Angle-Resolved Photoemission Study on the Band Structure of Organic Single Crystals
Abstract
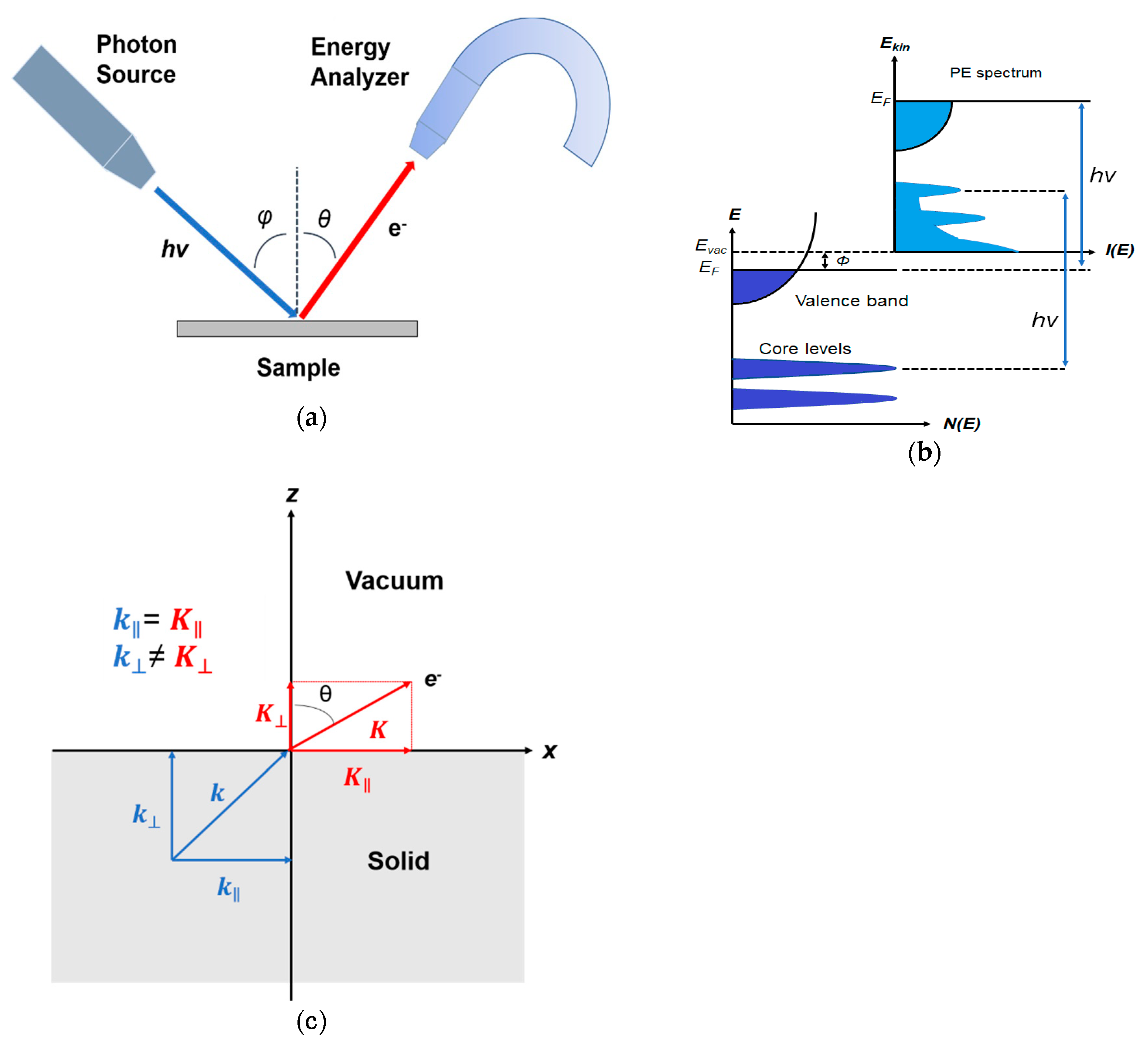
1. Introduction
2. Organic Charge Transfer Conductors
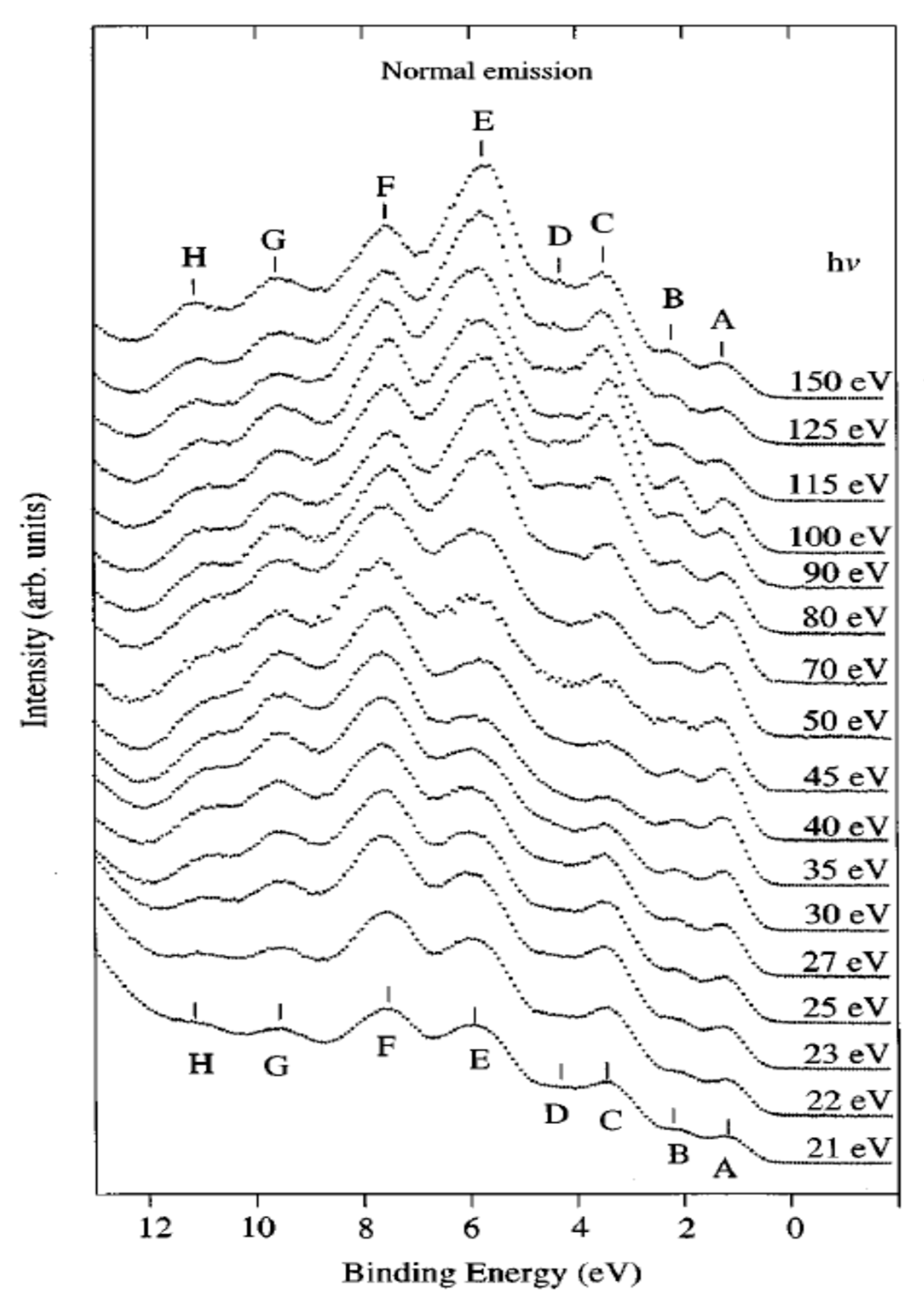
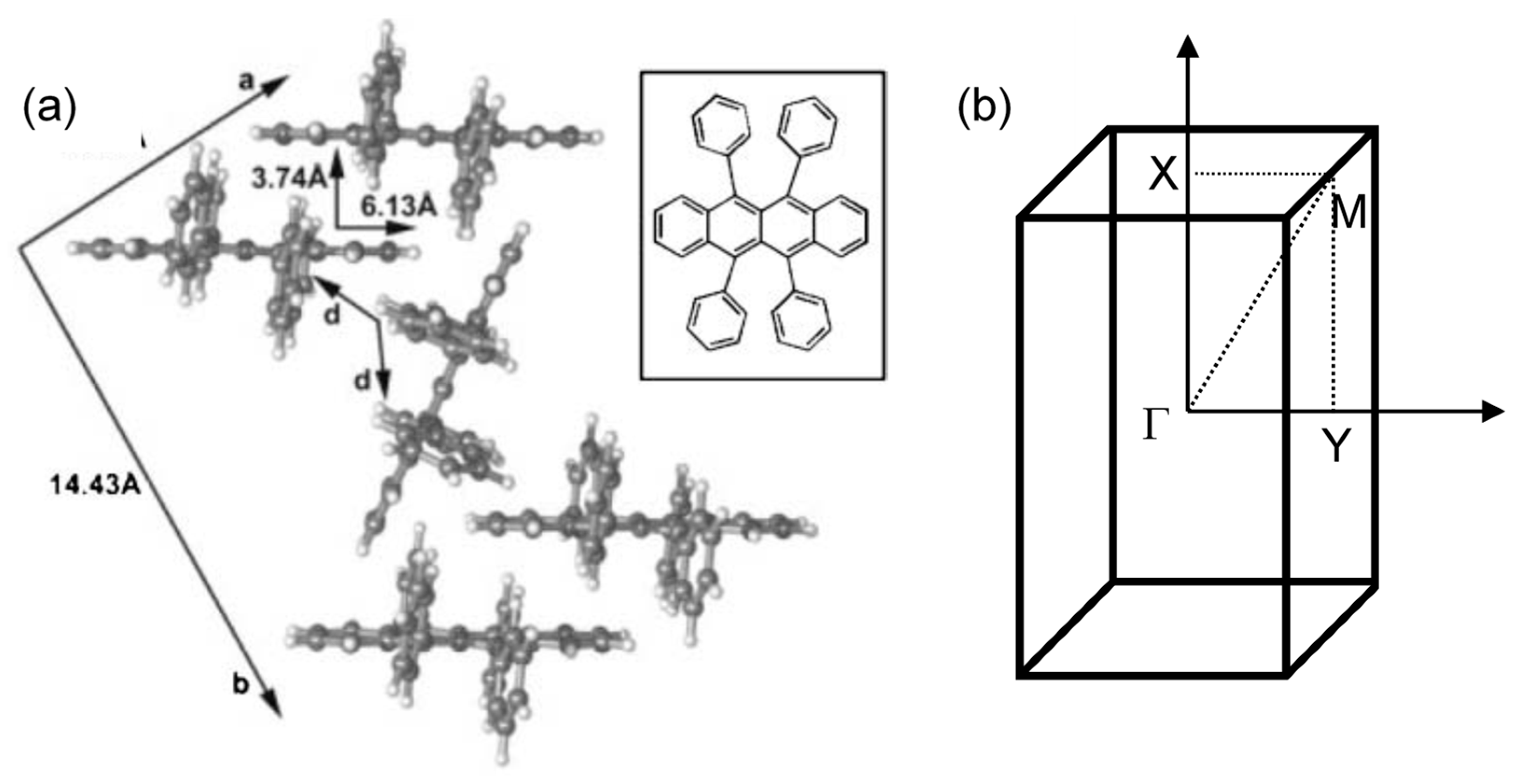
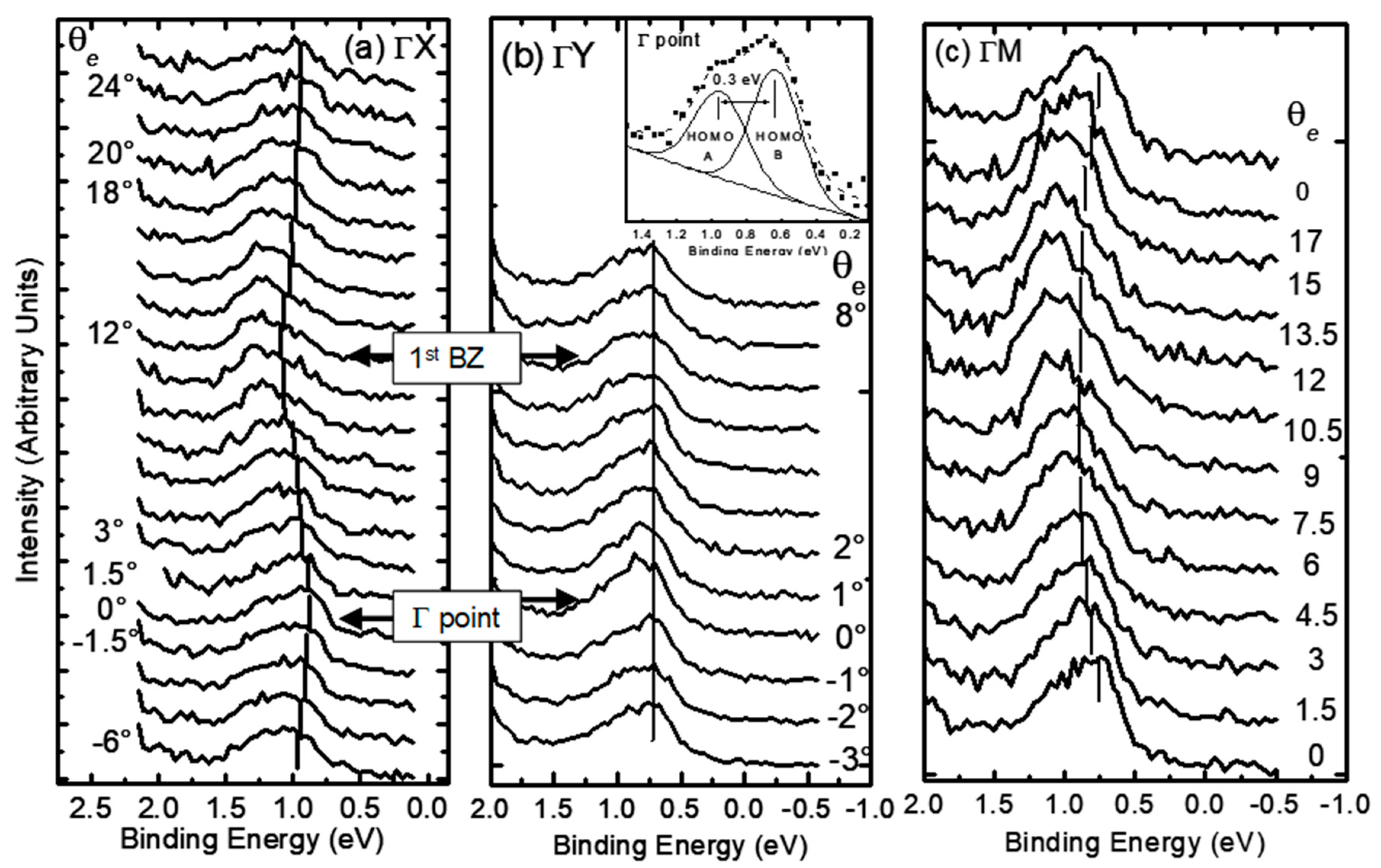
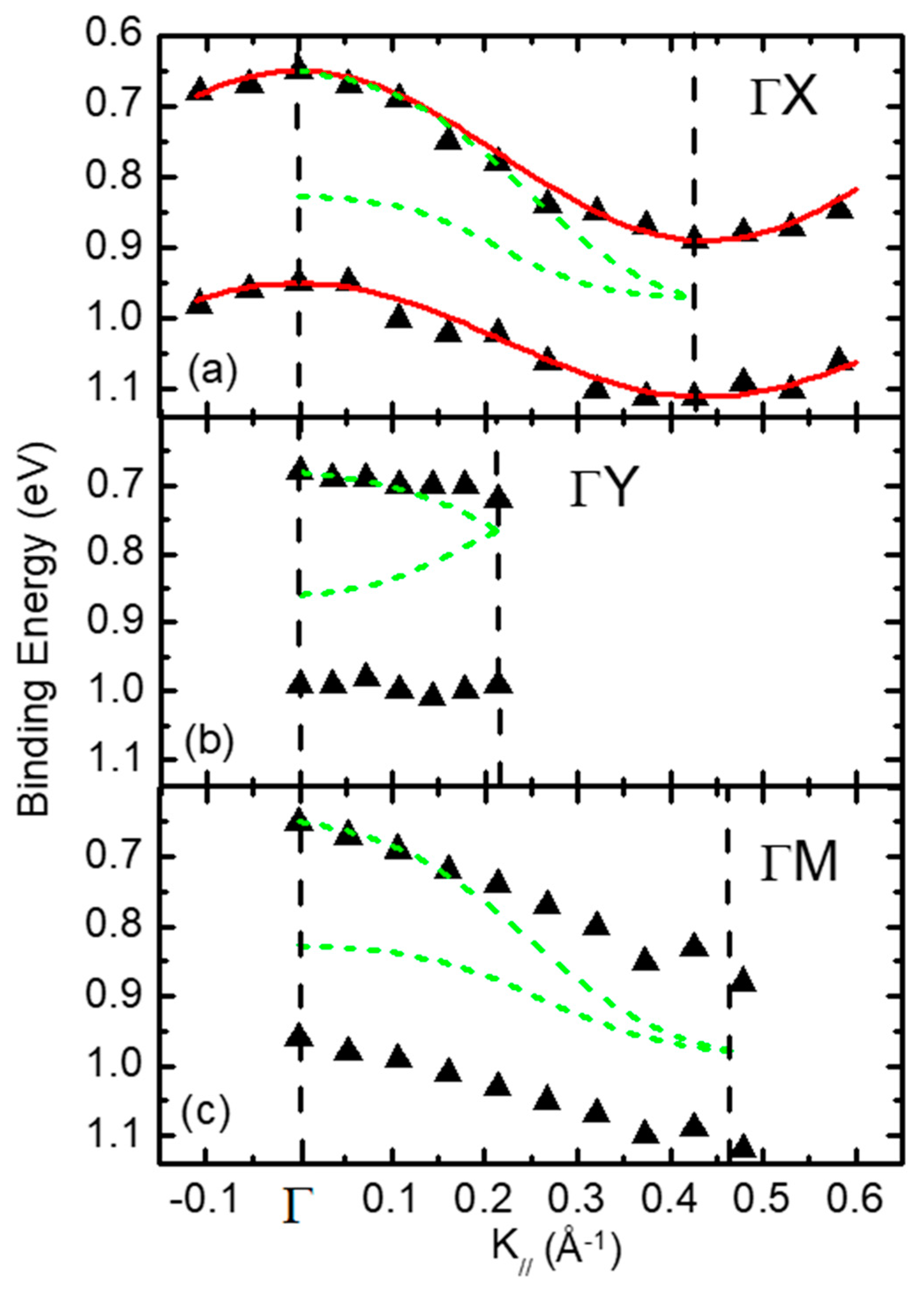
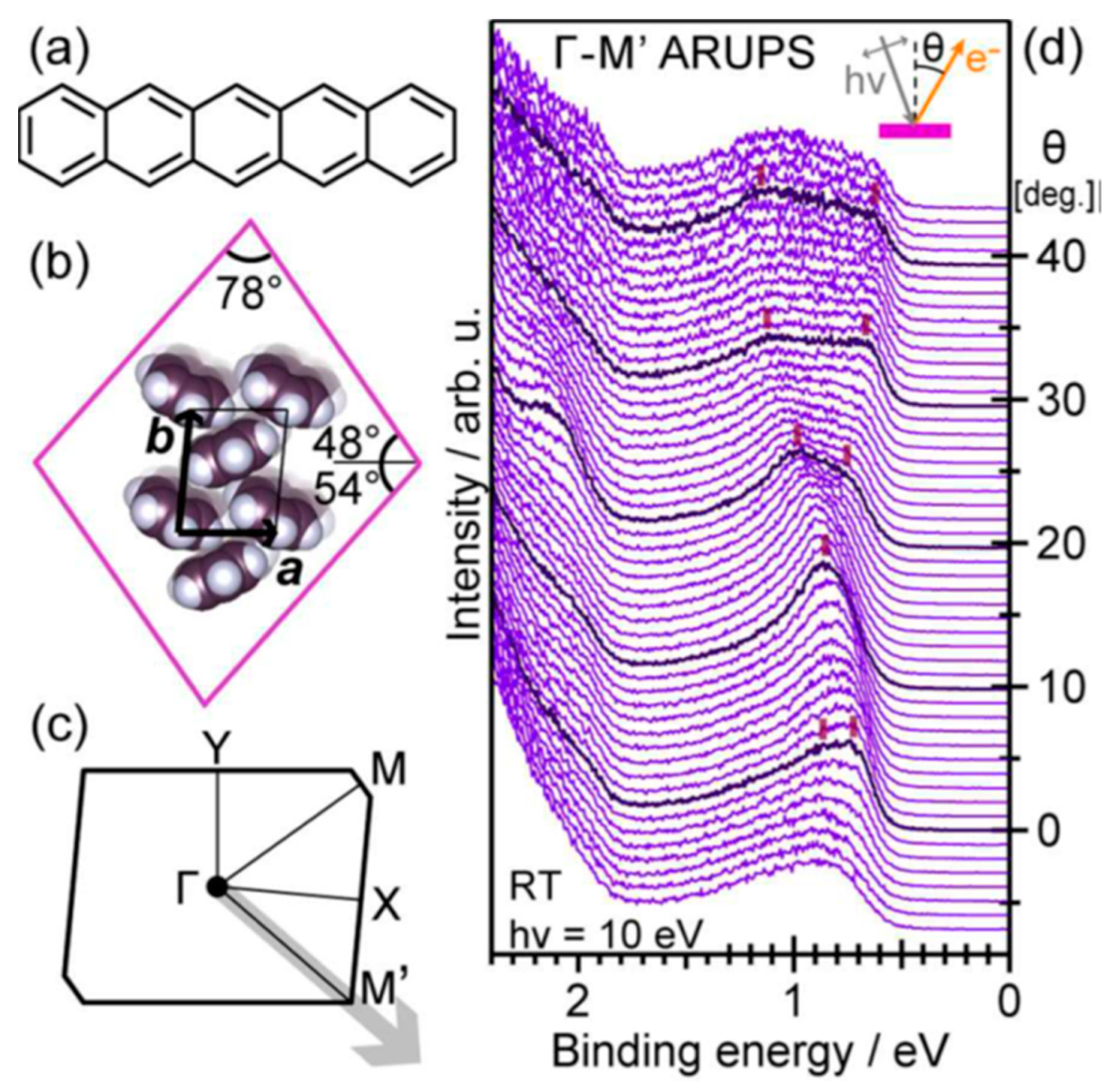
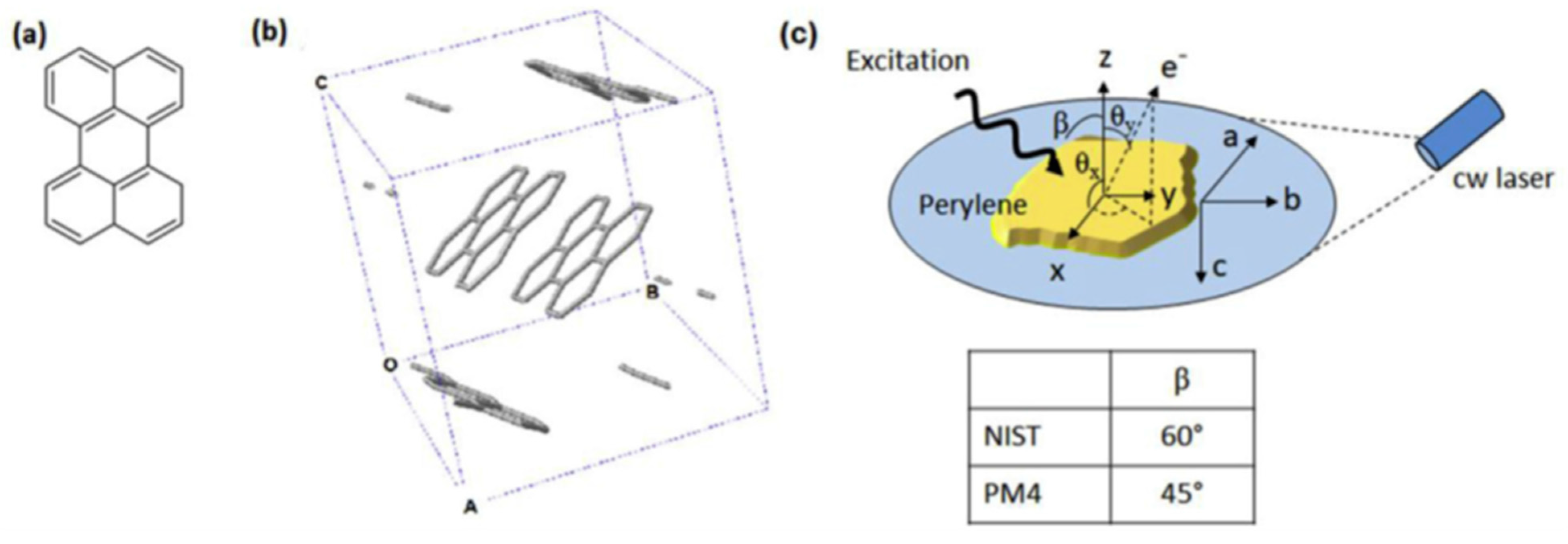
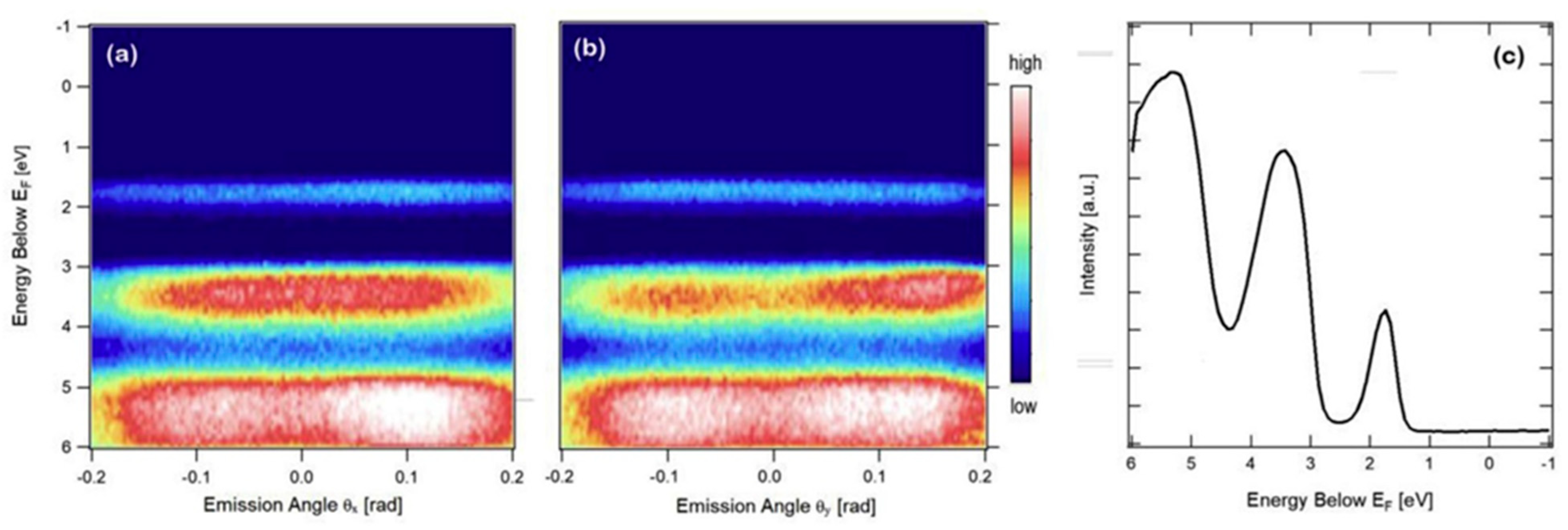
3. Organic Semiconductors
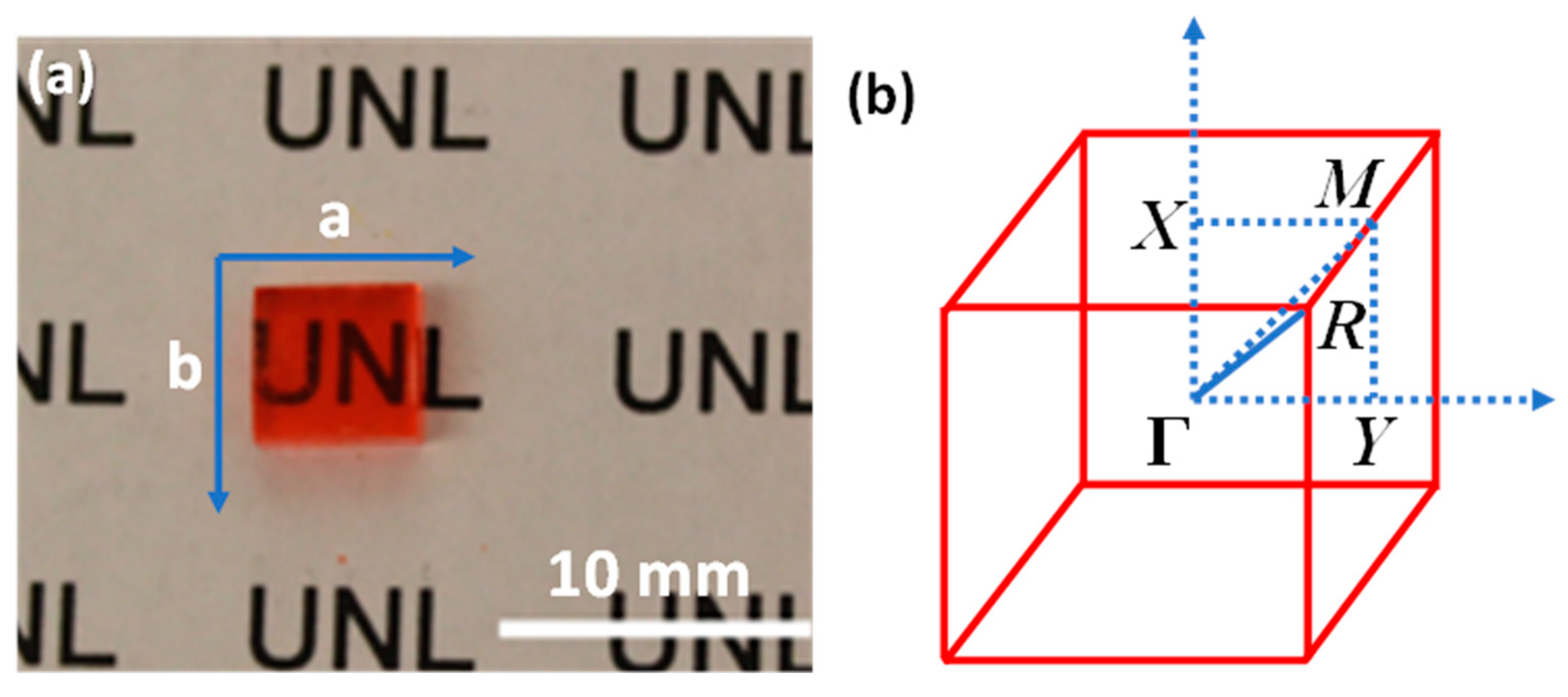
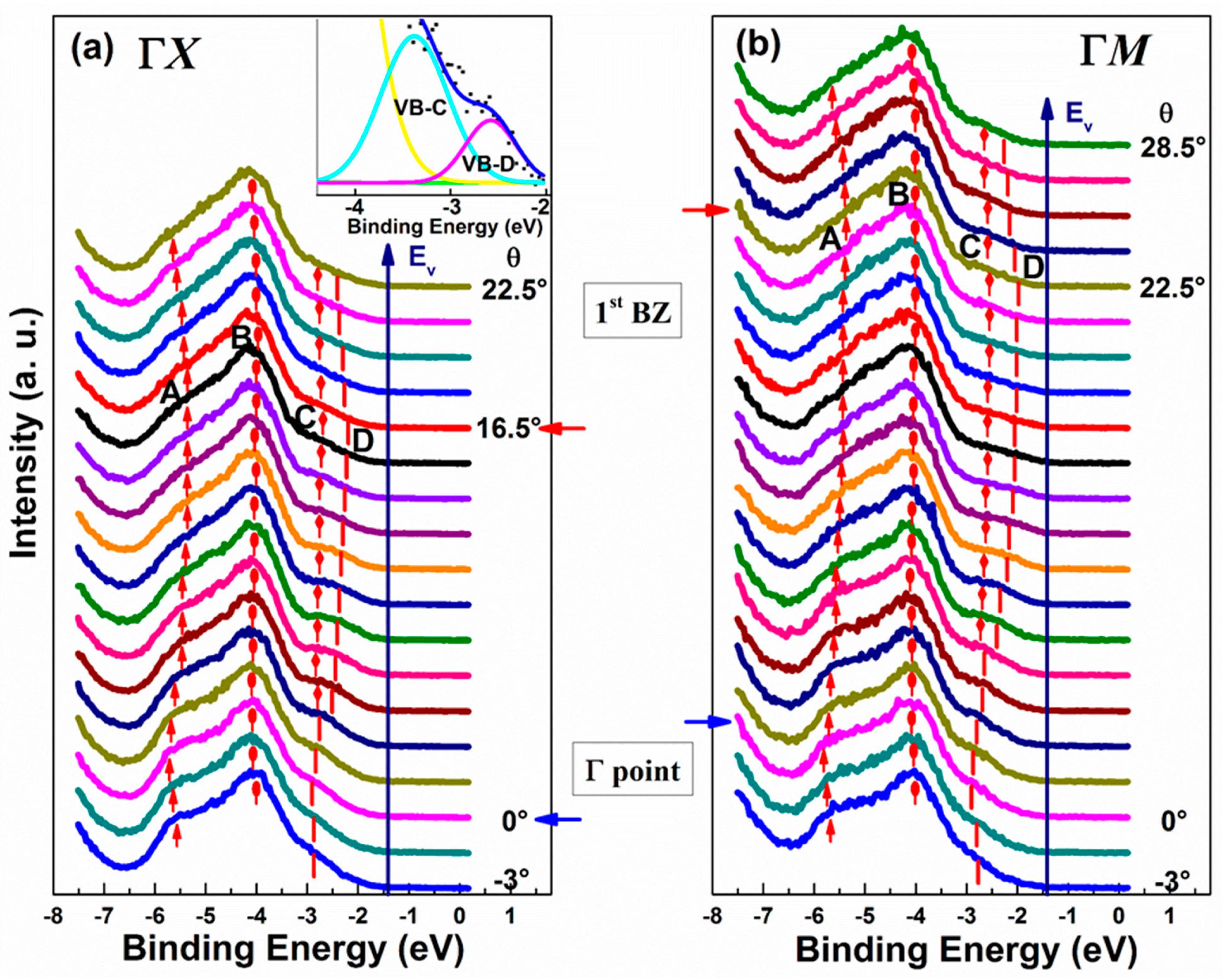
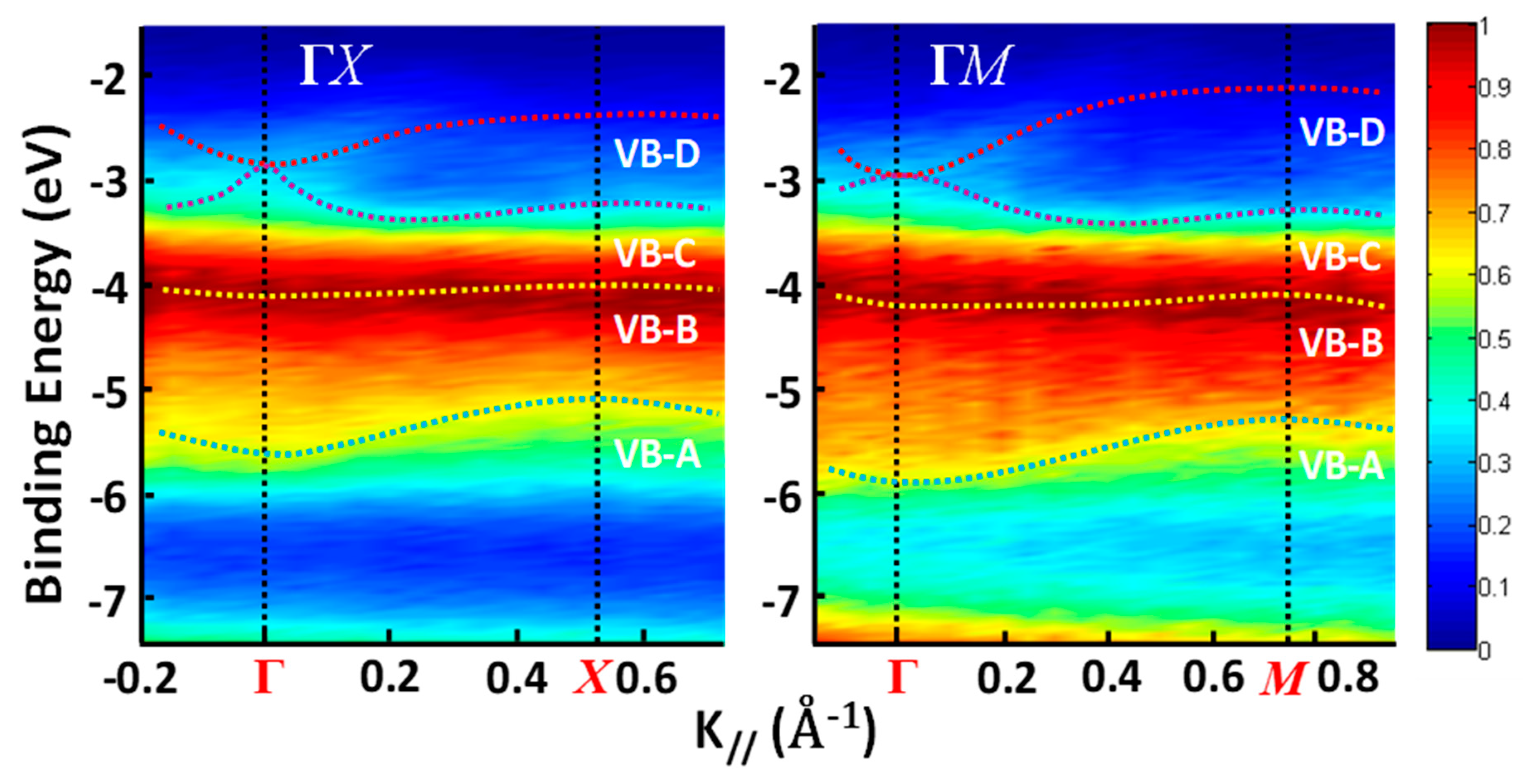
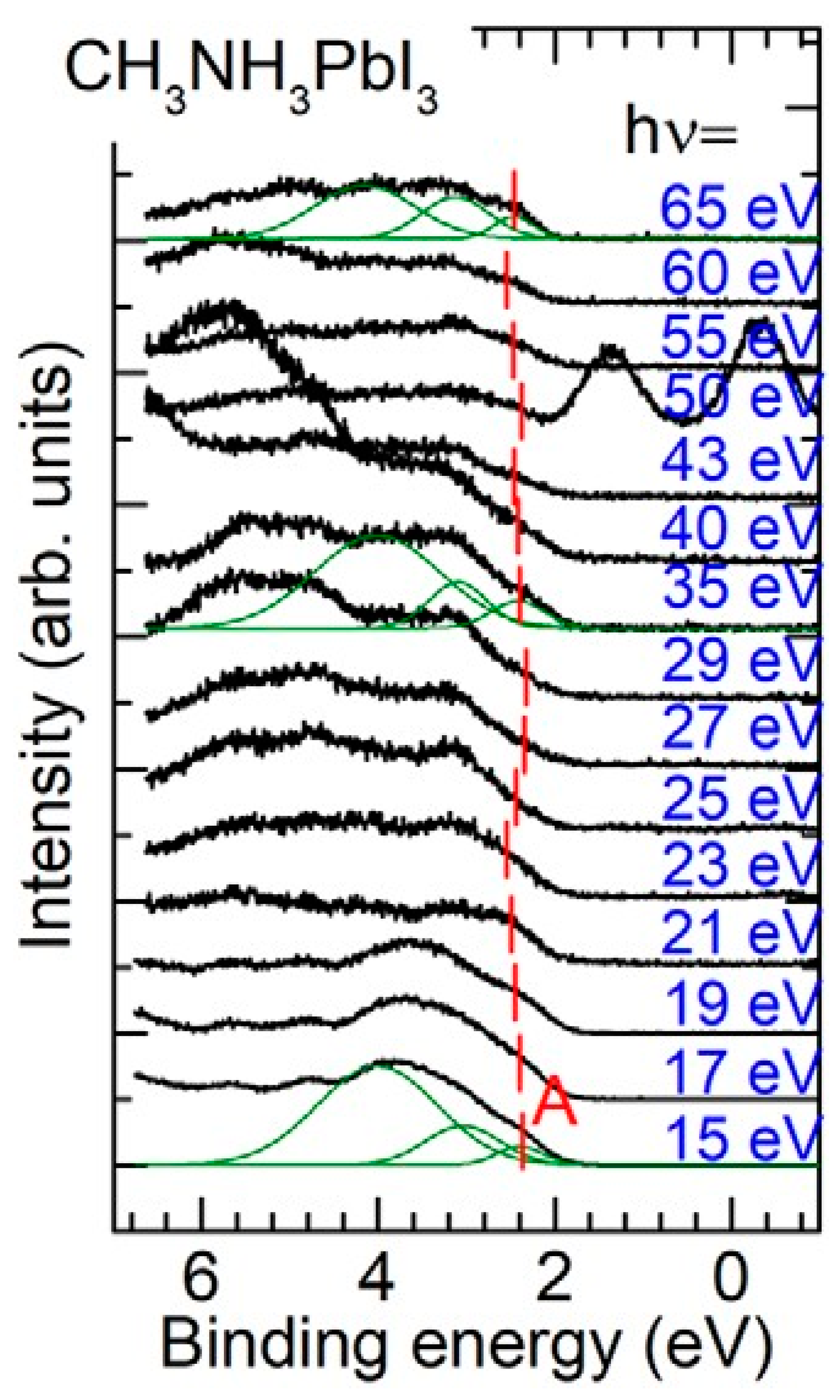
4. Organo-Metallic Perovskite Charge Transfer Crystals
5. Final Remarks
Funding
Conflicts of Interest
References
- Ashcroft, N.W.; Mermin, N.D. Solid State Physics; Harcourt: New York, NY, USA, 1976. [Google Scholar]
- Gao, Y. Surface analytical studies of interfaces in organic semiconductor devices. Mater. Sci. Eng. R Rep. 2010, 68, 39–87. [Google Scholar] [CrossRef]
- Gao, Y. Surface Analytical Studies of Interface Formation in Organic Light-Emitting Devices. Acc. Chem. Res. 1999, 32, 247–255. [Google Scholar] [CrossRef]
- Hwang, J.; Wan, A.; Kahn, A. Energetics of metal—Organic interfaces: New experiments and assessment of the field. Mater. Sci. Eng. R Rep. 2009, 64, 1–31. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy level alignment and interfacial electronic structures at organic metal and organic organic interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Koch, N. Energy levels at interfaces between metals and conjugated organic molecules. J. Phys. Condens. Matter 2008, 20, 184008. [Google Scholar] [CrossRef]
- Salaneck, W.R.; Stafstrom, S.; Bredas, J.-L. Conjugated Polymer Surfaces and Interfaces; Cambridge University Press: New York, NY, USA, 1996. [Google Scholar]
- Ueno, N.; Kera, S. Electron spectroscopy of functional organic thin films: Deep insights into valence electronic structure in relation to charge transport property. Prog. Surf. Sci. 2008, 83, 490–557. [Google Scholar] [CrossRef]
- Hüfner, S.; Hüfner, P.D.S. Photoelectron Spectroscopy; Springer Science and Business Media LLC: Berlin, Germany, 2003. [Google Scholar]
- Einstein, A. On a Heuristic Viewpoint Concerning the Production and Transformation of Light. Ann. Phys. 1905, 17, 132–148. [Google Scholar] [CrossRef]
- Park, R.L.; Lagally, M.G. Solid State Physics: Surfaces; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Reinert, F.; Hüfner, S. Photoemission spectroscopy—From early days to recent applications. New J. Phys. 2005, 7, 97. [Google Scholar] [CrossRef]
- Seah, M.; Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard data base for electron inelastic mean free paths in solids. Surf. Interface Anal. 1979, 1, 2. [Google Scholar] [CrossRef]
- Smith, K.E.; Kevan, S.D. The electronic structure of solids studied using angle resolved photoemission spectroscopy. Prog. Solid State Chem. 1991, 21, 49–131. [Google Scholar] [CrossRef]
- Damascelli, A.; Hussain, Z.; Shen, Z.-X. Angle-resolved photoemission studies of the cuprate superconductors. Rev. Mod. Phys. 2003, 75, 473–541. [Google Scholar] [CrossRef]
- Chiang, T.C.; Knapp, J.A.; Aono, M.; Eastman, D.E. Angle-resolved photoemission, valence band dispersions E(k), and electron and hole lifetimes for GaAs. Phys. Rev. B 1980, 21, 3513–3522. [Google Scholar] [CrossRef]
- Pope, M.; Swenberg, C.E. Electronic Processes in Organic Crystals and Polymers; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Hannewald, K.; Stojanović, V.M.; Schellekens, J.M.T.; Bobbert, P.A.; Kresse, G.; Hafner, J. Theory of polaron bandwidth narrowing in organic molecular crystals. Phys. Rev. B 2004, 69, 075211–075218. [Google Scholar] [CrossRef]
- Reddy, C.M.; Gundakaram, R.C.; Basavoju, S.; Kirchner, M.T.; Padmanabhan, K.A.; Desiraju, G.R. Structural basis for bending of organic crystals. Chem. Commun. 2005, 2005, 3945. [Google Scholar] [CrossRef]
- Arkhipov, S.G.; Losev, E.A.; Nguyen, T.T.; Rychkov, D.A.; Boldyreva, E. A large anisotropic plasticity of L-leucinium hydrogen maleate preserved at cryogenic temperatures. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 143–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zhang, S.; Zhou, X. Synthesis, Crystal Structure, and Solubility Analysis of a Famotidine Cocrystal. Crystal 2019, 9, 360. [Google Scholar] [CrossRef]
- Nicoud, L.; Licordari, F.; Myerson, A.S. Estimation of the Solubility of Metastable Polymorphs: A Critical Review. Cryst. Growth Des. 2018, 18, 7228–7237. [Google Scholar] [CrossRef]
- Mahon, J.K.; Zhou, T.X.S.R.; Forrest, S.R.; Schwambera, M.; Meyer, M. Organic VPD shows promise for OLED volume production (FPD). Solid State Technol. 2002, 45, 131–136. [Google Scholar]
- Andreasson, M.; Tengelin-Nilsson, M.; Ilver, L.; Kanski, J. Photoelectron spectroscopic studies of ultra-thin CuPc and PTCDA layers on Cu(100). Synth. Met. 2008, 158, 45–49. [Google Scholar] [CrossRef]
- Hasegawa, S.; Tanaka, S.; Yamashita, Y.; Inokuchi, H.; Fujimoto, H.; Kamiya, K.; Seki, K.; Ueno, N. Molecular orientation in thin films of bis(1,2,5-thiadiazolo)-p-quinobis(1,3-dithiole) on graphite studied by angle-resolved photoelectron spectroscopy. Phys. Rev. B 1993, 48, 2596–2600. [Google Scholar] [CrossRef]
- Hatch, R.C.; Höchst, H. Evolution of interface properties of the Pentacene/Bi(0001) system. Surf. Sci. 2010, 604, 1684–1687. [Google Scholar] [CrossRef]
- Huang, H.; Mao, H.; Chen, Q.; Yan, X.; Qian, H.; Zhang, J.; Li, H.; He, P.; Bao, S. The electronic states of ordered thin films of perylene on Ag(110). Phys. B Condens. Matter 2004, 352, 36–41. [Google Scholar]
- Huang, H.; Yan, X.; Mao, H.; Chen, Q.; Qian, H.; Zhang, J.; Li, H.; He, P.; Bao, S. The electronic states of ordered thin film of perylene on Ag(110). Acta Phys. Chim. Sin. 2004, 20, 892–896. [Google Scholar]
- Huang, J.; Kertesz, M. Electronic Structures and Charge Transport Properties of the Organic Semiconductor Bis[1,2,5]thiadiazolo-p-quinobis(1,3-dithiole), BTQBT, and Its Derivatives. J. Phys. Chem. B 2005, 109, 12891–12898. [Google Scholar] [CrossRef]
- Mao, H.Y.; Huang, H. The growth of perylene on Ru(0001). J. Chem. Phys. 2004, 121, 6972–6977. [Google Scholar]
- Chen, Q.; Mao, H.Y.; Huang, H.; Qian, H.Q.; Zhang, J.-H.; Li, H.Y.; He, P.-M.; Bao, S.-N.; Yan, X.-C. The structure and electronic state of the ordered thin film of perylene and tetracene on Ag(110) surface. Acta Phys. Sin. 2005, 54, 460–466. [Google Scholar]
- Nicoara, N.; Román, E.; Gómez-Rodríguez, J.; Martín-Gago, J.; Méndez, J. Scanning tunneling and photoemission spectroscopies at the PTCDA/Au(111) interface. Org. Electron. 2006, 7, 287–294. [Google Scholar] [CrossRef]
- Tengelin-Nilsson, M.; Ilver, L.; Kanski, J. Photoemission and low-energy electron diffraction studies of 3,4,9,10-perylene tetracarboxylic dianhydride layers on Si(111):H. Surf. Sci. 2000, 464, 265–271. [Google Scholar] [CrossRef]
- Tengelin-Nilsson, M.; Ilver, L.; Kanski, J. Photoelectron spectroscopic studies of thin PTCDA layers on TiSe2. Org. Electron. 2002, 3, 73–79. [Google Scholar] [CrossRef]
- Ueno, N. Angle-resolved UPS of ultrathin films of functional organic molecules with synchrotron radiation: Determination of molecular orientation by quantitative analysis of photoelectron angular distribution. J. Electron. Spectrosc. Relat. Phenom. 1996, 78, 345–350. [Google Scholar] [CrossRef]
- Ueno, N.; Kitamura, A.; Okudaira, K.K.; Miyamae, T.; Harada, Y.; Hasegawa, S.; Ishii, H.; Inokuchi, H.; Fujikawa, T.; Miyazaki, T.; et al. Angle-resolved ultraviolet photoelectron spectroscopy of thin films of bis(1,2,5-thiadiazolo)-p-quinobis (1,3-dithiole) on the MoS2 surface. J. Chem. Phys. 1997, 107, 2079–2088. [Google Scholar] [CrossRef]
- Vasseur, G.; Abadia, M.; Miccio, L.A.; Brede, J.; Garcia-Lekue, A.; de Oteyza, D.G.; Rogero, C.; Lobo-Checa, J.; Ortega, J.E. Pi Band Dispersion along Conjugated Organic Nanowires Synthesized on a Metal Oxide Semiconductor. J. Am. Chem. Soc. 2016, 138, 5685–5692. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Han, H.; Hong-Ying, M.; Xin-Guo, Y.; Jian-Hua, Z.; Mang, W.; Shi-Ning, B. Study on the interface of an organic semiconductor grown on Ru(0001) surface. Acta Phys. Sin. 2004, 53, 1604–1610. [Google Scholar]
- Yamane, H.; Kanai, K.; Ouchi, Y.; Ueno, N.; Seki, K. Impact of interface geometric structure on organic–metal interface energetics and subsequent films electronic structure. J. Electron. Spectrosc. Relat. Phenom. 2009, 174, 28–34. [Google Scholar] [CrossRef]
- Yamane, H.; Kera, S.; Okudaira, K.K.; Yoshimura, D.; Seki, K.; Ueno, N. Intermolecular energy-band dispersion in PTCDA multilayers. Phys. Rev. B 2003, 68, 033102–033106. [Google Scholar] [CrossRef]
- Yamane, H.; Kosugi, N. Systematic study on intermolecular valence-band dispersion in molecular crystalline films. J. Electron. Spectrosc. Relat. Phenom. 2015, 204, 61–67. [Google Scholar] [CrossRef]
- Fan, Y.; Xinguo, Y.; Yue, W.; Jianhua, Z.; Jingzhi, S.; Mang, W.; Haiyang, L.; Pimo, H.; Shining, B. Study on the stages of N,N-dibutyl–3,4,9,10-perylene tetracarboxylic diimide growth on Ru (0001) surface. Phys. B Condens. Matter 2003, 334, 244–249. [Google Scholar] [CrossRef]
- Yang, J.-P.; Bussolotti, F.; Kera, S.; Ueno, N. Origin and role of gap states in organic semiconductor studied by UPS: As the nature of organic molecular crystals. J. Phys. D Appl. Phys. 2017, 50, 423002. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Berland, K.; Cooper, V.R.; Lee, K.; Schröder, E.; Thonhauser, T.; Hyldgaard, P.; Lundqvist, B. van der Waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.K.; Kang, S.O.; Ko, J.; Yum, J.-H.; Fantacci, S.; De Angelis, F.; Di Censo, D.; Nazeeruddin, K.; Grätzel, M. Molecular Engineering of Organic Sensitizers for Solar Cell Applications. J. Am. Chem. Soc. 2006, 128, 16701–16707. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Langreth, D.C.; Lundqvist, B.; Chakarova-Käck, S.D.; Cooper, V.R.; Dion, M.; Hyldgaard, P.; Kelkkanen, A.; Kleis, J.; Kong, L.; Li, S.; et al. A density functional for sparse matter. J. Phys. Condens. Matter 2009, 21, 084203. [Google Scholar] [CrossRef] [PubMed]
- Usta, H.; Facchetti, A.; Marks, T.J. n-Channel Semiconductor Materials Design for Organic Complementary Circuits. Acc. Chem. Res. 2011, 44, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Amat, A.; Nazeeruddin, M.; Grätzel, M.; De Angelis, F. First-Principles Modeling of Mixed Halide Organometal Perovskites for Photovoltaic Applications. J. Phys. Chem. C 2013, 117, 13902–13913. [Google Scholar] [CrossRef]
- Djurovich, P.I.; Mayo, E.I.; Forrest, S.R.; Thompson, M. Measurement of the lowest unoccupied molecular orbital energies of molecular organic semiconductors. Org. Electron. 2009, 10, 515–520. [Google Scholar] [CrossRef]
- Gao, J.; Miao, J.; Li, Z.; Teng, W.Y.; Yang, L.; Zhao, Y.; Liu, B.; Zhang, Q. A p-type Ti(iv)-based metal–organic framework with visible-light photo-response. Chem. Commun. 2014, 50, 3786–3788. [Google Scholar] [CrossRef]
- Dauth, M.; Wiessner, M.; Feyer, V.; Scholl, A.; Puschnig, P.; Reinert, F.; Kummel, S. Angle resolved photoemission from organic semiconductors: Orbital imaging beyond the molecular orbital interpretation. New J. Phys. 2014, 16, 103005. [Google Scholar] [CrossRef]
- Söderholm, S.; Girard, R.T.; Schweitzer, D. Electronic structure of the organic conductor α-(BEDT-TTF)2I3 sstudied by angle-resolvedand core-level photoelectron spectroscopy. Phys. Rev. B 1997, 55, 4267–4274. [Google Scholar] [CrossRef]
- Hennig, I.; Bender, K.; Schweitzer, D.; Dietz, K.; Endres, H.; Keller, H.J.; Gleitz, A.; Helberg, H.W. α And β-((BEDT-TTF)2+I3−: Two Dimensional Organic Metals. Mol. Cryst. Liq. Cryst. 1985, 119, 337–341. [Google Scholar] [CrossRef]
- Yagubskii, E.B.; Shchegolev, I.F.; Laukhin, V.N.; Kononovich, P.A.; Karstovnik, M.V.; Zvarykina, A.V.; Buravov, L.I. Normal-pressure superconductivity in an organic metal (BEDT-TTF)2I3 [bis (ethylene dithiolo) tetrathiof ulvalene triiodide]. JETP Lett. 1984, 39, 12–16. [Google Scholar]
- Bender, K.; Hennig, I.; Schweitzer, D.; Dietz, K.; Endres, H.; Keller, H.J. Synthesis, Structure and Physical Properties of a Two-Dimensional Organic Metal, Di[bis(ethylenedithiolo)tetrathiofulvalene] triiodide, (BEDT-TTF)2+I3−. Mol. Cryst. Liq. Cryst. 1984, 108, 359–371. [Google Scholar] [CrossRef]
- Zwick, F.; Onellion, M.; Voit, J.; Jérome, D.; Margaritondo, G.; Grioni, M. Band Mapping and Quasiparticle Suppression in the One-Dimensional Organic Conductor TTF-TCNQ. Phys. Rev. Lett. 1998, 81, 2974–2977. [Google Scholar] [CrossRef]
- Sing, M.; Schwingenschlögl, U.; Claessen, R.; Blaha, P.; Carmelo, J.; Martelo, L.M.; Sacramento, P.D.; Dressel, M.; Jacobsen, C.S. Electronic structure of the quasi-one-dimensional organic conductor TTF-TCNQ. Phys. Rev. B 2003, 68, 125111–125121. [Google Scholar] [CrossRef]
- Kistenmacher, T.J.; Phillips, T.E.; Cowan, D.O. The crystal structure of the 1:1 radical cation–radical anion salt of 2,2’-bis-l,3-dithiole (TTF) and 7,7,8,8-tetracyanoquinodimethane (TCNQ). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1974, 30, 763–768. [Google Scholar] [CrossRef]
- Claessen, R.; Sing, M.; Schwingenschlögl, U.; Blaha, P.; Dressel, M.; Jacobsen, C.S. Spectroscopic Signatures of Spin-Charge Separation in the Quasi-One-Dimensional Organic Conductor TTF-TCNQ. Phys. Rev. Lett. 2002, 88, 096402. [Google Scholar] [CrossRef]
- Heeger, A.J.; Su, W.-P.; Kivelson, S.; Schrieffer, J.R. Solitons in conducting polymers. Rev. Mod. Phys. 1988, 60, 781–850. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burn, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Podzorov, V.; Menard, E.; Borissov, A.; Kiryukhin, V.; Rogers, J.A.; Gershenson, M.E. Intrinsic Charge Transport on the Surface of Organic Semiconductors. Phys. Rev. Lett. 2004, 93, 086602. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.C.; Zaumseil, J.; Podzorov, V.; Menard, E.; Willett, R.L.; Someya, T.; Gershenson, M.E.; Rogers, J.A. Elastomeric Transistor Stamps: Reversible Probing of Charge Transport in Organic Crystals. Science 2004, 303, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- single crystal with angle-resolved photoemission. Appl. Phys. Lett. 2010, 96, 222106. [CrossRef]
- Kakuta, H.; Hirahara, T.; Matsuda, I.; Nagao, T.; Hasegawa, S.; Ueno, N.; Sakamoto, K. Electronic Structures of the Highest Occupied Molecular Orbital Bands of a Pentacene Ultrathin Film. Phys. Rev. Lett. 2007, 98, 247601. [Google Scholar] [CrossRef]
- Hummer, K.; Draxl, C. Electronic properties of oligoacenes from first principles. Phys. Rev. B 2005, 72, 205205. [Google Scholar] [CrossRef]
- Troisi, A. Prediction of the Absolute Charge Mobility of Molecular Semiconductors: The Case of Rubrene. Adv. Mater. 2007, 19, 2000–2004. [Google Scholar] [CrossRef]
- Li, Z.Q.; Podzorov, V.; Sai, N.; Martín, M.C.; Gershenson, M.E.; Di Ventra, M.; Basov, D.N. Light Quasiparticles Dominate Electronic Transport in Molecular Crystal Field-Effect Transistors. Phys. Rev. Lett. 2007, 99, 016403. [Google Scholar] [CrossRef]
- Silva-Filho, D.; Kim, E.-G.; Bredas, J.-L. Transport Properties in the Rubrene Crystal: Electronic Coupling and Vibrational Reorganization Energy. Adv. Mater. 2005, 17, 1072–1076. [Google Scholar] [CrossRef]
- Reese, C.; Bao, Z. High-Resolution Measurement of the Anisotropy of Charge Transport in Single Crystals. Adv. Mater. 2007, 19, 4535–4538. [Google Scholar] [CrossRef]
- Machida, S.; Nakayama, Y.; Duhm, S.; Xin, Q.; Funakoshi, A.; Ogawa, N.; Kera, S.; Ueno, N.; Ishii, H. Highest-Occupied-Molecular-Orbital Band Dispersion of Rubrene Single Crystals as Observed by Angle-Resolved Ultraviolet Photoelectron Spectroscopy. Phys. Rev. Lett. 2010, 104, 156401. [Google Scholar] [CrossRef]
- Nakayama, Y.; Uragami, Y.; Machida, S.; Koswattage, K.R.; Yoshimura, D.; Setoyama, H.; Okajima, T.; Mase, K.; Ishii, H. Full Picture of Valence Band Structure of Rubrene Single Crystals Probed by Angle-Resolved and Excitation-Energy-Dependent Photoelectron Spectroscopy. Appl. Phys. Express 2012, 5, 111601. [Google Scholar] [CrossRef]
- Vollmer, A.; Ovsyannikov, R.; Gorgoi, M.; Krause, S.; Oehzelt, M.; Lindblad, A.; Mårtensson, N.; Svensson, S.; Karlsson, P.; Lundvuist, M.; et al. Two dimensional band structure mapping of organic single crystals using the new generation electron energy analyzer ARTOF. J. Electron. Spectrosc. Relat. Phenom. 2012, 185, 55–60. [Google Scholar] [CrossRef][Green Version]
- Koch, N.; Vollmer, A.; Salzmann, I.; Nickel, B.; Weiss, H.; Rabe, J.P. Evidence for Temperature-Dependent Electron Band Dispersion in Pentacene. Phys. Rev. Lett. 2006, 96, 156803. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Mori, T.; Imaeda, K.; Tanaka, S.; Yamashita, Y.; Inokuchi, H.; Fujimoto, H.; Seki, K.; Ueno, N. Intermolecular energy-band dispersion in oriented thin films of bis(1,2,5-thiadiazolo)-p-quinobis(1,3-dithiole) by angle-resolved photoemission. J. Chem. Phys. 1994, 100, 6969–6973. [Google Scholar] [CrossRef]
- Gavrila, G.; Mendez, H.; Kampen, T.; Zahn, D.R.; Vyalikh, D.V.; Braun, W. Energy band dispersion in well ordered N,N[sup ʹ]-dimethyl-3,4,9,10-perylenetetracarboxylic diimide films. Appl. Phys. Lett. 2004, 85, 4657. [Google Scholar] [CrossRef]
- Meier, H. Organic Semiconductors; Ebel, H.F., Ed.; Verlag Chemie: Winheim, Germany, 1974. [Google Scholar]
- Frohlich, H.; Sewell, G.L. Electric Conduction in Semiconductors. Proc. Phys. Soc. 1959, 74, 643–647. [Google Scholar] [CrossRef]
- Menard, E.; Marchenko, A.A.; Podzorov, V.; Gershenson, M.E.; Fichou, D.; Rogers, J.A. Nanoscale Surface Morphology and Rectifying Behavior of a Bulk Single-Crystal Organic Semiconductor. Adv. Mater. 2006, 18, 1552–1556. [Google Scholar] [CrossRef]
- Podzorov, V.; Pudalov, V.M.; Gershenson, M.E. Light-induced switching in back-gated organic transistors with built-in conduction channel. Appl. Phys. Lett. 2004, 85, 6039–6041. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mizuno, Y.; Hikasa, M.; Yamamoto, M.; Matsunami, M.; Ideta, S.; Tanaka, K.; Ishii, H.; Ueno, N. Single-Crystal Pentacene Valence-Band Dispersion and Its Temperature Dependence. J. Phys. Chem. Lett. 2017, 8, 1259–1264. [Google Scholar] [CrossRef]
- Jurchescu, O.D.; Baas, J.; Palstra, T.T.M. Effect of impurities on the mobility of single crystal pentacene. Appl. Phys. Lett. 2004, 84, 3061. [Google Scholar] [CrossRef]
- Xin, Q.; Duhm, S.; Bussolotti, F.; Akaike, K.; Kubozono, Y.; Aoki, H.; Kosugi, T.; Kera, S.; Ueno, N. Accessing Surface Brillouin Zone and Band Structure of Picene Single Crystals. Phys. Rev. Lett. 2012, 108, 226401. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Sato, N. Crystallographic and electronic structures of three different polymorphs of pentacene. Phys. Rev. B 2008, 77, 235205. [Google Scholar] [CrossRef]
- Vermeulen, D.; Zhu, L.; Goetz, K.P.; Hu, P.; Jiang, H.; Day, C.S.; Jurchescu, O.D.; Coropceanu, V.; Kloc, C.; McNeil, L. Charge Transport Properties of Perylene–TCNQ Crystals: The Effect of Stoichiometry. J. Phys. Chem. C 2014, 118, 24688–24696. [Google Scholar] [CrossRef]
- Pookpanratana, S.J.; Goetz, K.; Bittle, E.; Haneef, H.; You, L.; Hacker, C.A.; Robey, S.; Jurchescu, O.D.; Ovsyannikov, R.; Giangrisostomi, E. Electronic properties and structure of single crystal perylene. Org. Electron. 2018, 61, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, I.A.; Zhuravlev, Y.N.; Berveno, V.P. Structural and electronic properties of perylene from first principles calculations. J. Chem. Phys. 2013, 138, 94509. [Google Scholar] [CrossRef]
- Hodes, G. Perovskite-Based Solar Cells. Science 2013, 342, 317–318. [Google Scholar] [CrossRef]
- Lin, Q.; Armin, A.; Burn, P.L.; Meredith, P. Organohalide Perovskites for Solar Energy Conversion. Acc. Chem. Res. 2016, 49, 545–553. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2015, 28, 284–292. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Seok, S.I.; Lee, J.; Seo, J. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 2018, 3, 682–689. [Google Scholar] [CrossRef]
- Werner, J.; Weng, C.-H.; Walter, A.; Fesquet, L.; Seif, J.P.; De Wolf, S.; Niesen, B.; Ballif, C. Efficient Monolithic Perovskite/Silicon Tandem Solar Cell with Cell Area >1 cm2. J. Phys. Chem. Lett. 2015, 7, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Rech, B.; Saliba, M.; Correa-Baena, J.-P.; Lang, F.; Kegelmann, L.; Mews, M.; Steier, L.; Abate, A.; Rappich, J.; Korte, L.; et al. Monolithic perovskite/silicon-heterojunction tandem solar cells processed at low temperature. Energy Environ. Sci. 2016, 9, 81–88. [Google Scholar]
- Wei, H.; Fang, Y.; Mulligan, P.L.; Chuirazzi, W.; Fang, H.-H.; Wang, C.; Ecker, B.; Gao, Y.; Loi, M.A.; Cao, L.; et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photon. 2016, 10, 333–339. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.B.; Sadhanala, A.; Outón, L.M.P.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Deschler, F.; Price, M.B.; Pathak, S.; Klintberg, L.E.; Jarausch, D.-D.; Higler, R.; Hüttner, S.; Leijtens, T.; Stranks, S.D.; Snaith, H.J.; et al. High Photoluminescence Efficiency and Optically Pumped Lasing in Solution-Processed Mixed Halide Perovskite Semiconductors. J. Phys. Chem. Lett. 2014, 5, 1421–1426. [Google Scholar] [CrossRef]
- Wang, C.; Ecker, B.; Wei, H.; Huang, J.; Meng, J.-Q.; Gao, Y. Valence band dispersion measurements of perovskite single crystals using angle-resolved photoemission spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 5361–5365. [Google Scholar] [CrossRef]
- Jishi, R.A.; Ta, O.B.; Sharif, A.A. Modeling of Lead Halide Perovskites for Photovoltaic Applications. J. Phys. Chem. C 2014, 118, 28344–28349. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, S.; Yan, Y.; Yang, Y.; Luther, J.; Wei, S.-H.; Parilla, P.; Zhu, K. Electronic Structure and Optical Properties of α-CH3NH3PbBr3 Perovskite Single Crystal. J. Phys. Chem. Lett. 2015, 6, 4304–4308. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, C.; Leng, J.; Cui, R.; Jin, S. Visualizing Carrier Diffusion in Individual Single-Crystal Organolead Halide Perovskite Nanowires and Nanoplates. J. Am. Chem. Soc. 2015, 137, 12458–12461. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Niesner, D.; Wilhelm, M.; Levchuk, I.; Osvet, A.; Shrestha, S.; Brabec, C.J.; Fauster, T.; Batentschuk, M. Giant Rashba Splitting in CH3NH3PbBr3 Organic-Inorganic Perovskite. Phys. Rev. Lett. 2016, 117, 126401. [Google Scholar] [CrossRef]
- Etienne, T.; Mosconi, E.; De Angelis, F. Dynamical Origin of the Rashba Effect in Organohalide Lead Perovskites: A Key to Suppressed Carrier Recombination in Perovskite Solar Cells? J. Phys. Chem. Lett. 2016, 7, 1638–1645. [Google Scholar] [CrossRef]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.; Grätzel, M.; De Angelis, F. Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin–Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef]
- Lee, M.-I.; Barragán, A.; Nair, M.N.; Jacques, V.L.R.; Le Bolloc’H, D.; Fertey, P.; Jemli, K.; Lédée, F.; Trippé-Allard, G.; Deleporte, E.; et al. First determination of the valence band dispersion of CH3NH3PbI3 hybrid organic–inorganic perovskite. J. Phys. D Appl. Phys. 2017, 50, 26LT02. [Google Scholar] [CrossRef]
- Zu, F.; Amsalem, P.; Egger, D.A.; Wang, R.; Wolff, C.M.; Fang, H.-H.; Loi, M.A.; Neher, D.; Kronik, L.; Duhm, S.; et al. Constructing the Electronic Structure of CH3NH3PbI3 and CH3NH3PbBr3 Perovskite Thin Films from Single-Crystal Band Structure Measurements. J. Phys. Chem. Lett. 2019, 10, 601–609. [Google Scholar] [CrossRef]
- Nandi, P.; Pandey, S.K.; Giri, C.; Singh, V.; Petaccia, L.; Manju, U.; Mahanti, S.D.B.; Topwal, D. Probing the Electronic Structure of Hybrid Perovskites in the Orientationally Disordered Cubic Phase. J. Phys. Chem. Lett. 2020, 11, 5719–5727. [Google Scholar] [CrossRef]
- Yang, J.-P.; Meissner, M.; Yamaguchi, T.; Zhang, X.-Y.; Ueba, T.; Cheng, L.; Ideta, S.; Tanaka, K.; Zeng, X.; Ueno, N.; et al. Band Dispersion and Hole Effective Mass of Methylammonium Lead Iodide Perovskite. Sol. RRL 2018, 2, 1800132. [Google Scholar] [CrossRef]
- Zhang, P.; Richard, P.; Qian, T.; Xu, Y.-M.; Dai, X.; Ding, H. A precise method for visualizing dispersive features in image plots. Rev. Sci. Instrum. 2011, 82, 43712. [Google Scholar] [CrossRef] [PubMed]
- Brivio, F.; Butler, K.T.; Walsh, A.; Van Schilfgaarde, M. Relativistic quasiparticle self-consistent electronic structure of hybrid halide perovskite photovoltaic absorbers. Phys. Rev. B 2014, 89, 155204. [Google Scholar] [CrossRef]
- Lindroos, M.; Sahrakorpi, S.; Bansil, A. Matrix element effects in angle-resolved photoemission from Bi2Sr2CaCu2O8: Energy and polarization dependencies, final state spectrum, spectral signatures of specific transitions, and related issues. Phys. Rev. B 2002, 65, 054514. [Google Scholar] [CrossRef]
- Yang, J.-P.; Ren, S.-X.; Yamaguchi, T.; Meissner, M.; Cheng, L.-W.; Zhou, L.; Ideta, S.-I.; Tanaka, K.; Kera, S. Valence band dispersion measured in the surface normal direction of CH3NH3PbI3 single crystals. Appl. Phys. Express 2019, 13, 011009. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Ecker, B.; Gao, Y. Angle-Resolved Photoemission Study on the Band Structure of Organic Single Crystals. Crystals 2020, 10, 773. https://doi.org/10.3390/cryst10090773
Wang K, Ecker B, Gao Y. Angle-Resolved Photoemission Study on the Band Structure of Organic Single Crystals. Crystals. 2020; 10(9):773. https://doi.org/10.3390/cryst10090773
Chicago/Turabian StyleWang, Ke, Ben Ecker, and Yongli Gao. 2020. "Angle-Resolved Photoemission Study on the Band Structure of Organic Single Crystals" Crystals 10, no. 9: 773. https://doi.org/10.3390/cryst10090773
APA StyleWang, K., Ecker, B., & Gao, Y. (2020). Angle-Resolved Photoemission Study on the Band Structure of Organic Single Crystals. Crystals, 10(9), 773. https://doi.org/10.3390/cryst10090773






