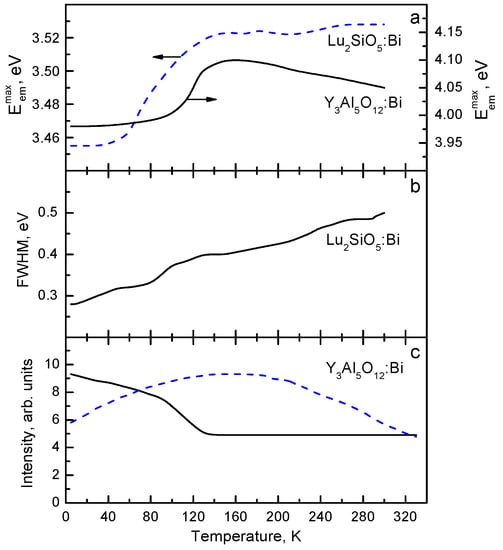
Figure 3.
(a) Temperature dependences of the UV emission band maximum position in Y3Al5O12:Bi (solid line) and Lu2SiO5:Bi (dashed line), (b) the FWHM of the UV emission band in Lu2SiO5:Bi, and (c) the maximum intensities of the UV (4.0 eV, solid line) and VIS (2.5 eV, dashed line) emissions in Y3Al5O12:Bi reported in [17,18,21], presented with the publisher’s permission.
At 4.2 K, the slow component with the decay time τSC ≈ 1.1 ms is observed in the decay kinetics of the UV emission of Y3Al5O12:Bi (Figure 4a). The decay time remains constant up to 100 K and then decreases (Figure 5). This dependence is characteristic for the radiative transitions from a triplet RES where the lowest-energy (metastable) level has much smaller radiative decay probability as compared to the upper (emitting) level (see also [3,68,80]). Indeed, at T < 100 K, the slow decay component is associated with transitions from the metastable level. As the temperature increases, the decay time shortens exponentially due to thermally stimulated transitions between the metastable and emitting levels and reaches a constant value at the temperatures (around 350 K), where the system achieves thermal equilibrium. At higher temperatures, the decay time decreases due to the luminescence thermal quenching. Analogous τSC(T) dependences were obtained, e.g., for the triplet emission of Pb2+ centers in alkali halides [131] and Bi3+ centers in CaO [85], alkaline-earth sulfides [87], and alkaline-earth fluorides [92].
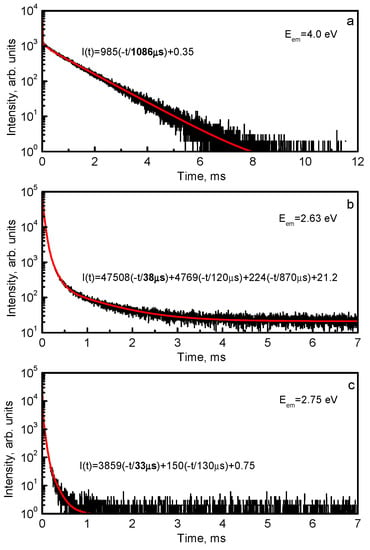
Figure 4.
Decay curves of the (a) 4.0 eV, (b) 2.63 eV and (c) 2.75 eV emissions of Y3Al5O12:Bi at 4.2 K.
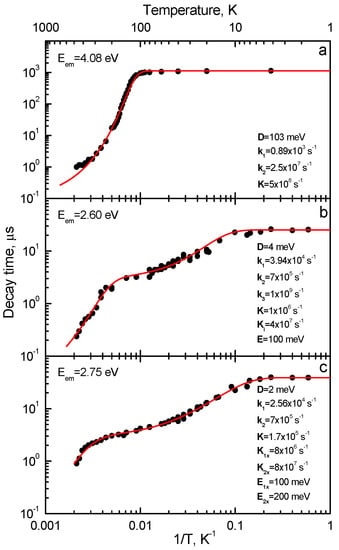
Figure 5.
Temperature dependences of decay times measured for (a) the UV emission, (b) the 2.6 eV emission and (c) the 2.75 eV emission of Lu3Al5O12:Bi. The circles are experimental data. Solid lines are the best fits of the two or three excited-state level models (Figure 6 and Figure 10) to the experimental data (for details see the text). The parameters of the fits are reported in the figures. (a) Eexc = 4.6 eV, Eem = 4.1 eV; (b) Eexc = 4.7 eV, Eem = 2.4 eV; (c) Eexc = 5.3 eV, Eem = 3.2 eV. See also [16,17,18]. Presented with the publisher’s permission.
2.2. Dynamics of the Triplet Excited State of Bi3+ Centers
Thermally stimulated transitions between the metastable and emitting minima of the triplet RES and between the excited and ground state of the luminescence centers responsible for the UV emission reveal themselves in the temperature dependences of the luminescence spectra and decay kinetics. The excited states dynamics of the luminescence center responsible for the UV emission are described within the phenomenological model sketched in Figure 6.
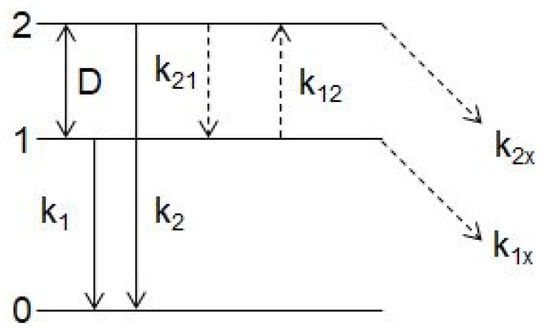
Figure 6.
Energy level diagram used for the description of the excited states dynamics of the Bi3+-related luminescence centers responsible for the UV emission. For details, see the text.
The time evolution of the populations N1, N2 of the excited levels 1 and 2, respectively, can be described by the following rate equations:
where k1, k2, k12, k21, and k1(2)x are radiative transition rates from levels 1,2, non-radiative rates of phonon assisted transitions between the radiative level 2 and metastable level 1 and the quenching channel from the level 1(2), respectively. Non-radiative transitions between levels 1,2 can be written as:
where K, n, D are the zero-temperature transition rate between the levels 1 and 2, the Bose–Einstein factor, and energy spacing between the levels, respectively. Non-radiative quenching channel is considered in the usual barrier form:
with K1(2)x being a frequency factor and E1(2)x the height of the barrier.
Application of the two-excited-level models on the temperature evolution of the UV luminescence intensity and decay times allowed determination of characteristic parameters of the corresponding triplet RES, e.g., the energy separation (D) between the emitting and metastable levels of the triplet RES, the rates of the radiative (k1, k2) and nonradiative (K) transitions from these levels, and activation energy E1(2)x for the luminescence thermal quenching (for more details, see Refs. [9,13,16,17,18,20,21,22,70,71]). Some parameters of the triplet RES corresponding to the UV emission of Bi3+-doped compounds are shown in Table 1.
As evident from Table 1, the triplet RES responsible for the UV emission is characterized by very large (~102 meV) energy distance D between the metastable and emitting levels of the triplet RES which can be explained by extremely large spin-orbit interaction energy characteristic for a free Bi3+ ion (ξ = 2.102 eV [4]). Therefore, the higher-energy (UV) emission of all the investigated materials can surely be ascribed to the electronic transitions from the triplet RES of Bi3+ corresponding to the 3P1,0 → 1S0 transitions of a free Bi3+ ion.
In some Bi3+-doped compounds, a fast (ns) component is observed at low temperatures in the UV luminescence decay. This component is associated with transitions from the emitting level of the triplet RES related to the 3P1 level of a free Bi3+ ion. The electronic transitions between the ground state (1S0) and the 3P1-related excited state are partly allowed due to mixing of the triplet 3P1 state with the singlet 1P1 state by the spin-orbit interaction. Due to a strong spin-orbit interaction, the probability of the radiative decay of the emitting level (k2) is relatively large (see Table 1). The radiative transitions from the metastable 3P0 -related state can occur due to mixing of the 3P1- and 3P0-related states by the vibronic interaction with the non-totally symmetric vibrations or by the hyperfine interaction (see, e.g., [132] and references therein). The only stable Bi isotope 209Bi has a nuclear spin of I = 9/2. Therefore, in Bi3+-doped compounds with a weak vibronic interaction, such as alkali-earth oxides, sulfides, fluorides (see, e.g., [84,86,88,89,90,91,92] and references therein), ScBO3:Bi [78], LuBO3:Bi [78], Cs2NaYBr6:Bi [97], Cs2NaLaCl6:Bi [97], Cs2NaYCl6:Bi [98], NaScO2:Bi, and YAl3B4O12:Bi (see also [68] and references therein), where the Stokes shift is extremely small (see Table 1) and even a vibronic structure of the emission and excitation spectra is observed at low temperatures, mainly the hyperfine interaction can be expected to be responsible for the radiative decay of the metastable 3P0-related level. The influence of the hyperfine interaction on the probability of the radiative 3P0 → 1S0 transitions in Bi3+-doped alkali-earth oxides was investigated in [133].
2.3. Relaxed Excited States Models
Hitherto, two models have been proposed to describe RES of the ns2-ion-doped ionic crystals with strongly different electron-phonon and spin-orbit interactions. The systems with a strong spin-orbit interaction and a very weak electron-phonon interaction can be described within the RES model, proposed by Seitz [134], which considers the spin-orbit interaction in RES being dominant. In this model, the excited states of the luminescence center originate from the 3P0, 3P1, 3P2, and 1P1 levels of a free ns2 ion, which are split in the crystal field of the corresponding symmetry. For the degenerate energy levels, the Jahn-Teller effect is taken into account as a perturbation. The totally symmetric 3P0 state is not degenerate, therefore it cannot be Jahn-Teller active. The configuration coordinates (q) of the 3P0 and 1S0 minima in this model should coincide.
The analysis of results obtained in a huge number of works (see, e.g., review papers [131,132,135]) have convincingly confirmed the suggestion of Seitz that the absorption processes in ns2-ion-doped compounds can be described in the approximation of a weak crystal field. This means that the spin-orbit interaction must be considered to be dominant in the unrelaxed excited state of the luminescence center. However, this model cannot adequately describe the luminescence characteristics and the relaxed excited state structure of the systems with a strong electron-phonon interaction.
For the systems of this type, a new RES model was proposed by Hizhnyakov [136], and the RES theory was developed in [135,137]. In this theory, the interaction of impurity optical electrons with non-totally symmetric vibrations is considered to be dominant in the relaxed excited state, while the spin-orbit, hyperfine, and other interactions are taken as small perturbations. As a result, the Jahn-Teller minima of different symmetries can be formed on the adiabatic potential energy surface of the singlet (1P) and triplet (3P) excited states. Due to the spin-orbit interaction, each Jahn-Teller minimum of the triplet RES is split into the upper emitting level and the lower metastable level, corresponding to the 3P1 and 3P0 levels of a free Bi3+ ion, respectively (Figure 7, solid lines). The applicability of this model was confirmed by the systematic experimental study of luminescence characteristics of ns2-ion-doped alkali halide crystals by the methods of time-resolved polarization spectroscopy in a wide temperature range, down to 0.4 K (see, e.g., [132,138]). It was also shown that in the Tl+, Pb2+, Bi3+ centers with a strong spin-orbit interaction, each metastable minimum of the triplet RES may not lie exactly under the corresponding emitting minimum, like in the Ga+, In+, Ge2+, Sn2+ centers with a weak spin-orbit interaction. Instead, it can be shifted towards smaller coordinate q values with respect to the emitting minimum (Figure 7). Due to that, the energy barriers for the thermally stimulated transitions between the metastable minima of various orientations can be much smaller than those between various emitting minima (see, e.g., [85,132,139] and references therein).
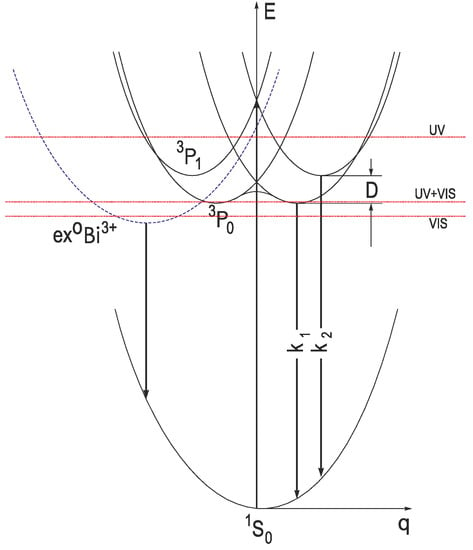
Figure 7.
Schematic configuration coordinate diagram of the Bi3+-related energy levels. The electronic transitions between the ground state, corresponding to the 1S0 level, and the excited states, corresponding to the 3P1 and 3P0 levels of a free Bi3+ ion and the triplet Bi3+-related localized exciton state (ex0Bi3+), are indicated by arrows. The straight red dotted lines indicate the position of the bottom of CB in the case where only the UV emission (UV), only the VIS emission (VIS), and both the UV and VIS emissions (UV+VIS) can appear in the luminescence spectrum of a Bi3+-doped compound.
Thus, in the ns2-ion-doped compounds characterized by a strong electron-phonon interaction, different models have to be used for the description of the structure and properties of the unrelaxed and relaxed excited states of a luminescence center.
Since a free Bi3+ ion is characterized by the largest spin-orbit interaction energy (ξ) among all ns2 ions, different models should also be used for the description of the triplet RES responsible for the UV luminescence of Bi3+ centers in the compounds with an extremely small electron-phonon interaction (e.g., in alkaline-earth oxides, sulfates, fluorides) with respect to the materials with a relatively strong electron-phonon interaction (large FWHM and S), such as alkali halides, oxyorthosilicates, etc. For the description of the latter type systems, the model [135,137] should be considered. However, usually only the model [134] is used in the literature for the description of the UV luminescence of all Bi3+-doped materials.
To investigate the applicability of the model [135,137] to the centers with extremely strong spin-orbit interaction, the luminescence characteristics of two Bi3+-doped crystals with strongly different electron-phonon interaction (KCl:Bi and CaO:Bi) were compared in [85]. As evident from Figure 8, the characteristics of KCl:Bi and CaO:Bi are different. In the emission spectrum of KCl:Bi at 4.2 K, the broad (FWHM = 0.4 eV) complex band located around 2.5 eV is observed (Figure 8a, curve 1) (see also [140]). The lowest-energy excitation band of this emission is located around 3.8 eV (curve 2), thus, S ≈ 1.3 eV.
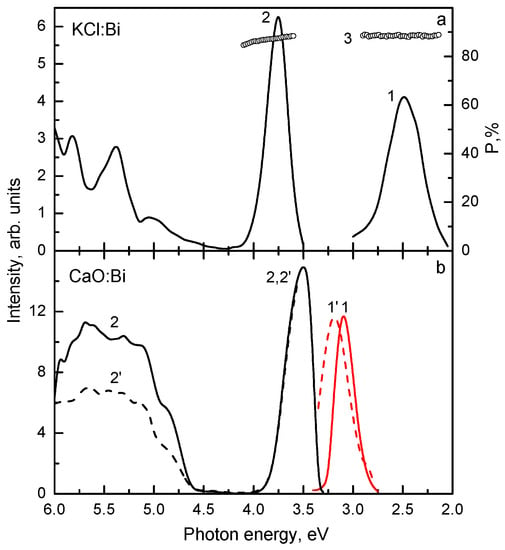
Figure 8.
Emission (curves 1,1’), excitation (curves 2,2’), and polarization (curve 3) spectra of (a) KCl:Bi and (b) CaO:Bi at 4.2 K. In (a), Eexc=3.7 eV (curves 1,3) and Eem=2.6 eV (curves 2,3). In (b), Eexc = 3.5 eV, slow component (curve 1), Eexc = 3.6 eV, fast component (curve 1’), Eem = 3.1 eV (curve 2), and Eem = 3.3 eV (curve 2’). Based on the data reported in [85]. Presented with the publisher’s permission.
In the emission spectrum of CaO:Bi at 4.2 K, the narrow (FWHM = 0.14 eV) strong 3.1 eV and weak 3.3 eV bands are observed (Figure 8b, curves 1,1’). Their excitation spectra coincide (curves 2,2’). The lowest-energy excitation band is located at 3.5 eV, i.e., S = 0.4 eV. The intensity of the 3.1 eV emission remains constant up to 100 K and then decreases. The reduction of the 3.1 eV emission is accompanied with the 3.3 eV emission enhancement. The intensity redistribution between the 3.1 eV and 3.3 eV emissions around 140 K points to the thermally stimulated transitions between the corresponding levels.
Two fast (17 ns and 27 ns) and two slow (1.38 ms and 2.8 ms) components were observed at 4.2 K in the KCl:Bi emission decay (Figure 9a). Their excitation spectra practically coincide. The emission spectra of two fast decay components are located at 2.54 eV and 2.46 eV, respectively, and the emission spectra of two slow decay components, at 2.84 eV and 2.68 eV, respectively. The decay times of the slow components are constant up to about 60 K and then decrease exponentially. The D value is estimated to be of the order of 102 meV.
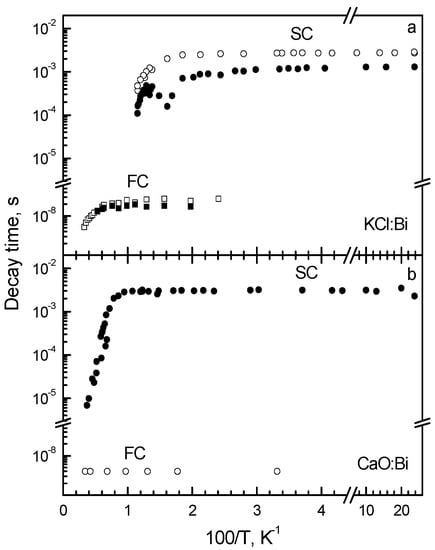
Figure 9.
Temperature dependences of the decay times of the slow (SC) and fast (FC) decay components of the triplet luminescence of (a) KCl:Bi and (b) CaO:Bi. In (a), Eexc = 3.7 eV, Eem = 2.68 eV (empty circles) and 2.84 eV (solid circles), Eem = 2.46 eV (empty squares) and 2.54 eV (solid squares). In (b), Eexc = 3.6 eV, Eem = 3.1 eV (solid circles) and Eem = 3.3 eV (empty circles). Based on the data reported in [85]. Presented with the publisher’s permission.
At 4.2 K, only the slow component (3.25 ms) is observed in the decay kinetics of the 3.1 eV emission of CaO:Bi and only the fast component (4 ns), in the decay kinetics of the 3.3 eV emission (Figure 9b). These components arise from RES, related to 3P0 and 3P1 excited levels of a free Bi3+ ion. Decay time of the slow component decreases exponentially at T > 100 K due to the thermally stimulated 3P0 → 3P1 transitions with the activation energy of about 152 meV.
Results obtained in [85] confirmed the conclusion [84] that the Seitz model [134], considering the spin-orbit interaction in the triplet RES being dominant, should be used for the description of the luminescence characteristics of CaO:Bi. These data also indicate that in each Jahn-Teller minimum of the triplet RES of KCl:Bi, the metastable minimum is located under emitting minimum (Figure 7). The radiative transitions from these minima result in appearance of the slow and fast decay component, respectively. The fast component is strongly polarized in the <100> direction [96]. The shift of the slow component emission spectra to higher energies with respect to those of the fast components, as well as a very small polarization degree of the slow decay component are caused by the shift of the metastable minima towards a smaller configuration coordinate q values with respect to the emitting minimum as well as the decrease of the energy barriers between the metastable minima of different orientation as compared with the emitting minima (see Figure 7). Analysis of these data allows to conclude that the theoretical model [135,137] is still valid in the case of KCl:Bi, despite the strong spin-orbit interaction.
Comparison of the Stokes shifts and FWHM of emission bands as well as the values of RES parameters presented in Table 1 indicate that the Bi3+ center in aluminum garnets and oxyorthosilicates can be considered as an intermediate case between Bi3+-doped CaO and KCl. These data also allow to conclude that the electron-phonon interaction in oxyorthosilicates and Y4Al2O9:Bi is noticeably stronger than that in garnets. Indeed, the larger probability (k1) of the radiative decay of the triplet RES metastable minima points to a stronger electron-phonon interaction in these compounds. In addition, a smaller value of the spin-orbit splitting energy (D) of the triplet RES of Bi3+ center in oxyorthosilicates and Y4Al2O9 as compared with aluminum garnets is caused by stronger suppression of the spin-orbit interaction by the electron-phonon interaction (see [135,137]). Thus, the structure and properties of the triplet RES of these materials and the characteristics of their luminescence might be described in terms of the theory [135,137] which considers a strong Jahn-Teller effect to be a dominant interaction in the triplet RES.
3. Visible Luminescence of Bi3+-Doped Compounds
3.1. Characteristics of the Visible Luminescence
Characteristics of the lower-energy luminescence (the VIS emission) in various Bi3+-doped materials are similar and presented in Table 2. Let us demonstrate them at an example of the Lu3Al5O12:Bi and Y3Al5O12:Bi single crystalline films investigated in [16,17,18].
In Lu3Al5O12:Bi, two broad VIS emission bands with large Stokes shifts are located at 2.6 eV and 2.75 eV (Figure 1a). In Y3Al5O12:Bi, analogous bands are observed at 2.63 eV and 2.75 eV (Figure 1b). Comparison of the UV and VIS emission spectra shows that FWHM and S values of the VIS emission are several times larger as compared with those of the UV emission. The lowest-energy excitation band of the VIS emission is always slightly shifted to lower-energies with respect to that of the UV emission (Figure 2b,c) (compare also Table 1 and Table 2). The VIS emission is much more effectively excited in the higher-energy absorption bands as compared with the UV emission. As the temperature increases, the intensity redistribution takes place between the UV emission and the lower-energy VIS emission of Lu3Al5O12:Bi and Y3Al5O12:Bi (see, e.g., Figure 3c).
The decay curves of the VIS emissions in Y3Al5O12:Bi measured at 4.2 K are shown in Figure 4b,c. At 4.2 K, the components with the decay times τSC ≈ 33 and 38 μs dominate in the decay kinetics of the 2.63 eV and 2.75 eV emissions, respectively. At T < 6 K, the decay times remain constant (Figure 5b,c) which means that the radiative transitions take place from the metastable levels. As the temperature increases, the decay times shorten exponentially due to thermally stimulated transitions between the metastable and emitting levels and reach a constant value at the temperatures (around 100 K), where the system achieves thermal equilibrium. Such temperature dependences are characteristic for the radiative transitions from the triplet RES of a luminescence center (see also [7,9,78,83,93]). At higher temperatures, the decay time decreases due to the luminescence thermal quenching. Analogous τSC(T) dependences were obtained, e.g., for the triplet emission of Ga+- and In+- doped alkali halides (see, e.g., [132]) due to a small spin-orbit interaction energy characteristic for free Ga+ and In+ ions (ξ ≈ 0.2–0.3 eV, see, e.g., [4]). Indeed, D = 0.33–0.67 meV was obtained in [132] for Ga+ centers and D = 2.15–3.04 meV, for In+ centers.
In Lu3Al5O12:Bi, as well as in some other Bi3+-doped materials (e.g., Lu2SiO5:Bi, Gd3Ga5O12:Bi), the participation of the singlet exciton state in the VIS luminescence decay kinetics is also evident. This is caused by the fact that in case of excitons, the singlet state is located close to the triplet state (Figure 10b). The probability (k3) of its radiative decay is found to be 108 – 5 × 109 s−1 and the energy distance between the singlet and triplet states E = 75–150 meV (see [9,16,21,74]).
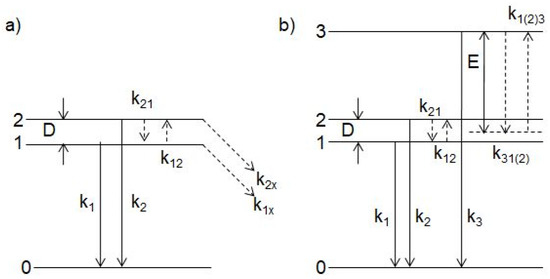
Figure 10.
Energy level diagrams used for the description of the excited states dynamics of the Bi3+-related luminescence centers responsible for the VIS emissions. In (a) two-excited-level diagram, in (b) three-excited-level diagram. For details, see the text.
3.2. Dynamics of the Bi3+-Related Exciton-Like States
Thermally stimulated transitions between the metastable and emitting minima of the triplet RES, triplet and singlet excited states, and excited and ground states of the luminescence center responsible for the VIS emission reveal themselves in the temperature dependences of the VIS emission spectra and decay kinetics. The phenomenological models are proposed to describe the excited-state dynamics of these centers. Application of the two- or three-excited-level models on the temperature evolution of the VIS luminescence decay times allows determination of characteristic parameters of the corresponding RES (the energy separations between the excited states and the rates of the radiative and non-radiative transitions from these states). The excited states dynamics of the luminescence center responsible for the VIS emission is described within the phenomenological model sketched in Figure 10.
In case of the two-excited-level model (Figure 10a), the time evolution of the populations N1, N2 of the excited levels 1 and 2, respectively, can be described by the rate equations given by Equation (1) with consideration of Equations (2) and (3). In case of the three-excited-level model (Figure 10b), the time evolution of the populations N1, N2, N3 of the excited levels 1,2 and 3, respectively, can be described by the following rate equations:
where analogous parameters have the same meaning as in (1). In addition, k3 is the radiative transition rate from the singlet level 3 and k3(2)1, k1(2)3, are non-radiative rates of transitions between the levels 3(2) and 1:
E is the energy distance between the triplet and singlet levels. Since D<<E we consider
The values of some parameters are presented in Table 2. A strong difference between the structure and parameters of RES responsible for the UV and VIS emissions clearly indicates their different origin.
As evident from Table 2, the VIS emission is characterized not only by the large Stokes shift and FWHM. In all the works [7,9,16,17,18,21,69,72,73,74,75,76,77,78,83,93,95] where the low-temperature luminescence decay kinetics was investigated, also a very small (~ 1 meV) energy distance D between the two lowest excited levels was reported.
3.3. On the Origin of the Excited States Responsible for the VIS Luminescence
In most of the studies, a possible origin of the unrelaxed excited states, responsible for the lowest-energy excitation band of the VIS emission, was mainly considered for various Bi3+-doped compounds (see, e.g., [7,64]). For example, the lowest energy excitation band of the VIS emission of YNbO4:Bi was ascribed to the Bi3+ (6s2) → Nb5+ (d0) MMCT (see also [141]). It should be noted that the consideration of absorption and emission bands of some Bi3+-doped complex oxides as an electron transfer between Bi3+ and host lattice transition metal ions was proposed about 50 years ago [1] (see also [6,141,142]).
In [65], an empirical model, proposed earlier for Pr3+- and Tb3+-doped d0 closed-shell transition metal compounds, was applied to predict the energy position of the MMCT bands in various Bi3+-doped closed-shell d0 transition metal (Mn+) complex oxides. The dependence of the energy positions of the Bi3+-related absorption bands Eabs on the ratio between the optical electronegativities Xopt (Mn+) of the d0 metal cations and the shortest Bi3+ - Mn+ interatomic distances was found to be linear. It was described by the following empirical equation:
Eabs (Bi3+, cm−1) = 46,000 – 27,000 {Xopt (Mn+)/d(Bi3+ − Mn+)}.
It was concluded that any Bi3+-related absorption band satisfying this equation is of the MMCT origin.
This model was developed further in [7] where the structural characteristics of the host lattice, anion relaxation resulting from Bi3+ doping, and electronegativities and coordination numbers of the Bi3+ and Mn+ ions in the compounds were taken into account. For the metals with the coordination number CN’ (Mn+) = 4, the Equation (1) was modified to:
where dcorr is the shortest distance between Bi3+ and Mn+ ions corrected to account for the effect of anion relaxation due to Bi doping.
MMCT(Bi3+, cm−1) = 70,000 – 52,000 {X4 (Mn+)/dcorr},
For the metals with the coordination numbers CN’ (Mn+) > 4, the following equation was proposed:
MMCT(Bi3+, cm−1) = 55,000 – 45,500 {XCN’>4 (Mn+)/dcorr}.
In [7,64,65], the energies of the 1S0 → 3P1 transitions of a Bi3+ ion and the Bi3+ → Mn+ MMCT transitions were calculated for many Bi3+-doped compounds. In a few cases, e.g., in YVO4:Bi, these energies were obtained to be very close (3.779 eV and 3.778 eV, respectively [65]). As both these energies are close to the energy of the lowest-energy excitation band of the VIS emission in YVO4:Bi (3.78 eV), in principle, both the 1S0 → 3P1 transitions of a Bi3+ ion and the Bi3+ → V5+ MMCT transitions could be considered as responsible for the lowest-energy Bi3+-related excitation band in YVO4:Bi. In [1,3], this band was ascribed to MMCT and in [76], to the 1S0 → 3P1 transitions. However, in most of the considered materials, the position of the lowest-energy excitation band of the VIS emission and the MMCT energy (see, e.g., [10,65]) are significantly different. For example, in Lu3Al5O12:Bi, the MMCT energy is 5.95 eV [10] while the lowest excitation band of the 2.6 eV emission is located at 4.6 eV. The same is true for Y3Al5O12:Bi. In YPO4:Bi, the MMCT energy (7.3 eV [10]) is also much higher as compared with the position (≈5.5 eV, Table 2) of the lowest excitation band.
In Bi3+-doped compounds, the UV emission is concluded to arise from the radiative decay of the triplet RES of a single Bi3+ ion, and the lowest-energy excitation band of this emission corresponds to the 1S0 → 3P1 transitions of Bi3+. According to [7,64], the MMCT occurs from the ground 1S0 state of a Bi3+ ion to the bottom of the conduction band (CB) formed by the energy levels of d0 or d10 host lattice ions, and the energy of the MMCT is defined as the energy difference between the 1S0 and MMCT states (Figure 11). Therefore, in general, the lowest-energy excitation bands of the UV and VIS emissions should not coincide as these bands arise due to processes of different origin. However, in all the Bi3+-doped compounds, where both the UV and VIS emissions are present, their lowest-energy excitation bands are close (see, e.g., Figure 2b,c and compare the Eexc values in Table 1 and Table 2). In some cases, they practically coincide (see, e.g., [24,120]), and the intensity redistribution is observed between the UV and VIS emissions (see, e.g., [9,17,18,93,114,115,120] and Figure 3c). For example, the lowest excitation bands of the UV and VIS emissions completely coincide in La2Zr2O7:Bi [120]. This indicates that the VIS emission of La2Zr2O7:Bi, ascribed in [120] to the impurity trapped exciton, is excited in the 1S0 → 3P1 absorption band of Bi3+ (4.32 eV) [7,64,65] even at 4.2 K, despite the fact that the lowest calculated MMCT energy in this material is 4.88 eV [65] or 5.06 eV [10]. In our opinion, the intensity redistribution between the UV and VIS emissions observed in this work can be caused by the thermally stimulated release of an electron from the 3P level of Bi3+ into CB. From the I(T) dependence in the T < 50 K temperature range, the activation energy of this process can be estimated as ≈ 2 meV. This value can correspond to the energy distance between the triplet RES of Bi3+ and CB. The reverse intensity redistribution observed in [120] at higher temperature can be caused by thermally stimulated transitions from the localized exciton state to the 3P1 state of Bi3+ over the energy barrier of about 27 meV.
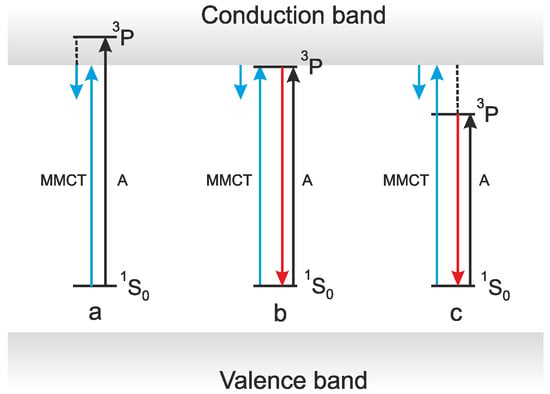
Figure 11.
Schematic presentation of energy levels in Bi3+-doped compounds proposed in [7].
According to [7], the UV emission appears when the 3P1 level of Bi3+ is located below CB. In this case, the energy of the MMCT should always be higher as compared with the energy of the 1S0 → 3P1 transition (Figure 11). However, in most of the investigated cases, the excitation band of the VIS emission is slightly shifted to lower energies as compared to that of the UV emission (see, e.g., [14,15,19,24,64,78,93,94,95,96,104,109,110,119,121,140,141] and Figure 2b,c).
Thus, the data considered above allow us to suggest that the electron transitions, corresponding to the 1S0 → 3P1 transition of a free Bi3+ ion, are most probably responsible for the lowest excitation band of both the UV and VIS emissions. The MMCT transitions as well as the 1S0 → 3P2 and 1S0 → 1P1 transitions of a Bi3+ ion, resulting in the electron release into CB, can be responsible for the higher-energy excitation bands of the VIS luminescence.
3.4. On the Origin of the Visible Luminescence
The VIS emission was identified in the literature as the emission corresponding to the 3P1,0 → 1S0 transitions of a free Bi3+ ion [3,26,28,29,30,36,39,40,42,43,124,127,130,143,144,145,146,147,148,149,150,151,152,153], D-state emission [63,114,115,120,128,154], charge transfer emission [1,10,65,114,115,154], impurity-trapped or impurity-bound exciton emission [12,69,83,120,122,123,128,154,155,156], MMCT emission [6,7,63,64,65,120,128,130,141,156,157], and emission of Bi3+ pairs and clusters [1,10,14,15,19,24,58,64,78,95,97,99,102,104,109,110,112,113,119,120,121,128,154,158,159] (see also review papers [7,10,11]). However, in most of these papers, the structure and parameters of RES, responsible for the VIS emission, were not determined, and conclusions about the origin of this emission were not confirmed by experimental data.
Detailed investigations of the VIS emission by the time-resolved spectroscopy methods in a wide temperature range (down to 0.4 K) and determination of the RES parameters carried out in [7,9,13,16,17,18,21,72,73,74,75,76,77,78,83,93,95,99,156] allow us to make a justified conclusion on the exciton-like origin of the emission in the considered Bi3+-doped compounds. The VIS emission was interpreted as the luminescence of an exciton localized around a Bi3+-related center. Indeed, since a free Bi3+ ion has the largest spin-orbit interaction energy among all the other ns2 ions (ξ = 2.102 eV [4]), a very small energy distance (D = 0.34–5.7 meV, see Table 2) between the metastable and emitting levels of the triplet RES responsible for the VIS emission can only be explained by the exciton-like origin of this emission. In addition to the small spin-orbit interaction energy, a strong exciton-phonon interaction is also characteristic for excitons which explains the large Stokes shift and FWHM of the VIS emission.
The structure and parameters of the triplet RES should be similar for all exciton-like emissions in the same host material. This was clearly demonstrated on the example of caesium iodides in [160,161] where similar RES parameters were obtained for the self-trapped excitons and for the excitons localized around various intrinsic and impurity defects. Therefore, in Table 3, available data on the exciton-like luminescence in some undoped materials are collected to compare them with corresponding characteristics of the Bi3+-related VIS emission.

Table 3.
Emission peak positions (Eem), full widths at half maxima (FWHM), the Stokes shifts (S), positions of the lowest-energy excitation band (Eexc), and decay times (τSC) (at 0.4 K for PbWO4 and 4.2 K for the other materials) of the triplet exciton-like emissions of the undoped compounds. The spin-orbit splitting energy (D) and the probabilities of the radiative decay of the metastable (k1) and emitting (k2) levels of the triplet RES.
Unfortunately, for most of these materials, the RES parameters were not determined since for that task investigations of the luminescence decay kinetics at temperatures well below 4.2 K are needed. For example, in PbWO4 the slow component decay time (τSC) reaches its maximum value only at T < 0.6 K [178,179,180].
Comparison of the data presented in Table 3 with those in Table 2 demonstrates that the exciton-like emissions in an undoped material and the lower-energy (VIS) emission of the same host material, Bi3+-doped have very close values of D, FWHM, and S. This is an additional confirmation of the exciton-like origin of the broad lower-energy emission bands in these compounds (see also [69,83]).
A drastic difference in the D values (up to two orders of magnitude), appearing in the decay kinetics of the luminescence arising from the triplet RES of an impurity ion with respect to that arising from the triplet state of an exciton localized around the impurity ion, was also clearly demonstrated for Tl+- [188,189] and Pb2+-doped [190,191,192] caesium halides. In these compounds both types of the impurity-related emission bands mentioned above were observed in a single system. The Stokes shifts and FWHM related to the two types of the emission bands were found to be considerably different as well.
Let us consider possible mechanisms of the processes resulting in the appearance of the exciton-like luminescence in Bi3+-doped compounds.
3.5. On Possible Mechanisms of Processes Responsible for the VIS Luminescence
We suggest that the VIS emission appears under excitation of a Bi3+ ion with the energy (hνexc) which allows an electron delocalization from the excited state of Bi3+ into CB and its subsequent immediate recombination with the hole remained at the Bi3+ ion (the Bi4+ hole center). For that, the lowest-energy relaxed excited state of Bi3+ (responsible for the UV emission) should be located inside CB (see Figure 7). As a result of the electron-hole recombination around the Bi3+ ion, a localized exciton (ex0Bi3+) is created. The radiative decay of the lowest-energy triplet localized exciton state results in the appearance of the Bi3+-related exciton-like emission (hνem) in case the corresponding RES is located below the bottom of CB:
Bi3+ + hνexc → e−(CB) … Bi4+ → (e− + e+) Bi3+ → ex0Bi3+ → Bi3+ + hνem
In the considered case, only the VIS emission can appear. In case the relaxed excited states of both the Bi3+ ion and the localized exciton ex0Bi3+ are located below or close to the bottom of CB, both the UV and VIS emissions can appear. The UV/VIS emission intensity ratio depends not only on the RES position with respect to the bottom of CB but also on the rate of vibronic relaxation in the Bi3+ excited state and the probability of an electron delocalization from this state. No VIS emission can appear in case the lowest-energy Bi3+-related level (corresponding to the 3P1 level of a free Bi3+ ion) is located well below the bottom of CB.
Similar mechanism of the appearance of the VIS emission was proposed in [155] (see also [120]), where this emission was ascribed to the impurity-bound exciton recombination. It was suggested that the emitting level of InBO3:Bi is situated either close to or inside CB of the host lattice, so that after excitation the luminescence center can get ionized. According to [155], this results in formation of an impurity-trapped exciton, with the hole located at the luminescence center and the electron located in its neighborhood.
It should be noted that the above-mentioned Bi3+-related center can be not only a single Bi3+ ion, but also a dimer {Bi3+ - Bi3+} or a Bi3+ ion located close to a crystal lattice defect d ({Bi3+ - d} center). In this case, the localized excitons of the type of ex0{Bi3+ - Bi3+} and ex0{Bi3+ - d} can also be created and their radiative decay can be accompanied by the VIS emission. This means that several overlapping emission bands of exciton-like origin can appear in Bi3+-doped compounds resulting in a complex structure of the VIS emission band.
The luminescence of the localized exciton of the type of ex0{Bi3+ - d} can appear with the highest probability in case the Bi3+ ion substitutes for a divalent or monovalent host lattice ion where the excess charge of Bi3+ should be compensated by some defect (d). Probably, this is the case of Sr3Ga4O9:Bi [58], where two emission bands could arise not only from single Bi3+ ions, substituting for Sr2+ ions in different lattice sites, but also from the localized excitons of the type of ex0Bi3+ and ex0{Bi3+ - d}. In the materials of this type, an electron transfer from the valence band to the Bi3+ ion, resulting in the formation of a stable Bi2+ center, is also possible. In more detail, this process was considered for PbWO4:Bi in Ref. [73] (see Appendix A). In this case, besides the radiative electron-hole recombination, resulting in the appearance of the Bi3+-related exciton-like luminescence, the electron Bi2+ centers and the self-trapped holes can also be optically created. These centers were indeed detected in EPR [73,193].
4. On the Dependence of the UV/VIS Emission Intensity Ratio on the Band-Gap and Band-Edge Energy
According to Figure 7, the UV/VIS emission intensity ratio should strongly depend on the position of the lowest-energy RES of Bi3+ with respect to the CB edge. Therefore, it could also depend on the band gap energy Eg of the host material and increase with increasing Eg [154]. The best materials to investigate these dependences could be multicomponent garnets where both the CB edge energy and Eg can be changed by variation of their composition (see, e.g., [194,195,196,197,198,199]). Let us consider some examples.
The UV/VIS emission intensity ratio was found to be much larger in Y3Al5O12:Bi (Eg ≈ 7.7 eV [10,154]) as compared to Lu3Al5O12:Bi (Eg ≈ 7.9 eV [10,154]) (compare Figure 1a and b). This could mean that the triplet RES of Bi3+ is located closer to the bottom of CB in Lu3Al5O12 as compared to Y3Al5O12, despite the larger band gap in Lu3Al5O12.
In Ref. [200], the effect of Ga3+ doping on the photoluminescence properties of Y3Al5-xGaxO12:Bi was studied. It was shown that the incorporation of the Ga3+ ions results in a strong reduction of the band gap edge (by 1 eV from Y3Al5O12:Bi to Y3Ga5O12:Bi). In [195,196,197,198,199], it was shown that the increasing Ga content also results in a drastic decrease of the CB bottom energy in different Ce3+-doped multicomponent garnets. The presence of two—UV and VIS—emission bands in Y3Al5O12:Bi with the close lowest-energy excitation bands indicates that the triplet RES of Bi3+ should be located close to the bottom of CB of Y3Al5O12 [7]. In this case, the reduction of Eg and the CB bottom energy with the increasing Ga content should result in the disappearance of the UV emission in Y3Ga5O12:Bi. Indeed, no UV emission was observed in [12]. However, according to [13,14,16,17], both in Y3Al5O12:Bi (Eg ≈ 7.7 eV [10,154]) and in Y3Ga5O12:Bi (Eg ≈ 6.6 eV [10]), the UV emission is much stronger than the VIS emission (compare Figure 12a and b). In [110], the UV/VIS emission intensity ratio is about 2. The reason of such strong difference in the experimental data [12], [13,14], and [110] is not clear.
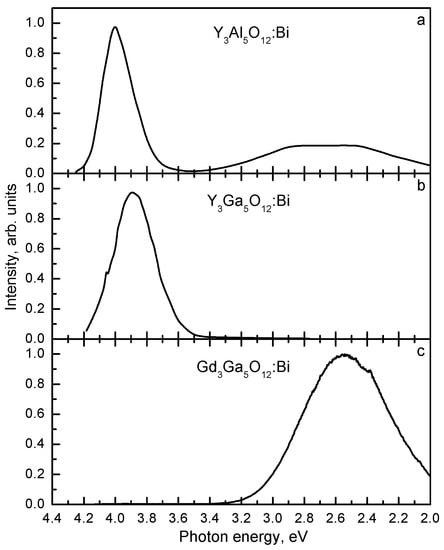
Figure 12.
Emission spectra of (a) Y3Al5O12:Bi [17], (b) Y3Ga5O12:Bi [13], and (c) Gd3Ga5O12:Bi [74]. T = 80 K. Based on the data reported in [13,17,74], presented with the publisher permission.
In Ce3+-doped multicomponent garnets [196], the influence of Gd on the Eg value and the position of the lowest-energy 5d1 excitation band of Ce3+ with respect to CB was found to be much weaker [194] and dependent on the Ga and Gd content [196,199]. In general, the 5d1 - CB energy distance slightly increases with the increasing Gd content. In case the same dependence is valid for the Bi3+-doped gallates, the luminescence spectra of Y3Ga5O12:Bi and Gd3Ga5O12:Bi should be similar (like in [12]). However, according to [13,14,74,75,110], in Bi3+-doped (Gd,Y)3Ga5O12 multicomponent garnets, the UV/VIS emission intensity ratio decreases drastically with the increasing Gd content (compare Figure 12b and c). The absence of the UV emission in Gd3Ga5O12:Bi [12,74,75] and the strongly dominating UV emission in Y3Ga5O12:Bi [13,14] could indicate that the RES of Bi3+ is located inside CB in Gd3Ga5O12:Bi and well below the bottom of CB, in Y3Ga5O12:Bi. However, the Eg values (≈ 6.6 eV and ≈ 6.4 eV [10]) in Y3Ga5O12:Bi and Gd3Ga5O12:Bi are close.
The data reported above indicate that the UV/VIS emission intensity ratio is not determined only by the band gap energy. Most probably, it depends much more on the probability ratio of the vibronic relaxation in the triplet excited state of Bi3+ center and the Bi3+ ionization followed by the formation of the exciton-like state. In some Gd-based compounds, the UV emission of Bi3+ centers can be absent due to an effective Bi3+ - Gd3+ energy transfer. In addition, in case the VIS emission arises from ex0{Bi3+ - Bi3+} or ex0{Bi3+ - d}, its intensity should also depend on the Bi3+ content and on the concentration of defects (d) in the investigated sample.
5. On the Luminescence Ascribed in Literature to {Bi3+ - Bi3+} Dimers and Bi3+ Clusters
In many papers (see, e.g., [1,10,14,15,19,24,58,64,78,95,97,99,102,104,109,110,112,113,119,120,121,128,154,158,159,201,202]), the lower-energy emission bands of Bi3+-doped compounds were ascribed to the {Bi3+ - Bi3+} pairs or the clusters of Bi3+ ions. The characteristics of this luminescence are presented in Table 4.

Table 4.
Emission peak positions (Eem), full widths at half maxima (FWHM), the Stokes shifts (S), positions of the lowest-energy excitation band (Eexc), and decay times (τSC) of the slow decay component at LHeT obtained for the luminescence ascribed in literature to dimer {Bi3+ - Bi3+} centers. The spin-orbit splitting energy (D) and the probabilities of the radiative decay of the metastable (k1) and emitting (k2) levels of the triplet RES.
The most comprehensive investigation of dimer impurity centers was performed in ns2-ion-doped alkali halides (see, e.g., [203,204,205,206,207] and references therein). Most of the studies were devoted to the dimer Tl+ centers. As Tl+ and Bi3+ ions have the same electronic configuration, the characteristics of the {Bi3+ - Bi3+} dimers in different compounds are expected to be similar to those of the Tl+ dimers in alkali halides. Let us consider the latter centers in more detail.
5.1. Ultraviolet Luminescence
The dimer impurity centers in alkali halides were found to appear only in highly doped crystals, and their concentration was usually much smaller as compared with the concentration of single impurity centers. The absorption coefficient in the single center absorption band shows a linear dependence on the impurity content. In the dimer-related absorption bands, the absorption coefficient quadratically increases with the increasing impurity concentration. However, for the luminescence intensity, the same concentration dependence can be obtained only under excitation in the absorption spectrum region, where the optical density (OD) does not exceed 0.2, since only at OD < 0.2, a linear dependence holds between the number of the absorbed and radiated quanta (see, e.g., [204] and references therein). Therefore, at OD < 0.2, the linear concentration dependence should be observed for the luminescence intensity of single impurity centers and quadratic, for the impurity dimers. At higher optical density (e.g., OD ≈ 0.5 - 1.0), the luminescence intensity dependence on the impurity concentration becomes sublinear for single impurity centers and superlinear for dimers. When OD > 2, the luminescence intensity becomes practically independent of the impurity concentration or even decreases due to the reabsorption, concentration quenching, or energy transfer processes.
The Stokes shift an FWHM of the dimer-related emission bands in alkali halides were found to be close or even smaller as compared with emission bands of the corresponding single centers [204]. The low-temperature luminescence decay kinetics was also found to be similar in the single and dimer Tl+ centers [206].
Two types of dimer Tl+ centers were detected in alkali halides, the centers of the D2h symmetry and the centers of the D4h symmetry (see, e.g., [203,204,205,206,207]). In some systems, the centers of both types coexist (see, e.g., [205]). In the case of the D2h-type centers, two close Tl+ ions can strongly perturb each other and can be considered as a quasimolecule (Tl+)2 consisting of two Tl+ ions. The electron states of the quasimolecule can be considered as molecular orbitals constructed from the electron states of the two Tl+ ions. In the case of the D4h-type centers, two Tl+ ions are separated by an anion (see, e.g., [206]). Such a {Tl+ - anion - Tl+} dimer center can be considered as a single Tl+ ion perturbed by the field of the second Tl+ ion. The absorption and emission bands of the dimer centers of both types arise from electronic transitions between the energy levels of these centers (for more details, see [203,204,207] and references therein).
According to [7,65,77,158], the Bi3+ - Bi3+ distances in the investigated materials are about 3−4 Å. Therefore, the same considerations could also be applied in case of dimer Bi3+ centers in more complicated materials. The analysis of literature data allows to suggest that the UV luminescence of such {Bi3+ - Bi3+} dimer centers was also observed in complex oxides, namely, in Y3Al5O12:Bi [201] and Lu2SiO5:Bi [9,21].
In [201], the spectra of Y3Al5O12:Bi with two different Bi3+ concentrations (0.13 and 0.27 at.%) were compared at room temperature (RT). Under excitation in the region of the lowest-energy (4.54 eV) absorption band of Bi3+, the presence of two types of Bi3+-related centers was revealed from the dependence of the UV emission band position on the excitation energy. The higher energy (4.045 eV) emission band was ascribed to single Bi3+ centers. The 3.995 eV emission band, slightly shifted to lower energies with respect to the former, was ascribed to Bi3+ pairs since it can be better distinguished with the increasing Bi3+ concentration. The decay kinetics is not too different for these UV emissions. It is slower for the 3.995 eV emission as compared to the 4.045 eV emission. The energy transfer between the single and dimer centers was suggested due to the overlap of the emission band of the single Bi3+ centers with the absorption band of dimers. However, no dependence of the UV emission band position on the Bi3+ content (varying from 0.07 to 0.18 at.%) was noticed in Lu3Al5O12:Bi [19].
In [21], two UV emission bands located at 3.45 eV and 3.30 eV and having similar characteristics (Table 5) were observed in Lu2SiO5:Bi with a large Bi content (2.24 at.%). The steady-state emission spectrum of Lu2SiO5:Bi is presented in Figure 13a, however, the presence of two emission bands becomes evident in the time-resolved emission spectra shown in Figure 13b. Comparison of the shapes of their excitation spectra (Figure 14) indicates that the center with the 3.30 eV emission competes with the dominating 3.45 eV emission center in the excitation light absorption process. The excitation spectrum of the 3.30 eV emission is shifted to lower energies with respect to that of the 3.45 eV emission and distorted due to a strong absorption arising from the main Bi3+ centers. Such behavior is characteristic for the dimer centers of the D4h symmetry in alkali halides (see, e.g., [204,205,206]). The decay kinetics of both these emissions is alike (Figure 15). From the temperature dependence of the decay times, the parameters of the corresponding RES were calculated and found to be similar (see Table 5).

Table 5.
Characteristics of two UV emission bands of Lu2SiO5:2.24 at.% Bi at 4.2 K.
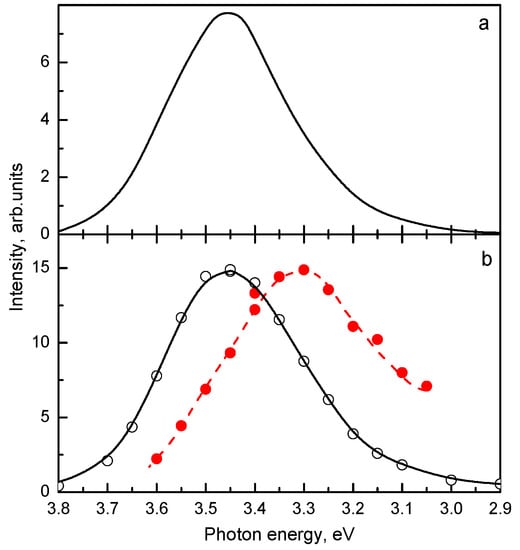
Figure 13.
(a) The steady-state UV emission spectrum of Lu2SiO5:Bi measured in [21] under Eexc = 4.15 eV and (b) time-resolved (t = 300 μs) emission spectra measured under Eexc = 4.15 eV (empty circles) and Eexc = 3.8 eV (solid circles). T = 4.2 K. Based on the data reported in [21], presented with the publisher permission.
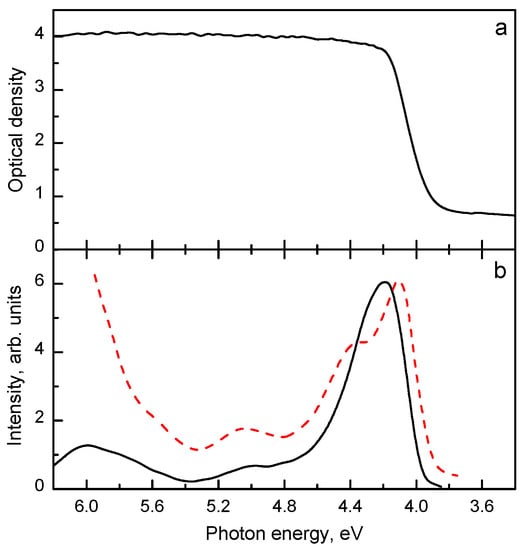
Figure 14.
(a) Absorption spectrum of Lu2SiO5:Bi at 295 K. (b) Normalized excitation spectra measured at 4.2 K for Eem = 3.5 eV (solid line) and Eem = 3.15 eV (dashed line). Based on the data reported in [21], presented with the publisher permission.
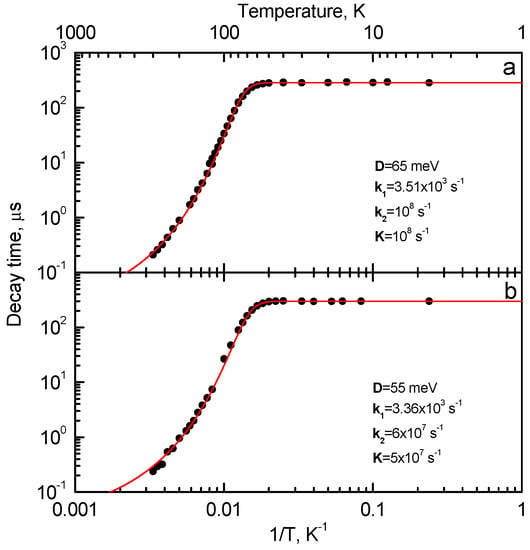
Figure 15.
Temperature dependences of the decay times of the (a) 3.5 eV and (b) 3.3 eV emissions of Lu2SiO5:Bi measured under Eexc = 4.15 eV and Eexc = 3.9 eV, respectively. Solid lines are the best fits to experimental data of the two excited-state level models shown in Figure 6 (for details, see the text). The parameters of the fit are reported in the figures. Based on the data reported in [21], presented with the publisher’s permission.
The obtained data indicate that both the strong 3.45 eV emission and weak 3.30 eV emission arise from the triplet RES of Bi3+-related centers. It is not excluded that the 3.30 eV emission of Lu2SiO5:Bi arises from dimer {Bi3+ - Bi3+} centers. It should be noted that in [21], the 3.30 eV emission was ascribed to the Bi3+ ions located in the Lu2 lattice sites (Bi2 centers). If so, the Bi2 centers should exist in Y2SiO5:Bi as well. However, no related emission was observed in [20] in the Y2SiO5:Bi sample with much smaller Bi3+ content (0.04 at.% Bi). This fact could confirm our suggestion on the dimer origin of the 3.30 eV emission in Lu2SiO5:Bi. Unfortunately, the concentration dependences of the luminescence intensity were not studied in [20,21] due to the absence of samples with various Bi3+ content.
The search for the UV emission of {Bi3+ - Bi3+} dimers should be carried out by the comparison of the emission spectra and decay kinetics under excitation in different regions of the lowest-energy (A) absorption band, as well as by the detailed study of the concentration dependence of the UV emission intensity.
5.2. Visible Luminescence
As mentioned above, in many works devoted to the investigation of the luminescence characteristics of the Bi3+-doped compounds, the lower-energy broad emission bands, usually located in the visible spectral range and having the characteristics (see Table 4) which are strongly different from the characteristics of the UV emission of the single Bi3+ centers (Table 1), were ascribed to the {Bi3+ - Bi3+} dimers or Bi3+ clusters (see, e.g., [158] and references therein). This conclusion was often based only on the spectra measured at room temperature, without precise measurements of the Bi3+ concentration dependence of the luminescence intensity. A strong difference in the luminescence characteristics (in particular, the values of S, FWHM, and D) of the single Bi3+ centers and the centers ascribed to {Bi3+ - Bi3+} dimers was not explained. For example, in [58,97,99,104,110,112,121], the lower-energy emission of Y3Ga5O12:Bi [110], (Y,Gd)2O2SO4:Bi and Li6(Y,Gd)(BO3)3:Bi [121], LaB3O6:Bi [104], La2O3:Bi [112], Cs2NaYBr6:Bi and Cs2NaLaCl6:Bi [97], YOCl:Bi and GdOCl:Bi [99], Sr3Ga4O9:Bi [58] was attributed to Bi3+ pairs or clusters based solely on their large FWHM and S.
In [14,15,19,24,58,78,95,102,109,113,119,202], the lower-energy emission band of Y3Ga5O12:Bi and Gd3Ga5O12:Bi [14], Y2Sn2O7:Bi [119], LaBO3:Bi [78,95], Y3Al5O12:Bi and LuAl5O12:Bi [15,19], YPO4:Bi [24,113], LuPO4:Bi [24], GdAlO3:Bi [109], LiLaP4O12:Bi [102], and Lu2SiO5:Bi [202] was attributed to Bi3+ pairs or clusters due to an increase of the VIS/UV emission intensity ratio with the increasing Bi3+ content. However, this effect cannot be used for the confirmation of the dimer-related origin of the VIS emission.
Indeed, the number of single Bi3+ centers responsible for the UV emission has to increase linearly with the increasing Bi3+ concentration. However, the emission spectra are usually measured under excitation in the absorption band maximum where OD is too large. In this case, the emission intensity can be practically independent of the impurity concentration due to the saturation effect (see, e.g., [14,19,119]). In many works even the decrease of the UV emission intensity of single Bi3+ centers with the increasing Bi3+ content was observed (see, e.g., [15,24,113]). This effect can be caused by various processes, such as increasing reabsorption or concentration quenching of the UV emission (in case of the small Stokes shift where the emission and absorption bands of single Bi3+ centers are overlapped), energy transfer to some other centers (e.g., to Gd3+ ions in Gd-containing materials [15,75,76,78,104,111,208] or to other Bi3+- related centers, see, e.g., [97,104,201]). For example, the absence of the Bi3+ emission in GdP3O9:Bi [111] and GdB3O6:Bi [104,208] was explained just by an effective Bi3+ → Gd3+ energy transfer as the Bi3+ emission band overlaps the Gd3+ 8S → 6P absorption lines. In LaB3O6:Bi, the Bi3+ emission is very weak due to the energy transfer between the centers responsible for the UV and VIS emissions [104]. The concentration quenching of the UV emission was reported, e.g., in [111] for ScP3O9:Bi and in [78] for LnBO3:Bi (Ln=Sc, Lu). It takes place in case the Bi3+ concentration exceeds the critical value needed for energy migration among the Bi3+ ions, due to that, the excitation energy can be transferred to quenching centers.
Unlike for the UV emission, for the VIS emission, the probability of its reabsorption, concentration quenching, and energy transfer is negligible due to its large Stokes shift and the absence of absorption bands in visible spectral region. Therefore, the VIS emission intensity always increases with the increasing Bi3+ content, which could explain the increasing VIS/UV ratio. However, the sublinear dependence of the emission intensity on the Bi3+ concentration is usually reported (see, e.g., [15,24,58,113,119]). For example, as the Bi3+ content in YPO4:Bi increases 40 times, the lower-energy (3.81 eV) emission intensity increases only about 4 times [113]. In our opinion, such concentration dependence does not allow to conclude that the 3.81 eV emission arises from the {Bi3+ - Bi3+} pairs.
The lower-energy broad emission bands of Bi3+-doped compounds were considered as arising from Bi3+ pairs also in [10,103,113,154,158,159]. A new mechanism for the {Bi3+ - Bi3+} emission was proposed in [103] and further investigated in [10,154,159]. It was suggested that in a pair of neighboring Bi3+ ions, an electron transfer from the excited state of one Bi3+ ion to a neighboring Bi3+ ion is possible. The electronic transitions within Bi3+ pairs were associated to an intervalence charge transfer (IVCT) of the type Bi3+(6s2),Bi3+(6s2) → Bi4+(6s1)Bi2+(6s2p1). The IVCT is only possible when the ground state of Bi2+ is located below the first excited state of Bi3+. In [103] was shown that this is the case of Li2BaP2O7:Bi. In [159], the vacuum referred binding energies of the electron in the ground state of Bi2+ and in the lowest-energy excited state of Bi3+ were compared for 15 compounds. It was found that the ground level of Bi2+ is always located below the 3P1 level of Bi3+. This means that the excitation of one Bi3+ ion in the {Bi3+ - Bi3+} pair can always result in the electron transfer toward another Bi3+ ion of the pair and formation of the {Bi4+ - Bi2+} pair. The electron back transfer in the {Bi4+ - Bi2+} pair was suggested to result in the appearance of the initial {Bi3+ - Bi3+} pair in the ground state. It was concluded that this process can be radiative resulting in the broad emission band in the visible region, which is the case, e.g., of LaBO3 [78], La2O3 [112], and YOCl [99]. In Li2BaP2O7:Bi, this process was assumed to be nonradiative and, as a result, the emission ascribed to dimers was absent [103]. Since also no emission of single Bi3+ centers was observed in this compound even at the lowest temperatures, it was concluded in [103,159] that IVCT between two neighboring Bi3+ ions can be responsible for the quenching of the triplet luminescence of single Bi3+ centers as well.
However, the absence of the UV luminescence of single Bi3+ centers in Li2BaP2O7:Bi (as well as in many other Bi3+-doped compounds, see, e.g., [12,58,72,73,74,75,76,77,99,102,104,109]) is most probably caused by the location of the lowest-energy relaxed excited level of Bi3+ inside CB. The same can also be true for the VIS emission ascribed in literature to Bi3+ dimers as well as for any other emission. In our opinion, the luminescence quenching in Bi3+-doped compounds considered in [103,159] can be caused by the location of the corresponding relaxed excited states inside the conduction band.
It should also be noted that in the Bi3+ - Bi3+ IVCT model, the perturbation of energy levels of one Bi3+ ion by another closely located Bi3+ ion as well as the possibility of the molecular bond formation between the two close Bi3+ ions were not taken into account. Under the lowest-energy excitation, the 1S0 → 3P1 transitions of the single Bi3+ ion were considered despite the presence of a closely located second Bi3+ ion. However, as the perturbation of a Bi3+ ion by another Bi3+ ion in the {Bi3+ - Bi3+} pair was considered to be negligible, it is not clear why the electron recombination with almost unperturbed Bi4+ ion results in the broad VIS emission with a large Stokes shift, but not in the slightly perturbed UV emission of a single Bi3+ center. As the considered IVCT process takes place in the dimer {Bi3+ - Bi3+} center, it is also not clear how it can explain the quenching of the UV emission of another, single Bi3+ center. In some works, the energy distances D between the emitting and metastable levels of the triplet RES were determined for the centers responsible for both the UV and VIS emissions of the same compound. From comparison of the data in Table 1 and Table 4 it is evident that the values of D differ by up to two orders of magnitude. However, it was not explained how the formation of the {Bi3+ - Bi3+} pair can result in such strong reduction of the spin-orbit splitting energy of the triplet RES of Bi3+ (e.g., from 55 meV to 5.7 meV in LaBO3:Bi, see [78]).
In [158], an empirical equation was proposed to estimate of the Bi3+ – Bi3+ IVCT energy, similar to that proposed in [7] for the calculation of the MMCT energy. However, for most of the Bi3+-doped compounds considered in [158], the experimental position of the lowest-energy excitation band of the VIS emission ascribed in the literature to {Bi3+ - Bi3+} centers (see Eexc values in Table 4) markedly differs from the calculated IVCT energy. Only in LaZr2O7:Bi (where, however, the VIS emission was ascribed to the impurity trapped exciton [120]), these energies were found to be close (4.27 eV [120,159] and 4.22 eV [158], respectively).
Thus, in our opinion, the presence of the luminescent {Bi3+ - Bi3+} pairs or clusters in Bi3+-doped compounds is not confirmed by experimental data. The data of [21,201] allow only to suggest that the lower-energy UV emission bands reported in these papers could arise from the {Bi3+ - Bi3+} dimers. The broad visible Bi3+-related emission bands with the large Stokes shifts presented in Table 2 and Table 4 are all of an exciton-like origin. These bands can arise from the excitons localized around different Bi3+-related centers, including also the {Bi3+ - Bi3+} dimers, i.e., from ex0Bi3+, ex0{Bi3+ - Bi3+}, ex0{Bi3+ - d).
In our opinion, in case the IVCT between two close Bi3+ ions can really take place, the electron-hole recombination in the optically created {Bi4+ - Bi2+} pair could result in the formation of an exciton localized around the {Bi3+ - Bi3+} pair. The radiative decay of ex0{Bi3+ - Bi3+} should result in the appearance of the broad emission band with the large Stokes shift characteristic for an exciton-like emission.
The luminescence origin of Bi3+-doped compounds can be confirmed only by the study of the luminescence decay kinetics in a wide temperature range which allows to determine the parameters of the corresponding RES. Only the dependence of the number of luminescence centers on the concentration of Bi3+ in the crystal can indicate, whether the single Bi3+ ions or the {Bi3+ - Bi3+} pairs are responsible for the investigated emission. As an example, in Figure 16 and Figure 17, the dependences of the luminescence intensity on the Bi3+ concentration inside the investigated samples are presented for the exciton-like VIS emission of Gd3Ga5O12:Bi [74,75] and the Bi3+-doped vanadates [76] and niobates [77]. These dependences were measured under excitation in the absorption band region where the optical density is surely small (OD < 0.5) (see Figure 16a). The superlinear dependence of the emission intensity on the Bi3+ content was found only for the VIS emission of the Bi3+-doped niobates [77] (Figure 17b). This allowed us to ascribe this band to the exciton localized around a dimer {Bi3+ - Bi3+} center: ex0{Bi3+ - Bi3+}. In other cases, the dependence was linear or sublinear (Figure 16b and Figure 17a). This indicates that the lower-energy exciton-like emission is connected with a single Bi3+ ion associated with a lattice defect (ex0 {Bi3+ - d}).
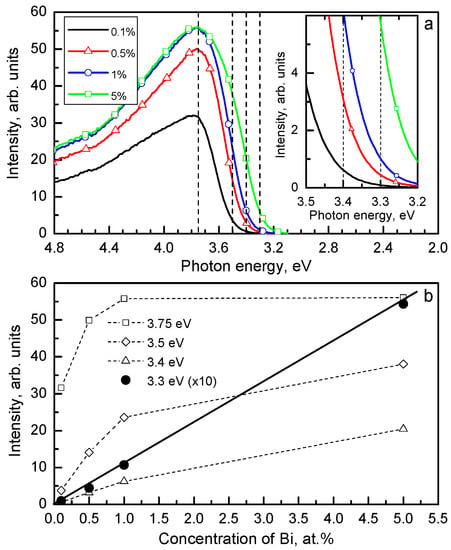
Figure 16.
(a) The excitation band of the Bi3+-related 2.19 eV emission measured at the same conditions at 295 K for the YVO4:Bi powders with different Bi3+ contents (shown in the legend). (b) The dependences of the 2.19 eV emission intensity, taken from Figure 16a for some selected excitation energies, on the Bi3+ content. See also [76]. Presented with the publisher’s permission.
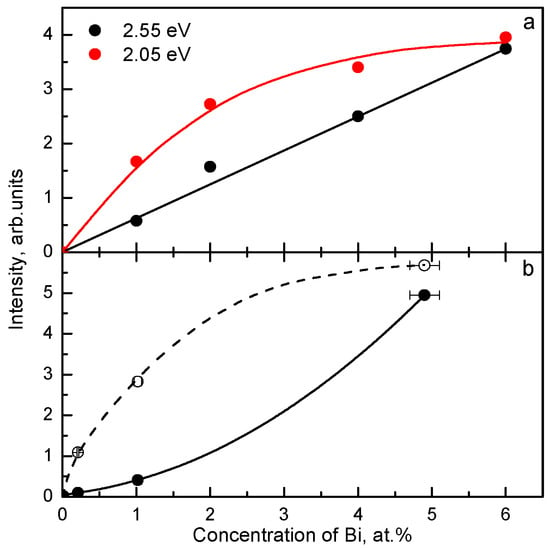
Figure 17.
Dependences of the maximum luminescence intensity on the Bi content inside the powder measured at 80 K (a) for the 2.55 eV and 2.05 eV emission bands of Gd3Ga5O12:Bi under excitation Eexc = 4.08 eV and Eexc = 3.4 eV, respectively and (b) for the 2.41 eV emission of YNbO4:Bi under excitation Eexc = 3.6 eV (solid line) and for the 2.53 eV emission of YNbO4:Bi under excitation in the absorption band maximum (Eexc = 4.09 eV, dashed line). Based on the data reported in [75,76,77]. Presented with the publisher’s permission.
It should be noted that the sublinear dependence of luminescence intensity on the impurity concentration, reaching the saturation (for example, similar to that presented in Figure 16b, empty squares), usually obtained under excitation in the absorption band maximum, is often interpreted as the luminescence concentration quenching. For example, the Bi3+-related emission intensity was found to increase with the increasing Bi content only up to ≈0.5 at.% in GdNbO4:Bi [125,157] and up to 0.5 at.% [153], 1 at.% [101], or 1.5–2 at.% [125,150] in YNbO4:Bi. At higher Bi content, it reaches the maximum and then decreases. However, such dependence could appear due to too large optical density in the chosen excitation region. As evident from Figure 16b and Figure 17a,b, no concentration quenching is observed in the considered Bi3+-doped compounds at least up to 6 at.% of Bi3+.
In many cases, the lowest excitation band of the VIS emission is shifted to lower energies with respect to the lowest excitation band of the UV emission of a Bi3+ center (see, e.g., [14,15,19,24,64,78,93,94,95,96,104,109,110,112,119,121,140,141] and compare Eexc values in Table 1, Table 2, and Table 4). It is not excluded that in this case the lower-energy part of the excitation band arises from {Bi3+ - Bi3+} dimers. Under excitation in this {Bi3+ - Bi3+}-related band, the VIS emission of ex0{Bi3+-Bi3+} can appear which is overlapped with the ex0Bi3+ emission. Indeed, in many cases, the VIS emission band is found to be complex (see, e.g., [9,16,17,18,21,74,75,77,95,102,128]). This means that it can consist of emission bands of the excitons localized around different Bi3+-related centers.
The mechanism of the appearance of the visible {Bi3+ - Bi3+}-related exciton-like emission could be the following. Like in the case of a single Bi3+ center (see equation (11)), the lowest-energy RES of a {Bi3+ - Bi3+} dimer can be located inside CB. Under excitation in the lowest-energy absorption band of the {Bi3+ - Bi3+} center, the electron from the {Bi3+ - Bi3+} center can be optically released into CB and then recombine with the hole remained at the dimer center. The electron-hole recombination results in the formation of ex0{Bi3+ - Bi3+} whose radiative decay results in the appearance of the {Bi3+ - Bi3+}-related exciton-like emission:
{Bi3+ - Bi3+} + hνexc → e−CB … {Bi4+ - Bi3+} → ex0{Bi3+ - Bi3+} → {Bi3+ - Bi3+} + hνem
6. The Bi2+, Bi+ and Bi0 Emission Centers
The richness of the Bi3+-based luminescence in various hosts is further enlarged by bismuth emitting centers with the valence lower than 3+, the research of which started systematically in the 1990s. In 1994, the red emission of Bi-doped SrB4O7 was explained by Blasse et al. [105] as due to the divalent Bi2+ based on the similarity of luminescence characteristics with isoelectronic Pb+ [209] and Tl0 [210,211] centers. In the same year, Bi2+ luminescence was briefly reported in alkali earth sulfates [212], followed by reports dealing with Bi2+ in crystalline hosts of Me2+BPO5 (Me = Ca, Sr, Ba) [213], BaB8O13 [214], BaSO4 [215], Sr2P2O7 [216], Ba2P2O7 [106], MeF2 (Me = Ca, Sr) [217], and CaAl12O19 [218].
Electron configuration (6s26p) of Bi2+ is split by spin-orbit coupling and crystal field into the 2P1/2 ground state and 2P3/2(1) and 2P3/2(2) excited states [105]. It gives rise to the emission transition 2P3/2(1) → 2P1/2 and two excitation maxima arising from 2P1/2 → 2P3/2(1), 2P3/2(2) ones, see Figure 18. The third excitation maximum in Figure 18 at shorter wavelength below 300 nm was ascribed to 2P1/2 → 2S1/2 (6s27s) allowed transition [105,213]. However, later on, based on theoretical calculations determining the intensity of transitions resulting from the admixture of parity-allowed 6s → 6p and 6p → 6d transitions [219], the 300 nm excitation maximum was attributed rather to a mixed state of 6s6p2, 6s26d, and 6s26p configurations.
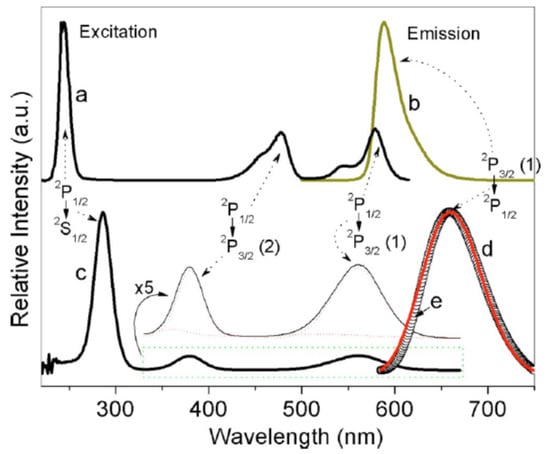
Figure 18.
Excitation and emission spectra of SrB4O7:0.5%Bi (a) em = 630 nm; (b) ex = 245 nm and SrB6O10:0.5%Bi (c) em = 690 nm; (d) ex = 380 nm. Reprinted from [220] with permission.
Vibrational structure at the high energy side of Bi2+ emission in SrB4O7 at 4.2 K observed already in [105] was subjected to theoretical calculations and ascribed to two totally symmetric off-center vibrations of Bi2+ [221]. The emission transition is parity forbidden (p-p), but this selection rule is relaxed by admixture of emitting level with higher lying 2S1/2 term and observed decay times are about 10 µs in SrB4O7 host [105]. In [103] the energy levels of Bi2+ were situated within the band gap of Li2BaP2O7 and it turns out that the emitting 2P3/2(1) state is about 1 eV below the bottom of CB providing sufficiently high energy barrier against the thermal ionization quenching. Furthermore, high thermal stability of Bi2+ emission in SrB4O7 (reported in 20–300 K) [222] and Sr2P2O7 (reported up to 500 K) [223] also points to very limited thermal quenching to the ground state within the temperature range studied.
Given the dominant p-character of the ground and lowest excited levels which are included in the radiative deexcitation of Bi2+, such a center becomes sensitive to the surrounding crystal field defined by the chemical composition and structure of the host. Currently available studies show the spread in the Bi2+ emission peak positions from about 550 nm in CaF2 [218] to about 716 nm in Ba2P2O7 [106]. In case of stable solid solutions, expected in (Ca,Sr)F2 [218], (Ca,Sr)BPO5 [213], or (Ca,Sr)SO4 [224] the peak position and FWHM could even be finely tuned. In case when Bi2+ shows the emission peak within 580–630 nm and its thermal stability is sufficient, application of such a phosphor for white LED sources was considered, e.g., in [220,222]. Occurrence of a broad excitation 2P1/2 → 2P3/2(2) band within 380–480 nm enables to use the blue or near UV LEDs as excitation sources. Nevertheless, due to partially forbidden character of this transition, its oscillator strength might be too low for practical application. It has been partially improved e.g., by admixing Ca into SrB4O7 host [222].
The discovery of a new broad ((FWHM 150 nm) emission peak at 1150 nm with the decay time of 650 µs in the Bi-doped SiO2 glass in 1999 [225] provided a new hot topic widely explored by many laboratories. Since amorphous hosts are out of the scope of this review, the reader is directed to a nice review on this subject published by Sun et al. in 2014 [8]. The charge state of bismuth and composition of related emission center in amorphous matrices were a subject of debate for a decade, briefly reviewed in [106]. In that work, the Bi0 center in Ba2P2O7 crystalline powder host was ascribed to a broad emission band in the near infrared region peaking at 1100 nm, having the FWHM of 140 nm, and decay time above 600 µs, see Figure 19.
Figure 19.
Near infrared emission spectra of Ba2P2O7:Bi excited at 586 nm, 723 nm (dotted lines: Gaussian peak fits), 838 nm, and 924 nm, respectively, and dependence of NIR emission intensity on nominal bismuth concentration (inset). Reprinted from [106] with permission.
This emission center can be formed and removed reversibly by annealing the sample at 1100 °C in the CO atmosphere or air, respectively. Its counter-appearance with the Bi2+ center in such annealing cycles proves bismuth with a valence lower than 2+ to be a responsible center. Based on analyses of the literature data and available lattice sites it has been attributed to radiative transition between to 2D3/2 → 4S3/2 levels of Bi0. In continuation of the search for such near infrared luminescence bismuth-based centers the sintered ceramic of Ba2B5O9Cl:Bi was prepared and treated under air or 95%N2/5%H2 atmospheres at 850 °C [107]. It follows from the set of excitation and emission spectra collected that there are at least two emission centers in the bismuth doped compound, which correspond to the emissions at 1030 nm and 1061 nm. Two different emission centers are due to the substitution of bismuth for Ba in two different sites, Ba(2) and Ba(1) in the Ba2B5O9Cl structure. The presence of two centers is also reflected in the decay curves of the emissions at 1030 nm and 1061 nm with the decay times of 30.2 µs and 35.9 µs, respectively, as illustrated in Figure 20.
Figure 20.
Decay and fit (with simple exponential decay equation) curves of Ba2(1-x)B5O9Cl: 2x%Bi (x = 0.7): (1) and (2) for the case of the emission at 1061 nm upon 478 nm excitation, and (3) and (4) for the emission at 1030 nm upon the excitation of 298 nm. Inset shows the dependence of lifetime on the bismuth content x%. Reprinted from [107] with permission.
When treating Ba2B5O9Cl:Bi alternatively in air and N2/H2 atmosphere, the above described near infrared (1030+1061 nm) and red 650 nm emission centers, the latter being Bi2+, can be removed and restored reversibly. With prolongation of treatment in N2/H2 the near infrared emission increases monotonically at the expense of that of 650 nm. With the same reasoning it proves bismuth with the valence lower than 2+ to be a center responsible for the infrared emission. By analysis of its excitation spectra peak positions it turns out that they cannot be ascribed to Bi+ [226]. Therefore, the most probable assignment becomes again Bi0 despite the decay time more than one order of magnitude shorter in the near infrared emission of Ba2B5O9Cl:Bi compared to the above described Ba2P2O7:Bi. Shorter decay time is explained by the enhanced electron-phonon interaction which promotes admixing of 4S3/2 with higher 4P, 2P, or 2D energy states. Consequently, it decreases the forbidden character of radiative transition 2D3/2 → 4S3/2 of Bi0 center.
Interestingly, the emission picture in Ba2B5O9Cl:Bi becomes even richer, if the changes in emission pattern are monitored more systematically during the annealing cycle [227]. As the annealing time increases from 0.5 h to 2.5 h, the Bi0 luminescence at 1055 nm gradually becomes more intense at the expense of the Bi2+ 660 nm emission and it reaches the maximum at the dwell time of 2.5 h. This reflects the change of bismuth valence state from +2 to 0 in the in situ reduction. At intermediate annealing times, besides already established excitation and emission peaks of Bi2+ and Bi0 centers, there are additional broad luminescence signals within 600 to 850 nm upon 330 nm excitation with maxima at 660 nm and 790 nm, Figure 21. The decay time of these emissions is much longer, of about 1.15 ms, i.e., strikingly different from those related to Bi2+ and Bi0 centers. Similar slow decay was also found for a new band at about 970 nm arising in the same intermediate phase of annealing cycle. All in all, the valence conversion most probably happens starting from Bi2+ via Bi+ to Bi0 in Ba2B5O9Cl:Bi by the in situ reduction process. The intermediate species shows extraordinarily broad luminescence from 600 to 1200 nm with the lifetime longer than 1ms, due to the cascade transitions from 3P2 and 3P1 to 3P0, and their emission characteristics are rather different from those of the Bi0 and Bi2+ centers in the Ba2B5O9Cl host.
Figure 21.
(a) Emission spectra (λex = 330 nm) of Ba1.99B5O9Cl: 1%Bi treated in N2/H2 for different time; (b) emission spectra (λex = 330 nm) of Ba2(1-x%)B5O9Cl: 2x%Bi (x = 0.1, 0.5, 1.0, 1.5, 5.0) treated in N2/H2 for 1h detected by visible and NIR photomultipliers; the spectra were rescaled and combined; (c) excitation spectra (λem = 790 nm, λem = 970 nm) of Ba1.99B5O9Cl:1% Bi treated in N2/H2 for 1h and 0h; (d) decay curves of Ba1.99B5O9Cl:1% Bi treated in H2/N2 for 1h. Reprinted from [227] with permission.
7. Conclusions
In Bi3+-doped compounds, the Bi3+-related luminescence of at least three types can be observed:
1. The UV emission band of single Bi3+ centers with the relatively small FWHM and S and the ms-decay time at 4.2 K. As the temperature increases, the decay time remains constant up to 40−100 K owing to a large (~102 meV) energy distance D between the emitting and metastable levels of the lowest-energy triplet RES of Bi3+ corresponding to the excited 3P1 and 3P0 levels of a free Bi3+ ion. Similar characteristics should also be observed for a Bi3+ ion perturbed by a crystal lattice defect (a {Bi3+ - d} center). The concentration dependence of the UV emission intensity should be linear.
2. The UV emission of dimer {Bi3+ - Bi3+} centers with the emission and excitation spectra slightly shifted to lower energies with respect to the spectral bands of the single Bi3+ centers, showing the superlinear dependence of the intensity on the Bi3+ concentration.
3. The lower-energy (usually VIS) emission bands of an exciton-like origin with the large FWHM and Stokes shift and with the temperature dependence of the decay time characteristic for the triplet RES with a very small (~1 meV) energy distance D between the emitting and metastable levels. These bands arise from the excitons localized around different Bi3+-related defects: ex0Bi3+, ex0{Bi3+ - d}, and ex0{Bi3+ - Bi3+}. In the latter case, the VIS emission intensity shows the superlinear dependence on the Bi3+ concentration.
The appearance of the exciton-like emission under excitation in the lowest-energy Bi3+-related absorption band can be explained in the following way:
Under this excitation, the electron transition takes place from the ground state of the Bi3+, {Bi3+ - d} or {Bi3+ - Bi3+} center into the triplet excited state of this center located inside CB. As a result, the electron in CB and the hole remained at the Bi3+, {Bi3+ - d}, or {Bi3+ - Bi3+} center are optically created. An immediate electron-hole recombination results in the formation of an exciton localized around the Bi3+, {Bi3+ - d}, or {Bi3+ - Bi3+} centers. The radiative decay of these localized excitons results in the appearance of the exciton-like emission bands related to the Bi3+, {Bi3+ - d}, or {Bi3+ - Bi3+} centers, respectively. The exciton-like origin of these emission bands is evident from the structure and parameters of the corresponding relaxed excited state (especially, from a very small values of the spin-orbit splitting energy D of the triplet RES) as well as from the large Stokes shift and FWHM of the emission band characteristic just for the luminescence of an exciton-like origin.
Luminescence spectra of some Bi3+-doped compounds contain the overlapping emission bands of excitons localized around different Bi3+-related centers. This explains the dependence of the intensity and position of the complex VIS emission band on the concentration of Bi3+ ions and crystal structure defects (d) in the investigated material.
The luminescence quenching observed in some Bi3+-doped compounds can be caused by the location of the corresponding relaxed excited states inside the conduction band.
From the comparison of the FWHM and S values of the UV emission bands and the parameters of the triplet RES of Bi3+ centers (Table 1), we conclude that the electron-phonon interaction of Bi3+ with its nearest surroundings noticeably increases in the following sequence of oxides: CaO:Bi → Y3Al5O12:Bi → YAlO3:Bi → Y2SiO5:Bi → Y4Al2O9:Bi → La2SO6:Bi. In the same sequence of oxides, the spin-orbit splitting energy (D) decreases due to suppression of the spin-orbit interaction by the increasing electron-phonon interaction.
The analysis of the experimental data on luminescence characteristics of Bi3+-doped materials reported above allows to conclude that for the correct interpretation of an emission band origin, the following investigations have to be carried out:
1. The measurement of temperature dependence of the investigated luminescence decay time in a wide temperature range (down to 4.2 K and lower temperatures) and the determination of the RES parameters, especially the D value. These measurements allow to separate the triplet emission of single (Bi3+, {Bi3+ - d}), and dimer ({Bi3+ - Bi3+}) centers from the triplet Bi3+-related luminescence of an exciton-like origin.
2. The measurement of the dependence of the investigated luminescence intensity on the concentration of Bi3+ under excitation in the absorption spectrum region where the optical density does not exceed 0.5. Only at these conditions, the luminescence intensity is proportional to the absorption intensity and, consequently, to the number of the corresponding luminescence centers. In case of absence of the re-absorption, concentration quenching and energy transfer from Bi3+ ions, the number of single Bi3+ centers should increase linearly with the increasing concentration of Bi3+ ions, while the superlinear (quadratic) concentration dependence is characteristic for the number of dimer Bi3+ related centers (both the {Bi3+ -Bi3+} centers and the ex0{Bi3+ - Bi3+}). These measurements allow to separate the single Bi3+- and the dimer {Bi3+ - Bi3+}-related luminescence centers.
Regarding the bismuth centers with the valence lower than 3+, the Bi2+ emission in the red spectral region was convincingly evidenced in a number of crystalline hosts. Pronounced treatment of some oxide or oxyhalide hosts with large divalent site in reduction atmosphere can decrease further the charge state of bismuth to zero and such a Bi0 center provides a broad infrared emission around 1000–1200 nm. As an intermediate state during the reduction atmosphere-air annealing cycles also the Bi+ center was proposed in Ba2B5O9Cl host to explain emission bands at 660 nm, 790 nm and 970 nm, but these assignments still need further verification in another host(s).
Besides the above-mentioned detailed investigations of Bi3+-doped compounds in a wide temperature range by the methods of the steady-state and time-resolved luminescence spectroscopy, the application of other investigation methods (e.g., electron paramagnetic resonance, nuclear magnetic resonance, thermally stimulated luminescence, etc.) could also be very useful for clarification of the geometrical structure of the Bi3+-related centers and determination of their excited states location with respect to the conduction band. In this respect, it is worth mentioning the recently published positioning of Bi3+ and Bi2+ ground states in YPO4 [228]. Further progress in the experimental research of these materials could surely be complemented by theoretical models of the Bi3+-related excited states, including into consideration the energy levels of Bi3+ ions and the host states and allowing to determine the energy level structure of Bi3+-related luminescence centers. These studies as well as the electronic band structure calculations could allow to predict the luminescence characteristics of new Bi3+-doped compounds suitable for different applications.
This is also valid for Bi ions of lower valence states. The knowledge of the valence state of bismuth ions as well as the host influence on the valence state and structure of Bi-related centers and on the luminescence origin in various Bi-activated materials is needed to understand the physical processes taking place in these materials. This could help to create novel materials for practical applications. In more detail, possible future directions for the search, fabrication, research, and development of Bi-activated photonic materials suitable for different applications are considered in the review paper [8].
Author Contributions
All the authors (A.K., E.M., M.N., S.Z., and Y.Z.) participated in the acquisition, analysis, and interpretation of the experimental data on luminescence of Bi3+-doped compounds, discussion and improvement of the manuscript and approved the submitted version. Y.Z. collected and analyzed the data presented in Section 1. The manuscript was written by M.N. (Section 1, Section 6, Section 7), S.Z. (Section 1, Section 2, Section 3, Section 4, Section 5, Section 7, and Appendix A), and E.M. (Section 2.2 and Section 3.2). E.M. elaborated phenomenological models for description of excited-state dynamics and calculated the parameters of the excited states of Bi3+-related luminescence centers. The figures for Section 2, Section 3, Section 4, Section 5 were prepared by A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Regional Development Fund in Estonia (Center of Excellence TK141, project No. 2014−2020.4.01.15−0011), Estonian Research Council grant (PUT PRG 619), Ministry of Education, Youth and Sports of Czech Republic (projects LO1409 and CZ.02.1.01/0.0/0.0/16_013/0001406), Polish National Science Centre (project no. 2018/31/B/ST8/00774).
Acknowledgments
The work was supported by the ERDF funding in Estonia granted to the Center of Excellence TK141 “Advanced materials and high-technology devices for sustainable energetics, sensorics and nanoelectronics“ (project No. 2014−2020.4.01.15−0011) and the Estonian Research Council grant (PUT PRG 619). Partial support from the Ministry of Education, Youth and Sports of Czech Republic under projects LO1409 and CZ.02.1.01/0.0/0.0/16_013/0001406 and from the Polish National Science Centre (project no. 2018/31/B/ST8/00774) are also gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Bi3+-Doped Alkali Halides
Luminescence characteristics of KCl:Bi were studied in [2,85,94,96,140,206,229,230,231]. In [85,94], different data were obtained on the emission and excitation bands positions, luminescence polarization characteristics, and decay kinetics (Table 1) indicating the possibility of formation of Bi3+ centers of different structure (e.g., Bi3+ ions located close to one or two cation vacancies, impurity anions like O2-, S2-, etc.). This effect is caused by a small solubility of trivalent ns2 ions as well as their twofold excess charge whose compensation can take place in different ways, depending on the conditions of the single crystal synthesis and quenching, the purification degree of the host salt, etc.
In [94], two emission bands were observed at 15 K, peaking at 3.19 eV and 2.88 eV. Only the fast component (≈15 ns) was observed in the 3.19 eV emission decay, while the slow component (0.55 ms) dominates in the decay kinetics of the 2.88 eV emission. The decay time of the slow component remains constant up to about 70 K and then decreases exponentially. The value of D = 59 meV was obtained. A noticeable difference in the excitation spectra of the two emissions as well as temperature variations of the emission spectra indicate that the 3.19 eV and 2.88 eV emission bands arise from different Bi3+ centers.
In [85], the complex emission band located around 2.5 eV was observed in the emission spectrum of KCl:Bi (Figure 8a). Two fast (17 ns and 27 ns) and two slow (1.38 ms and 2.8 ms) components were observed at 4.2 K in the emission decay (Figure 9a). Their excitation spectra practically coincide. The emission spectra of two fast decay components are shifted to lower energy with respect to the spectra of two slow components. The analysis of the temperature dependences of the decay times allows to conclude that the emission of Bi3+ centers arises from the triplet RES and that the theoretical model [135,137] is still valid in the case of KCl:Bi, despite the strong spin-orbit interaction.
Appendix A.2. Bi3+-Doped Alkaline-Earth Oxides, Sulfides, and Fluorides
Luminescence properties of all Bi3+-doped alkaline-earth oxides [79,84,85,88,89,91,133,232,233,234,235,236,237,238,239], sulfides [84,86,87,88,89,90] and fluorides [92] are similar (Table 1). The triplet emission of Bi3+ ions in these materials shows the extremely small Stokes shift and FWHM and the extremely large energy D of the spin-orbit splitting of the triplet RES (up to 265 meV in CaF2 [92]). In the luminescence decay kinetics, the slow decay component at low temperatures is much stronger than the fast component. This indicates that mainly the metastable minima of the triplet RES are populated in the process of the excited state relaxation. In many systems, vibronic structure of the emission band and the lowest-energy excitation band was observed at low temperatures (see, e.g., [84,86,88,89,90,91,92,133,234]). All the observed features are caused by an extremely weak electron-phonon interaction characteristic for these materials.
As an example, the characteristics of triplet luminescence of CaO:Bi are presented in Figure 8b and Figure 9b. At 4.2 K, the strong slow 3.1 eV emission and weak fast 3.3 eV emission were observed, arising from the radiative decay of two triplet RES, corresponding to the 3P0 and 3P1 excited levels of a Bi3+, respectively [85]. It was concluded that the model [134], considering the spin-orbit interaction being dominant in the triplet RES, should be used for the description of the luminescence characteristics of the systems of this type.
In addition to the triplet emission, a new 4.15 eV emission was found in [85] and ascribed to the electron transitions from the singlet excited state of a Bi3+ ion related to the 1P1 excited state of a free Bi ion. At the temperatures around 30 K, the intensity redistribution takes place between the 4.15 eV and 3.1 eV emissions indicating thermal transitions between the singlet and triplet excited levels of the same luminescence center. No such emission was observed in other Bi3+-doped compounds.
Appendix A.3. Bi3+-Doped Lanthanide Oxides
The luminescence of Y2O3:Bi, Lu2O3:Bi, and Gd2O3:Bi arises from the triplet RES of Bi3+ ions located in two different crystal lattice sites of the S6 and the C2 symmetry (see, e.g., [1,3,71,80,112] and references therein). The Bi(S6) and Bi(C2) centers reveal a very weak and a relatively strong electron-phonon interaction of an optical electron with the surrounding oxygen ion. This allowed to suggest [71] that these centers should be described within different models of RES: the Seitz model [134] for Bi(S6) centers and the model of Hizhnyakov [135] for Bi(C2) centers. The competition between the radiative and thermally stimulated and tunneling nonradiative processes in the triplet RES was considered in [71] to explain the low-temperature quenching of the triplet luminescence of Bi3+ found in [112], as well as the absence of the Bi3+-related exciton-like emission in Y2O3:Bi (for more details, see [71]).
In La2O3:Bi, the 2.63 eV emission band was ascribed to the 3P → 1S0 transitions of a free Bi3+ ion [3,80]. In [112], two emission bands, 2.58 eV and 2.18 eV, were observed and ascribed to single and dimer Bi3+ centers, respectively. However, it is not excluded that the 2.18 eV emission (S ≈ 1.47 eV) arises from excitons localized around Bi3+. If so, the presence of the exciton-like emission in La2O3:Bi, unlike in Y2O3:Bi and Lu2O3:Bi, and the absence of the low-temperature luminescence quenching in La2O3:Bi could be connected (see [71]).
Appendix A.4. Bi3+-Doped Garnets
In Y3Al5O12:Bi and Lu3Al5O12:Bi, the co-existence of the triplet UV emission of Bi3+ ions and the VIS emission of excitons localized around Bi3+ ions was found in [17,18].
The luminescence of Bi3+-doped gallate garnets Y3Ga5O12 and Gd3Ga5O12 was found in [12] and suggested to be of an exciton origin. In Gd3Ga5O12:Bi, no Bi3+-related UV emission was observed in [12,74,75,240,241]. The exciton-like origin of the VIS emission was confirmed in [74,75]. The close to linear dependence of the VIS emission intensity on the Bi3+ content indicates that two VIS emission bands observed in [75] are connected with the excitons localized around the single Bi3+ ions and {Bi3+ - d} centers.
Unlike the above-mentioned works, both the UV and VIS emissions were reported [14,110] for Y3Ga5O12:Bi and Gd3Ga5O12:Bi. The UV emission was ascribed to the 3P1,0 → 1S0 transitions of Bi3+ ions, while the VIS emission was attributed to Bi3+ pairs and/or clusters. In Y3Ga5O12:Bi, the UV/VIS emission intensity ratio was found to be ≈17 in [14] and ≈2 in [110]. No VIS emission was observed in [13] despite a large Bi content (10%).
In all these cases, the lowest excitation bands of the UV and VIS emissions were found to be close (compare Table 1, Table 2, and Table 4). In [10], this band was ascribed to the 1S0 → 3P1 transitions of Bi3+ ions and the VIS emission of Y3Ga5O12:Bi and Gd3Ga5O12:Bi, to the CT transitions. It was not excluded in [7] that the lowest-energy excitation band of Y3Ga5O12:Bi and Gd3Ga5O12:Bi arises from the MMCT transitions and that their VIS luminescence is of the MMCT origin. However, it should be noted that the VIS emission of Gd3Ga5O12:Bi is excited at much lower energy (4.0−4.3 eV [12,74,75]) as compared with the lowest-energy MMCT transition (4.59 eV [10]). The same is true also for Y3Al5O12:Bi and Lu3Al5O12:Bi. These data induce some doubts about the MMCT origin of the lowest excitation band of the VIS emission.
Appendix A.5. Bi3+-Doped Aluminum Perovskites YAlO3 and GdAlO3
The crystal structures of YAlO3 and GdAlO3 are different. The band gap Eg in YAlO3 is about 8.5 eV [154] or 8.8 eV [242], and in GdAlO3, Eg ≈ 7.8 eV [154]. In YAlO3:Bi, only the UV emission was observed, located at 4.2 K at 3.76 eV and excited at 4.43 eV [22]. In GdAlO3:Bi [109], both the UV emission (3.72 eV) and the VIS emissions (2.50 and 2.58 eV, see Table 4) were observed. In both papers, the UV emission was ascribed to the transitions from the triplet excited state of Bi3+ ions. In [109], the VIS emission was concluded to arise from Bi3+ dimers (although the concentration dependence of the emission intensity was not measured). In [10], the visible emission was ascribed to the CT transitions and in [154], it was interpreted in terms of IVCT in Bi3+ pairs. It is not excluded that the broad VIS emission band of GdAlO3:Bi consists of the emission bands of excitons localized around single and dimer Bi3+ centers. This could explain the dependence of its position and shape on the Bi3+ content.
Appendix A.6. Bi3+-Doped Oxyorthosilicates
For the first time, the luminescence of Lu2SiO5:Bi and Y2SiO5:Bi was detected in [20,21]. The characteristics of Bi3+-doped Lu2SiO5 and Y2SiO5 were found to be similar. In the emission spectrum of Lu2SiO5:Bi, a strong UV band (3.455 eV), much weaker 3.3 eV band, and two visible bands (2.2 eV and 2.3 eV) are observed at 4.2 K. In Y2SiO5:Bi, only the 3.56 eV band can surely be ascribed to Bi3+ centers. The low-temperature luminescence decay kinetics clearly indicates that the UV emission of both Lu2SiO5:Bi and Y2SiO5:Bi arises from the triplet RES of Bi3+ ions. The light sum of the VIS emission of Bi3+-doped silicates is in at least two orders of magnitude smaller as compared with the light sum of the UV emission of the main Bi3+ centers. Temperature dependences of decay times of both VIS emissions of Lu2SiO5:Bi are similar to those observed for the VIS emission of other Bi3+-doped compounds and allow to connect them with the Bi3+-related localized excitons.
Appendix A.7. Bi3+-Doped Tungstates
Spectroscopic studies of Bi3+-doped tungstates (except PbWO4) were carried out [122,123,124,146,147,148]. In these papers, the well-separated lowest-energy Bi3+-related absorption (excitation) band was ascribed to the 1S0 → 3P1 transitions of a free Bi3+ ion. In [124,146,147,148], the Bi3+-related luminescence was connected with the radiative decay of the triplet RES of Bi3+. From the analysis of the low-temperature decay kinetics of the 2.75 eV emission of CaWO4:Bi, the conclusion on the excitonic origin of this luminescence was made [122,123]. Similar decay kinetics was later observed for CaWO4:Bi [147] and for CdWO4:Bi [148].
The luminescence of PbWO4:Bi crystals was studied in detail in [73]. The 2.2 eV emission was ascribed to the excitons localized around the Bi3+-related centers of two types. Three possibilities were considered for the interpretation of the lowest absorption (excitation) band of PbWO4:Bi:
(i) This band arises from the electron transitions from the ground state to the lowest-energy triplet excited state of Bi3+, corresponding to the 1S0 → 3P1 transitions of a free Bi3+ ion. As the emission from the triplet RES of Bi3+ is absent, the 3P1-related level of Bi3+ should be located inside CB, and the electron transitions result in the ionization of Bi3+ and release of electrons into CB. Similarly to the undoped PbWO4 crystal, the subsequent fast electron-hole recombination followed by vibronic relaxation results in the formation of the (WO4)2--type exciton localized around the Bi3+ ion. The optically released electrons can also be trapped at different traps (e.g., around oxygen vacancies VO or at Bi3+ and Bi5+ ions), producing stable paramagnetic electron {Pb+ - VO}, Bi2+, and Bi4+ centers.
(ii) Similar absorption band observed in PbWO4:Pr [243] was explained by the Pr3+/W6+ → Pr4+/W5+ electron-transfer transitions proposed in [142]. The Bi3+ → W6+ charge-transfer transitions in Y2WO6:Bi were suggested to occur in [1]. In tungstates, the transfer of an electron from the ground state of a Bi3+ ion to the nearest host W6+ ion: Bi3+(6s2)/W6+(5d0) → Bi4+(6s1)/W5+(5d1) was concluded to result in the formation of the hole Bi4+ and electron W5+ centers. Their subsequent fast recombination and the following relaxation results in the creation of the exciton localized around the Bi3+ ion and its subsequent radiative decay. With the use of the equation presented in [7], the MMCT energy in PbWO4:Bi was estimated to be ≈4.2 eV. This value is close to the suggested position of the Bi3+-related absorption band maximum (≈4 eV).
(iii) A trivalent Bi3+ ion in the PbWO4 crystal lattice, substituting for a divalent Pb2+ ion, has an excess positive charge. By analogy with some other materials (see, e.g., [244]), it is not excluded that under photoexcitation of PbWO4:Bi, the photostimulated electron transfer from the valence band (VB) to the ground state of a single Bi3+ ion can also take place, resulting in creation of an electron Bi2+ center and a mobile hole in VB. The hole can be trapped by oxygen ions located close to the Bi3+ - related center and/or lead vacancy VPb. The subsequent fast recombination in close pairs of the optically created electron and hole centers is suggested to result in the creation of excitons localized around Bi3+-related centers. The presence of two types of excitons in PbWO4:Bi, probably, ex0Bi3+ and ex0{Bi3+ - VPb} with strongly different thermal stabilities is suggested. The radiative decay of these excitons is accompanied with the 2.2 eV emission. Similar processes were proposed to explain the appearance of the localized exciton emission and creation of the impurity-related electron centers (Tl0, Pb+) and self-trapped holes (VK centers) in the UV-irradiated Tl+- and Pb2+-doped caesium halides (see, e.g., [245] and references therein).
The data obtained by the TSL and EPR methods on the origin of stable electron and hole centers created under photoexcitation of PbWO4:Bi crystals in the lowest-energy absorption band [73,193] indicate that, in principle, all the above-mentioned processes can take place. As both the single Bi3+ ions and the Bi3+ ions associated with lead vacancies can exist in PbWO4, the process (ii) seems to be more preferable for {Bi3+ - VPb} centers with a negative excess charge, but the process (iii), for single Bi3+ centers with a positive excess charge.
It should be noted that the same processes can also take place in other materials where a Bi3+ ion is substituting for mono- or divalent host lattice ion.
Appendix A.8. Bi3+-Doped Phosphates
In Bi3+-doped LiLaP4O12 glasses and powders, two emission bands were registered at 4.2 K. The 3.02 eV emission was observed under the 5.28 eV excitation while the 2.78 eV emission was observed under the 4.95 eV excitation. In [102], these bands were ascribed to single Bi3+ ions and Bi3+ dimers, respectively. In [246], both these bands were ascribed to single Bi3+ ions. To explain this fact, the presence of two crystallographic positions for a Bi3+ ion in the phosphate host was suggested. However, a later work [247] on the single crystal X-ray diffraction excluded such possibility.
In [72], the LiLaP4O12:Bi with different Bi contents and the undoped LiLaP4O12 were investigated. Only the 2.95 eV emission of LiLaP4O12:Bi was shown to arise from Bi3+-related centers and ascribed to an exciton localized around a single Bi3+ ion. The characteristics of this emission are similar to the characteristics of the 2.8 eV emission of undoped LiLaP4O12. The absence of the UV emission was explained by the location of the lowest-energy excited state of Bi3+ inside CB. Owing to a close position of the lowest excitation band of the 2.95 eV emission (5.4 eV) to the estimated (according to [7]) value of the Bi3+ → La3+ charge transfer energy (≈ 5.5 eV), it was suggested that under the 5.4 eV excitation, an electron transfer from the ground state of a Bi3+ ion to the nearest host La3+ ion, Bi3+(6s2)/La3+(5d0) → Bi4+(6s1)/La2+(5d1), takes place. The subsequent fast electron-hole recombination at the Bi3+ ion and the following relaxation result in the formation of the exciton localized around Bi3+ whose radiative decay is accompanied with the 2.95 eV emission.
The same interpretation can be given to both broad visible emission bands of LiLaP4O12:Bi located at 2.78 eV (S = 2.17 eV) and 3.02 eV (S = 2.25 eV) and ascribed to the triplet emission of Bi3+ [102]. Based on the concentration dependence reported in [102], the 2.78 eV emission can be assumed to arise from an exciton localized around a Bi3+ dimer.
In YPO4:Bi, LuPO4:Bi, two emission bands, an intense narrow band at about 5.12−5.17 eV and a weak broad band at about 3.7−3.8 eV, were observed under excitation around 5.37−5.45 eV. The higher-energy band was ascribed to isolated Bi3+ ions [7,24,113,128,154,248] while the lower-energy band, to Bi3+ dimers [24,113,128], MMCT transitions [7,128], or to the electron transfer in the Bi2+ - Bi4+ pairs [154]. In our opinion, the lower-energy broad 3.7−3.8 eV emission band with the large Stokes shift (S = 1.75 - 1.79 eV) can arise from the excitons localized around Bi3+-related centers.
From the temperature dependence of the decay time of the broad (FWHM = 0.6 eV) 2.75 eV emission band of LaPO4:Bi with the large Stokes shift (S = 2.4 eV) measured in [83], the value of D = 2.05 meV was obtained. Such small D value allows to make a reliable conclusion that the 2.75 eV emission of LaPO4:Bi arises from an exciton localize around a Bi3+ ion.
In [111], the Bi3+-doped metaphosphates LnP3O9 (Ln=Sc, Lu, Y, Gd, La) were studied. In ScP3O9:Bi, LuP3O9:Bi, and YP3O9:Bi with relatively small Stokes shifts (0.56 eV, 0.86 eV, and 0.92 eV, respectively) the excitation band was ascribed to the 1S0 → 3P1 transitions and the emission, to the 3P1,0 → 1S0 transitions of Bi3+. However, the 2.72 eV emission of LaP3O9:Bi has much larger Stokes shift (S = 2.54 eV). This means that this emission can arise from the exciton localized around a Bi3+ ion.
Appendix A.9. Bi3+-Doped Rare-Earth Orthovanadates
The study of luminescence characteristics of the undoped and Bi3+-doped orthovanadates started in [1,3,166]. In [166], temperature dependences of the luminescence decay time were measured for YVO4 and YVO4:Bi and found to be similar. The decay time of the Bi3+-related emission decreases drastically from 165 μs at 4 K to 4.5 μs at ~20 K. This effect was explained by the presence of two close excited state levels separated by only about 0.62 meV. However, the origin of these states was not discussed. In [3,26,29,36,39,40,42,43,143,144,145], the Bi3+-related spectral bands of YVO4:Bi were ascribed to the electronic transitions between the ground and excited states of a Bi3+ ion, while in [7,27,33,34,38,41,44,128,141,156,249,250,251,252,253], to the charge transfer transitions between Bi and V ions. In the latter set of works, the lowest-energy absorption band was connected with the Bi3+(6s2) → V5+(3d0) transitions and the emission band, with the V4+(3d1) → Bi4+(6s1) transitions. Luminescence of LuVO4:Bi and GdVO4:Bi was much less studied and mainly at 295 K (see, e.g., [3,28,29,35,37,38,41,64,65,143,144]).
In [76], the conclusion on the exciton-like origin of both the intrinsic blue emission and the Bi3+-related yellow emission in Bi3+-doped rare-earth orthovanadates was made based on the analysis of temperature dependences of their decay times. It was suggested that the Bi3+-related absorption (excitation) band around 3.73–3.79 eV arises from the electronic transition from the ground 1S0 state to the excited 3P1 state of Bi3+ located inside CB. The excitation in this band was suggested to result in an electron release from the 3P1 level into CB and its subsequent immediate recombination with the hole remained at the Bi3+ ion. As a result, an exciton localized around the single Bi3+ ion is created whose radiative decay is accompanied with the 2.12–2.19 eV emission. No other VIS emissions, which could arise, e.g., from the excitons localized around dimer {Bi3+ - Bi3+} centers, and no UV emission, which could arise from the triplet excited state of Bi3+ ions, were found in YVO4:Bi, LuVO4:Bi, and GdVO4:Bi.
Appendix A.10. Bi3+-Doped Lanthanide Niobates
Luminescence of Bi3+-doped niobates was studied mainly at 295 K. Not only the interpretation of experimental results but also positions of the emission and excitation bands reported in different papers were different. For YNbO4:Bi, the MMCT origin of the spectral bands was suggested in [1,7,64,65,157]. The lowest-energy absorption band was connected with the Bi3+(6s2) → Nb5+(3d0) transitions and the emission band, with the Nb4+(3d1) → Bi4+(6s1) transitions. However, in [149,150,151,152,153], the excitation and emission bands of YNbO4:Bi were ascribed to the electron transitions between the ground state (1S0) and the triplet excited state (3P1) of a single Bi3+ ion. In [254], the absorption of YNbO4:Bi was ascribed to the charge transfer transitions from O2- 2p to the excited 6p levels of Bi3+. The energies of the 1S0 → 3P1 transitions (3.91 eV) and the MMCT transitions (3.91 eV) calculated in [65] were found to be close to the position of the lowest-energy excitation band maximum in YNbO4:Bi and GdNbO4:Bi. This means that, in principle, the appearance of both these types of transitions is possible in the absorption (and luminescence) spectra of YNbO4:Bi.
In [77], two Bi3+-related emission bands were observed in YNbO4:Bi. Based on detailed and systematic investigation of the intrinsic and Bi3+-related luminescence in YNbO4:Bi powders with different Bi content, carried out by the methods of the steady-state and time-resolved luminescence spectroscopy in the 4.2–500 K temperature range, the conclusion on their exciton-like origin was made. The most intense emission band peaking at about 2.53 eV and excited around 4.09 eV was ascribed to the radiative decay of an exciton localized around a single Bi3+ ion. The weaker 2.41 eV emission with the superlinear dependence of intensity on the impurity concentration was ascribed to an exciton localized around a dimer Bi3+ center. No ultraviolet emission arising from the 3P1,0 → 1S0 transition of a Bi3+ ion was found. This fact as well as the exciton-like origin of the Bi3+-related emission bands indicate that the triplet excited level of Bi3+ ion is located inside CB of YNbO4.
Appendix A.11. Bi3+-Doped Borates
In [1,78,95], the luminescence characteristics of Bi3+-doped lanthanide orthoborates LnBO3 (Ln: Sc, Y, La, Gd, Lu) of different structure and coordination numbers were investigated. In ScBO3:Bi, the appearance of the vibronic structure of the emission and excitation spectra of Bi3+ centers at low temperatures and the small Stokes shift (0.22 eV) indicate a very small electron-phonon interaction. The energy distance between the emitting and metastable minima of the triplet RES is large (D = 120 meV) indicating a strong spin-orbit interaction. For the UV (3.46 eV) emission of LaBO3:Bi, which also arises from Bi3+ centers, the Stokes shift is much larger (1.16 eV), the decay time at 4.2 K is several times shorter, and the D value is about two times smaller (55 meV). These features are caused by much stronger electron-phonon interaction with respect to the spin-orbit interaction in this compound as well as an increase of the probability of the radiative decay of the metastable minima of the triplet RES. Thus, the variation of luminescence characteristics in the sequence of the orthoborates ScBO3 → LuBO3 → YBO3 → LaBO3 is caused by the increasing electron-phonon interaction. The broad VIS emission of LaBO3:Bi (2.69 eV) with the large Stokes shift (1.84 eV) ascribed in [78] to Bi3+ pairs or clusters arises most probably from the Bi3+-related localized exciton. Indeed, as follows from the temperature dependence of its decay time, the D value for the corresponding triplet RES is in an order of magnitude smaller (5.7 meV) as compared to that of the UV emission (55 meV) and is characteristic for the exciton-like states.
Similar broad emission band of InBO3:Bi with the large Stokes shift (1.41 eV) was ascribed to the impurity-bound exciton [155]. It was suggested that the lowest excited level of Bi3+ is located inside or close to CB of InBO3. The excitation of Bi3+ results in the release of an electron into CB with the subsequent recombination with the hole on the luminescence center and formation of an impurity-trapped exciton.
References
- Blasse, G.; Bril, A. Investigations on Bi3+-activated phosphors. J. Chem. Phys. 1968, 48, 217–222. [Google Scholar] [CrossRef]
- Lushchik, C.; Gindina, R.; Zazubovich, S.; Lushchik, N. Nature of the luminescence centers in ionic crystals. Czechoslov. J. Phys. 1970, 20B, 585–604. [Google Scholar] [CrossRef]
- Boulon, G. Processus de photoluminescence dans les oxydes et les orthovanadates de terres rares polycristallins actives par l’ion Bi3+. J. de Physique (France) 1971, 32, 333–347. [Google Scholar] [CrossRef]
- Boulon, G. Spectroscopy of post-transition metal ions. In Spectroscopy of Solid-State Laser-Type Materials; Di Bartolo, B., Ed.; Plenum Press: New York, NY, USA, 1987; Volume 30, pp. 223–266. [Google Scholar]
- Blasse, G. Luminescence of inorganic solids: From isolated centers to concentrated systems. Prog. Solid State Chem. 1988, 18, 79–171. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin, Germany, 1994. [Google Scholar]
- Boutinaud, P. Revisiting the spectroscopy of the Bi3+ ion in oxide compounds. Inorg. Chem. 2013, 52, 6028–6038. [Google Scholar] [CrossRef]
- Sun, H.-T.; Zhou, J.; Qui, J. Recent advances in bismuth activated photonic materials. Prog. Mater. Sci. 2014, 64, 1–72. [Google Scholar] [CrossRef]
- Zazubovich, S.; Krasnikov, A.; Zorenko, Y.; Gorbenko, V.; Babin, V.; Mihokova, E.; Nikl, M. Luminescence of Pb- and Bi-related centers in aluminum garnet, perovskite and orthosilicate single crystalline films. In Nanocomposite, Ceramic and Thin Film Scintillators; Nikl, M., Ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2016; Chapter 6; pp. 227–302. [Google Scholar]
- Awater, R.H.P.; Dorenbos, P. The Bi3+ 6s and 6p electron binding energies in relation to the chemical environment of inorganic compounds. J. Lumin. 2017, 184, 221–231. [Google Scholar] [CrossRef]
- Swart, H.C.; Kroon, R.E. Ultraviolet and visible luminescence from bismuth doped materials. Opt. Mater. X 2019, 2, 100025. [Google Scholar] [CrossRef]
- Ilmer, M.; Grabmaier, B.C.; Blasse, G. Luminescence of Bi3+ in gallate garnets. Chem. Mat. 1994, 6, 204–206. [Google Scholar] [CrossRef]
- Nikl, M.; Novoselov, A.; Mihokova, E.; Polak, K.; Dusek, M.; McClune, B.; Yoshikawa, A.; Fukuda, T. Photoluminescence of Bi3+ in Y3Ga5O12 single-crystal host. J. Phys. Condens. Matter 2005, 17, 3367–3375. [Google Scholar] [CrossRef]
- Setlur, A.A.; Srivastava, A.M. The nature of Bi3+ luminescence in garnet hosts. Opt. Mater. 2006, 29, 410–415. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Voznyak, T.; Vistovsky, V.; Nedilko, S.; Nikl, M. Luminescence of Bi3+ ions in Y3Al5O12:Bi single crystalline films. Radiat. Meas. 2007, 42, 882–886. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Krasnikov, A.; Makhov, A.; Mihokova, E.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Origin of Bi3+-related luminescence centers in Lu3Al5O12:Bi and Y3Al5O12:Bi single crystalline films and the structure of their relaxed excited states. Phys. Status Solidi B 2012, 249, 1039–1045. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Krasnikov, A.; Makhov, A.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Photoluminescence of Lu3Al5O12:Bi and Y3Al5O12:Bi single crystalline films. Radiat. Meas. 2010, 45, 331–335. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Krasnikov, A.; Makhov, A.; Nikl, M.; Polak, K.; Zazubovich, S.; Zorenko, Y. Peculiarities of excited state structure and photoluminescence in Bi3+-doped Lu3Al5O12 single-crystalline films. J. Phys. Condens. Matter 2009, 21, 415502. [Google Scholar] [CrossRef]
- Zorenko, Y.; Mares, J.A.; Kucerkova, R.; Gorbenko, V.; Savchun, V.; Voznyak, T.; Nikl, M.; Beitlerova, A.; Jurek, K. Optical, luminescence and scintillation characteristics of Bi-doped LuAG and YAG single crystalline films. J. Phys. D Appl. Phys. 2009, 42, 075501. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Krasnikov, A.; Mihokova, E.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Photoluminescence and excited state structure in Bi3+-doped Y2SiO5 single crystalline films. Radiat. Meas. 2013, 56, 90–93. [Google Scholar] [CrossRef]
- Gorbenko, V.; Krasnikov, A.; Mihokova, E.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Photoluminescence and excited state structure of Bi3+-related centers in Lu2SiO5:Bi single crystalline films. J. Lumin. 2013, 134, 469–476. [Google Scholar] [CrossRef]
- Krasnikov, A.; Lipińska, L.; Mihokova, E.; Nikl, M.; Shalapska, T.; Suchocki, A.; Zazubovich, S.; Zhydachevskii, Y. Time-resolved photoluminescence and excited state structure of Bi3+ center in YAlO3. Opt. Mater. 2014, 36, 1705–1708. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, H.; Cheng, J.; Song, P.; Yang, G.; Zhang, G.; Wu, C. Site-selective luminescence of Bi3+ in the YBO3 host under vacuum ultraviolet excitation at low temperature. J. Lumin. 2008, 128, 2027–2030. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Camardello, S.J. Concentration dependence of the Bi3+ luminescence in LnPO4 (Ln = Y3+, Lu3+). Opt. Mater. 2015, 39, 130–133. [Google Scholar] [CrossRef]
- Mu, Z.; Hu, Y.; Chen, L.; Wang, X. Enhanced luminescence of Dy3+ in Y3Al5O12 by Bi3+ co-doping. J. Lumin. 2011, 131, 1687–1691. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.J.; Lin, C.C.; Chu, C.I.; Hu, S.F.; Lee, M.H.; Liu, R.S. Combinatorial approach to the development of a single mass YVO4:Bi3+,Eu3+ phosphor with red and green dual colors for high color rendering white light-emitting diodes. J. Comb. Chem. 2010, 12, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Chen, D.; Yang, M.; Ying, T. Synthesis and luminescence properties of YVO4:Eu3+, Bi3+ phosphor with enhanced photoluminescence by Bi3+ doping. J. Phys. Chem. Sol. 2010, 71, 175–180. [Google Scholar] [CrossRef]
- Kang, F.; Peng, M.; Zhang, Q.; Qiu, J. Abnormal anti-quenching and controllable multi-transitions of Bi3+ luminescence by temperature in a yellow-emitting LuVO4:Bi3+ phosphor for UV-converted white LEDs. Chem. Eur. J. 2014, 20, 11522–11530. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Peng, M.; Yang, X.; Dong, G.; Nie, G.; Liang, W.; Xu, S.; Qiu, J. Broadly tuning Bi3+ emission via crystal field modulation in solid solution compounds (Y,Lu,Sc)VO4:Bi for ultraviolet converted white LEDs. J. Mater. Chem. C 2014, 2, 6068–6076. [Google Scholar] [CrossRef]
- Kang, F.; Yang, X.; Peng, M.; Wondraczek, L.; Ma, Z.; Zhang, Q.; Qiu, J. Red photoluminescence from Bi3+ and the influence of the oxygen-vacancy perturbation in ScVO4: A combined experimental and theoretical study. J. Phys. Chem. C 2014, 118, 7515–7522. [Google Scholar] [CrossRef]
- Kang, F.; Zhang, Y.; Wondraczek, L.; Zhu, J.; Yang, X.; Peng, M. Processing-dependence and the nature of the blue-shift of Bi3+-related photoemission in ScVO4 at elevated temperatures. J. Mater. Chem. 2014, 2, 9850–9857. [Google Scholar]
- Kang, F.; Zhang, Y.; Peng, M. Controlling the energy transfer via multiluminescent centers to achieve white light/tunable emissions in a single-phased X2-type Y2SiO5:Eu3+,Bi3+ phosphor for ultraviolet converted LEDs. Inorg. Chem. 2015, 54, 1462–1473. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, J.X.; Yu, D.C.; Ye, S.; Zhang, Q.Y.; Sun, X.W. Spectral conversion for solar cell efficiency enhancement using YVO4:Bi3+,Ln3+ (Ln = Dy, Er, Ho, Eu, Sm, and Yb) phosphors. J. Appl. Phys. 2011, 109, 113526. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, P.; Lin, H.; Shan, Z.; Zeng, L.; Yang, A.; Wang, Y. Color-tunable luminescence for Bi3+/Ln3+:YVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphors excitable by near-ultraviolet light. Phys. Chem. Chem. Phys. 2010, 12, 7775–7778. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tang, Q.; Lin, B.; Zhou, H.; Liu, G.; Li, Y.; Chen, D. Fabrication of Bi3+/Ln3+:LuVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphors and its color-tunable optical performance. J. Lumin. 2018, 194, 667–674. [Google Scholar] [CrossRef]
- Park, W.J.; Jung, M.K.; Im, S.J.; Yoon, D.H. Photoluminescence characteristics of energy transfer between Bi3+ and Eu3+ in LnVO4: Eu, Bi (Ln = Y, La, Gd). Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 373–377. [Google Scholar] [CrossRef]
- Lenczewska, K.; Tomala, R.; Hreniak, D. Near-UV sensitized NIR emission in Nd3+ and Bi3+ co-doped GdVO4 phosphors. Opt. Mater. 2017, 74, 12–15. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Lin, B.; Tang, Q.; Zhou, H.; Zeng, J.; Liu, G.; Chen, D. Rational design of Bi3+/Ln3+:GdVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphor: Synthesis, characterization and color-tunable property. Opt. Mater. 2018, 77, 204–210. [Google Scholar] [CrossRef]
- Natarajan, V.; Dhobale, A.R.; Lu, C.-H. Preparation and characterization of tunable YVO4: Bi3+, Sm3+ phosphors. J. Lumin. 2009, 129, 290–293. [Google Scholar] [CrossRef]
- Zhao, M.; Li, G.; Zheng, J.; Li, L.; Yang, L. Fabrication of assembled-spheres YVO4: (Ln3+, Bi3+) towards optically tunable emission. CrystEngComm. 2012, 14, 2062–2070. [Google Scholar] [CrossRef]
- Lenczewska, K.; Gerasymchuk, Y.; Vu, N.; Liem, N.Q.; Boulon, G.; Hreniak, D. The size effect on the energy transfer in Bi3+–Eu3+ co-doped GdVO4 nanocrystals. J. Mater. Chem. C 2017, 5, 3014–3023. [Google Scholar] [CrossRef]
- Matos, M.G.; Rocha, L.A.; Nassar, E.J.; Verelst, M. Influence of Bi3+ ions on the excitation wavelength of the YVO4:Eu3+ matrix. Opt. Mater. 2016, 62, 12–18. [Google Scholar]
- Yang, L.; Mi, X.; Su, J.; Zhang, X.; Bai, Z.; Wang, N.; Lin, J. Tunable luminescence and energy transfer properties in YVO4:Bi3+,Ln3+ (Ln = Dy, Sm, Eu) phosphors prepared by microwave sintering method. J. Mater. Sci. Mater. Electron. 2018, 29, 7941–7951. [Google Scholar] [CrossRef]
- Cavalli, E.; Angiuli, F.; Belletti, A.; Boutinaud, P. Luminescence spectroscopy of YVO4:Ln3+, Bi3+ (Ln3+ = Eu3+, Sm3+, Dy3+) phosphors. Opt. Mater. 2014, 36, 1642–1648. [Google Scholar] [CrossRef]
- Park, W.J.; Jung, M.K.; Yoon, D.H. Influence of Eu3+, Bi3+ co-doping content on photoluminescence of YVO4 red phosphors induced by ultraviolet excitation. Sens. Actuators B 2007, 126, 324–327. [Google Scholar] [CrossRef]
- Zhydachevskii, Y.; Lipińska, L.; Baran, M.; Berkowski, M.; Suchocki, A.; Reszka, A. Broadband down-conversion in Bi3+-Yb3+-codoped yttrium and yttrium-aluminum oxides. Mater. Chem. Phys. 2014, 143, 622–628. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, Q.Y. Near-infrared quantum cutting via cooperative energy transfer in Gd2O3:Bi3+, Yb3+ phosphors. J. Appl. Phys. 2010, 107, 063505. [Google Scholar] [CrossRef]
- Zhydachevskyy, Y.; Tsiumra, V.; Baran, M.; Lipińska, L.; Sybilski, P.; Suchocki, A. Quantum efficency of the down-conversion process in Bi3+ - Yb3+ co-doped Gd2O3. J. Lumin. 2018, 196, 169–173. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ueda, J.; Tanabe, S. Photochromism and white long-lasting persistent luminescence in Bi3+-doped ZnGa2O4 ceramics. Opt. Mater. Express 2012, 2, 1378–1383. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Zhou, D.; Qui, J.; Xu, X.; Yu, X. Long persistent properties of CaGa2O4:Bi3+ at different ambient temperature. J. Am. Ceram. Soc. 2017, 100, 3514–3521. [Google Scholar] [CrossRef]
- Wang, T.; Xu, X.; Zhou, D.; Yang, Y.; Qui, J.; Yu, X. Effect of defect distribution on the optical storage properties of strontium gallates with a low-dimensional chain structure. Inorg. Chem. 2016, 55, 894–901. [Google Scholar] [CrossRef]
- Jia, D.D.; Zhu, J.; Wu, B.Q. Improvement of persistent phosphorescence of Ca0.9Sr0.1S:Bi3+ by codoping Tm3+. J. Lumin. 2000, 91, 59–65. [Google Scholar] [CrossRef]
- Sun, W.; Pang, R.; Li, H.; Li, D.; Jiang, L.; Zhang, S.; Fu, J.; Li, C. Investigation of a novel color tunable long afterglow phosphor KGaGeO4:Bi3+: Luminescence properties and mechanism. J. Mater. Chem. C 2017, 5, 1346–1355. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Chen, L.; Wang, X.; Ju, G.; Mu, Z. Persistent luminescence in Bi3+ doped CaWO4 matrix. Radiat. Meas. 2013, 51–52, 18–24. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, J.; Wang, T.; Wu, H.; Qiu, J.; Song, Z.; Zhou, D. Ultraviolet long afterglow emission in Bi3+ doped CdSiO3 phosphors. Mater. Express 2014, 4, 172–176. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Meng, C.Z.Y.; Tang, J.; Qiu, Q. An efficient UV converted blue-emitting Lu2CaGeO6:Bi3+ persistent phosphor for potential application in photocatalysis. Ceramics. Int. 2018, 44, 14712–14716. [Google Scholar] [CrossRef]
- Dou, X.; Xiang, H.; Wei, P.; Zhang, S.; Ju, G.; Meng, Z.; Chen, L.; Hu, Y.; Li, Y. A novel phosphor CaZnGe2O6:Bi3+ with persistent luminescence and photo-stimulated luminescence. Mater. Res. Bull. 2018, 105, 226–230. [Google Scholar] [CrossRef]
- Wang, X.; Boutinaud, P.; Li, L.; Cao, J.; Xiong, P.; Li, X.; Luo, H.; Peng, M. Novel persistent and tribo-luminescence from bismuth ion pairs doped strontium gallate. J. Mater. Chem. C 2018, 6, 10367–10375. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Z.; He, X.; Wei, Y.; Zou, Z.; Zhang, J.; Wang, Z.; Zhang, Z.; Wang, Y. How to design ultraviolet emitting persistent materials for potential multifunctional applications: A living example of a NaLuGeO4:Bi3+, Eu3+ phosphor. J. Mater. Chem. C 2017, 5, 4310–4318. [Google Scholar] [CrossRef]
- Feng, P.; Wei, Y.; Wang, Y.; Zhang, J.; Li, H.; Ci, Z.; Xie, R. Long persistent phosphor CdSiO3:Gd3+,Bi3+ and potential photocatalytic application of CdSiO3:Gd3+,Bi3+@TiO2 in dark. J. Amer. Ceram. Soc. 2016, 99, 2368–2375. [Google Scholar] [CrossRef]
- Lai, S.; Yang, Z.; Liao, J.; Qiu, J.; Song, Z.; Yang, Y.; Zhou, D. Investigation of persistent luminescence property of Bi3+, Dy3+ co-doped CdSiO3 phosphor. Mater. Res. Bull. 2014, 60, 714–718. [Google Scholar] [CrossRef]
- Xiong, P.; Peng, M. Recent advances in ultraviolet persistent phosphors. Opt. Mater. X 2019, 2, 100022. [Google Scholar] [CrossRef]
- Back, M.; Ueda, J.; Xu, J.; Asami, K.; Amidani, L.; Trave, E.; Tanabe, S. Uncovering the origin of the emitting states in Bi3+-activated CaMO3 (M = Zr, Sn, Ti) perovskites: Metal-to-metal charge transfer versus s−p transitions. J. Phys. Chem. C 2019, 123, 14677–14688. [Google Scholar] [CrossRef]
- Boutinaud, P.; Cavalli, E. Predicting metal-to-metal charge transfer in closed-shell transition metal oxides doped with Bi3+ or Pb2+. Chem. Phys. Lett. 2011, 503, 239–243. [Google Scholar] [CrossRef]
- Amer, M.; Boutinaud, P. On the character of the optical transitions in closed-shell transition metal oxides doped with Bi3+. Phys. Chem. Chem. Phys. 2017, 19, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Eucken, A. (Ed.) Landolt-Börnstein Zahlenwerte und Funktionen, I Band, Atom- und Molekularphysik, I Teil, Atome und Ionen; Springer: Berlin, Germany, 1950; p. 439. [Google Scholar]
- Lushchik, N.E.; Lushchik, C.B. Spectroscopy of luminescence centers in alkali halides activated by gomological rows of ions. Sov. Phys. Opt. Spectrosc. 1960, 8, 839–846. [Google Scholar]
- Blasse, G.; van der Steen, A.C. Luminescence characteristics of Bi3+-activated oxides. Solid St. Commun. 1979, 31, 993–994. [Google Scholar] [CrossRef]
- Moncorge, R.; Jacquier, B.; Boulon, G. Temperature dependent luminescence of Bi4Ge3O12: Discussion of possible models. J. Lumin. 1976, 14, 337–348. [Google Scholar]
- Babin, V.; Lipińska, L.; Mihokova, E.; Nikl, M.; Shalapska, T.; Suchocki, A.; Zazubovich, S.; Zhydachevskii, Y. Time-resolved spectroscopy of Bi3+ centers in Y4Al2O9. Opt. Mater. 2015, 46, 104–108. [Google Scholar] [CrossRef]
- Babin, V.; Chernenko, K.; Lipińska, L.; Mihokova, E.; Nikl, M.; Schulman, L.S.; Shalapska, T.; Suchocki, A.; Zazubovich, S.; Zhydachevskii, Y. Luminescence and excited state dynamics of Bi3+ centers in Y2O3. J. Lumin. 2015, 167, 268–277. [Google Scholar] [CrossRef]
- Babin, V.; Chernenko, K.; Demchenko, P.; Mihokova, E.; Nikl, M.; Pashuk, I.; Shalapska, T.; Voloshinovskii, A.; Zazubovich, S. Luminescence and excited state dynamics in Bi3+-doped LiLaP4O12 phosphates. J. Lumin. 2016, 176, 324–330. [Google Scholar] [CrossRef]
- Buryi, M.; Bohacek, P.; Chernenko, K.; Krasnikov, A.; Laguta, V.V.; Mihokova, E.; Nikl, M.; Zazubovich, S. Luminescence and photo-thermally stimulated defect-creation processes in Bi3+-doped single crystals of lead tungstate. Phys. Status Solidi B 2016, 253, 895–910. [Google Scholar] [CrossRef]
- Krasnikov, A.; Luchechko, A.; Mihokova, E.; Nikl, M.; Syvorotka, I.I.; Zazubovich, S.; Zhydachevskii, Y. Origin of Bi3+-related luminescence in Gd3Ga5O12:Bi epitaxial films. J. Lumin. 2017, 190, 81–88. [Google Scholar] [CrossRef]
- Tsiumra, V.; Krasnikov, A.; Zazubovich, S.; Zhydachevskyy, Y.; Vasylechko, L.; Baran, M.; Wachnicki, L.; Lipinska, L.; Nikl, M.; Suchocki, A. Crystal structure and luminescence studies of microcrystalline GGG:Bi3+ and GGG:Bi3+, Eu3+ as UV-to-VIS converting phosphors for white LEDs. J. Lumin. 2019, 213, 278–289. [Google Scholar] [CrossRef]
- Krasnikov, A.; Tsiumra, V.; Vasylechko, L.; Zazubovich, S.; Zhydachevskyy, Y. Photoluminescence origin in Bi3+-doped YVO4, LuVO4, and GdVO4 orthovanadates. J. Lumin. 2019, 212, 52–60. [Google Scholar] [CrossRef]
- Baran, M.; Kissabekova, A.; Krasnikov, A.; Lushchik, A.; Suchocki, A.; Tsiumra, V.; Vasylechko, L.; Zazubovich, S.; Zhydachevskyy, Y. Exciton-like luminescence of Bi3+ - doped yttrium niobate. Nucl. Instrum. Meth. Phys. Res. B 2020, 463, 7–15. [Google Scholar] [CrossRef]
- Wolfert, A.; Oomen, E.W.J.L.; Blasse, G. Host lattice dependence of the Bi3+ luminescence in orthoborates LnBO3 (with Ln = Sc, Y, La, Gd, or Lu). J. Solid St. Chem. 1985, 59, 280–290. [Google Scholar] [CrossRef]
- Hughes, A.E.; Pells, G.P. The luminescence spectra of Bi3+ ions in MgO and CaO. Phys. Status Solidi B 1975, 71, 707–718. [Google Scholar] [CrossRef]
- Boulon, G.; Pedrini, C.; Guidoni, M.; Pannel, C. Étude de la cinétique des centres luminogènes Bi3+ dans les cristaux. J. de Physique (France) 1975, 36, 267–278. [Google Scholar] [CrossRef]
- Pedrini, C.; Boulon, G.; Gaume-Mahn, F. Bi3+ and Pb2+ centres in alkaline-earth antimonate phosphors. Phys. Status Solidi A 1973, 15, K15–K18. [Google Scholar] [CrossRef]
- Moncorge, R.; Boulon, G.; Jacquier, B. Analysis of fluorescence processes of bismuth (3+) centers incorporated into lanthanum oxysulfate (La2SO6). Compt. Rend. Acad. Sci. Paris Ser. B 1976, 282, 239–242. [Google Scholar]
- Moncorge, R.; Boulon, G.; Denis, J. Fluorescence properties of bismuth-doped LaPO4. J. Phys. C Solid State Phys. 1979, 12, 1165–1171. [Google Scholar] [CrossRef]
- Ellervee, A.F. Luminescence of Pb2+ and Bi3+ centres in alkali-earth sulphides and oxides. Phys. Status Solidi B 1977, 82, 91–98. [Google Scholar] [CrossRef]
- Aceves, R.; Barboza Flores, M.; Maaroos, A.; Nagirnyi, V.; Perez Salas, R.; Tsuboi, T.; Zazubovich, S.; Zepelin, V. RES structure of Bi3+ centres in KCl:Bi, S and CaO:Bi crystals. Phys. Status Solidi B 1996, 194, 619–631. [Google Scholar] [CrossRef]
- Asano, S.; Nakao, Y.; Yamashita, N.; Matsuyama, I. Luminescence et transition non radiative 3T1u→3A1u dans le luminophore BaS:Bi3+. Phys. Status Solidi B 1986, 133, 333–344. [Google Scholar] [CrossRef]
- Donker, H.; Yamashita, N.; Smit, W.M.A.; Blasse, G. Luminescence decay times of the Sb2+, Pb2+, and Bi3+ ions in alkaline-earth sulfides. Phys. Status Solidi B 1989, 156, 537–544. [Google Scholar] [CrossRef]
- Asano, S.; Yamashita, N. Luminescence et interaction phonon-electron dans le luminophore MgS:Bi3+. Phys. Status Solidi B 1981, 105, 305–310. [Google Scholar] [CrossRef]
- Asano, S.; Yamashita, N. Effect of magnetic field on luminescence of MgS, CaS and CaO phosphors activated with Bi3+ ions. Phys. Lett. A 1981, 86, 191–193. [Google Scholar] [CrossRef]
- Yamashita, N.; Iwasaki, S.; Asano, S.; Ohishi, M.; Ohmori, K. Investigation of the luminescence in the SrS:Bi3+ phosphor by time-resolved emission spectroscopy. J. Phys. Soc. Japan 1984, 53, 4425–4431. [Google Scholar] [CrossRef]
- Yamashita, N.; Ikeda, S.; Asano, S. Photoluminescence and excitation spectra of the SrO:Bi3+ phosphor. Phys. Lett. A 1987, 121, 94–96. [Google Scholar] [CrossRef]
- Oboth, K.P.; Lohmeier, F.J.; Fisher, F. VUV and UV spectroscopy of Pb2+ and Bi3+ centres in alkaline-earth fluorides. Phys. Status Solidi B 1989, 154, 789–803. [Google Scholar] [CrossRef]
- Wolfert, A.; Blasse, G. Luminescence of Bi3+-activated LaOBr, a system with emission from different states. J. Lumin. 1985, 33, 213–226. [Google Scholar] [CrossRef]
- Kang, J.G.; Yoon, H.M.; Chun, G.M.; Kim, Y.D.; Tsuboi, T. Spectroscopic studies of Bi3+ colour centres in KCl single crystals. J. Phys. Condens. Matter 1994, 6, 2101–2116. [Google Scholar] [CrossRef]
- Wolfert, A.; Oomen, E.W.J.L.; Blasse, G. Host lattice dependence of the Bi3+ luminescence in orthoborates LnBO3. J. Lumin. 1984, 31–32, 308–310. [Google Scholar] [CrossRef]
- Zazubovich, S.G.; Lushchik, N.E.; Lushchik, C.B. Polarized luminescence of KCl:Bi phosphor. Trudy IFA AN EstSSR 1961, 14, 292–293. [Google Scholar]
- Wolfert, A.; Blasse, G. Luminescence of Bi3+-doped crystals of Cs2NaYBr6 and Cs2NaLaCl6. J. Solid State Chem. 1985, 59, 133–142. [Google Scholar] [CrossRef]
- van der Steen, A.C. Luminescence of Cs2NaYCl6-Bi3+ (6s2). Phys. Status. Solidi B 1980, 100, 603–611. [Google Scholar] [CrossRef]
- Wolfert, A.; Blasse, G. Luminescence of the Bi3+ ion in compounds LnOCI (Ln = La, Y, Gd). Mater. Res. Bull. 1984, 19, 67–75. [Google Scholar] [CrossRef]
- Boulon, G.; Jorgensen, C.K.; Reisfeld, R. The two luminescent levels of Bi3+ in solids. Chem. Phys. Lett. 1980, 75, 24–26. [Google Scholar] [CrossRef]
- Xiao, X.; Yan, B. Hybrid precursors synthesis and optical properties of LnNbO4:Bi3+ blue phosphors and Bi3+ sensitizing of on Dy3+’s luminescence in YNbO4 matrix. J. Alloy. Compd. 2006, 421, 252–257. [Google Scholar] [CrossRef]
- Blasse, G.; Dirksen, G.J. The luminescence of broad-band emitters in LiLaP4O12. Phys. Status Solidi B 1982, 110, 487–494. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Dorenbos, P. X-ray induced valence change and vacuum referred binding energies of Bi3+ and Bi2+ in Li2BaP2O7. J. Phys. Chem. C 2016, 120, 15114–15118. [Google Scholar] [CrossRef]
- Zhiran, H.; Blasse, G. Energy transfer phenomena in luminescent materials based on GdB3O6. Mater. Chem. Phys. 1985, 12, 257–274. [Google Scholar] [CrossRef]
- Blasse, G.; Meijerink, A.; Nomes, M.; Zuidema, J. Unusual Bismuth Luminescence in Strontium Tetraborate (SrB4O7:Bi). J. Phys. Chem. Sol. 1994, 55, 171–174. [Google Scholar] [CrossRef]
- Peng, M.; Sprenger, B.; Schmidt, M.A.; Schwefel, H.; Wondraczek, L. Broadband NIR photoluminescence from Bi-doped Ba2P2O7 crystals: Insights into the nature of NIR-emitting bismuth centers. Opt. Express 2010, 18, 12852–12863. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Peng, M.; Kang, F.; Cao, R.; Ma, Z.; Dong, G.; Qiu, J.; Xu, S. Broadband NIR luminescence from a new bismuth doped Ba2B5O9Cl crystal: Evidence for the Bi0 model. Opt. Express 2012, 20, 22569–22578. [Google Scholar] [CrossRef] [PubMed]
- Zorenko, Y.; Gorbenko, V.; Voznyak, T.; Nikl, M.; Beitlerova, A.; Jary, V. Bi3+- Ce3+ energy transfer and luminescent properties of LuAG:Bi,Ce and YAG:Bi,Ce single crystalline films. J. Lumin. 2013, 134, 539–543. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Comanzo, H.A. The ultraviolet and visible luminescence of Bi3+ in the orthorhombic perovskite, GdAlO3. Opt. Mater. 2017, 63, 118–121. [Google Scholar] [CrossRef]
- Zhu, N.-F.; Li, Y.-X.; Yu, X.-F. Luminescence spectra of YGG:RE3+, Bi3+ RE=Eu and Tb) and energy transfer from Bi3+ to RE3+. Chin. Phys. Lett. 2008, 25, 703–706. [Google Scholar]
- Oomen, E.W.J.L.; Blasse, G. Luminescence of Bi3+ in the metaphosphates LnP309 (Ln = Sc, Lu, Y, Gd, La). J. Solid St. Chem. 1988, 75, 201–204. [Google Scholar] [CrossRef]
- van de Craats, A.M.; Blasse, G. The quenching of bismuth(III) luminescence in yttrium oxide (Y2O3). Chem. Phys. Lett. 1995, 243, 559–563. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Niemeijer-Berghuijs, L.C.; Dorenbos, P. Luminescence and charge carrier trapping in YPO4:Bi. Opt. Mater. 2017, 66, 351–355. [Google Scholar] [CrossRef]
- Srivastava, A.M. Luminescence of Bi3+ in the orthorhombic perovskites CaB4+O3 (B4+ = Zr, Sn): Crossover from localized to D-state emission. Opt. Mater. 2016, 58, 89–92. [Google Scholar] [CrossRef]
- Srivastava, A.M. Luminescence of Bi3+ in the orthorhombic perovskites, SrB4+O3 (B4+ = Zr, Sn). Opt. Mater. 2017, 72, 313–315. [Google Scholar] [CrossRef]
- Srivastava, A.M. Luminescence of Bi3+ in LaGaO3. Mater. Res. Bull. 1999, 34, 1391–1396. [Google Scholar] [CrossRef]
- van Steensel, L.I.; Bokhove, S.G.; van de Craats, A.M.; de Blank, J.; Blasse, G. The luminescence of Bi3+ in LaInO3 and some other perovskites. Mater. Res. Bull. 1995, 30, 1359–1362. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Comanzo, H.A.; Brik, M.G. The nature of Bi3+ luminescence in the double perovskite La2LiSbO6. J. Lumin. 2017, 192, 620–625. [Google Scholar] [CrossRef]
- Srivastava, A.M. On the luminescence of Bi3+ in the pyrochlore Y2Sn2O7. Mater. Res. Bull. 2002, 37, 745–751. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Beers, W.W. On the impurity trapped exciton luminescence in La2Zr2O7:Bi3+. J. Lumin. 1999, 81, 293–300. [Google Scholar] [CrossRef]
- Kiliaan, H.S.; Blasse, G. A study of the sensitizer in the luminescent systems (Y,Gd)2O2SO4:Bi,Tb and Li6(Y,Gd)(BO3)3:S,Tb (S = Ce3+, Pr3+ or Bi3+). Mater. Chem. Phys. 1987, 18, 155–170. [Google Scholar] [CrossRef]
- Nagirnyi, V.; Feldbach, E.; Jönsson, L.; Kirm, M.; Kotlov, A.; Svensson, G.; Asberg-Dahlborg, M. Relaxation of electronic excitations in PbWO4 and CaWO4:Bi crystals. In Proceedings of the Fifth Int. Conf. on Inorganic Scintillators and Their Applications (SCINT99), Moscow, Russia, 16–20 August 1999; Mikhailin, V., Ed.; Moscow State University Press: Moscow, Russia, 2000; pp. 315–320. [Google Scholar]
- Nagirnyi, V.; Kotlov, A.; Jönsson, L.; Kirm, M.; Lushchik, A. Emission decay kinetics in a CaWO4:Bi crystal. Nucl. Instrum. Methods Phys. Res. A 2005, 537, 61–65. [Google Scholar] [CrossRef]
- Zorenko, Y.; Pashkovsky, M.; Voloshinovskii, A.; Kuklinski, B.; Grinberg, M. The luminescence of CaWO4:Bi single crystals. J. Lumin. 2006, 116, 43–51. [Google Scholar] [CrossRef]
- Park, T.-K.; Ahn, H.-C.; Mho, S.-I. High concentration of Bi3+ incorporated into RNbO4:Eu3+ (R = La, Y, Gd) as red phosphors for white LED applications. J. Korean. Phys. Soc. 2008, 52, 431–434. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Comanzo, H.A.; Camardello, S.J. On the “Bi3+–Ti4+” charge transfer transition in the pyrochlore Y2Ti2O7:Bi3+. Opt. Mater. 2015, 48, 31–35. [Google Scholar] [CrossRef]
- Cao, R.; Fu, T.; Xu, H.; Luo, W.; Peng, D.; Chen, Z.; Fu, J. Synthesis and luminescence enhancement of CaTiO3:Bi yellow phosphor by codoping Al3+/B3+ ions. J. Alloy. Comp. 2016, 674, 51–55. [Google Scholar] [CrossRef]
- Cavalli, E.; Angiuli, F.; Mezzadri, F.; Trevisani, M.; Bettinelli, M.; Boutinaud, P.; Brik, M. Tunable luminescence of Bi3+-doped YPxV1−xO4 (0 ≤ x ≤1). J. Phys. Condens. Matter 2014, 26, 385503. [Google Scholar] [CrossRef] [PubMed]
- Madej, A.; Witkowski, M.E.; Wojtowicz, A.J.; Zych, E. Photo- and radioluminescent properties of undoped and Bi-doped Lu2WO6 powders at 10–300K. J. Lumin. 2015, 160, 50–56. [Google Scholar] [CrossRef]
- Cao, R.; Quan, G.; Shi, Z.; Luo, Z.; Hu, Q.; Guo, S. A double perovskite Ca2MgWO6:Bi yellow-emitting phosphor: Synthesis and luminescence properties. J. Lumin. 2017, 181, 332–336. [Google Scholar] [CrossRef]
- Jacobs, P.W.M. Alkali halide crystals containing impurity ions with the ns2 ground-state electronic configuration. J. Phys. Chem. Solids 1991, 52, 35–67. [Google Scholar] [CrossRef]
- Zazubovich, S. Polarization spectroscopy of ns2 impurity ions in alkali halides. Int. J. Mod. Phys. B 1994, 8, 985–1031. [Google Scholar] [CrossRef]
- Zavt, G.S.; Ellervee, A.F. Pb2+ and Bi3+ impurity centres in alkali-earth oxides: Vibronic spectra, lattice dynamics, and electron-phonon interaction. Phys. Status Solidi B 1979, 94, 757–768. [Google Scholar] [CrossRef]
- Seitz, F. Interpretation of the properties of alkali halide-thallium phosphors. J. Chem. Phys. 1938, 6, 150–162. [Google Scholar] [CrossRef]
- Hizhnyakov, V.V.; Kristoffel, N.N. Jahn-Teller mercury-like impurities in ionic crystals. In The Dynamical Jahn-Teller Effect in Localized Systems; Perlin, Y., Wagner, M., Eds.; Elsevier Publ. Co.: Amsterdam, The Netherlands, 1984; Chapter 9; pp. 383–437. [Google Scholar]
- Hizhnyakov, V.; Zazubovich, S.; Soovik, T. Kinetics and temperature dependences of polarized emission of anisotropic tin centres in alkali halides. Phys. Status Solidi B 1974, 66, 727–738. [Google Scholar] [CrossRef]
- Hizhnyakov, V.V. Adiabatic Potential Energy Surface and Luminescence CHARACTERISTICS of Impurity Centers with Two Optical Electrons; Preprint FI-36; Institute of Physics: Tartu, Estonia, 1975; 27p. [Google Scholar]
- Zazubovich, S.G.; Hizhnyakov, V.V. Polarized luminescence and a new model of mercury-like centers in ionic crystals. Izv. Akad. Nauk SSSR Ser. Fiz. 1985, 49, 1874–1879. [Google Scholar]
- Nagirnyi, V.; Soovik, T.; Vaino, P.; Zazubovich, S.; Jaanson, N. Triplet relaxed excited state structure and luminescence of thallium-doped alkali halides. Phys. Status Solidi B 1991, 164, 493–502. [Google Scholar] [CrossRef]
- Zazubovich, S.G.; Lushchik, N.E. Spectra of luminescence centres of crystals doped with isoelectronic ions. Trudy IFA AN EstSSR 1961, 14, 283–285. [Google Scholar]
- Blasse, G. Optical electron transfer between metal ions and its consequences. In Complex Chemistry (Structure and Bonding Series); Springer: Berlin, Germany, 1991; Volume 76, pp. 153–187. [Google Scholar]
- Reut, E.G.; Ryskin, A.I. Virtual recharge: Mechanism of radiationless transition in scheelite and fergusonite type crystals doped with rare-earth ions. Phys. Status Solidi A 1973, 17, 47–57. [Google Scholar] [CrossRef]
- Anitha, M.; Ramakrishnan, P.; Chatterjee, A.; Alexander, G.; Singh, H. Spectral properties and emission efficiencies of GdVO4 phosphors. Appl. Phys. A 2002, 74, 153–162. [Google Scholar] [CrossRef]
- Mahalley, B.N.; Dhoble, S.J.; Pode, R.B.; Alexander, G. Photoluminescence in GdVO4:Bi3+,Eu3+ red phosphor. Appl. Phys. A Mater. Sci. Process. 2000, 70, 39–45. [Google Scholar] [CrossRef]
- Xie, W.; Tian, C.; Lyu, F.; Wang, Z.; Zou, C.; Kang, F.; Dong, H.; Sun, G. Toward temperature-dependent Bi3+-related tunable emission in the YVO4:Bi3+ phosphor. J. Am. Ceram. Soc. 2019, 102, 3488–3497. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Zorenko, T.; Savchun, V.; Voloshinovskii, A. Luminescent and scintillation properties of CaWO4 and CaWO4:Bi single crystalline films. In Proceedings of the International Conference on Oxide Materials for Electronic Engineering—Fabrication, Properties and Applications, Lviv, Ukraine, 26–30 May 2014; pp. 253–254. [Google Scholar]
- Kang, F.; Peng, M.M. A new study on the energy transfer in the color-tunable phosphor CaWO4:Bi. Dalton. Trans. 2014, 43, 277–284. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Voznyak, T.; Konstankevych, I.; Savchyn, V.; Batentschuk, M.; Winnacker, A.; Brabec, C.J. Scintillators based on CdWO4 and CdWO4:Bi single crystalline films. IEEE Trans. Nucl. Sci. 2012, 59, 2281–2285. [Google Scholar] [CrossRef]
- Shin, S.H.; Jeon, D.Y.; Suh, K.S. Charge-transfer nature in luminescence of YNbO4:Bi blue phosphor. J. Appl. Phys. 2001, 90, 5986–5990. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, X.; Gou, J.; Duan, L.; Su, X.; Fan, G.; Duan, Y. Design, luminescence and energy transfer of single-phased color-tunable YNbO4:Bi3+, Eu3+, phosphor for UV pumped white light-emitting diodes. J. Mater. Sci. Mater. Electron. 2017, 28, 3630–3636. [Google Scholar] [CrossRef]
- Zhou, R.; Kou, Y.; Wei, X.; Duan, C.; Chen, Y.; Yin, M. Broadband downconversion based near-infrared quantum cutting via cooperative energy transfer in YNbO4:Bi3+, Yb3+ phosphor. Appl. Phys. B 2012, 107, 483–487. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Salamo, G.J.; Li, Y.; He, L.; Yang, G.; Gao, Y.; Liu, Q. Sensitized intense near-infrared downconversion quantum cutting three-photon luminescence phenomena of the Tm3+ ion activator in Tm3+Bi3+:YNbO4 powder phosphor. Opt. Express 2015, 23, A51–A61. [Google Scholar] [CrossRef]
- Bang, J.H.; Oh, E.S.; Seo, S.H.; Kim, J.S.; Lee, M.; Park, H.L.; Lee, C.-I.; Kim, C.C.; Kim, K.J. Cathodoluminescence study of Bi3+-doped YNbO4 phosphor. Phys. Status Solidi A 2002, 191, 291–295. [Google Scholar] [CrossRef]
- Dorenbos, P. Charge transfer bands in optical materials and related defect level location. Opt. Mater. 2017, 69, 8–22. [Google Scholar] [CrossRef]
- Blasse, G.; de Mello Donega, C.; Berezovskaja, I.; Dotsenko, V. The luminescence of bismuth (III) in indium orthoborate. Solid St. Commun. 1994, 91, 29–31. [Google Scholar] [CrossRef]
- Tsiumra, V.; Zhyshkovych, A.; Malyi, T.; Chornodolskyy, Y.; Vistovskyy, V.; Syrotyuk, S.; Zhydachevskyy, Y.; Suchocki, A.; Voloshinovskii, A. Localized exciton luminescence in YVO4:Bi3+. Opt. Mater. 2019, 89, 480–487. [Google Scholar] [CrossRef]
- Jing, X.; Gibbons, C.; Nicholas, D.; Silver, J.; Vecht, A.; Frampton, C.S. Blue luminescence in yttrium and gadolinium niobates caused by bismuth. The importance of non-bonding ns2 valence orbital electrons. J. Mater. Chem. 1999, 9, 2913–2918. [Google Scholar] [CrossRef]
- Boutinaud, P. On the luminescence of Bi3+ pairs in oxidic compounds. J. Lumin. 2018, 197, 228–232. [Google Scholar] [CrossRef]
- Awater, R.H.P.; Dorenbos, P.P. Towards a general concentration quenching model of Bi3+ luminescence. J. Lumin. 2017, 188, 487–489. [Google Scholar] [CrossRef]
- Babin, V.; Fabeni, P.; Mihokova, E.; Nikl, M.; Stolovich, A.; Pazzi, G.P.; Zazubovich, S. Time-resolved spectroscopy of exciton states in undoped and doped CsI crystals. In Proceedings of the Fifth International Conference on Inorganic Scintillators and Their Applications (SCINT99), Moscow, Russia, 16–20 August 1999; Mikhailin, V., Ed.; Moscow State University Press: Moscow, Russia, 2000; pp. 544–549. [Google Scholar]
- Zazubovich, S. Physics of halide scintillators. Radiat. Meas. 2001, 33, 699–704. [Google Scholar] [CrossRef]
- Buth, A.H.; Blasse, G. Luminescence and energy transfer in yttrium niobate (YNbO4). Phys. Status Solidi A 1981, 64, 669–676. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, H.; Liu, W.; Sun, D.; Zhang, Q. Experimental and first principle investigation the electronic and optical properties of YNbO4 and LuNbO4 phosphors. J. Mater. Sci. Mater. Electron. 2018, 29, 11878–11885. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Li, S.; Dai, Y.; Guo, H.; Tang, X.; Xie, Y.; Yan, L. Host-sensitized and tunable luminescence of GdNbO4:Ln3+ (Ln3+ = Eu3+/Tb3+/Tm3+) nanocrystalline phosphors with abundant color. Inorg. Chem. 2016, 55, 10383–10396. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Luminescence phenomena in compounds with fergusonite structure. J. Lumin. 1970, 3, 109–131. [Google Scholar] [CrossRef]
- Moncorge, R.; Boulon, G. Investigations of the absorption and emission properties along with energy transfer in pure and Bi3+ doped YVO4. J. Lumin. 1979, 18–19, 376–380. [Google Scholar] [CrossRef]
- Zorenko, Y.; Voloshinovskii, A.; Savchyn, V.; Voznyak, T.; Nikl, M.; Nejezchleb, K.; Mikhailin, V.; Kolobanov, V.; Spassky, D. Exciton and antisite defect-related luminescence in Lu3Al5O12 and Y3Al5O12 garnets. Phys. Status Solidi B 2007, 244, 2180–2189. [Google Scholar] [CrossRef]
- Babin, V.; Bichevin, V.; Gorbenko, V.; Kink, M.; Makhov, A.; Maksimov, Y.; Nikl, M.; Stryganyuk, G.; Zazubovich, S.; Zorenko, Y. Time-resolved spectroscopy of exciton-related states in single crystals and single crystalline films of Lu3Al5O12 and Lu3Al5O12:Ce. Phys. Status Solidi B 2011, 248, 1505–1512. [Google Scholar] [CrossRef]
- Babin, V.; Blazek, K.; Krasnikov, A.; Nejezchleb, K.; Nikl, M.; Savikhina, T.; Zazubovich, S. Luminescence of undoped LuAG and YAG crystals. Phys. Status Solidi 2005, C 2, 97–100. [Google Scholar] [CrossRef]
- Mürk, V.; Yaroshevich, N. Exciton and recombination processes in YAG crystals. J. Phys. Condens. Matter 1995, 7, 5857–5864. [Google Scholar] [CrossRef]
- Zorenko, Y.; Zorenko, T.; Vistovskyy, V.; Grinberg, M.; Łukasiewicz, T. Time-resolved spectroscopy of intrinsic luminescence of Y3Ga5O12 and (LaLu)3Lu2Ga3O12 single crystals. Opt. Mater. 2009, 31, 1835–1838. [Google Scholar] [CrossRef][Green Version]
- Lammers, M.J.J.; Severin, J.M.; Blasse, G. The luminescence properties of unactivated and Tb3+-activated Gd3Ga5O12. J. Electrochem. Soc. Solid State Sci. Technol. 1987, 134, 2356–2358. [Google Scholar]
- Babin, V.; Gorbenko, V.; Kondakova, I.; Kärner, T.; Laguta, V.V.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Time-resolved spectroscopy of exciton states in single crystals and single crystalline films of YAlO3 and YAlO3:Ce. J. Phys. D Appl. Phys. 2011, 44, 315402. [Google Scholar] [CrossRef]
- Korzhik, M.V.; Trower, W.P. Origin of scintillation in cerium-doped oxide crystals. Appl. Phys. Lett. 1995, 66, 2327–2328. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Voloshinovskii, A.; Vistovskii, V.; Nikl, M.; Mihokova, E.; Nejezchleb, K. Intrinsic and Ce3+-related luminescence of single crystals and single crystalline films of YAP perovskites: New results. IEEE Trans. Nucl. Sci. 2008, 55, 1186–1191. [Google Scholar] [CrossRef]
- Zorenko, T.; Gorbenko, V.; Petrosyan, A.; Gieszczyk, W.; Bilski, P.; Zorenko, Y. Intrinsic and defect-related luminescence of YAlO3 and LuAlO3 single crystals and films. Opt. Mater. 2018, 86, 376–381. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Voznyak, T.; Mikhailin, V.; Kolobanov, V.; Spassky, D.; Nikl, M. Intrinsic and Ce3+-related luminescence in single crystalline films and single crystals of LuAP and LuAP:Ce perovskites. IEEE Trans. Nucl. Sci. 2008, 55, 1192–1196. [Google Scholar] [CrossRef]
- Nikl, M.; Bohacek, P.; Mihokova, E.; Kobayashi, M.; Ishii, M.; Usuki, Y.; Babin, V.; Stolovich, A.; Zazubovich, S.; Bacci, M. Excitonic emission of scheelite tungstates AWO4 (A = Pb, Ca, Ba, Sr). J. Lumin. 2000, 87–89, 1136–1139. [Google Scholar] [CrossRef]
- Babin, V.; Bohacek, P.; Bender, E.; Krasnikov, A.; Mihokova, E.; Nikl, M.; Senguttuvan, N.; Stolovits, A.; Usuki, Y.; Zazubovich, S. Decay kinetics of the green emission in tungstates and molybdates. Radiat. Meas. 2004, 38, 533–537. [Google Scholar] [CrossRef]
- Babin, V.; Bohachek, K.; Krasnikov, A.; Nikl, M.; Stolovits, A.; Zazubovich, S. Origin of green luminescence in PbWO4 crystals. J. Lumin. 2007, 124, 113–119. [Google Scholar] [CrossRef]
- Cooke, D.W.; Bennett, B.L.; Muenchausen, R.E.; Lee, J.-K.; Nastasi, M.A. Intrinsic ultraviolet luminescence from Lu2O3, Lu2SiO5 and Lu2SiO5:Ce3+. J. Lumin. 2004, 106, 125–132. [Google Scholar] [CrossRef]
- Jacobsohn, L.G.; Lee, J.-K.; Bennett, B.L.; Muenchausen, R.E.; Nastasi, M.; Cooke, D.W. Effects of ion beam irradiation on self-trapped defects in single-crystal Lu2SiO5. J. Lumin. 2007, 124, 5–9. [Google Scholar] [CrossRef]
- Zorenko, Y.; Zorenko, T.; Voznyak, T.; Sidletskiy, O. Intrinsic luminescence of Lu2SiO5 (LSO) and Y2SiO5 (YSO) orthosilicates. J. Lumin. 2013, 137, 204–207. [Google Scholar] [CrossRef]
- Meunier-Beillard, P.; Moine, B.; Dujardin, C.; Cieren, X.; Pedrini, C.; Huguenin, D.; Archambault, V. Exciton trapping in LaPO4 doped with trivalent cerium and/or terbium ions. Rad. Eff. Def. Solids 1999, 149, 25–30. [Google Scholar] [CrossRef]
- Wisniewski, D.; Tavernier, S.; Wojtowicz, A.J.; Wisniewska, M.; Bruyndonckx, P.; Dorenbos, P.; van Loef, E.; van Eijk, C.W.E.; Boatner, L.A. LuPO4:Nd and YPO4:Nd – new promising VUV scintillation materials. Nucl. Instrum. Meth. Phys. Res. A 2002, 486, 239–243. [Google Scholar] [CrossRef]
- Mikhailin, V.V.; Spassky, D.A.; Kolobanov, V.N.; Meotishvili, A.A.; Permenov, D.G.; Zadneprovski, B.I. Luminescence study of the LuBO3 and LuPO4 doped with RE3+. Radiat. Meas. 2010, 45, 307–310. [Google Scholar] [CrossRef]
- Knitel, M.J.; Dorenbos, P.; van Eijk, C.W.E.; Plasteig, B.; Viana, B.; Kahn-Harari, A.; Vivien, D. Photoluminescence, and scintillation/thermoluminescence yields of several Ce3+ and Eu2+ activated borates. Nucl. Instrum. Meth. Phys. Res. A 2000, 443, 364–374. [Google Scholar] [CrossRef]
- Nagirnyi, V.; Stolovich, A.; Zazubovich, S.; Zepelin, V.; Mihokova, E.; Nikl, M.; Pazzi, G.P.; Salvini, L. Peculiarities of the triplet relaxed excited-state structure and luminescence of a CsI:Tl crystal. J. Phys. Condens. Matter 1995, 7, 3637–3653. [Google Scholar] [CrossRef]
- Mihokova, E.; Nagirnyi, V.; Nikl, M.; Stolovich, A.; Pazzi, G.P.; Zazubovich, S.; Zepelin, V. Relaxed excited state structure and luminescence of thallium-doped caesium chloride and bromide. J. Phys. Condens. Matter 1996, 8, 4301–4314. [Google Scholar] [CrossRef]
- Aceves, R.; Babin, V.; Barboza Flores, M.; Fabeni, P.; Mihokova, E.; Nagirnyi, V.; Nikl, M.; Nitsch, K.; Pazzi, G.P.; Perez Salas, R.; et al. Coexistence of the impurity and perturbed exciton levels in the relaxed excited state of CsCl:Pb crystal. J. Phys. Condens. Matter 1998, 10, 5449–5461. [Google Scholar] [CrossRef]
- Aceves, R.; Babin, V.; Barboza Flores, M.; Fabeni, P.; Mihokova, E.; Nikl, M.; Nitsch, K.; Pazzi, G.P.; Perez Salas, R.; Zazubovich, N.; et al. Relaxed excited states origin and structure in lead-doped caesium bromide. Phys. Status Solidi B 2001, 223, 745–756. [Google Scholar] [CrossRef]
- Babin, V.; Krasnikov, A.; Nikl, M.; Nitsch, K.; Stolovitš, A.; Zazubovich, S. Luminescence and relaxed excited state origin in CsI:Pb crystals. J. Lumin. 2003, 101, 219–226. [Google Scholar] [CrossRef]
- Vazhenin, V.A.; Potapov, A.P.; Asatryan, G.R.; Nikl, M. Photosensitive bismuth ions in lead tungstate. Phys. Solid St. 2013, 55, 803–806. [Google Scholar] [CrossRef]
- Yadav, S.K.; Uberuaga, B.P.; Nikl, M.; Jiang, C.; Stanek, C.R. Band-gap and band-edge engineering of multicomponent garnet scintillators from first principles. Phys. Rev. Appl. 2015, 4, 054012. [Google Scholar] [CrossRef]
- Babin, V.; Buryi, M.; Chlan, V.; Kamada, K.; Laguta, V.V.; Nikl, M.; Pejchal, J.; Štěpánková, H.; Yoshikawa, A.; Fomichov, Y.; et al. Influence of gallium content on Ga3+ position and photo- and thermally stimulated luminescence in Ce3+-doped multicomponent (Y,Lu,)3GaxAl5−xO12 garnets. J. Lumin. 2018, 200, 141–150. [Google Scholar] [CrossRef]
- Babin, V.; Hanus, M.; Krasnikov, A.; Kučera, M.; Nikl, M.; Zazubovich, S. Determination of the position of the 5d excited levels of Ce3+ ions with respect to the conduction band in the epitaxial films of the multicomponent (Lu,Gd)3(Ga,Al)5O12:Ce garnets. Opt. Mat. 2016, 62, 465–474. [Google Scholar] [CrossRef]
- Babin, V.; Chernenko, K.; Kučera, M.; Nikl, M.; Zazubovich, S. Photostimulated luminescence and defects creation processes in Ce3+-doped epitaxial films of multicomponent Lu3-xGdxGayAl5-yO12 garnets. J. Lumin. 2016, 179, 487–495. [Google Scholar] [CrossRef]
- Ogiegło, J.M.; Katelnikovas, A.; Zych, A.; Jüstel, T.; Meijerink, A.; Ronda, C.R. Luminescence and luminescence quenching in Gd3(Ga,Al)5O12 scintillators doped with Ce3+. J. Phys. Chem. A 2013, 117, 2479–2484. [Google Scholar] [CrossRef]
- Nikl, M.; Kamada, K.; Kurosawa, S.; Yokota, Y.; Yoshikawa, A.; Pejchal, J.; Babin, V. Luminescence and scintillation mechanism in Ce3+ and Pr3+ doped (Lu,Y,Gd)3(Ga,Al)5O12 single crystal scintillators. Phys. Status Solidi C 2013, 10, 172–175. [Google Scholar] [CrossRef]
- Yousif, A.; Vinod, K.; Seed Ahmed, H.A.A.; Som, S.; Noto, L.L.; Ntwaeaborwa, O.M.; Swart, H.C. Effect of Ga3+ doping on the photoluminescence properties of Y3Al5-xGaxO12:Bi3+ phosphor. ECS J. Solid St. Sci. Technol. 2014, 3, R222–R227. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Voznyak, T.; Jary, V.; Nikl, M. Luminescence spectroscopy of the Bi3+ single and dimer centers in Y3Al5O12:Bi single crystalline films. J. Lumin. 2010, 130, 1963–1969. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Zorenko, T.; Malinowski, P.; Jary, V.; Kucerkova, R.; Beitlerova, A.; Mares, J.A.; Nikl, M.; Fedorov, A. Luminescent and scintillation properties of Bi3+ doped Y2SiO5 and Lu2SiO5 single crystalline films. J. Lumin. 2014, 154, 525–530. [Google Scholar] [CrossRef]
- Fukuda, A. Absorption bands of paired-ion centers (Ga+)2, (In+)2 and (Tl+)2 in KI. J. Phys. Soc. Jpn. 1969, 26, 1006–1013. [Google Scholar] [CrossRef]
- Ermoshkin, A.; Evarestov, R.; Gindina, R.; Maaroos, A.; Osminin, V.; Zazubovich, S. Monovalent mercury-like ion dimer centres in alkali halide crystals. Phys. Status Solidi B 1975, 70, 749–758. [Google Scholar] [CrossRef]
- Tsuboi, T. Geometrical structure of (Tl+)2 luminescent center in alkali halides: Presence of D2h and D4h dimer centers. Phys. Rev. B 1984, 29, 1022–1029. [Google Scholar] [CrossRef]
- Zazubovich, S.; Lushchik, N.E.; Lushchik, C.B. Optical structure of luminescence centres in ionic crystals doped with mercury-like ions. Soviet Phys. Opt. Spectrosc. 1963, 15, 381–388. [Google Scholar]
- Tsuboi, T.; Jacobs, P.W.M. Optical studies of s2-ion dimer centers in alkali halide crystals. J. Phys. Chem. Solids 1991, 52, 69–80. [Google Scholar] [CrossRef]
- de Vries, A.J.; Blasse, G. Energy migration in gadolinium compounds. J. de Phys. Colloq. 1985, 46, C7–C109. [Google Scholar] [CrossRef]
- Nagli, L.E.; Dyachenko, S.V. Influence of a vc− Vacancy on Luminescence of Pb+ Centres in Alkali Halides. Phys. Stat. Sol. B 1988, 146, 295–301. [Google Scholar] [CrossRef]
- Mollenauer, L.F.; Vieira, N.D.; Szeto, L. Optical properties of the Tl0(1) center in KCl. Phys. Rev. B 1983, 27, 5332–5346. [Google Scholar] [CrossRef]
- Fockele, M.; Ahlers, F.J.; Lohse, F.; Spaeth, J.-M.; Bartram, R.H. Optical properties of atomic thallium centres in alkali halides. J. Phys. C Solid State Phys. 1985, 18, 1963. [Google Scholar] [CrossRef]
- Hamstra, M.A.; Folkerts, H.F.; Blasse, G. Red bismuth emission in alkaline-earth-metal sulfates. J. Mater. Chem. 1994, 4, 1349–1350. [Google Scholar] [CrossRef]
- Srivastava, A.M. Luminescence of divalent bismuth in M2+BPO5 (M2+ = Ba2+, Sr2+ and Ca2+). J. Lumin. 1998, 78, 239–243. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, T.; Pei, Z.; Qiang, S.U. Luminescence of unusual bismuth in barium borates (BaB8O13:Bi). J. Mater. Sci. Techn. 1999, 15, 282. [Google Scholar]
- Gaft, M.; Reisfeld, R.; Panczer, G.; Boulon, G.; Saraidarov, T.; Erlish, S. The luminescence of Bi, Ag and Cu in natural and synthetic barite BaSO4. Opt. Mater. 2001, 16, 279–290. [Google Scholar] [CrossRef]
- Peng, M.; Wondraczek, L. Photoluminescence of Sr2P2O7:Bi2+ as a red phosphor for additive light generation. Opt. Lett. 2010, 35, 2544–2546. [Google Scholar] [CrossRef]
- Cao, R.; Shang, F.; Liao, C.; Qiu, J. Yellow-to-orange emission from Bi2+-doped RF2 (R = Ca and Sr) phosphors. Opt. Express 2013, 21, 15728–15733. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Peng, M. A New Red Aluminate Phosphor CaAl12O19 Activated by Bi2+ for White LEDs. Sci. Adv. Mater. 2017, 9, 485–489. [Google Scholar] [CrossRef]
- Jong, M.; Meijerink, A.; Gordon, R.A.; Barandiaran, Z.; Seijo, L. Is Bi2+ Responsible for the Red-Orange Emission of Bismuth-Doped SrB4O7? J. Phys.Chem. C 2014, 118, 9696–9705. [Google Scholar] [CrossRef]
- Peng, M.; Wondraczek, L. Bi2+-doped strontium borates for white-light-emitting diodes. Opt. Lett. 2009, 34, 2885–2887. [Google Scholar] [CrossRef]
- Jong, M.; Meijerink, A.; Barandiaran, Z.; Seijo, L. Structure and Hindered Vibration of Bi2+ in the Red-Orange Phosphor SrB4O7:Bi. J. Phys. Chem. C 2014, 118, 17932–17939. [Google Scholar] [CrossRef]
- Peng, M.; Wondraczek, L. Orange-to-Red Emission from Bi2+ and Alkaline Earth Codoped Strontium Borate Phosphors for White Light Emitting Diodes. J. Am. Ceram. Soc. 2010, 93, 1437–1442. [Google Scholar] [CrossRef]
- Li, L.; Peng, M.; Viana, B.; Wang, J.; Lei, B.; Liu, Y.; Zhang, Q.; Qiu, J. Unusual Concentration Induced Antithermal Quenching of the Bi2+ Emission from Sr2P2O7:Bi2+. Inorg. Chem. 2015, 54, 6028–6034. [Google Scholar] [CrossRef]
- Cao, R.; Peng, M.; Qiu, J. Luminescence from Bi2+-doped BaSO4 for White LEDs. Opt. Express 2012, 20, A977–A983. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Fujimoto, Y.; Kanabe, T.; Fujita, H.; Nakatsuka, M. Bi-doped SiO as a new laser material for an intense laser. Fusion Eng. Des. 1999, 44, 437–439. [Google Scholar] [CrossRef]
- Bjerrum, N.; Boston, C.; Smith, G. Lower oxidation states of bismuth. Bi+ and Bi53+ in molten salt solutions. Inorg. Chem. 1967, 6, 1162–1172. [Google Scholar] [CrossRef]
- Zheng, J.; Tan, L.; Wang, L.; Peng, M.; Xu, S. Superbroad visible to NIR photoluminescence from Bi+ evidenced in Ba2B5O9Cl:Bi crystal. Opt. Express 2016, 24, 2830–2835. [Google Scholar] [CrossRef]
- Lyu, T.; Dorenbos, P. Bi3+ acting both as an electron and as a hole trap in La-,Y-, and LuPO4. J. Mater. Chem. C 2018, 6, 6240–6249. [Google Scholar] [CrossRef]
- Zazubovich, S.G.; Lushchik, N.E. Alkali halide crystals doped with bismuth and antimony. Trudy IFA AN EstSSR 1961, 17, 50–66. [Google Scholar]
- Lushchik, C.B.; Gindina, R.I.; Zazubovich, S.G.; Lushchik, N.E. Polarization characteristics of some alkali halide phosphors. Trudy IFA AN EstSSR 1961, 17, 38–49. [Google Scholar]
- Zazubovich, S.G. Polarized luminescence of alkali halides doped with mercury-like ions. Trudy IFA AN EstSSR 1969, 36, 109–153. [Google Scholar]
- Ewles, J.; Curry, C. Resolution and analysis of low temperature luminescent spectra of Bi and Pb activated solids of simple crystal structure. Proc. Phys. Soc. A 1950, 63, 708–715. [Google Scholar] [CrossRef]
- Runciman, W.A. Absorption and emission spectra of bismuth-activated phosphors. Proc. Phys. Soc. A 1955, 68, 647–649. [Google Scholar] [CrossRef]
- Lushchik, C.; Lushchik, N.; Muuga, I. Vibronic spectra of crystals activated with mercury-like ions. Trudy Ins. Fiz. Astron. Akad. Nauk EstSSR 1963, 23, 22–37. [Google Scholar]
- Hughes, A.E.; Runciman, W.A. Stress and Zeeman spectra in bismuth-doped and undoped calcium oxide. J. Phys. C Solid State Phys. 1969, 2, 37–45. [Google Scholar] [CrossRef]
- Lehmann, W. Calcium oxide phosphors. J. Lumin. 1973, 6, 455–470. [Google Scholar] [CrossRef]
- Hughes, A.E.; Pells, G.P. Absorption and luminescence of bismuth ions implanted into CaO and MgO single crystals. Phys. Status Solidi A 1974, 25, 437–443. [Google Scholar] [CrossRef]
- Runciman, W.A.; Manson, N.B.; Marshall, M. Magnetic effect on the emission of bismuth-doped calcium oxide. J. Lumin. 1976, 12/13, 413–417. [Google Scholar] [CrossRef]
- van der Steen, A.C.; Dijcks, L.T.F. The luminescence properties of alkaline-earth oxides activated with 6s2 ions. Phys. Status Solidi B 1981, 104, 283–292. [Google Scholar] [CrossRef]
- Randoshkin, V.V.; Vasil’eva, N.V.; Kolobanov, V.N.; Mikhailin, V.V.; Petrovin, N.N.; Spasskii, D.A.; Sysoev, N.N. Effect of Gd2O3 concentration in Bi-containing high-temperature solutions on the luminescence of epitaxial Gd3Ga5O12 films. Inorg. Mater. 2009, 45, 418–422. [Google Scholar] [CrossRef]
- Luchechko, A.; Syvorotka, I.I.; Zakharko, Y.; Syvorotka, I.M. Growing features and luminescence of Bi3+ ions in Gd3Ga5O12 epitaxial films. Solid State Phenom. 2013, 200, 215–219. [Google Scholar]
- Lushchik, C.; Feldbach, E.; Frorip, A.; Kirm, M.; Lushchik, A.; Maaroos, A.; Martinson, I. Multiplication of electronic excitations in CaO and YAlO3 crystals with free and self-trapped excitons. J. Phys. Condens. Matter 1994, 6, 11177–11187. [Google Scholar] [CrossRef]
- Auffrey, E.; Korjik, M.; Shalapska, T.; Zazubovich, S. Photoluminescence and excited states dynamics in PbWO4:Pr3+ crystals. J. Lumin. 2014, 154, 381–386. [Google Scholar] [CrossRef]
- Laguta, V.V.; Nikl, M.; Zazubovich, S. Photothermally stimulated creation of electron and hole centers in Ce3+-doped Y2SiO5 single crystals. Opt. Mater. 2014, 36, 1636–1641. [Google Scholar] [CrossRef]
- Babin, V.; Kalder, K.; Krasnikov, A.; Zazubovich, S. Luminescence and defects creation under photoexcitation of CsI:Tl crystals in Tl+-related absorption bands. J. Lumin. 2002, 96, 75–85. [Google Scholar] [CrossRef]
- Verwey, J.W.M.; Blasse, G. Luminescence efficiency of ions with broad-band excitation in lithium lanthanum phosphate glass. Chem. Mater. 1990, 2, 458–463. [Google Scholar] [CrossRef]
- Boujelbene, M.; Mhiri, T. Synthesis and crystal structure of LiLaP4O12. X-ray Struct. Anal. Online 2011, 27, 21–22. [Google Scholar] [CrossRef][Green Version]
- Jüstel, T.; Huppertz, P.; Mayr, W.; Wiechert, D.U. Temperature-dependent spectra of YPO4:Me (Me = Ce, Pr, Nd, Bi). J. Lumin. 2004, 106, 225–233. [Google Scholar] [CrossRef]
- Dorenbos, P.; Krumpel, A.H.; van der Kolk, E.; Boutinaud, P.; Bettinelli, M.; Cavalli, E. Lanthanide level location in transition metal complex compounds. Opt. Mater. 2010, 32, 1681–1685. [Google Scholar] [CrossRef]
- Newport, A.; Silver, J.; Vecht, A. The synthesis of fine particle yttrium vanadate phosphors from spherical powder precursors using urea precipitation. J. Electrochem. Soc. 2000, 147, 3944–3947. [Google Scholar] [CrossRef]
- Takeshita, S.; Isobe, T.; Sawayama, T.; Niikura, S. Effects of the homogeneous Bi3+ doping process on photoluminescence properties of YVO4:Bi3+, Eu3+ nanophosphor. J. Lumin. 2009, 129, 1067–1072. [Google Scholar] [CrossRef]
- Boutinaud, P. Optical processes in (Y,Bi)VO4 doped with Eu3+ or Pr3+. J. Phys. Condens. Matter 2014, 26, 405501. [Google Scholar] [CrossRef] [PubMed]
- Mahlik, S.; Amer, M.; Boutinaud, P. Energy level structure of Bi3+ in zircon and scheelite polymorphs of YVO4. J. Phys. Chem. 2016, 120, 8261–8265. [Google Scholar] [CrossRef]
- Lee, S.K.; Chang, H.; Han, C.H.; Kim, H.J.; Jang, H.G.; Park, H.D. Electronic structures and luminescence properties of YNbO4 and YNbO4:Bi. J. Solid St. Chem. 2001, 156, 267–273. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).