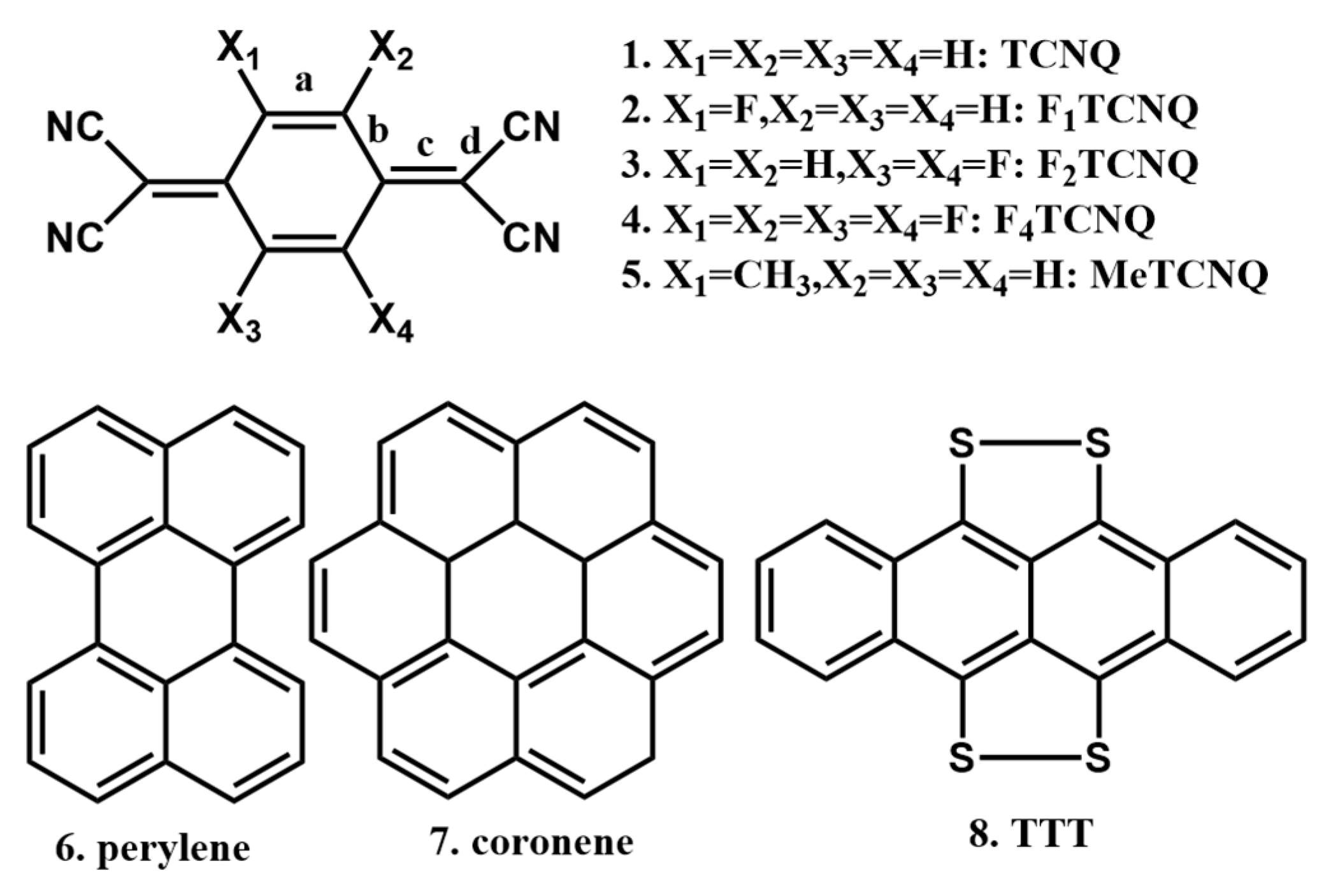
The Stoichiometry of TCNQ-Based Organic Charge-Transfer Cocrystals
Abstract
1. Introduction
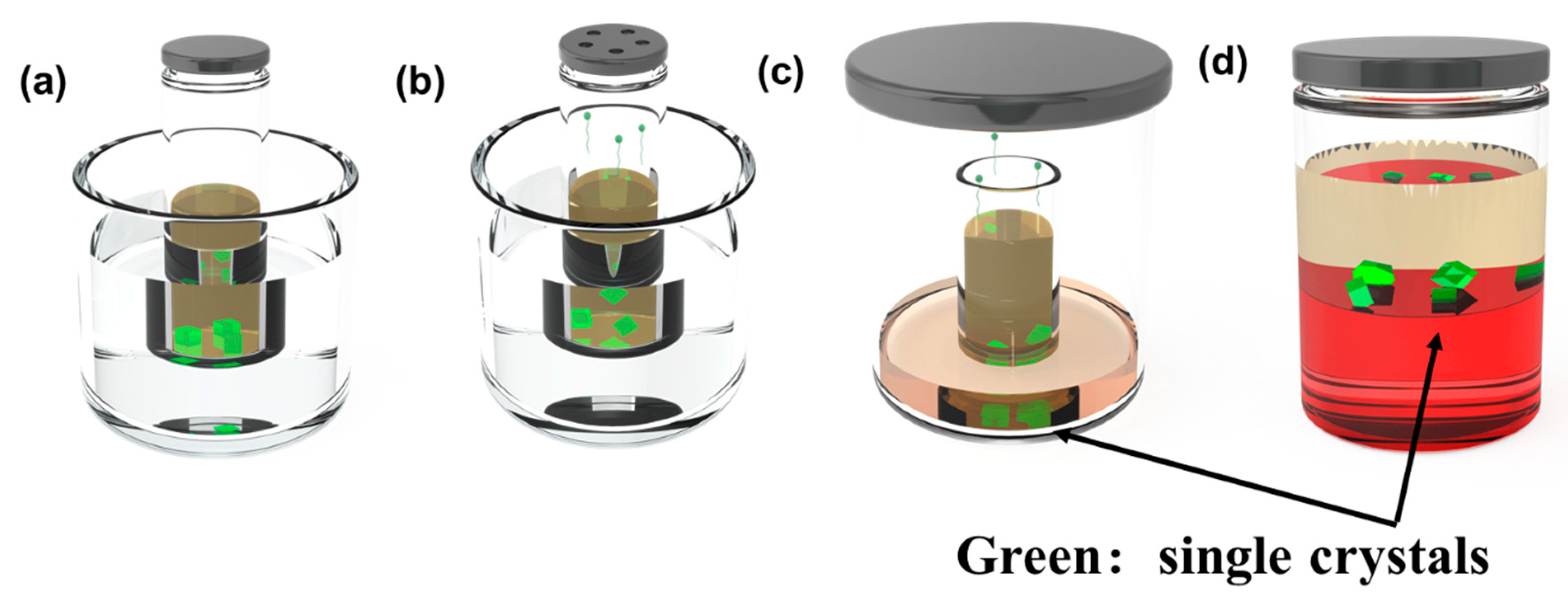
2. Single-Crystal Growth of Organic Charge-Transfer Cocrystals with Different Stoichiometry
3. Electronic Properties of Charge-Transfer Cocrystals with Different Stoichiometry
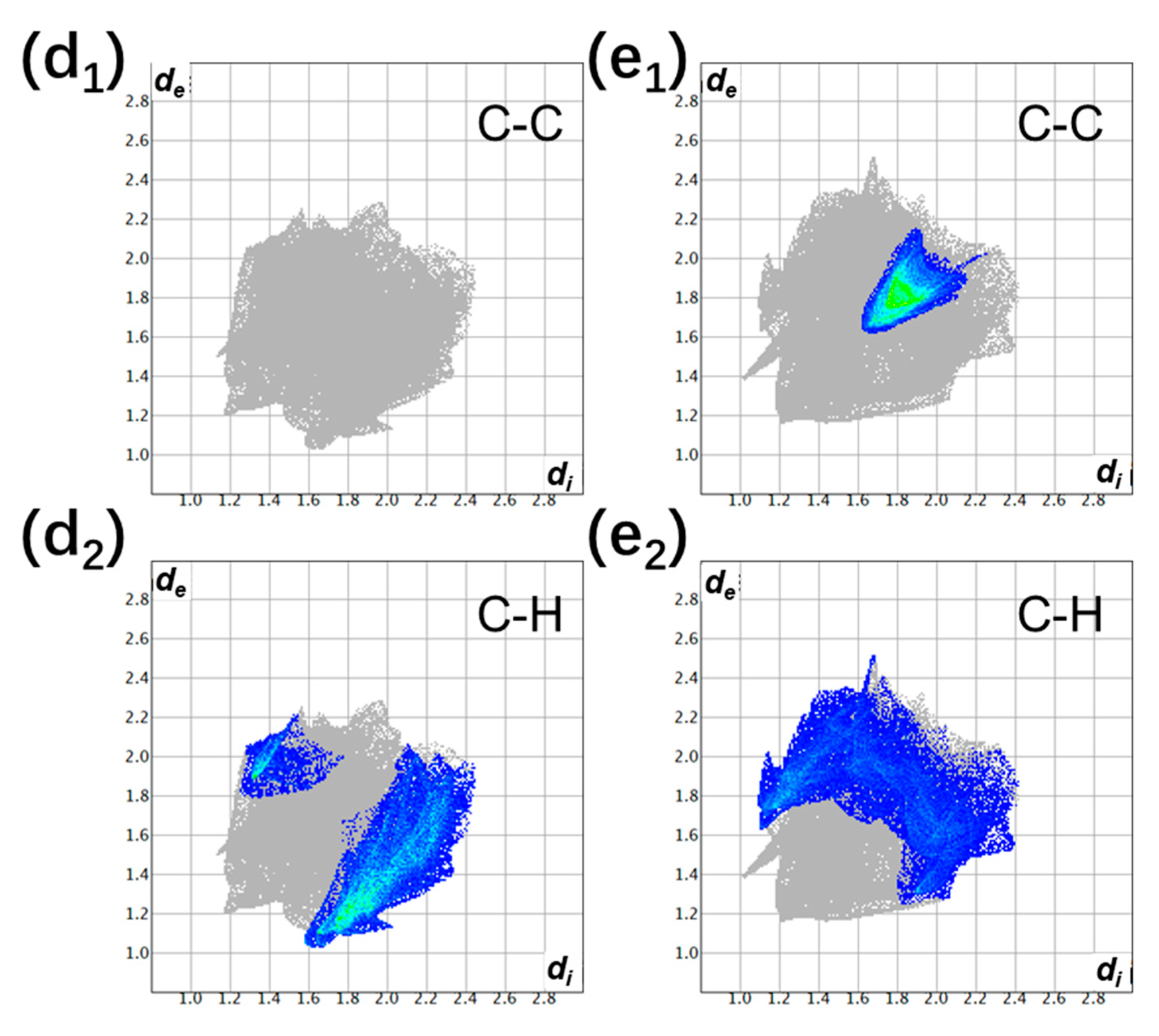
3.1. The Degree of Charge Transfer
3.2. The Electrical Conductivity and Field-Effect Mobility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, H.; Hu, W. The Emergence of Organic Single-Crystal Electronics. Angew. Chem. Int. Ed. 2020, 59, 1408–1428. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.; Sheng, P.; Zhao, G.; Zhu, D. Organic Donor-Acceptor Complexes as Novel Organic Semiconductors. Acc. Chem. Res. 2017, 50, 1654–1662. [Google Scholar] [CrossRef]
- Singleton, J. Why do physicists love charge-transfer salts? J. Solid State Chem. 2002, 168, 675–689. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Dong, H.; Zhang, X.; Li, R.; Hu, W. Organic Cocrystals: New Strategy for Molecular Collaborative Innovation. Top. Curr. Chem. 2016, 374, 83. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ye, J.; Jiang, H. Which isomer is better for charge transport: Anti- or syn-? J. Mater. Chem. C 2019, 7, 5858–5873. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Zhang, K.K.; Long, Y.; Hu, W.; Kloc, C. Tuning of the degree of charge transfer and the electronic properties in organic binary compounds by crystal engineering: A perspective. J. Mater. Chem. C 2018, 6, 1884–1902. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, W.; Yang, F.; Li, B.; Ren, X.; Zhang, X.; Hu, W. Molecular cocrystals: Design, charge-transfer and optoelectronic functionality. Phys. Chem. Chem. Phys. 2018, 20, 6009–6023. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, F.; Zhang, X.; Hu, W. Stimuli-responsive behaviors of organic charge transfer cocrystals: Recent advances and perspectives. Mater. Chem. Front. 2020, 4, 715–728. [Google Scholar] [CrossRef]
- Wöhler, F. Untersuchungen über das Chinon. Justus Liebigs Ann. Chem. 1844, 51, 145–163. [Google Scholar] [CrossRef]
- Epstein, A.J.; Etemad, S.; Garito, A.F.; Heeger, A.J. Metal-Insulator Transition and Antiferromagnetism in a One-Dimensional Organic Solid. Phys. Rev. B 1972, 5, 952–977. [Google Scholar] [CrossRef]
- Odom, S.A.; Caruso, M.M.; Finke, A.D.; Prokup, A.M.; Ritchey, J.A.; Leonard, J.H.; White, S.R.; Sottos, N.R.; Moore, J.S. Restoration of Conductivity with TTF-TCNQ Charge-Transfer Salts. Adv. Funct. Mater. 2010, 20, 1721–1727. [Google Scholar] [CrossRef]
- Klots, C.E.; Compton, R.N.; Raaen, V.F. Electronic and ionic properties of molecular TTF and TCNQ. J. Chem. Phys. 1974, 60, 1177–1178. [Google Scholar] [CrossRef]
- Anderson, P.W.; Lee, P.A.; Saitoh, M. Remarks on Giant Conductivity in TTF-TCNQ. Solid State Commun. 1973, 13, 595–598. [Google Scholar] [CrossRef]
- Groff, R.P.; Suna, A.; Merrifie, R. Temperature-Dependence of Conductivity of Tetrathiafulvalene-Tetracyanoquinodimethane (TTF-TCNQ) Single-Crystals. Phys. Rev. Lett. 1974, 33, 418–421. [Google Scholar] [CrossRef]
- Wu, H.-D.; Wang, F.-X.; Xiao, Y.; Pan, G.-B. Preparation and ambipolar transistor characteristics of co-crystal microrods of dibenzotetrathiafulvalene and tetracyanoquinodimethane. J. Mater. Chem. C 2013, 1, 2286–2289. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nakayama, J. The Crystal-Structure of the Charge-Transfer Complex of Dibenzotetrathiafulvalene-Tetracyanoquinodimethane, DBTTF-TCNQ. Bull. Chem. Soc. Jpn. 1981, 54, 2408–2411. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W. Control and interconversion of cocrystal stoichiometry in grinding: Stepwise mechanism for the formation of a hydrogen-bonded cocrystal. CrystEngComm 2009, 11, 470–481. [Google Scholar] [CrossRef]
- Paul, M.; Chakraborty, S.; Desiraju, G.R. Six-Component Molecular Solids: ABC[D1–(x+y)ExFy]2. J. Am. Chem. Soc. 2018, 140, 2309–2315. [Google Scholar] [CrossRef]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Four- and five-component molecular solids: Crystal engineering strategies based on structural inequivalence. IUCrJ 2016, 3, 96–101. [Google Scholar] [CrossRef]
- El-Zaria, M.E. Spectrophotometric study of the charge transfer complexation of some porphyrin derivatives as electron donors with tetracyanoethylene. Spectrochim. Acta A 2008, 69, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lei, Y.; Liao, L.; Hu, W. Competition between Arene–Perfluoroarene and Charge-Transfer Interactions in Organic Light-Harvesting Systems. Angew. Chem. Int. Ed. 2017, 56, 10352–10356. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Horiuchi, S.; Kagawa, F.; Ogawa, N.; Kurumaji, T.; Tokura, Y.; Kawasaki, M. Shift current photovoltaic effect in a ferroelectric charge-transfer complex. Nat. Commun. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ye, J.; He, X.; Du, K.; Zhang, K.K.; Wang, X.; Xiong, Q.; Liu, Z.; Jiang, H.; Kloc, C. Control of Radiative Exciton Recombination by Charge Transfer Induced Surface Dipoles in MoS2 and WS2 Monolayers. Sci. Rep. 2016, 6, 24105. [Google Scholar] [CrossRef] [PubMed]
- Yakuphanoglu, F.; Arslan, M. Determination of thermo-optic coefficient, refractive index, optical dispersion and group velocity parameters of an organic thin film. Phys. B Condens Matter 2007, 393, 304–309. [Google Scholar] [CrossRef]
- Tayi, A.S.; Shveyd, A.K.; Sue, A.C.H.; Szarko, J.M.; Rolczynski, B.S.; Cao, D.; Kennedy, T.J.; Sarjeant, A.A.; Stern, C.L.; Paxton, W.F.; et al. Room-temperature ferroelectricity in supramolecular networks of charge-transfer complexes. Nature 2012, 488, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, M.; Lei, Y. Organic materials for rechargeable sodium-ion batteries. Mater. Today 2018, 21, 60–78. [Google Scholar] [CrossRef]
- Hu, P.; He, X.; Ng, M.F.; Ye, J.; Zhao, C.; Wang, S.; Tan, K.; Chaturvedi, A.; Jiang, H.; Kloc, C.; et al. Trisulfide-Bond Acenes for Organic Batteries. Angew. Chem. Int. Ed. 2019, 58, 13513–13521. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Xu, H.; Zhang, Q.; Huang, W. Recent progress on organic donor–acceptor complexes as active elements in organic field-effect transistors. J. Mater. Chem. C 2018, 6, 3485–3498. [Google Scholar] [CrossRef]
- Lamport, Z.A.; Haneef, H.F.; Anand, S.; Waldrip, M.; Jurchescu, O.D. Tutorial: Organic field-effect transistors: Materials, structure and operation. J. Appl. Phys. 2018, 124, 071101. [Google Scholar] [CrossRef]
- Goetz, K.P.; Vermeulen, D.; Payne, M.E.; Kloc, C.; McNeil, L.E.; Jurchescu, O.D. Charge-transfer complexes: New perspectives on an old class of compounds. J. Mater. Chem. C 2014, 2, 3065–3076. [Google Scholar] [CrossRef]
- Hu, P.; Jiao, H.; Wang, C.H.; Wang, X.M.; Ye, S.; Jing, X.P.; Zhao, F.; Yue, Z.X. Influence of thermal treatments on the low frequency conductivity and microwave dielectric loss of CaTiO3 ceramics. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2011, 176, 401–405. [Google Scholar] [CrossRef]
- Henderson, J.; Masino, M.; Hatcher, L.E.; Kociok-Köhn, G.; Salzillo, T.; Brillante, A.; Raithby, P.R.; Girlando, A.; Da Como, E. New Polymorphs of Perylene:Tetracyanoquinodimethane Charge Transfer Cocrystals. Cryst. Growth Des. 2018, 18, 2003–2009. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Pearson: London, UK, 2005. [Google Scholar]
- Mori, T. Organic Conductors with Unusual Band Fillings. Chem. Rev. 2004, 104, 4947–4970. [Google Scholar] [CrossRef]
- Suzuki, K.; Kiyota, Y.; Yoo, D.; Uekusa, T.; Kawamoto, T.; Mori, T. A Large Variety of Crystal Structures and Conducting Properties in Dimethylbenzoimidazolium Salts of Tetracyanoquinodimethanes. Cryst. Growth Des. 2020, 20, 5940–5946. [Google Scholar] [CrossRef]
- Mori, T. Principles that Govern Electronic Transport in Organic Conductors and Transistors. Bull. Chem. Soc. Jpn. 2016, 89, 973–986. [Google Scholar] [CrossRef]
- Vermeulen, D.; Zhu, L.Y.; Goetz, K.P.; Hu, P.; Jiang, H.; Day, C.S.; Jurchescu, O.D.; Coropceanu, V.; Kloc, C.; McNeil, L.E. Charge Transport Properties of Perylene–TCNQ Crystals: The Effect of Stoichiometry. J. Phys. Chem. C 2014, 118, 24688–24696. [Google Scholar] [CrossRef]
- Chappell, J.S.; Bloch, A.N.; Bryden, W.A.; Maxfield, M.; Poehler, T.O.; Cowan, D.O. Degree of Charge-Transfer in Organic Conductors by Infrared-Absorption Spectroscopy. J. Am. Chem. Soc. 1981, 103, 2442–2443. [Google Scholar] [CrossRef]
- Rubtsov, I.V.; Kang, Y.K.; Redmore, N.P.; Allen, R.M.; Zheng, J.; Beratan, D.N.; Therien, M.J. The Degree of Charge Transfer in Ground and Charge-Separated States Revealed by Ultrafast Visible Pump/Mid-IR Probe Spectroscopy. J. Am. Chem. Soc. 2004, 126, 5022–5023. [Google Scholar] [CrossRef]
- Mendez, H.; Heimel, G.; Winkler, S.; Frisch, J.; Opitz, A.; Sauer, K.; Wegner, B.; Oehzelt, M.; Rothel, C.; Duhm, S.; et al. Charge-transfer crystallites as molecular electrical dopants. Nat. Commun. 2015, 6, 8560. [Google Scholar] [CrossRef]
- Hu, P.; Wang, S.; Chaturvedi, A.; Wei, F.; Zhu, X.; Zhang, X.; Li, R.; Li, Y.; Jiang, H.; Long, Y.; et al. Impact of C–H⋯X (X = F, N) and π–π Interactions on Tuning the Degree of Charge Transfer in F6TNAP-Based Organic Binary Compound Single Crystals. Cryst. Growth Des. 2018, 18, 1776–1785. [Google Scholar] [CrossRef]
- Hu, P.; Li, H.; Li, Y.; Jiang, H.; Kloc, C. Single-crystal growth, structures, charge transfer and transport properties of anthracene-F4TCNQ and tetracene-F4TCNQ charge-transfer compounds. CrystEngComm 2017, 19, 618–624. [Google Scholar] [CrossRef]
- Silva, R.A.L.; Santos, I.C.; Rabaça, S.; Lopes, E.B.; Gama, V.; Almeida, M.; Belo, D. Synthesis and Characterization of Charge Transfer Salts Based on [M(dcdmp)2] (M = Au, Cu and Ni) with TTF Type Donors. Crystals 2018, 8, 141. [Google Scholar] [CrossRef]
- Feng, Q.; Huan, W.; Wang, J.; Guo, F.; Lu, J.; Diao, G.; Shan, Y. Cocrystal Assembled by Pyrene Derivative and 1,4-Diiodotetrafluorobenzene via a C=O⋯I Halogen Bond. Crystals 2018, 8, 392. [Google Scholar] [CrossRef]
- Ding, X.; Tuikka, M.; Rissanen, K.; Haukka, M. Extended Assemblies of Ru(bpy)(CO)2 × 2 (X = Cl, Br, I) Molecules Linked by 1,4-Diiodotetrafluoro-Benzene (DITFB) Halogen Bond Donors. Crystals 2019, 9, 319. [Google Scholar] [CrossRef]
- Decato, D.A.; Riel, A.M.S.; Berryman, O.B. Anion Influence on the Packing of 1,3-Bis(4-Ethynyl-3-Iodopyridinium)-Benzene Halogen Bond Receptors. Crystals 2019, 9, 522. [Google Scholar] [CrossRef]
- Higashino, T.; Akiyama, Y.; Kojima, H.; Kawamoto, T.; Mori, T. Organic Semiconductors and Conductors with tert-Butyl Substituents. Crystals 2012, 2, 1222–1238. [Google Scholar] [CrossRef]
- Nakazawa, Y.I.S.; Matsumura, Y.; Yamashita, S.; Akutsu, H. Thermodynamic Picture of Dimer-Mott Organic Superconductors Revealed by Heat Capacity Measurements with External and Chemical Pressure Control. Crystals 2018, 8, 143. [Google Scholar] [CrossRef]
- Corpinot, M.K.; Bučar, D.-K. A Practical Guide to the Design of Molecular Crystals. Cryst. Growth Des. 2019, 19, 1426–1453. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Han, Q.; Ge, C.; Cui, S.; Zhang, L.; Zheng, X.; Liu, G.; Liu, J.; Liu, D.; et al. Microspacing In-Air Sublimation Growth of Organic Crystals. Chem. Mater. 2018, 30, 412–420. [Google Scholar] [CrossRef]
- Munshi, T.; Redha, B.; Feeder, N.; Meenan, P.; Blagden, N. Impact of Mixed Solvent on Co-Crystal Solubility, Ternary Phase Diagram, and Crystallization Scale Up. Cryst. Growth Des. 2016, 16, 1817–1823. [Google Scholar] [CrossRef]
- Jiang, H.; Kloc, C. Single-crystal growth of organic semiconductors. MRS Bull. 2013, 38, 28–33. [Google Scholar] [CrossRef]
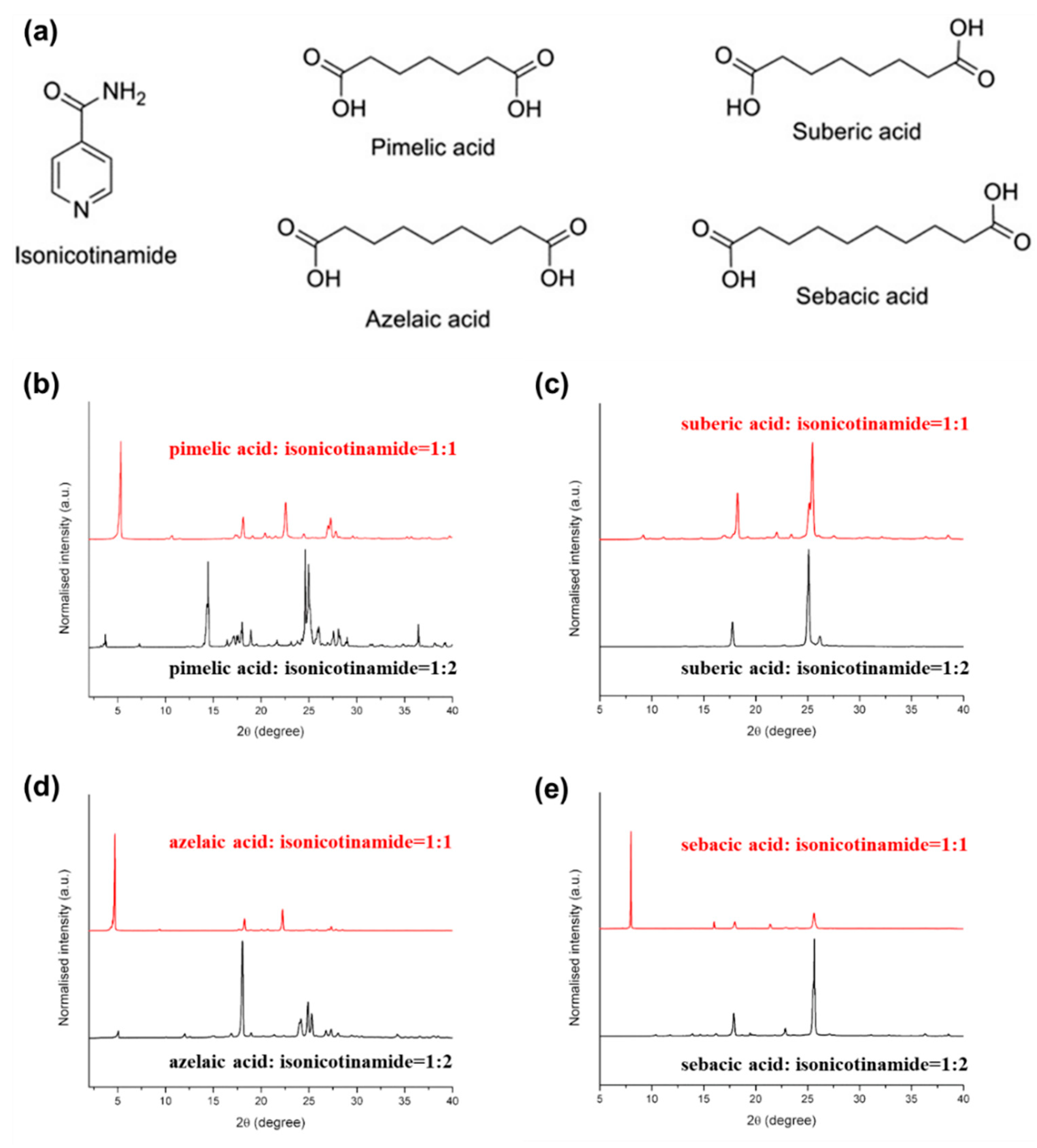
- Tothadi, S.; Phadkule, A. Does stoichiometry matter? Cocrystals of aliphatic dicarboxylic acids with isonicotinamide: Odd–even alternation in melting points. CrystEngComm 2019, 21, 2481–2484. [Google Scholar] [CrossRef]
- Miyao, K.; Funabiki, A.; Takahashi, K.; Mochida, T.; Uruichi, M. Reversible iodine absorption of nonporous coordination polymer Cu(TCNQ). New J. Chem. 2014, 38, 739–743. [Google Scholar] [CrossRef]
- Funabiki, A.; Mochida, T.; Takahashi, K.; Mori, H.; Sakurai, T.; Ohta, H.; Uruichi, M. Reversible iodine absorption by alkali-TCNQ salts with associated changes in physical properties. J. Mater. Chem. 2012, 22, 8361–8366. [Google Scholar] [CrossRef]
- Chen, R.; Gokus, M.K.; Pagola, S. etrathiafulvalene: A Gate to the Mechanochemical Mechanisms of Electron Transfer Reactions. Crystals 2020, 10, 482. [Google Scholar] [CrossRef]
- Ida, T.; Yakushi, K.; Kuroda, H. Pressure dependence of the polarized reflectance spectrum of the solid charge-transfer complex, perylene–TCNQ: Estimation of microscopic parameters. J. Chem. Phys. 1989, 91, 3450–3455. [Google Scholar] [CrossRef]
- Ishii, K.; Yakushi, K.; Kuroda, H.; Inokuchi, H. Reflection and Photoconduction Spectra of the Single Crystals of Perylene-TCNQ 1:1 and 3:1 Molecular Complexes. Bull. Chem. Soc. Jpn. 1984, 57, 3043–3047. [Google Scholar] [CrossRef]
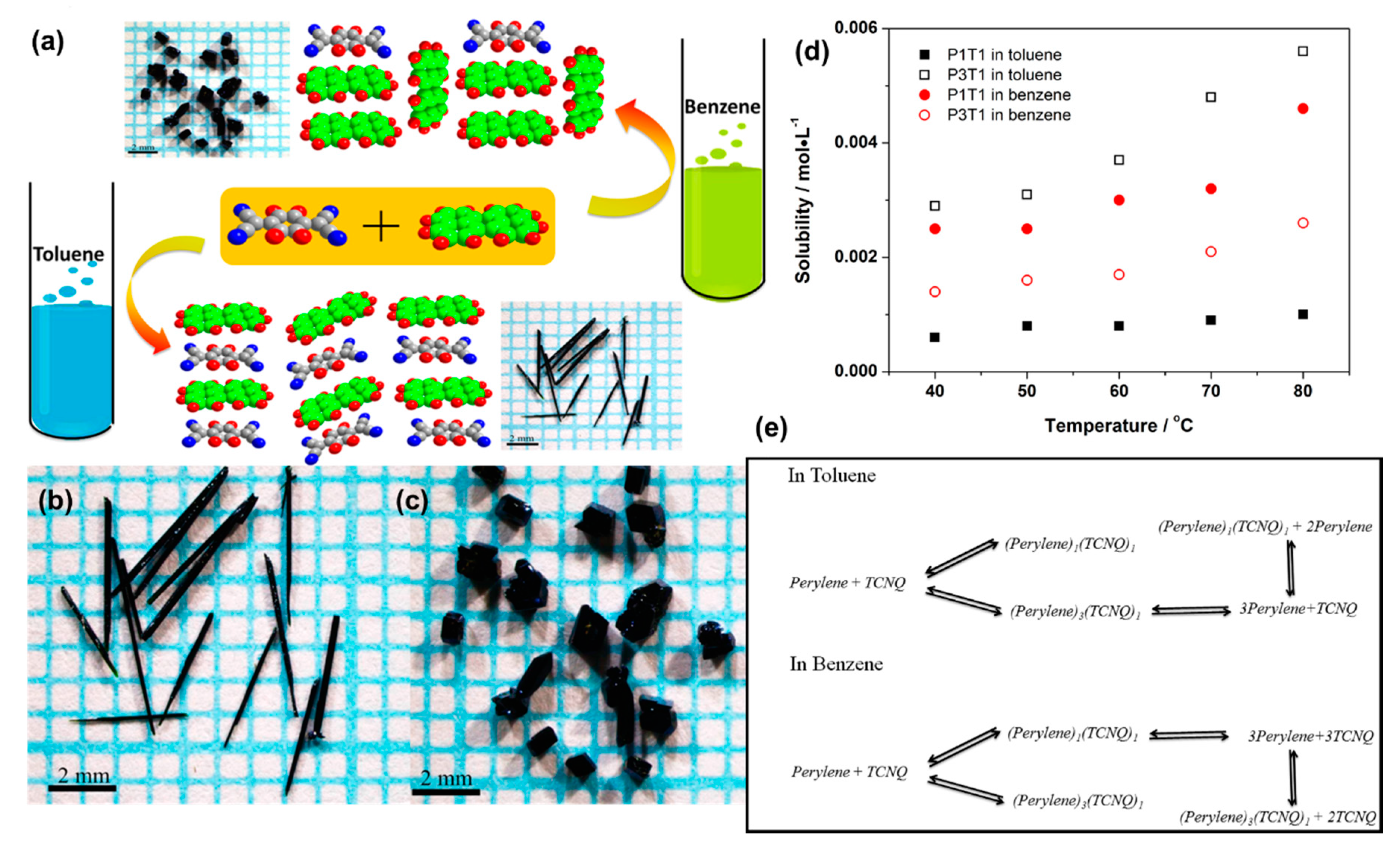
- Hu, P.; Ma, L.; Tan, K.J.; Jiang, H.; Wei, F.; Yu, C.; Goetz, K.P.; Jurchescu, O.D.; McNeil, L.E.; Gurzadyan, G.G.; et al. Solvent-Dependent Stoichiometry in Perylene–7,7,8,8-Tetracyanoquinodimethane Charge Transfer Compound Single Crystals. Cryst. Growth Des. 2014, 14, 6376–6382. [Google Scholar] [CrossRef]
- Konarev, D.V.; Lyubovskaya, R.N. Donor-acceptor complexes and radical ionic salts based on fullerenes. Russ. Chem. Rev. 1999, 68, 19–38. [Google Scholar] [CrossRef]
- Delhaès, P.; Flandrois, S.; Keryer, G.; Dupuis, P. Specific heat and paramagnetism of TTT-TCNQ and TTT-TCNQ2 charge transfer complexes. Mater. Res. Bull. 1975, 10, 825–829. [Google Scholar] [CrossRef]
- Yoshida, Y.; Shimizu, Y.; Yajima, T.; Maruta, G.; Takeda, S.; Nakano, Y.; Hiramatsu, T.; Kageyama, H.; Yamochi, H.; Saito, G. Molecular Rotors of Coronene in Charge-Transfer Solids. Chem. Eur. J. 2013, 19, 12313–12324. [Google Scholar] [CrossRef] [PubMed]
- Truong, K.D.; Bandrauk, A.D. A new TCNQ complex: (Perylene)3 TCNQ. Chem. Phys. Lett. 1976, 44, 232–235. [Google Scholar] [CrossRef]
- Ni, W.; Gurzadyan, G.G.; Ma, L.; Hu, P.; Kloc, C.; Sun, L. Ultrafast Tuning of Various Photochemical Pathways in Perylene–TCNQ Charge-Transfer Crystals. J. Phys. Chem. C 2020, 124, 13894–13901. [Google Scholar] [CrossRef]
- Zhu, W.; Yi, Y.; Zhen, Y.; Hu, W. Precisely Tailoring the Stoichiometric Stacking of Perylene-TCNQ Co-Crystals towards Different Nano and Microstructures with Varied Optoelectronic Performances. Small 2015, 11, 2150–2156. [Google Scholar] [CrossRef]
- Salzillo, T.; Della Valle, R.G.; Venuti, E.; Brillante, A.; Kociok-Köhn, G.; Di Nuzzo, D.; Masino, M.; Girlando, A. (Perylene)3-(TCNQF1)2: Yet Another Member in the Series of Perylene–TCNQFx Polymorphic Charge Transfer Crystals. Crystals 2020, 10, 177. [Google Scholar] [CrossRef]
- Hu, P.; Du, K.; Wei, F.; Jiang, H.; Kloc, C. Crystal Growth, HOMO–LUMO Engineering, and Charge Transfer Degree in Perylene-FxTCNQ (x = 1, 2, 4) Organic Charge Transfer Binary Compounds. Cryst. Growth Des. 2016, 16, 3019–3027. [Google Scholar] [CrossRef]
- Salzillo, T.; Masino, M.; Kociok-Köhn, G.; Di Nuzzo, D.; Venuti, E.; Della Valle, R.G.; Vanossi, D.; Fontanesi, C.; Girlando, A.; Brillante, A.; et al. Structure, Stoichiometry, and Charge Transfer in Cocrystals of Perylene with TCNQ-Fx. Cryst. Growth Des. 2016, 16, 3028–3036. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kumagai, Y.; Mizuno, M.; Saito, G. Structure–Property Relationship of Supramolecular Rotators of Coronene in Charge-Transfer Solids. Cryst. Growth Des. 2015, 15, 1389–1394. [Google Scholar] [CrossRef]
- Kataeva, O.; Khrizanforov, M.; Budnikova, Y.; Islamov, D.; Burganov, T.; Vandyukov, A.; Lyssenko, K.; Mahns, B.; Nohr, M.; Hampel, S.; et al. Crystal Growth, Dynamic and Charge Transfer Properties of New Coronene Charge Transfer Complexes. Cryst. Growth Des. 2016, 16, 331–338. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kumagai, Y.; Mizuno, M.; Isomura, K.; Nakamura, Y.; Kishida, H.; Saito, G. Improved Dynamic Properties of Charge-Transfer-Type Supramolecular Rotor Composed of Coronene and F4TCNQ. Cryst. Growth Des. 2015, 15, 5513–5518. [Google Scholar] [CrossRef]
- Mahns, B.; Kataeva, O.; Islamov, D.; Hampel, S.; Steckel, F.; Hess, C.; Knupfer, M.; Büchner, B.; Himcinschi, C.; Hahn, T.; et al. Crystal Growth, Structure, and Transport Properties of the Charge-Transfer Salt Picene/2,3,5,6-Tetrafluoro-7,7,8,8-tetracyanoquinodimethane. Cryst. Growth Des. 2014, 14, 1338–1346. [Google Scholar] [CrossRef]
- Hoekstra, A.; Spoelder, T.; Vos, A. The crystal structure of rubidium–7,7,8,8-tetracyanoquinodimethane, Rb–TCNQ, at −160 °C. Acta Crystallogr. B 1972, 28, 14–25. [Google Scholar] [CrossRef]
- Coppens, P.; Row, T.N.G. X-ray Diffraction Measurement of Net Atomic and Molecular Charges. Ann. N. Y. Acad. Sci. 1978, 313, 244–255. [Google Scholar] [CrossRef]
- Flandrois, S.; Chasseau, D. Longueurs de liaison et transfert de charge dans les sels du tétracyanoquinodiméthane (TCNQ). Acta Crystallogr. B 1977, 33, 2744–2750. [Google Scholar] [CrossRef]
- Dobrowolski, M.A.; Garbarino, G.; Mezouar, M.; Ciesielski, A.; Cyranski, M.K. Structural diversities of charge transfer organic complexes. Focus on benzenoid hydrocarbons and 7,7,8,8-tetracyanoquinodimethane. CrystEngComm 2014, 16, 415–429. [Google Scholar] [CrossRef]
- Kistenmacher, T.J.; Emge, T.J.; Bloch, A.N.; Cowan, D.O. Structure of the Red, Semiconducting Form of 4,4′,5,5′-Tetramethyl-Delta-2,2′-Bi-1,3-Diselenole-7,7,8,8-Tetracyano-Para-Quinodimethane, TMTSF-TCNQ. Acta Crystallogr. B 1982, 38, 1193–1199. [Google Scholar] [CrossRef]
- Uruichi, M.; Yakushi, K.; Mochida, T. Phase Separation in the Monovalent-to-Divalent Phase Transition of Biferrocenium-(F1TCNQ)3. J. Low Temp. Phys. 2006, 142, 667–670. [Google Scholar] [CrossRef]
- Meneghetti, M.; Pecile, C. Charge–transfer organic crystals: Molecular vibrations and spectroscopic effects of electron–molecular vibration coupling of the strong electron acceptor TCNQF4. J. Chem. Phys. 1986, 84, 4149–4162. [Google Scholar] [CrossRef]
- Salzillo, T.; Della Valle, R.G.; Venuti, E.; Kociok-Köhn, G.; Masino, M.; Girlando, A.; Brillante, A. Solution equilibrium between two structures of Perylene-F2TCNQ charge transfer co-crystals. J. Cryst. Growth 2019, 516, 45–50. [Google Scholar] [CrossRef]
- Masino, M.; Castagnetti, N.; Girlando, A. Phenomenology of the Neutral-Ionic Valence Instability in Mixed Stack Charge-Transfer Crystals. Crystals 2017, 7, 108. [Google Scholar] [CrossRef]
- Goud, N.R.; Matzger, A.J. Impact of Hydrogen and Halogen Bonding Interactions on the Packing and Ionicity of Charge-Transfer Cocrystals. Cryst. Growth Des. 2017, 17, 328–336. [Google Scholar] [CrossRef]
- Jaeger, C.D.; Bard, A.J. Electrochemical behavior of donor-tetracyanoquinodimethane electrodes in aqueous media. J. Am. Chem. Soc. 1980, 102, 5435–5442. [Google Scholar] [CrossRef]
- Buravov, L.I.; Eremenko, O.N.; Lyubovskii, R.B.; Rozenberg, L.P.; Khidekel, M.L.; Shibaeva, R.P.; Shchegolev, I.F.; Yagubskii, E.B. Structure and Electromagnetic Properties of a New High-Conductivity Complex (TTT)+(TCNQ. JETP Lett. 1974, 20, 208–209. [Google Scholar]
- Huang, Y.-E.; Wang, X.-Z.; Hu, P.; Qi, X.-H.; Huang, X.-Y.; Kloc, C.; Wu, X.; Du, K.-Z. Single-photon upconversion in 6-pentaceneone crystal from bulk to ultrathin flakes. Nanoscale 2020, 12, 6227–6232. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. B 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. CrystEngComm 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17 (2017). University of Western Australia. Available online: https://hirshfeldsurface.net (accessed on 1 September 2020).

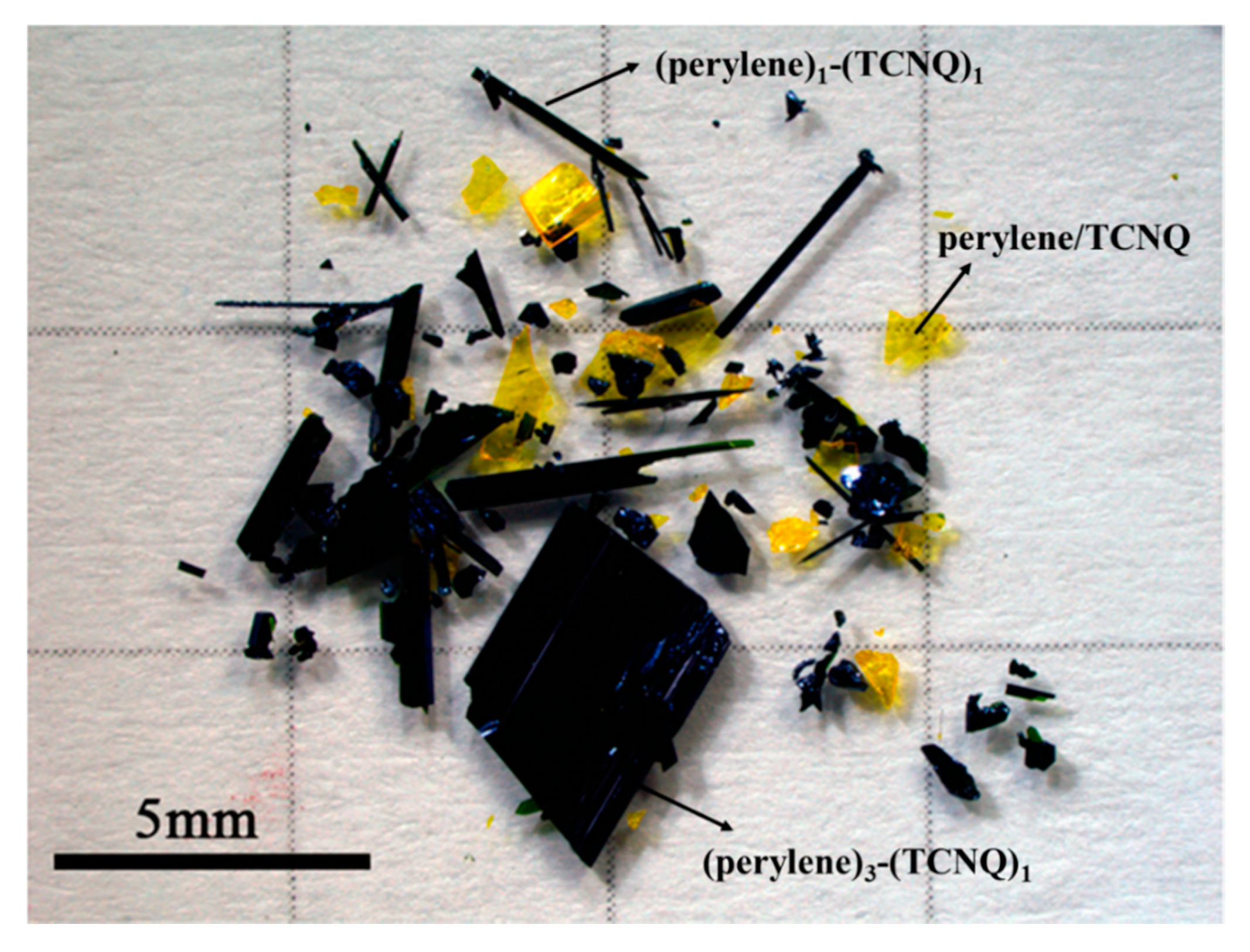
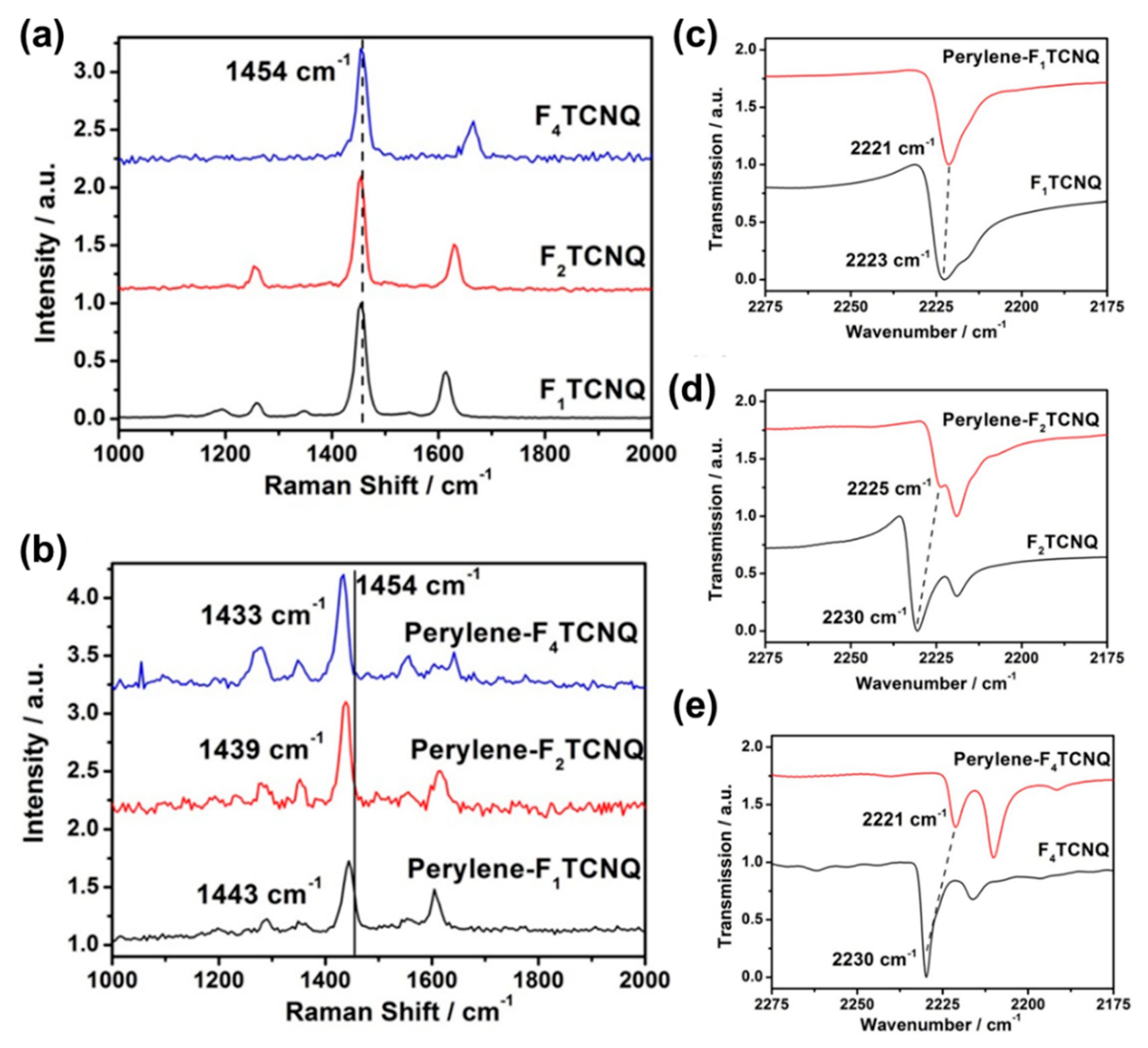
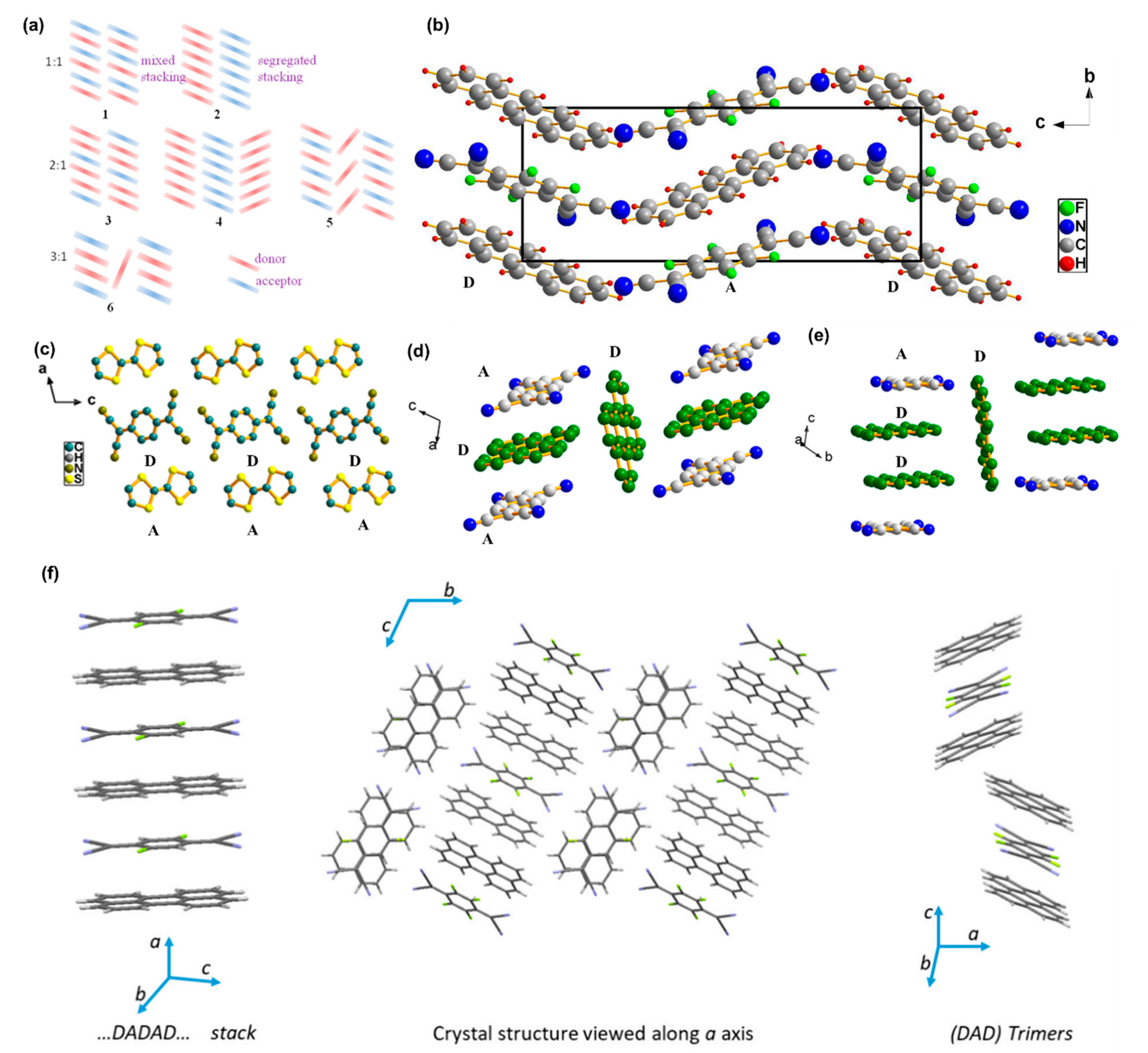
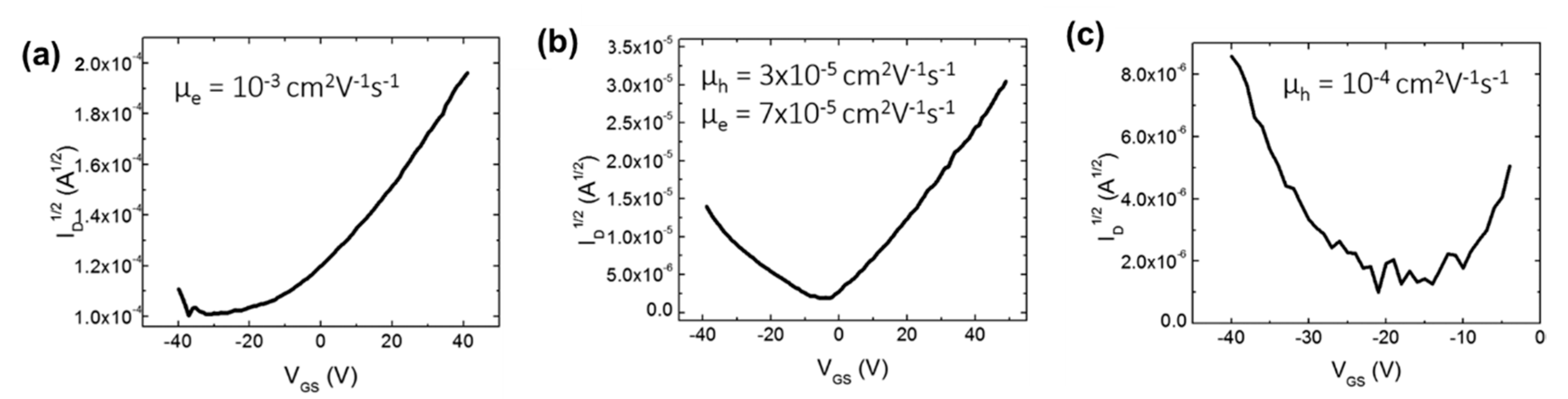


| Donor | Acceptor | Stoichiometry | Method | Solvent ** | Ref. |
|---|---|---|---|---|---|
| perylene | TCNQ | 1:1 | slow cooling | benzene:acetonitrile = 5:1 | [63] |
| perylene | TCNQ | 1:1 | gas-phase open system | N.A. | [37,64] |
| perylene | TCNQ | 1:1 | slow cooling | toluene | [59] |
| perylene | TCNQ | 1:1 | slow solvent evaporation | acetonitrile | [65] |
| perylene | TCNQ | 2:1 | gas-phase open system | N.A. | [37] |
| perylene | TCNQ | 3:1 | vapor diffusion | benzene | [63,64] |
| perylene | TCNQ | 3:1 | gas-phase open system | N.A. | [37] |
| perylene | TCNQ | 3:1 | slow cooling | benzene | [59] |
| perylene | TCNQ | 3:1 | slow evaporation | acetonitrile | [65] |
| perylene | F1TCNQ | 1:1 | gas-phase close system | N.A. | [66] |
| perylene | F1TCNQ | 1:1 | gas-phase open system | N.A. | [67] |
| perylene | F1TCNQ | 3:2 | gas-phase close system | N.A. | [66] |
| perylene | F2TCNQ | 1:1 | slow cooling | toluene:acetonitrile = 5:1 | [68] |
| perylene | F2TCNQ | 3:2 | gas-phase open system | N.A. | [67] |
| perylene | F2TCNQ | 3:2 | gas-phase close system | N.A. | [68] |
| perylene | F4TCNQ | 1:1 | slow cooling | toluene:acetonitrile = 5:1 | [68] |
| perylene | F4TCNQ | 1:1 | gas-phase open system | N.A. | [67] |
| perylene | F4TCNQ | 3:2 | gas-phase close system | N.A. | [68] |
| TTT * | TCNQ | 1:1 | slow cooling | methanol | [61] |
| TTT * | TCNQ | 1:2 | slow cooling | nitrobenzene | [61] |
| coronene | TCNQ | 1:1 | slow solvent evaporation | dichloromethane | [62] |
| coronene | TCNQ | 3:1 | slow cooling | benzene | [62] |
| coronene | MeTCNQ | 1:1 | slow solvent evaporation | dichloromethane | [62] |
| coronene | MeTCNQ | 2:1 | slow solvent evaporation | dichloromethane | [62] |
| coronene | F4TCNQ | 1:1 | diffusion | dichloromethane:pentane = 1:5 | [69] |
| coronene | F4TCNQ | 2:1 | gas-phase close system | N.A. | [70,71] |
| Donor | Acceptor | Stoichiometry | Degree of Charge Transfer | ||
|---|---|---|---|---|---|
| Bond Length | IR | Raman | |||
| perylene | TCNQ | 1:1 | 0.01 ± 0.07 [37] | - | 0.04 ± 0.02 [37] |
| perylene | TCNQ | 2:1 | 0.12 ± 0.07 [37] | - | 0.13 ± 0.02 [37] |
| perylene | TCNQ | 3:1 | 0.23 ± 0.06 [37] | - | 0.17 ± 0.02 [37] |
| coronene | F4TCNQ | 1:1 | - | 0.1 [62] | - |
| coronene | F4TCNQ | 2:1 | - | 0.15 [71] | - |
| perylene | F1TCNQ | 1:1 | 0.021 ± 0.061 [67] | 0.08 ± 0.05 [67] | - |
| perylene | F1TCNQ | 3:2 | - | 0.1 [66] | - |
| perylene | F2TCNQ | 1:1 | - | 0.13 [68] 0.17 ± 0.03 [67] | - |
| perylene | F2TCNQ | 3:2 | 0.17 ± 0.01 [67] | 0.15 [68] | - |
| perylene | F4TCNQ | 1:1 | 0.30 ± 0.02 [67] | 0.29 [68] 0.25 ± 0.03 [67] | - |
| perylene | F4TCNQ | 3:2 | - | 0.29 [68] | - |
| Donor | Acceptor | Stoichiometry | Conductivity (σ)/S·cm−1 | Ref. |
|---|---|---|---|---|
| perylene | TCNQ | 1:1 | 10−10~10−8 | [6,37] |
| perylene | TCNQ | 2:1 | 10−11~10−9 | [6,37] |
| perylene | TCNQ | 3:1 | 10−11~10−9 | [6,37] |
| TTT | TCNQ | 1:1 | 1 | [83] |
| TTT | TCNQ | 1:2 | 100 | [83] |
| Donor | Acceptor | Stoichiometry | Carrier Type | Mobility/cm2 V−1 s−1 | Ref. | |
|---|---|---|---|---|---|---|
| perylene | TCNQ | 1:1 | n | 10−3 | [37] | |
| 0.05 | [65] | |||||
| perylene | TCNQ | 2:1 | ambipolar | n | 7 × 10−5 | [37] |
| p | 3 × 10−5 | [37] | ||||
| perylene | TCNQ | 3:1 | p | 10−4 | [37] | |
| 0.03 | [65] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhai, H.; Hu, P.; Jiang, H. The Stoichiometry of TCNQ-Based Organic Charge-Transfer Cocrystals. Crystals 2020, 10, 993. https://doi.org/10.3390/cryst10110993
Gao J, Zhai H, Hu P, Jiang H. The Stoichiometry of TCNQ-Based Organic Charge-Transfer Cocrystals. Crystals. 2020; 10(11):993. https://doi.org/10.3390/cryst10110993
Chicago/Turabian StyleGao, Jiaoyang, Huifei Zhai, Peng Hu, and Hui Jiang. 2020. "The Stoichiometry of TCNQ-Based Organic Charge-Transfer Cocrystals" Crystals 10, no. 11: 993. https://doi.org/10.3390/cryst10110993
APA StyleGao, J., Zhai, H., Hu, P., & Jiang, H. (2020). The Stoichiometry of TCNQ-Based Organic Charge-Transfer Cocrystals. Crystals, 10(11), 993. https://doi.org/10.3390/cryst10110993







