Factor Analysis of XRF and XRPD Data on the Example of the Rocks of the Kontozero Carbonatite Complex (NW Russia). Part II: Geological Interpretation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Description
2.2. Analytical Techniques
2.3. Data Processing
3. Results and Discussion
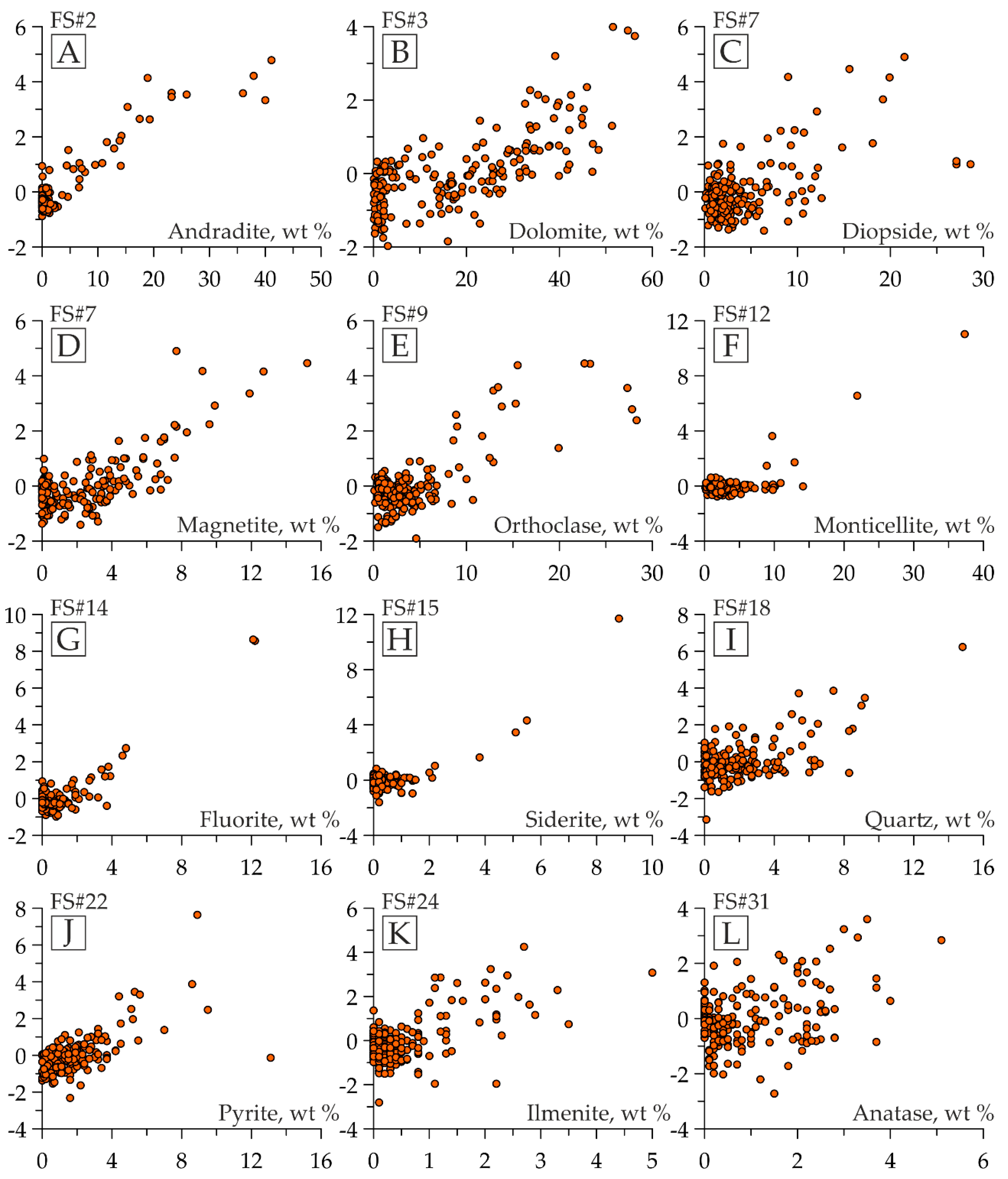
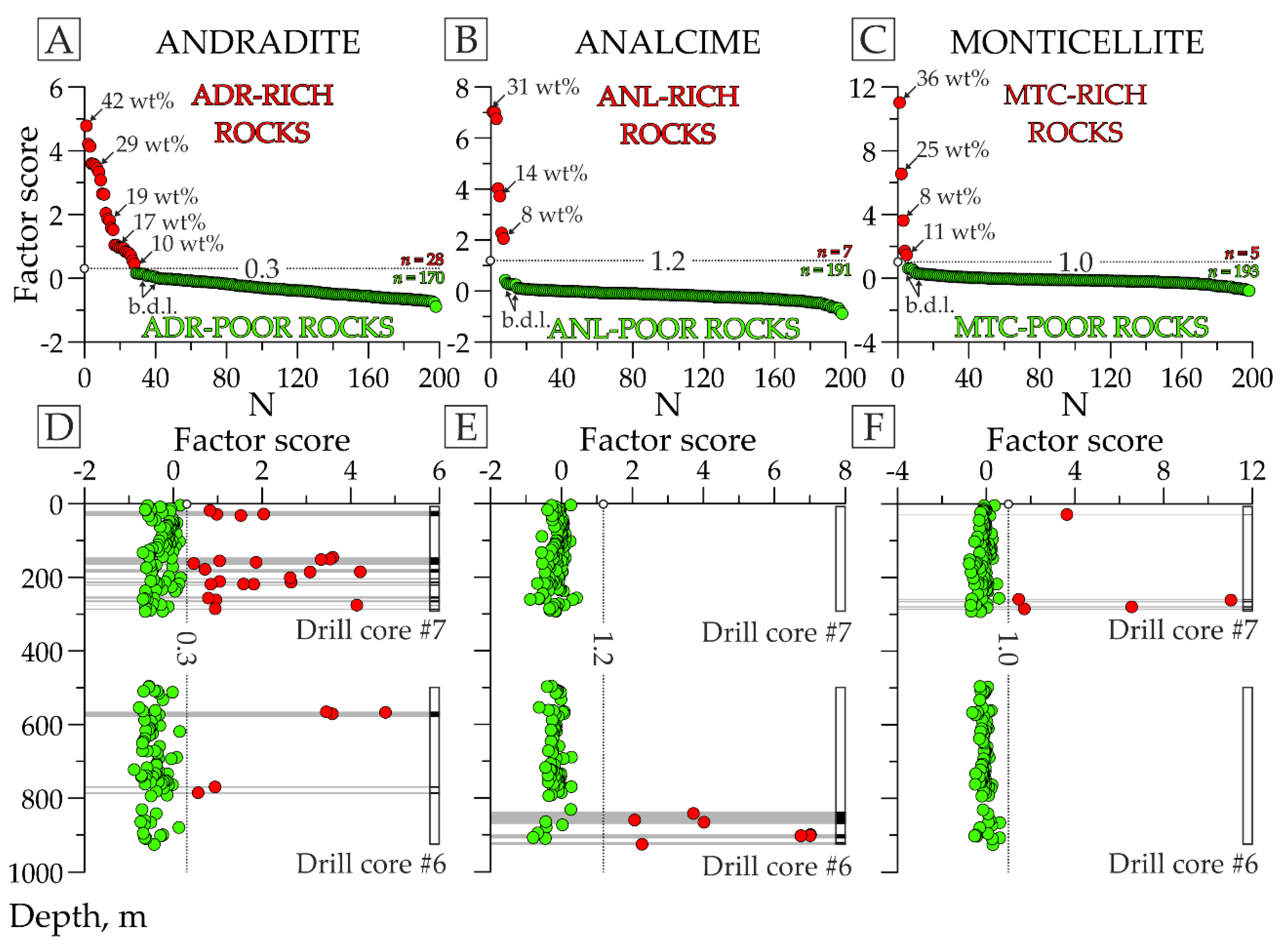
3.1. Use of Factor Scores for Qualitative and Semiquantitative Mineralogical Analysis
- GROUP I represents the samples/diffraction patterns with peak intensity in the considered region above half the maximum peak for the studied sample set (in Figure 5B,C, fragments of the diffraction patterns and the figurative points of the samples included in this group are red-colored);
- GROUP II has peak intensities from a quarter to half of the maximum peak height (in Figure 5B,C, the elements related to this group are orange-colored);
- GROUP III has a peak intensity of less than a quarter of the maximum peak height (in Figure 5B,C, the elements related to this group are green-colored);
- GROUP IV has no peaks in the considered area (in Figure 5B,C, the details concerning this group are blue-colored).
3.2. The Application of Factor Scores for Mineralogical Interpretation of Geochemical Data
- The influence of vector 1, related to high content of L.O.I., Ca, and Sr in the rock, led to a shift of the figurative points into group 1;
- High contents of both Si (+Ti) and Na–K–Al (+Zr), with an essential contribution of Ba–Sr–S, caused a shift of points along vector 2 (towards group 2); and
- High contents of Si (+Ti) and Mg–Fe (+Mn) resulted in the shifting of points along vector 3 (towards group 3).
- Vector 1 (and the corresponding group) was associated with calcite content in the rock. Consequently, group 1 predominantly represented calciocarbonatites (apparently being rich in strontium);
- Vector 2 was due to the presence of albite, orthoclase, and sometimes analcime (±natrolite) in the rock. Thus, group 2 included samples rich in alkaline salic minerals; and
- Vector 3 responded to the presence of diopside, magnetite, andradite, monticellite, amphibole, serpentine (secondary phase), and/or biotite. Thus, group 3 included rocks rich in femic minerals.
- Titanium oxides (anatase and ilmenite) gravitated toward group 2, being rich in alkaline sialic minerals. However, titanium did not participate in the discrimination of groups 2 and 3. This suggests that the rocks of both groups were rich in titanium. However, in group 3, phases hosting Ti were not oxides but silicates such as biotite and/or garnet (as confirmed by EPMA);
- The position of the “dolomite” variable (as well as the “apatite” variable) near the origin in the coordinate system suggests that the presence/absence of this mineral was irrelevant in the division into the groups noted above. This may indicate that all studied rocks of the complex were equally subjected to dolomite mineralization and, therefore, this process was superimposed;
- None of the considered factors tracked the presence of quartz (supposedly, its content is nearly equal in all types of rocks). Several mineral phases (e.g., apatite, baryte, strontianite, burbankite, and fluorite) exhibited a barely noticeable relationship to the considered separation of samples into groups. Nevertheless, the FSs of these phases were related to other factors identified by classical FA. For example, the scores of apatite and pyrite factors were close to the positive pole of the previously mentioned “phosphate” Factor III (FIIILAp = 0.77 and FIIILPy = 0.37; see Figure 8A,B). The scores of the burbankite, baryte, strontianite, fluorite, and quartz factors formed a shift vector towards the positive pole of the “baryte” Factor IV (Figure 8C,D). In the studied rocks, these minerals constitute a specific mineral paragenesis superimposed onto the earlier associations.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wang, X.; Sanei, H.; Dai, S.; Ardakani, O.H.; Isinguzo, N.; Kondla, D.; Tang, Y. A novel method to estimate mineral compositions of mudrocks: A case study for the Canadian unconventional petroleum systems. Mar. Pet. Geol. 2016, 73, 322–332. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Z.; Lavoie, D.; Percival, J.B.; Kabanov, P. Mineral carbon MinC(%) from Rock-Eval analysis as a reliable and cost-effective measurement of carbonate contents in shale source and reservoir rocks. Mar. Pet. Geol. 2017, 83, 184–194. [Google Scholar] [CrossRef]
- Nistor, M.M.; Har, N.; Dori, S.M.; Bigi, S.; Gualtieri, A.F. Progress in mineralogical quantitative analysis of rock samples: Application to quartzites from Denali National Park, Alaska Range (USA). Powder Diffr. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Chayes, F. Petrographie Modal Analysis: An Elementary Statistical Appraisal; John Wiley & Sons, Inc.: New York, NY, USA, 1956. [Google Scholar]
- Davis, B.L.; Walawender, M.J. Quantitative mineralogical analysis of granitoid rocks: A comparison of X-ray and optical technique. Am. Mineral. 1982, 67, 1135–1143. [Google Scholar]
- Warlo, M.; Wanhainen, C.; Bark, G.; Butcher, A.R.; McElroy, I.; Brising, D.; Rollinson, G.K. Automated Quantitative Mineralogy Optimized for Simultaneous Detection of (Precious/Critical) Rare Metals and Base Metals in A Production-Focused Environment. Minerals 2019, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y. Automated Scanning Electron Microscope Based Mineral Liberation Analysis An Introduction to JKMRC/FEI Mineral Liberation Analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Hrstka, T.; Gottlieb, P.; Skála, R.; Breiter, K.; Motl, D. Automated mineralogy and petrology—Applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Sindern, S.; Meyer, F.M. Automated Quantitative Rare Earth Elements Mineralogy by Scanning Electron Microscopy. Phys. Sci. Rev. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Gottlieb, P.; Wilkie, G.; Sutherland, D.; Ho-Tun, E.; Suthers, S.; Perera, K.; Jenkins, B.; Spencer, S.; Butcher, A.; Rayner, J. Using quantitative electron microscopy for process mineralogy applications. JOM 2000, 52, 24–25. [Google Scholar] [CrossRef]
- Hoal, K.O.; Stammer, J.G.; Appleby, S.K.; Botha, J.; Ross, J.K.; Botha, P.W. Research in quantitative mineralogy: Examples from diverse applications. Miner. Eng. 2009, 22, 402–408. [Google Scholar] [CrossRef]
- Madsen, I.C.; Scarlett, N.V.Y.; Webster, N.A.S. Quantitative Phase Analysis. In Uniting Electron Crystallography and Powder Diffraction. NATO Science for Peace and Security Series B: Physics and Biophysics; Kolb, U., Shankland, K., Meshi, L., Avilov, A., David, W.I.F., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 207–218. [Google Scholar]
- Lutterotti, L. Quantitative Phase Analysis: Method Developments. In Uniting Electron Crystallography and Powder Diffraction. NATO Science for Peace and Security Series B: Physics and Biophysics; Kolb, U., Shankland, K., Meshi, L., Avilov, A., David, W.I.F., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 233–242. [Google Scholar]
- Pruseth, K.L. Calculation of the CIPW norm: New formulas. J. Earth Syst. Sci. 2009, 118, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, L.; Trépanier, S.; Daigneault, R. CONSONORM_HG: A new method of norm calculation for mid- to high-grade metamorphic rocks. J. Metamorph. Geol. 2016, 34, 1–15. [Google Scholar] [CrossRef]
- Cross, W.; Iddings, J.P.; Pirsson, L.V.; Washington, H.S. A Quantitative Chemico-Mineralogical Classification and Nomenclature of Igneous Rocks. J. Geol. 1902, 10, 555–690. [Google Scholar] [CrossRef]
- Fomina, E.; Kozlov, E.; Ivashevskaja, S. Study of diffraction data sets using factor analysis: A new technique for comparing mineralogical and geochemical data and rapid diagnostics of the mineral composition of large collections of rock samples. Powder Diffr. 2019, 34, S59–S70. [Google Scholar] [CrossRef]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef] [Green Version]
- Bulakh, A.G.; Ivanikov, V.V.; Orlova, M.P. Overview of carbonatite-phoscorite complexes of the Kola Alkaline Province. In Phoscorites and Carbonatites from Mantle to Mine; Wall, F., Zaitsev, A.N., Eds.; Mineralogical Society of Great Britain and Ireland: London, UK, 2004; pp. 1–43. ISBN 9780903056229. [Google Scholar]
- Kramm, U.; Kogarko, L.N.; Kononova, V.A.; Vartiainen, H. The Kola Alkaline Province of the CIS and Finland: Precise Rb-Sr ages define 380–360 Ma age range for all magmatism. Lithos 1993, 30, 33–44. [Google Scholar] [CrossRef]
- Kirichenko, L.A. Kontozero Formation of Carboniferous Rocks in the Kola Peninsula; Nedra: Leningrad, Russia, 1970. [Google Scholar]
- Borodin, L.S.; Gladkikh, V.S. New petrographic and geochemical data on the volcanogenic alkaline rocks of the Kontozero formation. In New Data on the Geology, Mineralogy, and Geochemistry of Alkaline Rocks; Borodin, L.S., Ed.; Nauka: Moscow, Russia, 1973; pp. 48–55. [Google Scholar]
- Pyatenko, I.K.; Saprykina, L.G. Petrological features of alkali basaltic rocks and volcanic carbonatites of the Russian Platform. In Petrology and Petrochemistry of Ore-Bearing Igneous Rock Associations; Bogatikov, O.A., Simon, A.K., Eds.; Nauka: Moscow, Russia, 1981; pp. 233–255. [Google Scholar]
- Pyatenko, I.K.; Osokin, E.D. Geochemical features of the Kontozero carbonatite paleovolcano in the Kola Peninsula. Geokhimiya 1988, 25, 723–737. [Google Scholar]
- Arzamastsev, A.A.; Petrovsky, M.N. Alkaline volcanism in the Kola Peninsula, Russia: Paleozoic Khibiny, Lovozero and Kontozero calderas. Proc. MSTU 2012, 15, 277–299. [Google Scholar]
- Petrovsky, M.N.; Savchenko, E.A.; Kalachev, V.Y. Formation of eudialyte-bearing phonolite from Kontozero carbonatite paleovolcano, Kola Peninsula. Geol. Ore Depos. 2012, 54, 540–556. [Google Scholar] [CrossRef]
- Website of IM UB RAS (Miass, Russia). Available online: http://www.mineralogy.ru (accessed on 3 July 2020).
- Amosova, A.A.; Panteeva, S.V.; Chubarov, V.M.; Finkelshtein, A.L. Determination of major elements by wavelength-dispersive X-ray fluorescence spectrometry and trace elements by inductively coupled plasma mass spectrometry in igneous rocks from the same fused sample (110 mg). Spectrochim. Acta Part B At. Spectrosc. 2016, 122, 62–68. [Google Scholar] [CrossRef]
- Website of IG SB RAS (Irkutsk, Russia). Available online: http://www.igc.irk.ru (accessed on 3 July 2020).
- Qualx2 Software. Available online: http://www.ba.ic.cnr.it/softwareic/qualx/ (accessed on 3 July 2020).
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0 : A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- MAUD Software. Available online: http://maud.radiographema.eu/ (accessed on 3 July 2020).
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A friendly Java program for materials analysis using diffraction. Int. Union Crystallogr. Comm. Powder Diffr. Newsl. 1999, 21, 14–15. [Google Scholar]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Fomina, E.; Kozlov, E.; Bazai, A. Factor Analysis of XRF- and XRPD-data on the Case Study of the Rocks of the Kontozero Carbonatite Complex (NW Russia). Part I: Algorithm. Crystals 2020. [Google Scholar]
- Davis, B.L.; Kath, R.; Spilde, M. The Reference Intensity Ratio: Its Measurement and Significance. Powder Diffr. 1990, 5, 76–78. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Stephens, P.W. Rietveld Refinement. In Uniting Electron Crystallography and Powder Diffraction. NATO Science for Peace and Security Series B: Physics and Biophysics; Kolb, U., Shankland, K., Meshi, L., Avilov, A., David, W.I.F., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 15–26. [Google Scholar]
- Kozlov, E.; Fomina, E. Geological interpretation of the results of factor analysis of XRF- and XRD-data on carbonatite and aluminosilicate rocks of the Kontozero alkaline complex (Kola Peninsula, NW Russia). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; in press. [Google Scholar]
- Bish, D.L.; Post, J.E. Quantitative mineralogical analysis using the Rietveld full-pattern fitting method. Am. Mineral. 1993, 78, 932–940. [Google Scholar]
- Jöreskog, K.G.; Klovan, J.E.; Reyment, R.A. Geological Factor Analysis; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Quartieri, S.; Boscherini, F.; Chaboy, J.; Dalconi, M.C.; Oberti, R.; Zanetti, A. Characterization of trace Nd and Ce site preference and coordination in natural melanites: A combined X-ray diffraction and high-energy XAFS study. Phys. Chem. Miner. 2002, 29, 495–502. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, E.; Fomina, E.; Khvorov, P. Factor Analysis of XRF and XRPD Data on the Example of the Rocks of the Kontozero Carbonatite Complex (NW Russia). Part II: Geological Interpretation. Crystals 2020, 10, 873. https://doi.org/10.3390/cryst10100873
Kozlov E, Fomina E, Khvorov P. Factor Analysis of XRF and XRPD Data on the Example of the Rocks of the Kontozero Carbonatite Complex (NW Russia). Part II: Geological Interpretation. Crystals. 2020; 10(10):873. https://doi.org/10.3390/cryst10100873
Chicago/Turabian StyleKozlov, Evgeniy, Ekaterina Fomina, and Pavel Khvorov. 2020. "Factor Analysis of XRF and XRPD Data on the Example of the Rocks of the Kontozero Carbonatite Complex (NW Russia). Part II: Geological Interpretation" Crystals 10, no. 10: 873. https://doi.org/10.3390/cryst10100873
APA StyleKozlov, E., Fomina, E., & Khvorov, P. (2020). Factor Analysis of XRF and XRPD Data on the Example of the Rocks of the Kontozero Carbonatite Complex (NW Russia). Part II: Geological Interpretation. Crystals, 10(10), 873. https://doi.org/10.3390/cryst10100873





