LaxPr4−xNi3O10−δ: Mixed A-Site Cation Higher-Order Ruddlesden-Popper Phase Materials as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Syntheses and Characterisation
2.2. Cell Preparation
3. Results and Discussion
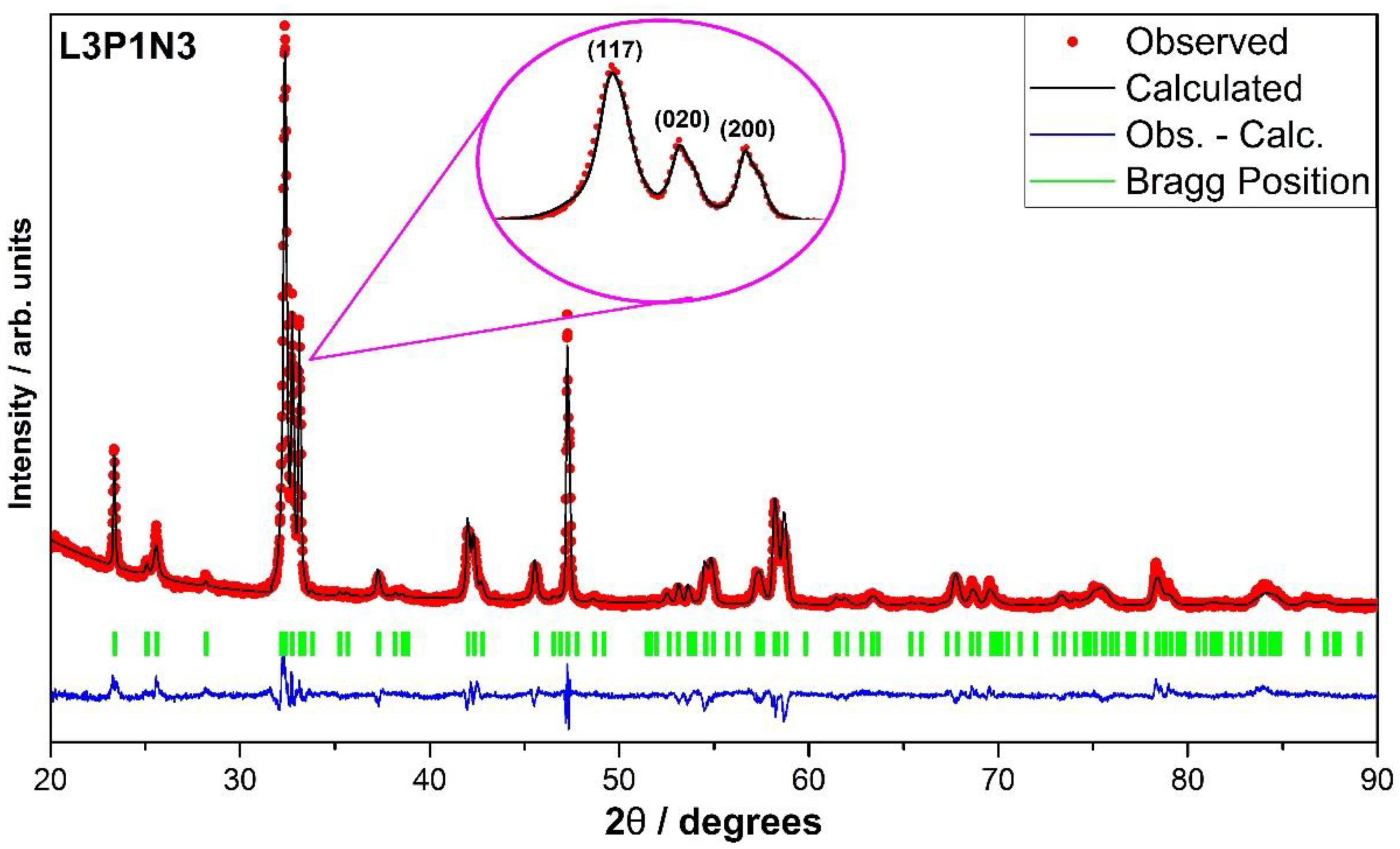
3.1. X-ray Diffraction
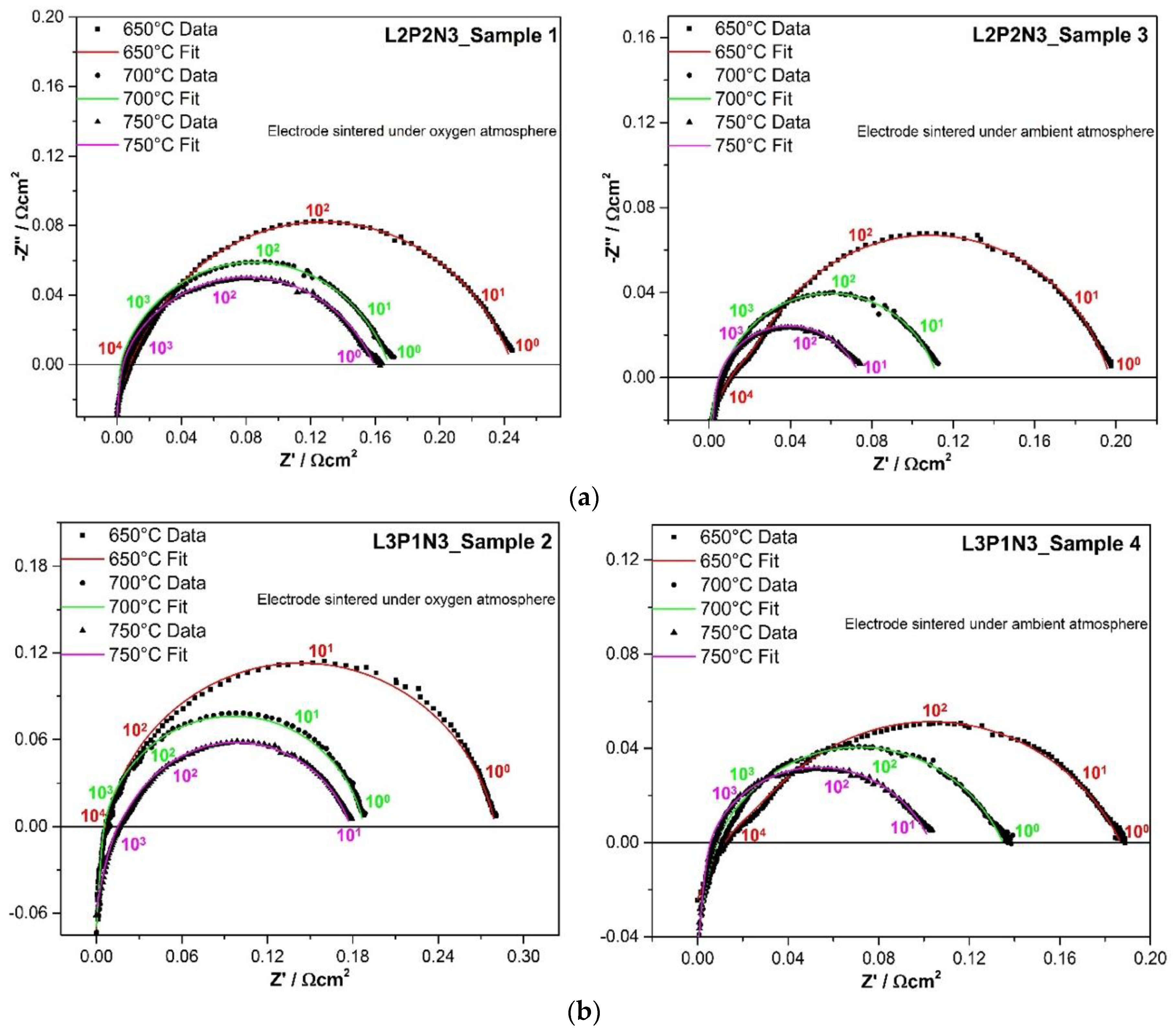
3.2. Symmetrical Cell Performance Testing
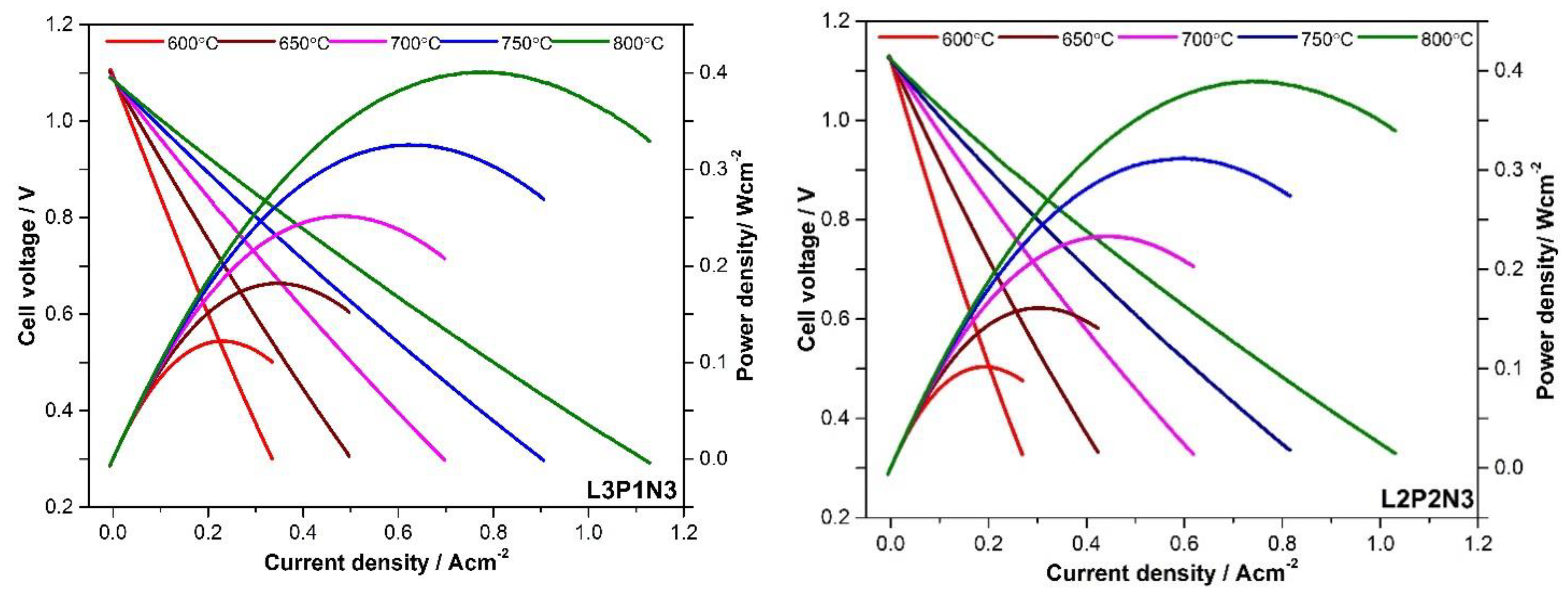
3.3. Single Button Cell Testing
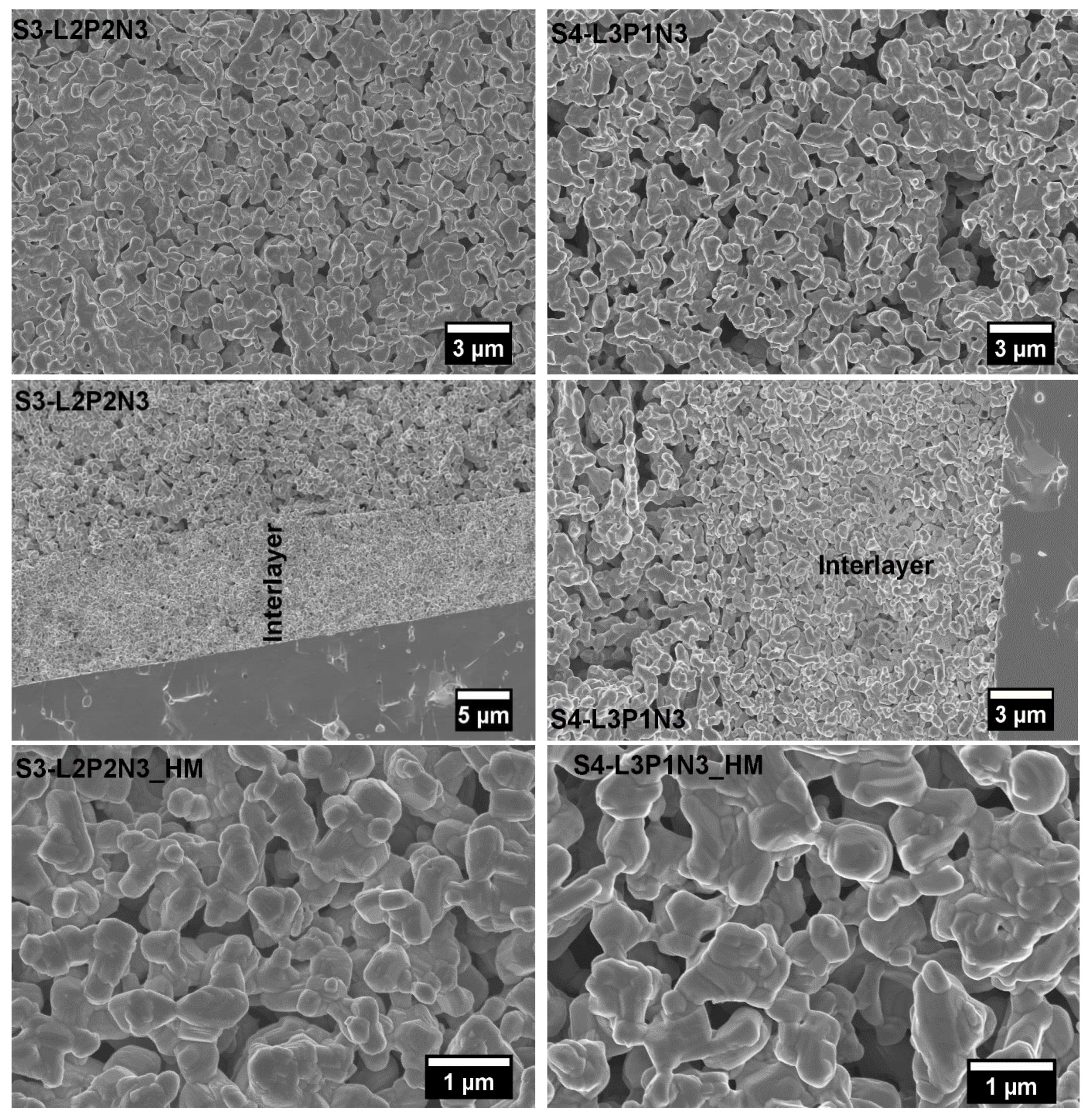
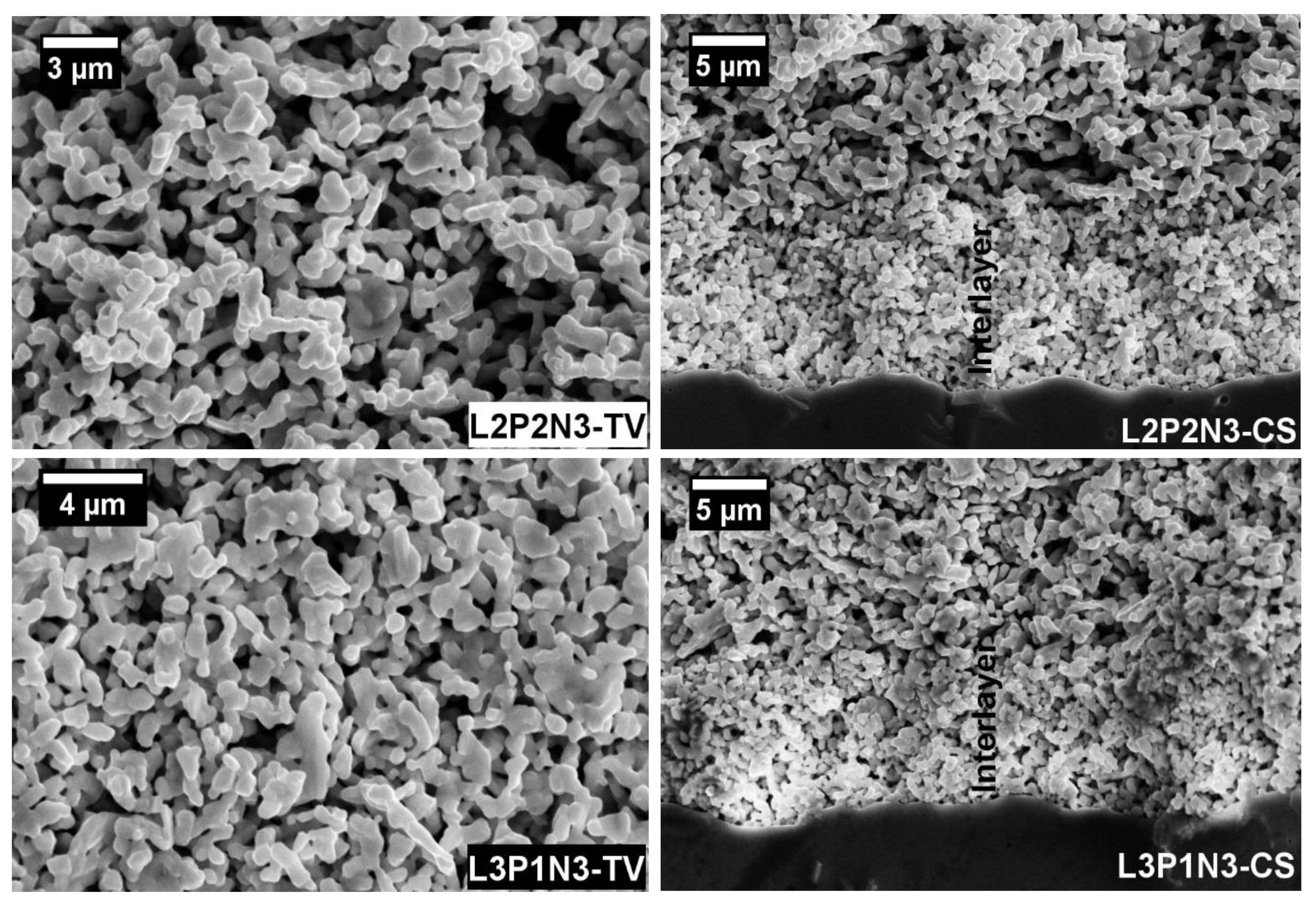
3.4. Post-Test Microstructural Studies
3.4.1. Symmetrical Cells
3.4.2. Single Fuel Cell Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruddlesden, S.N.; Popper, P. The Compound Sr3Ti2O7 and its structure. Acta Cryst. 1958, 11, 54–55. [Google Scholar] [CrossRef]
- Beznosikov, B.V.; Aleksandrov, K.S. Perovskite-like crystals of the Ruddlesden-Popper series. Crystallogr. Rep. 2000, 45, 792–798. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Kovalevsky, A.V.; Naumovich, E.N.; Marques, F.M.B. Ionic transport in oxygen-hyperstoichiometric phases with K2NiF4-type structure. Solid State Ionics 2001, 143, 337–353. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Naumovich, E.N.; Marques, F.M.B. Oxygen ion transport in La2NiO4-based ceramics. J. Mater. Chem. 1999, 9, 2623–2629. [Google Scholar] [CrossRef]
- Skinner, S.J. Characterisation of La2NiO4+δ using in-situ high temperature neutron powder diffraction. Solid State Sci. 2003, 5, 419–426. [Google Scholar] [CrossRef]
- Demourgues, A.; Wattiaux, A.; Grenier, J.C.; Pouchard, M.; Soubeyroux, J.L.; Dance, J.M.; Hagenmuller, P. Electrochemical Preparation and Structural Characterization of La2NiO4+δ Phases (0 ≤ δ ≤ 0.25). J. Solid State Chem. 1993, 105, 458–468. [Google Scholar] [CrossRef]
- Paulus, W.; Cousson, A.; Dhalenne, G.; Berthon, J.; Revcolevschi, A.; Hosoya, S.; Treutmann, W.; Heger, G.; Le Toquin, R. Neutron diffraction studies of stoichiometric and oxygen intercalated La2NiO4 single crystals. Solid State Sci. 2002, 4, 565–573. [Google Scholar] [CrossRef]
- Read, M.S.D.; Islam, M.S.; Watson, G.W.; Hancock, F.E. Surface structures and defect properties of pure and doped La2NiO4. J. Mater. Chem. 2001, 11, 2597–2602. [Google Scholar] [CrossRef]
- Minervini, L.; Grimes, R.W.; Kilner, J.A.; Sickafus, K.E. Oxygen migration in La2NiO4+δ. J. Mater. Chem. 2000, 10, 2349–2354. [Google Scholar] [CrossRef]
- Frayret, C.; Villesuzanne, A.; Pouchard, M. Application of Density Functional Theory to the Modeling of the Mixed Ionic and Electronic Conductor La2NiO4+δ: Lattice Relaxation, Oxygen Mobility, and Energetics of Frenkel Defects. Chem. Mater. 2005, 17, 6538–6544. [Google Scholar] [CrossRef]
- Yan, L.; Niu, H.J.; Duong, G.V.; Suchomel, M.R.; Bacsa, J.; Chalker, P.R.; Hadermann, J.; van Tendeloo, G.; Rosseinsky, M.J. Cation ordering within the perovskite block of a six-layer Ruddlesden-Popper oxide from layer-by-layer growth—Artificial interfaces in complex unit cells. Chem. Sci. 2011, 2, 261–272. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2−xSrxNiO4+δ. Solid State Ionics 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ionics 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Amow, G.; Skinner, S.J. Recent developments in Ruddlesden–Popper nickelate systems for solid oxide fuel cell cathodes. J. Solid State Electrochem. 2006, 10, 538–546. [Google Scholar] [CrossRef]
- Amow, G.; Davidson, I. Preliminary investigation of the higher order Ruddlesden-Popper phases for IT-SOFC cathodes, Lan+1NinO3n+1 (n=2 and 3). Proc. Electrochem. Soc. 2005, PV 2005-07, 1745–1750. [Google Scholar] [CrossRef]
- Amow, G.; Davidson, I.; Skinner, S.J. A comparative study of the Ruddlesden-Popper series, Lan+1NinO3n+1 (n = 1, 2 and 3), for solid-oxide fuel-cell cathode applications. Solid State Ionics 2006, 177, 1205–1210. [Google Scholar] [CrossRef]
- Aguadero, A.; Alonso, J.A.; Escudero, M.J.; Daza, L. Evaluation of the La2Ni1−xCuxO4+δ system as SOFC cathode material with 8YSZ and LSGM as electrolytes. Solid State Ionics 2008, 179, 393–400. [Google Scholar] [CrossRef]
- Escudero, M.J.; Fuerte, A.; Daza, L. La2NiO4+δ potential cathode material on La0.9Sr0.1Ga0.8Mg0.2O2.85 electrolyte for intermediate temperature solid oxide fuel cell. J. Power Sources 2011, 196, 7245–7250. [Google Scholar] [CrossRef]
- Rieu, M.; Sayers, R.; Laguna-Bercero, M.A.; Skinner, S.J.; Lenormand, P.; Ansart, F. Investigation of graded La2NiO4+d cathodes to improve SOFC electrochemical performance. J. Electrochem. Soc. 2010, 157, B477–B480. [Google Scholar] [CrossRef]
- Sayers, R.; Liu, J.; Rustumji, B.; Skinner, S.J. Novel K2NiF4-Type Materials for Solid Oxide Fuel Cells: Compatibility with Electrolytes in the Intermediate Temperature Range. Fuel Cells 2008, 8, 338–343. [Google Scholar] [CrossRef]
- Sayers, R.; Rieu, M.; Lenormand, P.; Ansart, F.; Kilner, J.A.; Skinner, S.J. Development of lanthanum nickelate as a cathode for use in intermediate temperature solid oxide fuel cells. Solid State Ionics 2011, 192, 531–534. [Google Scholar] [CrossRef]
- Ferchaud, C.; Grenier, J.-C.; Zhang-Steenwinkel, Y.; van Tuel, M.M.A.; van Berkel, F.P.F.; Bassat, J.-M. High performance praseodymium nickelate oxide cathode for low temperature solid oxide fuel cell. J. Power Sources 2011, 196, 1872–1879. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Yan, J.; Cai, Q.; Jung, H.Y.; Xing, Z.; Liu, Z.; Goettler, R.W.; Zhou, X.-D. Activity and Stability of (Pr1-xNdx)2NiO4+δ as Cathodes for Oxide Fuel Cells: Part VI. The Role of Cu Dopant on the Structure and Electrochemical Properties. J. Electrochem. Soc. 2017, 164, F3131–F3139. [Google Scholar] [CrossRef]
- Bassat, J.-M.; Vibhu, V.; Nicollet, C.; Flura, A.; Fourcade, S.; Grenier, J.-C.; Rougier, A. Comparison of Pr-Based Cathodes for IT-SOFCs in the Ruddlesden-Popper Family. ECS Trans. 2017, 78, 655–665. [Google Scholar] [CrossRef]
- Vibhu, V.; Bassat, J.-M.; Flura, A.; Nicollet, C.; Grenier, J.-C.; Rougier, A. Influence of La/Pr Ratio on the Ageing Properties of La2-xPrxNiO4+δ as cathodes in IT-SOFCs. ECS Trans. 2015, 68, 825–835. [Google Scholar] [CrossRef]
- Amow, G.; Whitfield, P.S.; Davidson, I.J.; Hammond, R.P.; Munnings, C.N.; Skinner, S.J. Structural and sintering characteristics of the La2Ni1−xCoxO4+δ series. Ceram. Inter. 2004, 30, 1635–1639. [Google Scholar] [CrossRef]
- Zhang, Z.; Greenblatt, M. Synthesis, structure, and properties of Ln4Ni3O10-δ (Ln= La, Pr, and Nd). J. Solid State Chem. 1995, 117, 236–246. [Google Scholar] [CrossRef]
- Berger, C.; Bucher, E.; Egger, A.; Schrodl, N.; Lammer, J.; Gspan, C.; Merkle, R.; Grogger, W.; Maier, J.; Sitte, W. Oxygen surface exchange kinetics and electronic conductivity of the third-order Ruddlesden-Popper phase Pr4Ni2.7Co0.3O10-d. Solid State Ionics 2020, 348, 115282. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Aguadero, A.; Skinner, S.J. LaPr3Ni3O9.76 as a candidate solid oxide fuel cell cathode: Role of microstructure and interface structure on electrochemical performance. APL Mater. 2019, 7, 013204. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to form a Capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS) Los Alamos National Laboratory Report LAUR; Los Almos National Laboratory: Los Almos, NM, USA, 2004; pp. 86–748. [Google Scholar]
- Hong, J.-E.; Ida, S.; Ishihara, T. Effects of transition metal addition on sintering and electrical conductivity of La-doped CeO2 as buffer layer for doped LaGaO3 electrolyte film. Solid State Ionics 2014, 262, 374–377. [Google Scholar] [CrossRef]
- Ling, C.D.; Argyriou, D.N.; Wu, G.; Neumeier, J.J. Neutron Diffraction Study of La3Ni2O7: Structural Relationships among n=1, 2, and 3 Phases Lan+1NinO3n+1. J. Solid State Chem. 2000, 152, 517–525. [Google Scholar] [CrossRef]
- Olafsen, A.; Fjellvåg, H.; Hauback, B.C. Crystal structure and properties of Nd4Co3O10+δ and Nd4Ni3O10+δ. J. Solid State Chem. 2000, 151, 46–55. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Du, Z.; Zhao, H.; Aguadero, A.; Skinner, S.J. La2Pr2Ni3O10±δ Ruddlesden-Popper phase as potential intermediate temperature-solid oxide fuel cell cathodes. Solid State Ionics 2018, 320, 148–151. [Google Scholar] [CrossRef]
- Bassat, J.M.; Allancon, C.; Odier, P.; Loup, J.P. Electronic properties of Pr4Ni3O10±δ. Eur. J. Solid State Lnorg. Chem. 1998, 35, 173–188. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Vibhu, V.; Rougier, A.; Nicollet, C.; Flura, A.; Fourcade, S.; Penin, N.; Grenier, J.-C.; Bassat, J.-M. Pr4Ni3O10+δ: A new promising oxygen electrode material for solid oxide fuel cells. J. Power Sources 2016, 317, 184–193. [Google Scholar] [CrossRef]
- Ishihara, T.; Shibayama, T.; Honda, M.; Nishiguchi, H.; Takita, Y. Intermediate temperature solid oxide fuel cells using LaGaO3 electrolyte II. Improvement of oxide ion conductivity and power density by doping Fe for Ga site of LaGaO3. J. Electrochem. Soc. 2000, 147, 1332–1337. [Google Scholar] [CrossRef]
- Woolley, R.J. Ruddlesden-Popper Phases as Solid Oxide Fuel Cell Cathodes: Electrochemical Performance and In Situ Characterisation. Ph.D. Thesis, Imperial College London, London, UK, 2013. [Google Scholar]
- Woolley, R.J.; Skinner, S.J. Novel La2NiO4+delta and La4Ni3O10-delta composites for solid oxide fuel cell cathodes. J. Power Sources 2013, 243, 790–795. [Google Scholar] [CrossRef]

| Sample | Composition | Electrode Sintering Conditions |
|---|---|---|
| 1 | L2P2N3 | 1150 °C/1 h, followed by cooling under air for 6 h |
| 2 | L3P1N3 | 1150 °C/1 h, followed by cooling under air for 6 h |
| 3 | L2P2N3 | 1050 °C/2.5 h, under ambient conditions |
| 4 | L3P1N3 | 1050 °C/2.5 h, under ambient conditions |
| Composition | a(Å) | b(Å) | c(Å) | β(°) | Space Group | Ref. |
|---|---|---|---|---|---|---|
| La4Ni3O10−δ | 5.415(1) | 5.465(1) | 27.959(9) | 90 | Fmmm | [27] |
| La3PrNi3O10−δ | 5.406(1) | 5.466(1) | 27.873(9) | 90 | Fmmm | This work |
| La2Pr2Ni3O10−δ | 5.392(3) | 5.465(3) | 27.7901(2) | 90 | Fmmm | [36] |
| LaPr3Ni3O10−δ | 5.383(2) | 5.463(2) | 27.676(2) | 90.280(4) | P21/a | [29] |
| Pr4Ni3O10.10 | 5.3714(2) | 5.4611(2) | 27.5271(3) | 90 | Fmmm | [39] |
| − | L3P1N3 Electrode ASR | L2P2N3 Electrode ASR | ||
|---|---|---|---|---|
| Sample 2 | Sample 4 | Sample 1 | Sample 3 | |
| 650 °C | 0.28 Ω cm2 | 0.20 Ω cm2 | 0.24 Ω cm2 | 0.19 Ω cm2 |
| 700 °C | 0.19 Ω cm2 | 0.14 Ω cm2 | 0.17 Ω cm2 | 0.11 Ω cm2 |
| 750 °C | 0.17 Ω cm2 | 0.10 Ω cm2 | 0.15 Ω cm2 | 0.07 Ω cm2 |
| Maximum Power Density/mWcm−2 | |||||
|---|---|---|---|---|---|
| Sample | 800 °C | 750 °C | 700 °C | 650 °C | 600 °C |
| L2P2N3 | 390 | 310 | 230 | 160 | 100 |
| L3P1N3 | 400 | 325 | 250 | 180 | 120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatoo, M.A.; Du, Z.; Yang, Z.; Zhao, H.; Skinner, S.J. LaxPr4−xNi3O10−δ: Mixed A-Site Cation Higher-Order Ruddlesden-Popper Phase Materials as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes. Crystals 2020, 10, 428. https://doi.org/10.3390/cryst10060428
Yatoo MA, Du Z, Yang Z, Zhao H, Skinner SJ. LaxPr4−xNi3O10−δ: Mixed A-Site Cation Higher-Order Ruddlesden-Popper Phase Materials as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes. Crystals. 2020; 10(6):428. https://doi.org/10.3390/cryst10060428
Chicago/Turabian StyleYatoo, Mudasir A., Zhihong Du, Zhang Yang, Hailei Zhao, and Stephen J. Skinner. 2020. "LaxPr4−xNi3O10−δ: Mixed A-Site Cation Higher-Order Ruddlesden-Popper Phase Materials as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes" Crystals 10, no. 6: 428. https://doi.org/10.3390/cryst10060428
APA StyleYatoo, M. A., Du, Z., Yang, Z., Zhao, H., & Skinner, S. J. (2020). LaxPr4−xNi3O10−δ: Mixed A-Site Cation Higher-Order Ruddlesden-Popper Phase Materials as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes. Crystals, 10(6), 428. https://doi.org/10.3390/cryst10060428







