Cocrystal Formation through Solid-State Reaction between Ibuprofen and Nicotinamide Revealed Using THz and IR Spectroscopy with Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Storage of Tablets
2.2.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Measurements
2.2.3. Terahertz Spectroscopy Measurements
2.2.4. Multivariate Analysis
3. Results and Discussion
3.1. FT-IR and THz Spectra
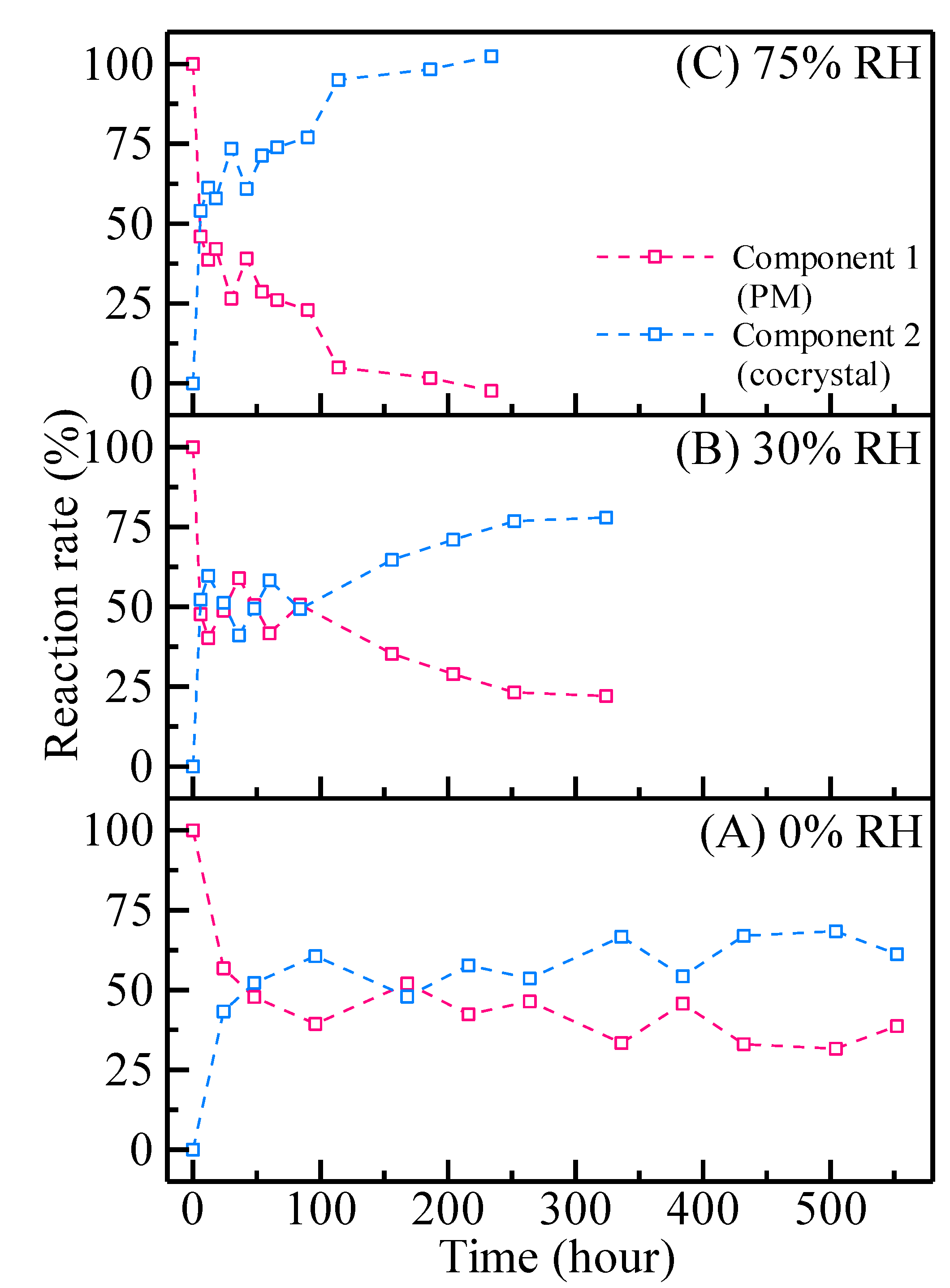
3.2. FT-IR Spectra and MCR-ALS Analysis
3.3. THz Spectra and MCR-ALS Analysis
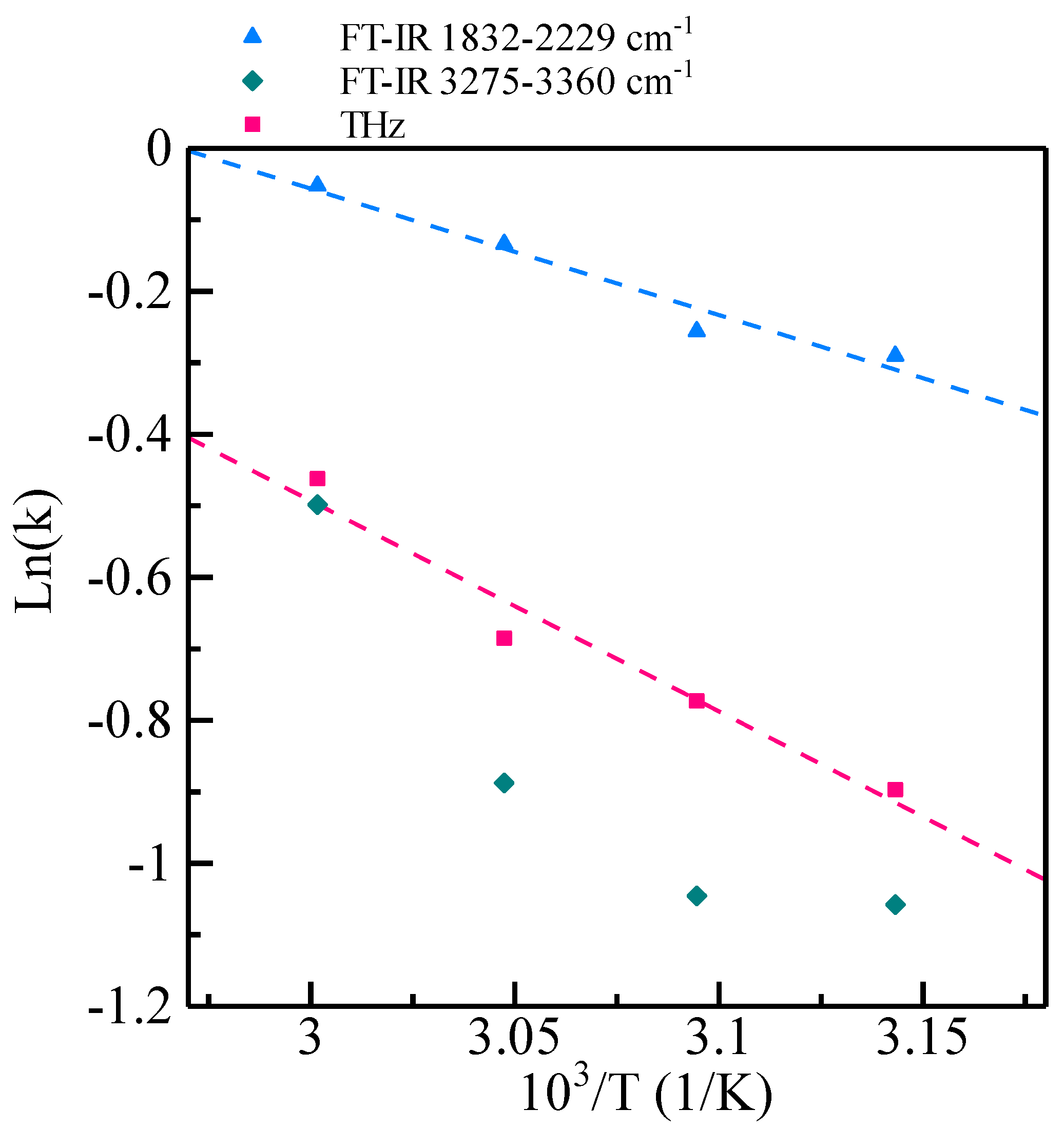
3.4. Contributions of Thermal Energy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Food and Drug Administratio Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry. Available online: https://www.fda.gov/media/81824/download (accessed on 28 August 2020).
- European Medicines Agency Reflection Paper on the Use of Cocrystals of Active Substances in Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-use-cocrystals-active-substances-medicinal-products_en.pdf (accessed on 28 August 2020).
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Ueto, T.; Takata, N.; Muroyama, N.; Nedu, A.; Sasaki, A.; Tanida, S.; Terada, K. Polymorphs and a Hydrate of Furosemide–Nicotinamide 1:1 Cocrystal. Cryst. Growth Des. 2012, 12, 485–494. [Google Scholar] [CrossRef]
- Takata, N.; Shiraki, K.; Takano, R.; Hayashi, Y.; Terada, K. Cocrystal Screening of Stanolone and Mestanolone Using Slurry Crystallization. Cryst. Growth Des. 2008, 8, 3032–3037. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Roy, L.; Rodríguez-Hornedo, N.; Velaga, S.P. pH-Dependent Solubility of Indomethacin–Saccharin and Carbamazepine–Saccharin Cocrystals in Aqueous Media. Mol. Pharm. 2012, 9, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Trask, A.V.; Jones, W.; Motherwell, W.D.S. Screening for Inclusion Compounds and Systematic Construction of Three-Component Solids by Liquid-Assisted Grinding. Angew. Chem. 2006, 118, 7708–7712. [Google Scholar] [CrossRef]
- Chun, N.-H.; Wang, I.-C.; Lee, M.-J.; Jung, Y.-T.; Lee, S.; Kim, W.-S.; Choi, G.J. Characteristics of indomethacin–saccharin (IMC–SAC) co-crystals prepared by an anti-solvent crystallization process. Eur. J. Pharm. Biopharm. 2013, 85, 854–861. [Google Scholar] [CrossRef]
- Wichianphong, N.; Charoenchaitrakool, M. Statistical optimization for production of mefenamic acid–nicotinamide cocrystals using gas anti-solvent (GAS) process. J. Ind. Eng. Chem. 2018, 62, 375–382. [Google Scholar] [CrossRef]
- Nishimaru, M.; Kudo, S.; Takiyama, H. Cocrystal production method reducing deposition risk of undesired single component crystals in anti-solvent cocrystallization. J. Ind. Eng. Chem. 2016, 36, 40–43. [Google Scholar] [CrossRef]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced methodologies for cocrystal synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Müllers, K.C.; Paisana, M.; Wahl, M.A. Simultaneous Formation and Micronization of Pharmaceutical Cocrystals by Rapid Expansion of Supercritical Solutions (RESS). Pharm. Res. 2015, 32, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Padrela, L.; Rodrigues, M.A.; Tiago, J.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Insight into the Mechanisms of Cocrystallization of Pharmaceuticals in Supercritical Solvents. Cryst. Growth Des. 2015, 15, 3175–3181. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Kaialy, W.; Buckton, G.; Gill, H.; Nokhodchi, A.; Velaga, S.P. Theophylline Cocrystals Prepared by Spray Drying: Physicochemical Properties and Aerosolization Performance. AAPS PharmSciTech 2013, 14, 265–276. [Google Scholar] [CrossRef]
- Jayasankar, A.; Somwangthanaroj, A.; Shao, Z.J.; Rodríguez-Hornedo, N. Cocrystal Formation during Cogrinding and Storage is Mediated by Amorphous Phase. Pharm. Res. 2006, 23, 2381–2392. [Google Scholar] [CrossRef]
- Chieng, N.; Hubert, M.; Saville, D.; Rades, T.; Aaltonen, J. Formation Kinetics and Stability of Carbamazepine−Nicotinamide Cocrystals Prepared by Mechanical Activation. Cryst. Growth Des. 2009, 9, 2377–2386. [Google Scholar] [CrossRef]
- Ervasti, T.; Aaltonen, J.; Ketolainen, J. Theophylline–nicotinamide cocrystal formation in physical mixture during storage. Int. J. Pharm. 2015, 486, 121–130. [Google Scholar] [CrossRef]
- Fischer, F.; Heidrich, A.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. Polymorphism of Mechanochemically Synthesized Cocrystals: A Case Study. Cryst. Growth Des. 2016, 16, 1701–1707. [Google Scholar] [CrossRef]
- Hu, Y.; Gniado, K.; Erxleben, A.; McArdle, P. Mechanochemical Reaction of Sulfathiazole with Carboxylic Acids: Formation of a Cocrystal, a Salt, and Coamorphous Solids. Cryst. Growth Des. 2014, 14, 803–813. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying Hot-Stage Microscopy to Co-Crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.L.; Sun, C.C. Simultaneously Improving the Mechanical Properties, Dissolution Performance, and Hygroscopicity of Ibuprofen and Flurbiprofen by Cocrystallization with Nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Yuliandra, Y.; Zaini, E.; Syofyan, S.; Pratiwi, W.; Putri, L.N.; Pratiwi, Y.S.; Arifin, H. Cocrystal of Ibuprofen–Nicotinamide: Solid-State Characterization and In Vivo Analgesic Activity Evaluation. Sci. Pharm. 2018, 86, 23. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Hattori, Y.; Otsuka, M. MCR-ALS analysis of IR spectroscopy and XRD for the investigation of ibuprofen-nicotinamide cocrystal formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 221, 117142. [Google Scholar] [CrossRef]
- Soares, F.L.F.; Carneiro, R.L. Green Synthesis of Ibuprofen–Nicotinamide Cocrystals and In-Line Evaluation by Raman Spectroscopy. Cryst. Growth Des. 2013, 13, 1510–1517. [Google Scholar] [CrossRef]
- Hattori, Y.; Sato, M.; Otsuka, M. Initial dissolution kinetics of cocrystal of carbamazepine with nicotinamide. J. Pharm. Pharmacol. 2015, 67, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Kaneniwa, N.; Imagawa, K.; Otsuka, M. Effect of tabletting on the degree of crystallinity and on the dehydration and decomposition points of cephalexin crystalline powder. Chem. Pharm. Bull. 1985, 33, 802–809. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.E.; Timmins, P.; Williams, A.C.; York, P. Characterization of Dihydrates Prepared from Carbamazepine Polymorphs. J. Pharm. Sci. 1996, 85, 1064–1069. [Google Scholar] [CrossRef]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J. Pharm. Pharmacol. 2009, 61, 971–988. [Google Scholar] [CrossRef]
- Takeshima, R.; Hattori, Y.; Managaki, S.; Otsuka, M. Analysis of the dehydration process of caffeine using backscattering and transmission Raman spectroscopy. Int. J. Pharm. 2017, 530, 256–262. [Google Scholar] [CrossRef]
- Otsuka, M.; Nishizawa, J.-I.; Shibata, J.; Ito, M. Quantitative Evaluation of Mefenamic Acid Polymorphs by Terahertz-Chemometrics. J. Pharm. Sci. 2010, 99, 4048–4053. [Google Scholar] [CrossRef]
- Sasaki, T.; Sakamoto, T.; Otsuka, M. Detection of Impurities in Organic Crystals by High-Accuracy Terahertz Absorption Spectroscopy. Anal. Chem. 2018, 90, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Heilweil, E.; Hussain, A.; Khan, M. Process analytical technology (PAT): Effects of instrumental and compositional variables on terahertz spectral data quality to characterize pharmaceutical materials and tablets. Int. J. Pharm. 2007, 343, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Alcalá, M.; González, J.M.; Torras, E. A process analytical technology approach based on near infrared spectroscopy: Tablet hardness, content uniformity, and dissolution test measurements of intact tablets. J. Pharm. Sci. 2006, 95, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, C.; Abatzoglou, N.; Simard, J.-S.; McDermott, L.; Léonard, G.; Cartilier, L. Cohesive, multicomponent, dense powder flow characterization by NIR. Int. J. Pharm. 2007, 336, 292–301. [Google Scholar] [CrossRef] [PubMed]
- De Beer, T.; Burggraeve, A.; Fonteyne, M.; Saerens, L.; Remon, J.P.; Vervaet, C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int. J. Pharm. 2011, 417, 32–47. [Google Scholar] [CrossRef]
- Dohi, M.; Momose, W.; Yoshino, H.; Hara, Y.; Yamashita, K.; Hakomori, T.; Sato, S.; Terada, K. Application of terahertz pulse imaging as PAT tool for non-destructive evaluation of film-coated tablets under different manufacturing conditions. J. Pharm. Biomed. Anal. 2016, 119, 104–113. [Google Scholar] [CrossRef]
- Hattori, Y.; Otsuka, M. Modeling of feed-forward control using the partial least squares regression method in the tablet compression process. Int. J. Pharm. 2017, 524, 407–413. [Google Scholar] [CrossRef]
- Suto, K.; Sasaki, T.; Tanabe, T.; Saito, K.; Nishizawa, J.; Ito, M. GaP THz wave generator and THz spectrometer using Cr:Forsterite lasers. Rev. Sci. Instrum. 2005, 76, 123109. [Google Scholar] [CrossRef]
- Tauler, R.; Kowalski, B.; Fleming, S. Multivariate curve resolution applied to spectral data from multiple runs of an industrial process. Anal. Chem. 1993, 65, 2040–2047. [Google Scholar] [CrossRef]
- Garrido, M.; Rius, F.X.; Larrechi, M.S. Multivariate curve resolution–alternating least squares (MCR-ALS) applied to spectroscopic data from monitoring chemical reactions processes. Anal. Bioanal. Chem. 2008, 390, 2059–2066. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Takeuchi, M.; Tanaka, H. Dry Mechanochemical Synthesis of Caffeine/Oxalic Acid Cocrystals and Their Evaluation by Powder X-ray Diffraction and Chemometrics. J. Pharm. Sci. 2017, 106, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Duggirala, N.K.; Hattori, Y.; Otsuka, M.; Suryanarayanan, R. Formation of Indomethacin–Saccharin Cocrystals during Wet Granulation: Role of Polymeric Excipients. Mol. Pharm. 2020, 17, 274–283. [Google Scholar] [CrossRef] [PubMed]
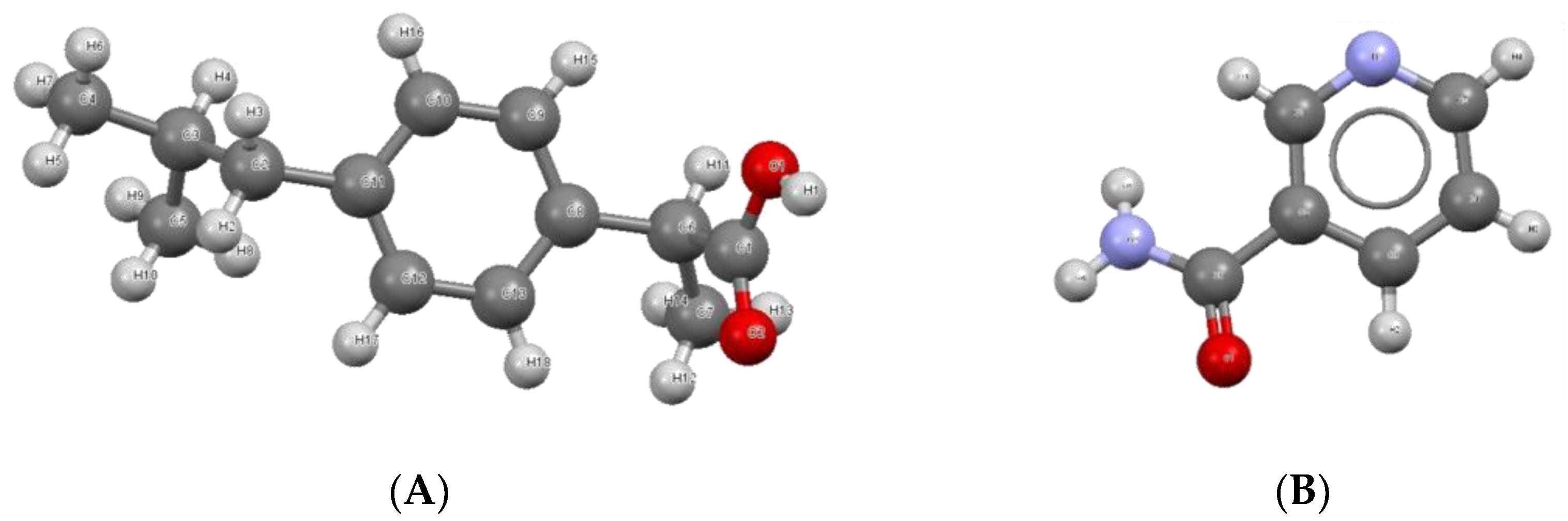
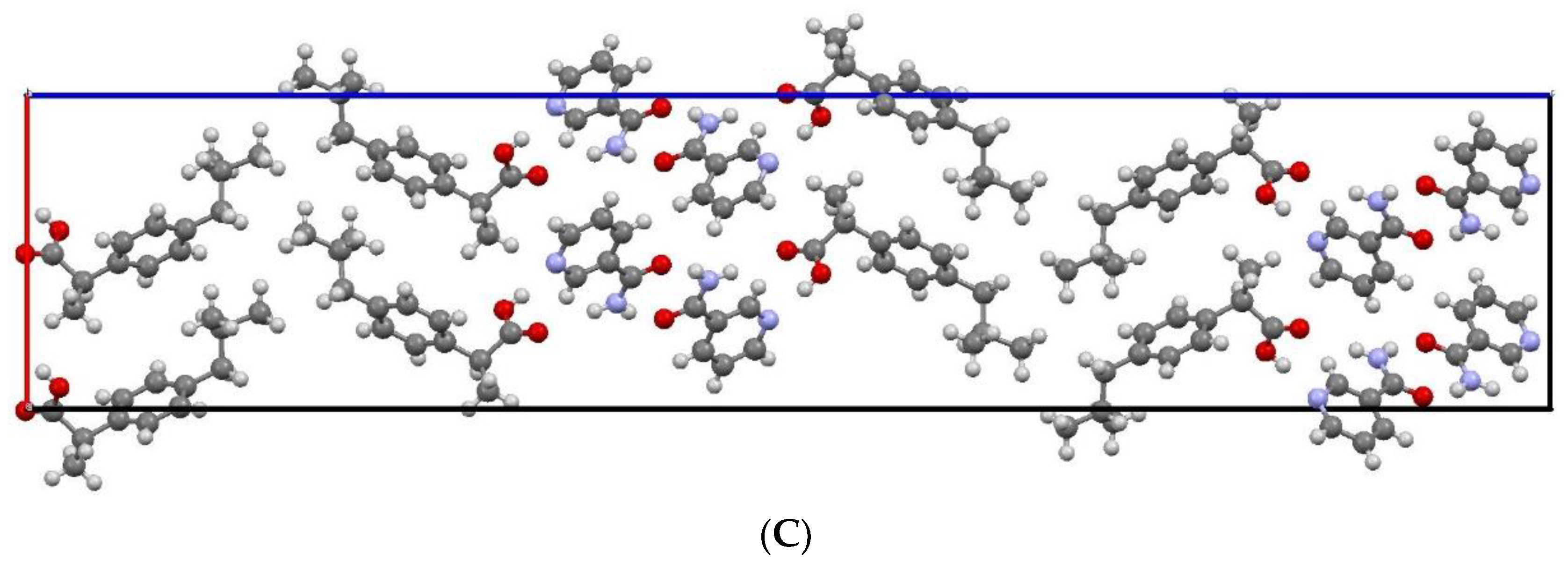

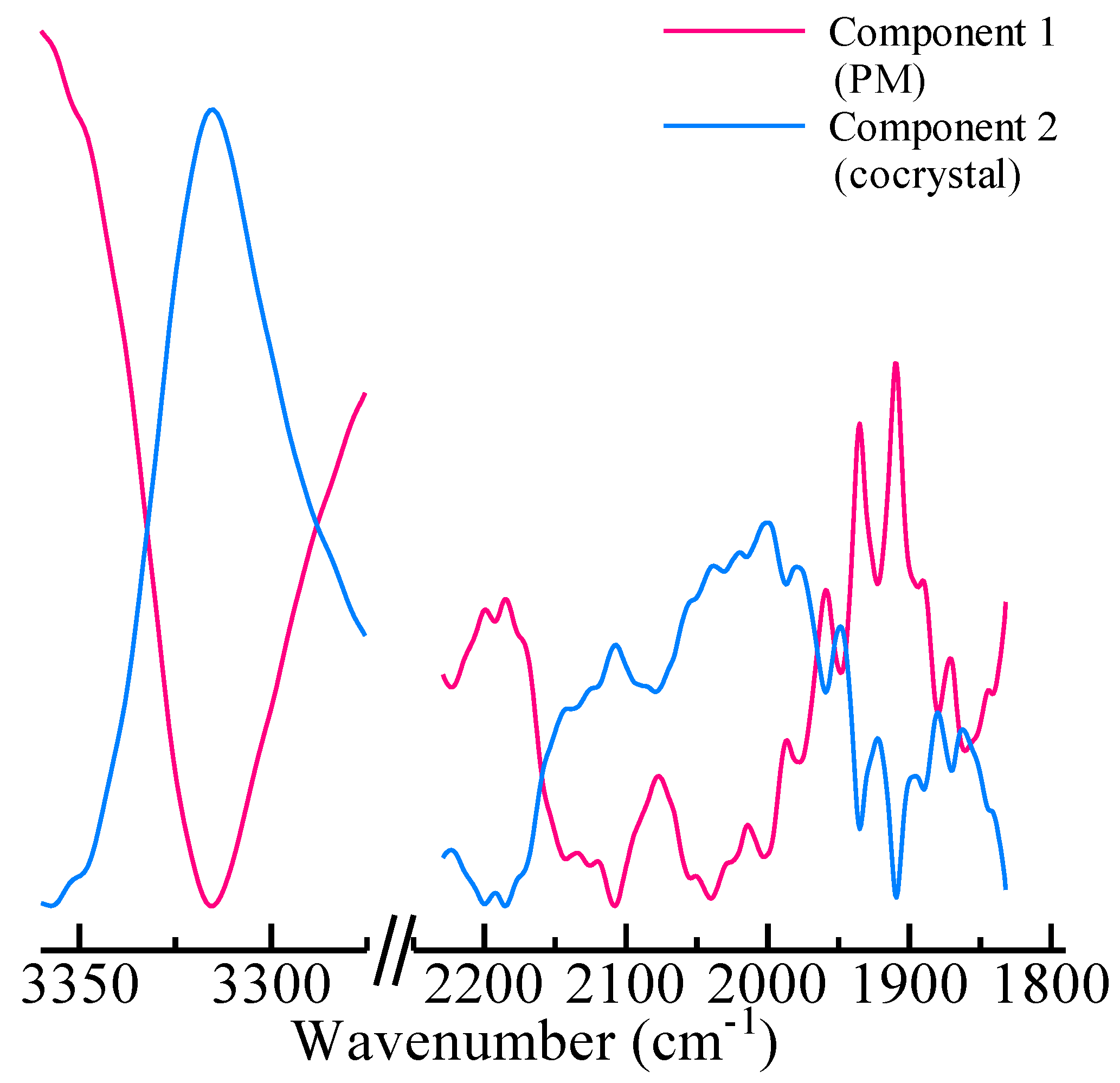
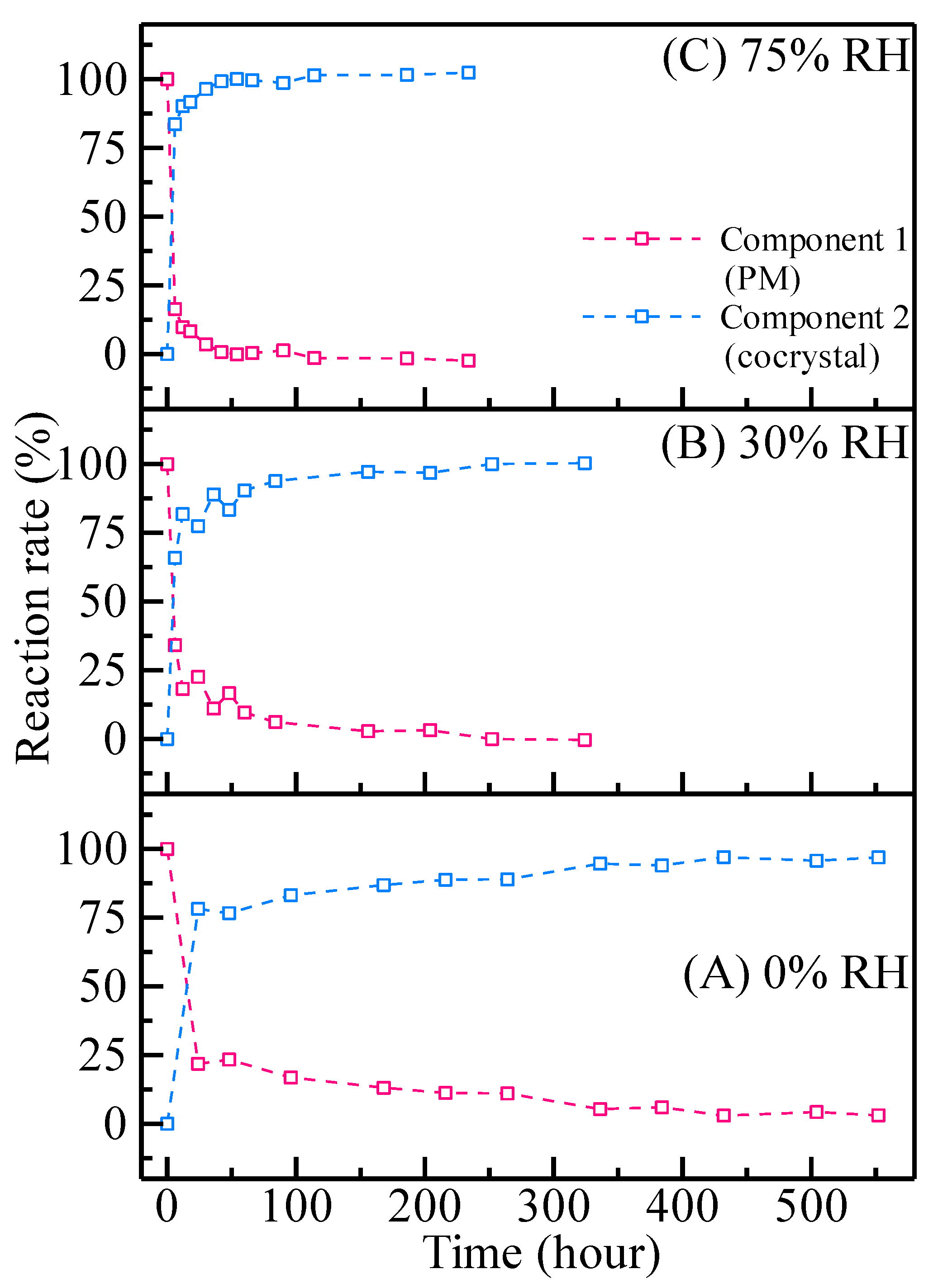


| Sample Number | 0% RH | 30% RH | 75% RH |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 24 | 6 | 6 |
| 3 | 48 | 12 | 12 |
| 4 | 96 | 24 | 18 |
| 5 | 168 | 36 | 30 |
| 6 | 216 | 48 | 42 |
| 7 | 264 | 60 | 54 |
| 8 | 336 | 84 | 66 |
| 9 | 384 | 156 | 90 |
| 10 | 432 | 204 | 114 |
| 11 | 504 | 252 | 186 |
| 12 | 552 | 324 | 234 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, S.; Hattori, Y.; Otsuka, M.; Sasaki, T. Cocrystal Formation through Solid-State Reaction between Ibuprofen and Nicotinamide Revealed Using THz and IR Spectroscopy with Multivariate Analysis. Crystals 2020, 10, 760. https://doi.org/10.3390/cryst10090760
Ishihara S, Hattori Y, Otsuka M, Sasaki T. Cocrystal Formation through Solid-State Reaction between Ibuprofen and Nicotinamide Revealed Using THz and IR Spectroscopy with Multivariate Analysis. Crystals. 2020; 10(9):760. https://doi.org/10.3390/cryst10090760
Chicago/Turabian StyleIshihara, Sae, Yusuke Hattori, Makoto Otsuka, and Tetsuo Sasaki. 2020. "Cocrystal Formation through Solid-State Reaction between Ibuprofen and Nicotinamide Revealed Using THz and IR Spectroscopy with Multivariate Analysis" Crystals 10, no. 9: 760. https://doi.org/10.3390/cryst10090760
APA StyleIshihara, S., Hattori, Y., Otsuka, M., & Sasaki, T. (2020). Cocrystal Formation through Solid-State Reaction between Ibuprofen and Nicotinamide Revealed Using THz and IR Spectroscopy with Multivariate Analysis. Crystals, 10(9), 760. https://doi.org/10.3390/cryst10090760





