GDC Buffer Layer Synthesized by Reactive Magnetron Sputtering: Effect of Total Pressure and Thickness on SOFC Performances
Abstract
1. Introduction
2. Experimental Details
2.1. Elaboration of GDC Buffer Layer
2.2. Structural and Morphologic Characterization
2.3. Fuel Cell Performances
3. Results and Discussion
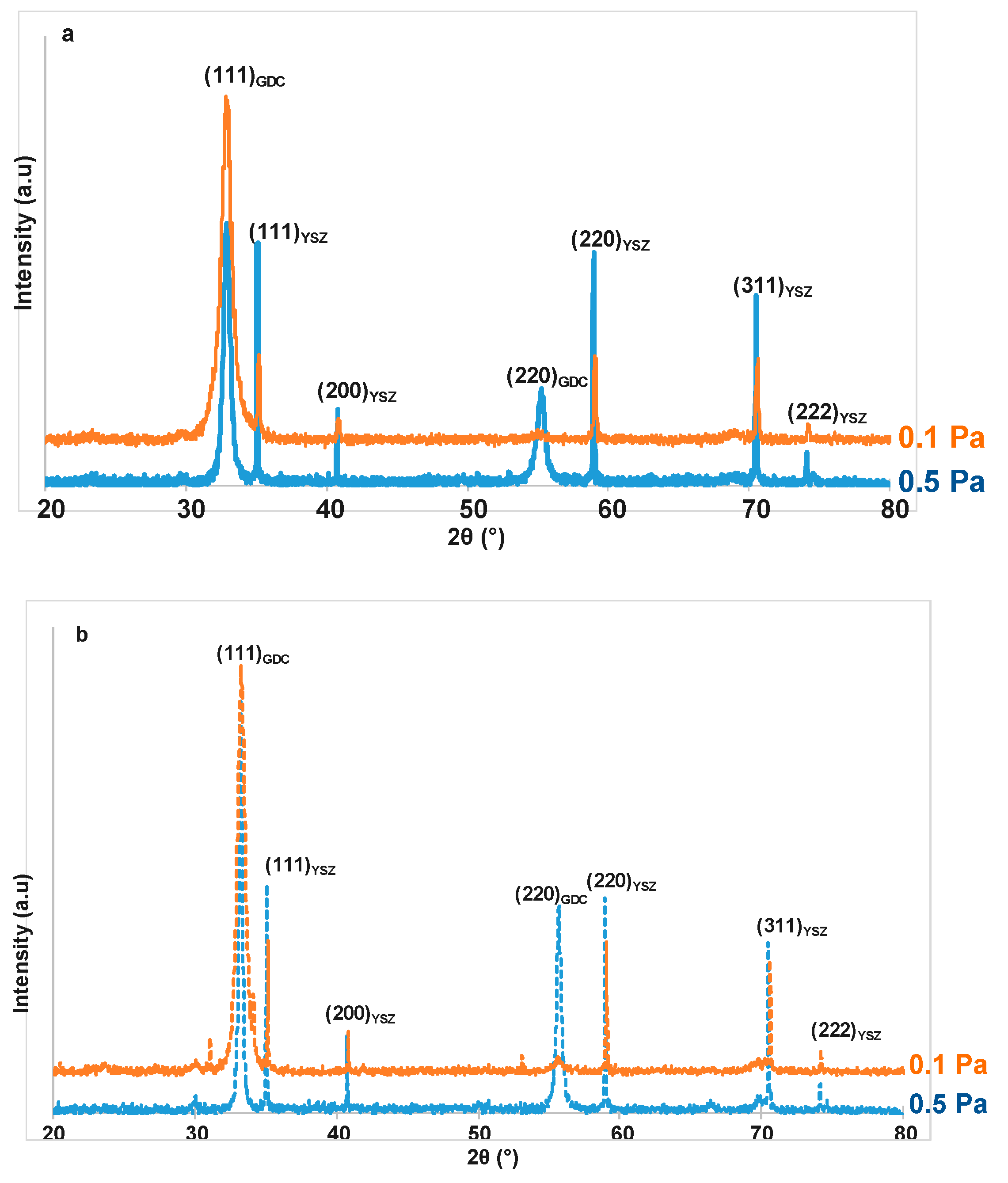
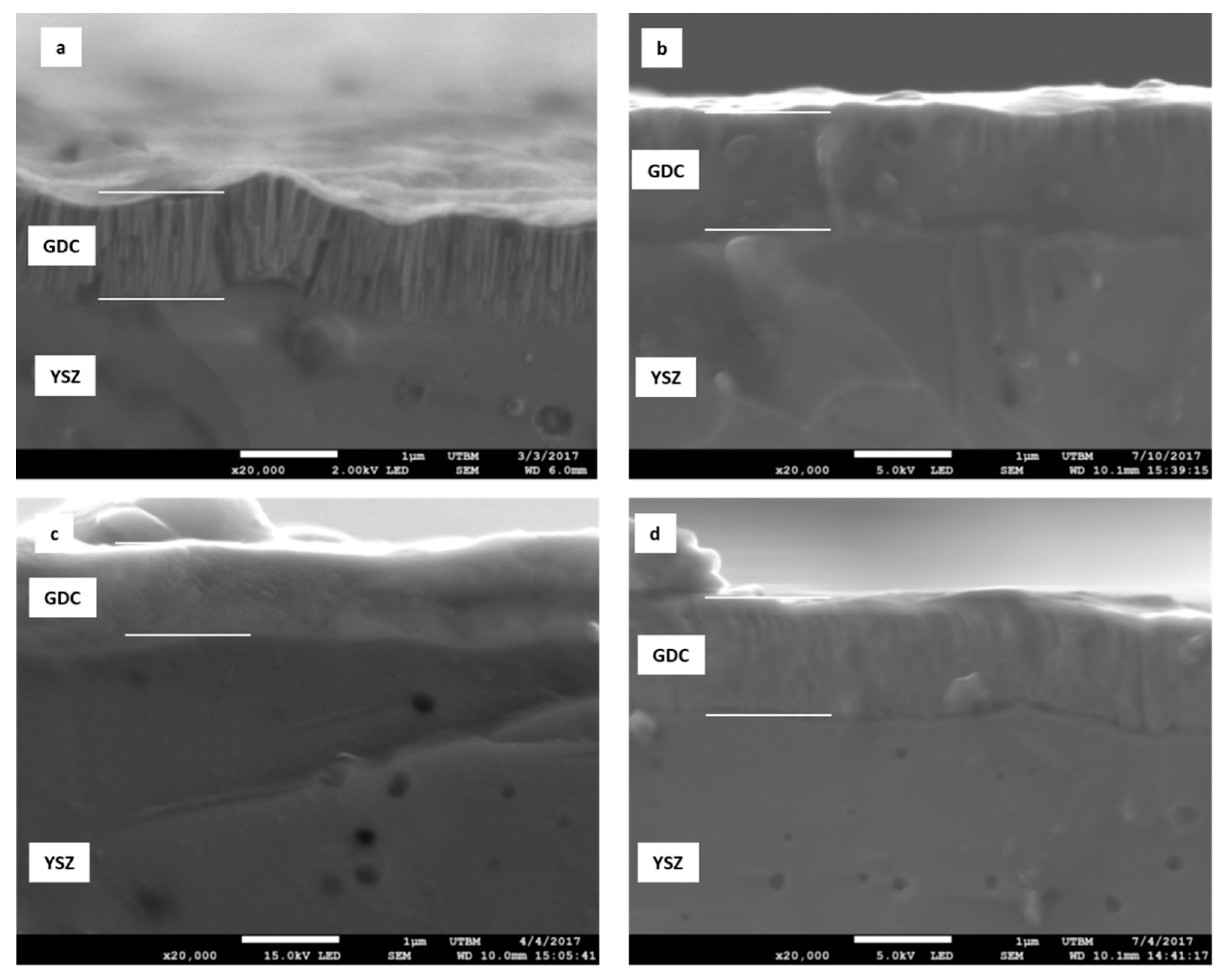
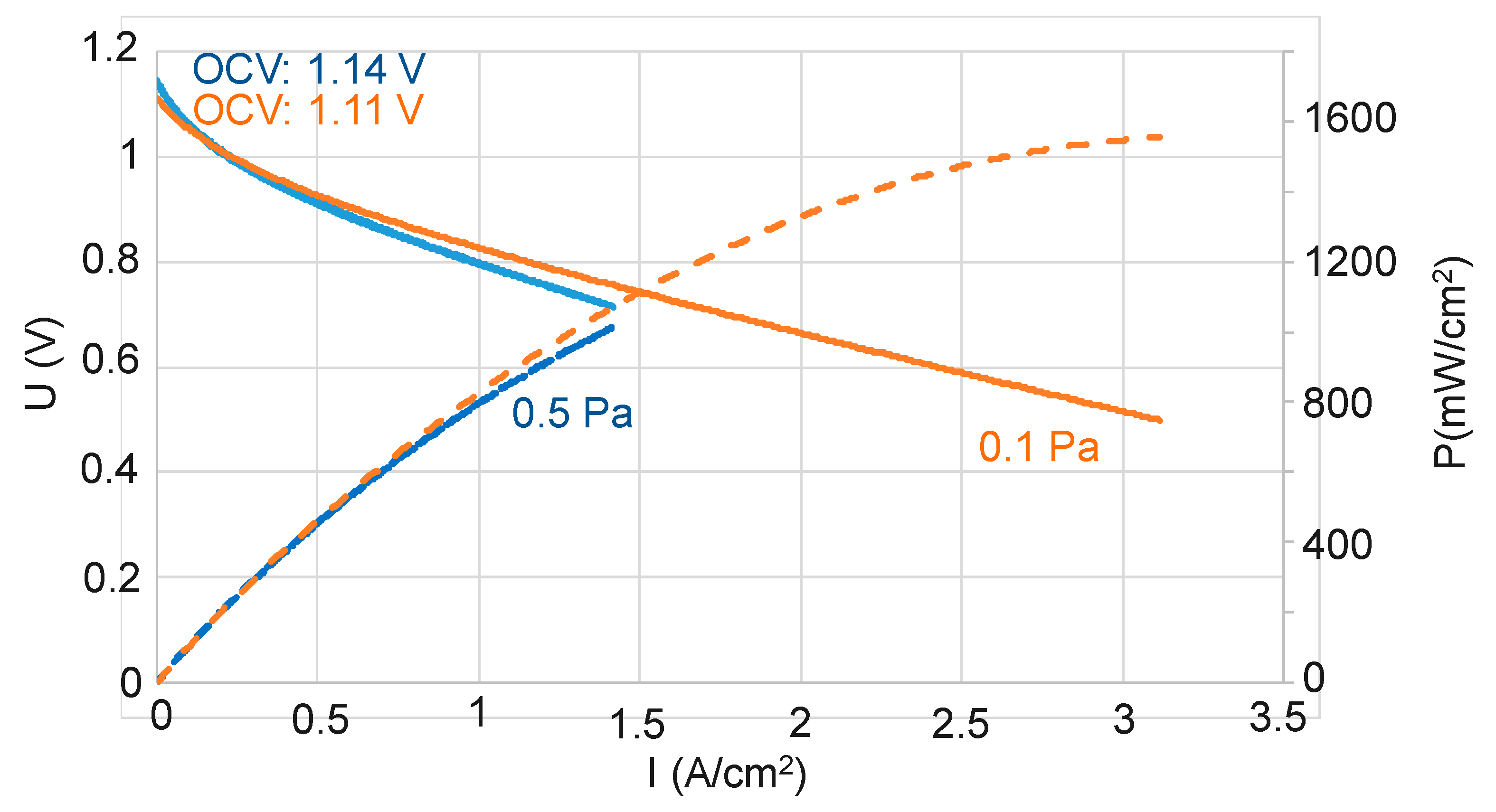
3.1. Influence of the Total Pressure
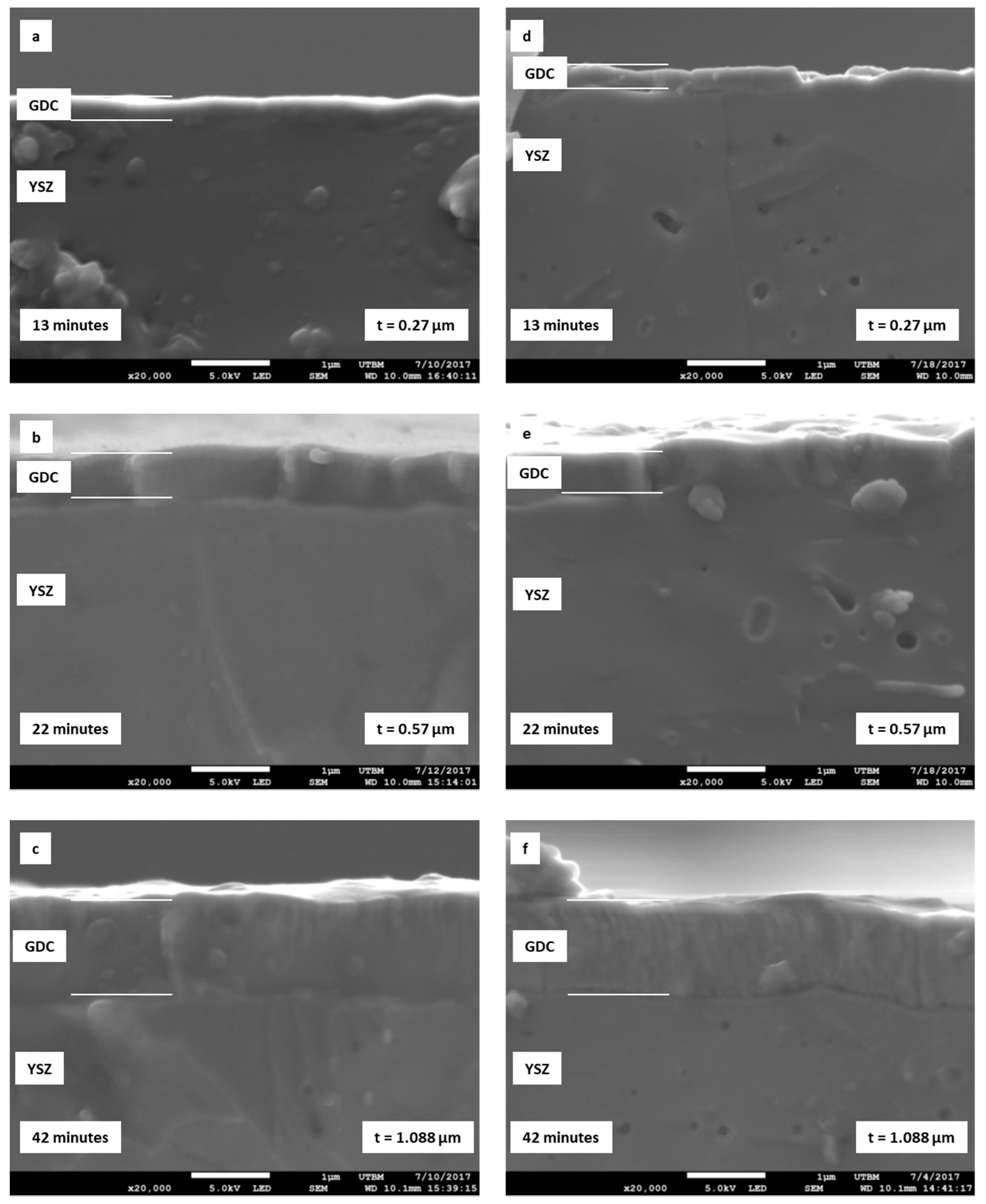
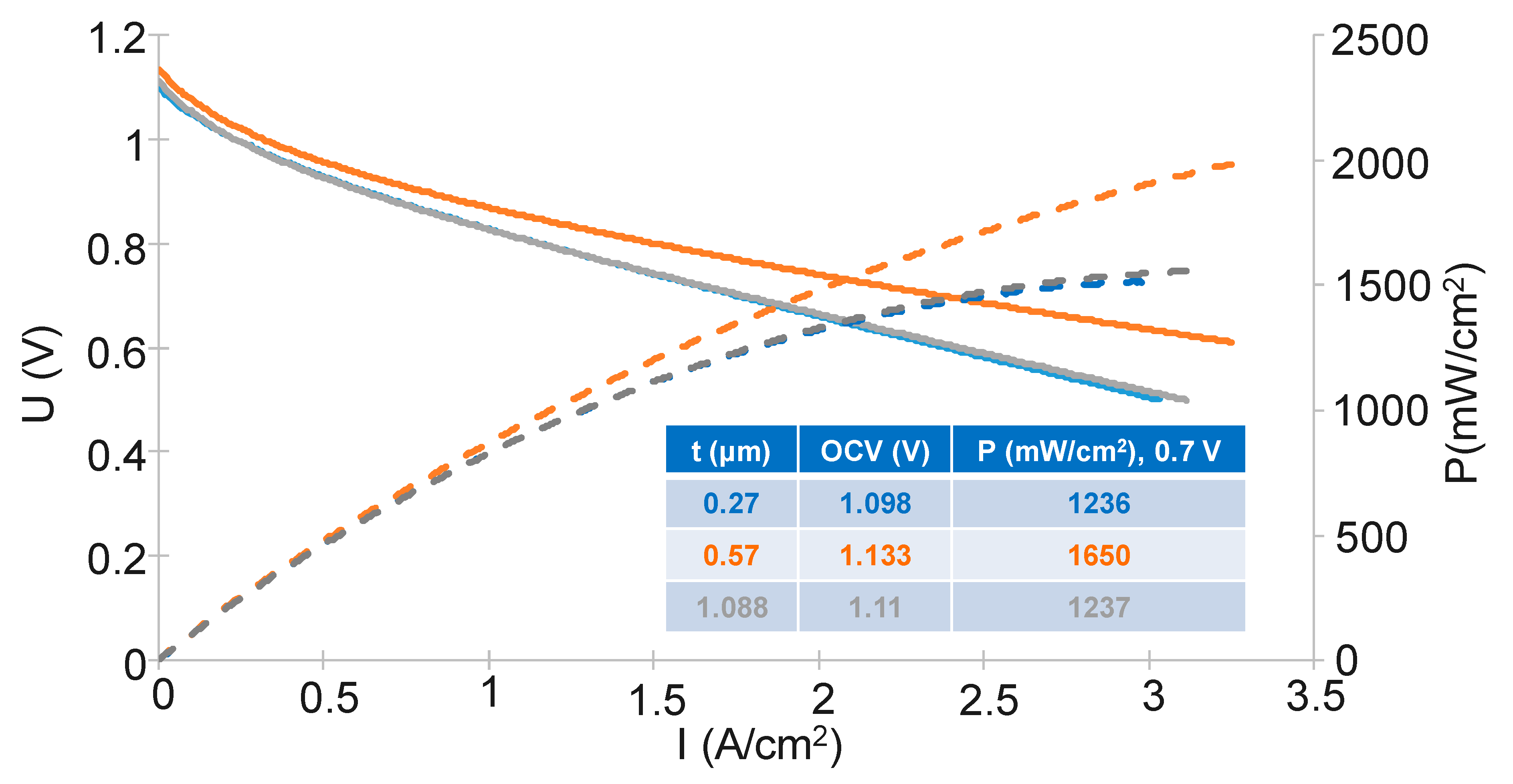
3.2. Effect of GDC Layer Thickness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huijsmans, J.P.P.; Van Berkel, F.P.F.; Christie, G.M. Intermediate temperature SOFC—A promise for the 21st century. J. Power Sources 1998, 71, 107–110. [Google Scholar] [CrossRef]
- Mc Evoy, A.J. Thin SOFC electrolytes and their interfaces—A near-term research strategy. Solid State Ion. 2000, 132, 159–165. [Google Scholar] [CrossRef]
- Boudghene Stambouli, A.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Harboe, S.; Sohn, Y.J.; Guillon, O.; Menzler, N.H. Investigation of LSM-8YSZ cathode within an all ceramic SOFC. Part I: Chemical interactions. J. Eur. Ceram. Soc. 2020, 40, 3608–3617. [Google Scholar] [CrossRef]
- Wang, W.; Hu, S.; Han, Y.; Pan, X.; Xu, Q.; Zhu, J. Interaction of Zr with oxidized and partially reduced ceria thin films. Surf. Sci. 2016, 653, 205–210. [Google Scholar] [CrossRef]
- Eguchi, K.; Setoguchi, T.; Inoue, T.; Arai, H. Electrical properties of ceria-based oxides and their application to solid oxide fuel cells. Solid State Ion. 1992, 52, 165–172. [Google Scholar] [CrossRef]
- Choi, J.; Park, D.; Seon, B.; Bae, H. Low-temperature preparation of dense (Gd,Ce)O2−δ–Gd2O3 composite buffer layer by aerosol deposition for YSZ electrolyte-based SOFC. Int. J. Hydrog. Energy 2012, 37, 9809–9815. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Weber, A.; Herbstritt, D. Materials and technologies for SOFC-components. J. Eur. Ceram. Soc. 2001, 21, 1805–1811. [Google Scholar] [CrossRef]
- Constantin, G.; Rossignol, C.; Briois, P.; Billard, A.; Dessemond, L.; Djurado, E. Efficiency of a dense thin CGO buffer layer for solid oxide fuel cell operating at intermediate temperature. Solid State Ion. 2013, 249–250, 98–104. [Google Scholar] [CrossRef]
- Choi, H.; Na, Y.; Kwak, M.; Kim, T.W.; Seo, D.; Woo, S.; Kim, S. Development of solid oxide cells by co-sintering of GDC diffusion barriers with LSCF air electrode. Ceram. Int. 2017, 43, 13653–13660. [Google Scholar] [CrossRef]
- Fu, Y.-P.; Chen, S.-H. Preparation and characterization of neodymium-doped ceria electrolyte materials for solid oxide fuel cells. Ceram. Int. 2010, 36, 483–490. [Google Scholar] [CrossRef]
- Kang, Y.J.; Choi, G.M. The effect of alumina and Cu addition on the electrical properties and the SOFC performance of Gd-doped CeO2 electrolyte. Solid State Ion. 2009, 180, 886–890. [Google Scholar] [CrossRef]
- Zha, S. Ni-Ce0.9Gd0.1O1.95 anode for GDC electrolyte-based low-temperature SOFCs. Solid State Ion. 2004, 166, 241–250. [Google Scholar] [CrossRef]
- Nagamori, M.; Shimonosono, T.; Sameshima, S.; Hirata, Y.; Matsunaga, N.; Sakka, Y. Densification and Cell Performance of Gadolinium-Doped Ceria (GDC) Electrolyte/NiO-GDC Anode Laminates. J. Am. Ceram. Soc. 2009, 92, S117–S121. [Google Scholar] [CrossRef]
- Sammes, N.M.; Tompsett, G.A.; Cai, Z. The chemical reaction between ceria and fully stabilised zirconia. Solid State Ion. 1999, 121, 121–125. [Google Scholar] [CrossRef]
- Tsoga, A.; Gupta, A.; Nikolopoulos, P. Gadolinia-doped ceria and yttria stabilized zirconia interfaces: Regarding their application for SOFC technology. Acta Mater. 2000, 48, 4709–4714. [Google Scholar] [CrossRef]
- Constantin, G.; Rossignol, C.; Barnes, J.-P.; Djurado, E. Interface stability of thin, dense CGO film coating on YSZ for solid oxide fuel cells. Solid State Ion. 2013, 235, 36–41. [Google Scholar] [CrossRef]
- Breaz, E.; Aubry, E.; Billard, A.; Coton, N.; Coquoz, P.; Pappas, A.; Diethelm, S.; Ihringer, R.; Briois, P. Influence of the Bias Substrate Power on the GDC Buffer Layer. ECS Trans. 2017, 78, 807–816. [Google Scholar] [CrossRef]
- Thornton, J.A.; Hoffman, D.W. Stress related effect in thin films. Thin Solid Film 1989, 171, 5–31. [Google Scholar] [CrossRef]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Film 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
| Bias Power (W) | OCV (V) |
|---|---|
| 20 | 1.139 |
| 40 | 1.135 |
| 60 | 1.07 |
| 80 | 1.143 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouleau, L.; Coton, N.; Coquoz, P.; Ihringer, R.; Billard, A.; Briois, P. GDC Buffer Layer Synthesized by Reactive Magnetron Sputtering: Effect of Total Pressure and Thickness on SOFC Performances. Crystals 2020, 10, 759. https://doi.org/10.3390/cryst10090759
Bouleau L, Coton N, Coquoz P, Ihringer R, Billard A, Briois P. GDC Buffer Layer Synthesized by Reactive Magnetron Sputtering: Effect of Total Pressure and Thickness on SOFC Performances. Crystals. 2020; 10(9):759. https://doi.org/10.3390/cryst10090759
Chicago/Turabian StyleBouleau, Lara, Noelia Coton, Pierre Coquoz, Raphael Ihringer, Alain Billard, and Pascal Briois. 2020. "GDC Buffer Layer Synthesized by Reactive Magnetron Sputtering: Effect of Total Pressure and Thickness on SOFC Performances" Crystals 10, no. 9: 759. https://doi.org/10.3390/cryst10090759
APA StyleBouleau, L., Coton, N., Coquoz, P., Ihringer, R., Billard, A., & Briois, P. (2020). GDC Buffer Layer Synthesized by Reactive Magnetron Sputtering: Effect of Total Pressure and Thickness on SOFC Performances. Crystals, 10(9), 759. https://doi.org/10.3390/cryst10090759





