Investigation of Structure, Optical, and Electrical Properties of CuS Thin Films by CBD Technique
Abstract
1. Introduction
2. Methodology and Characterization
3. Results and Discussion
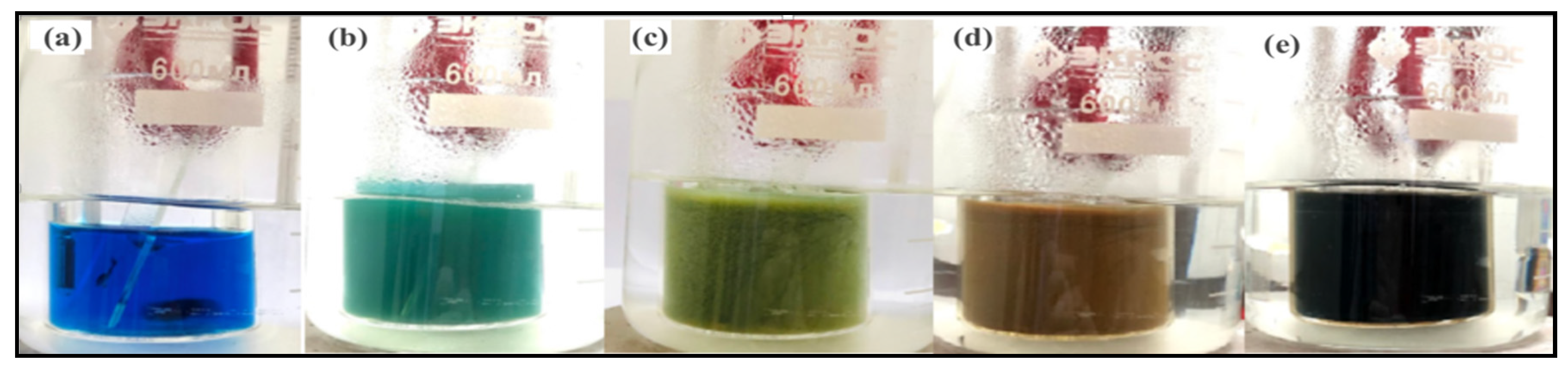
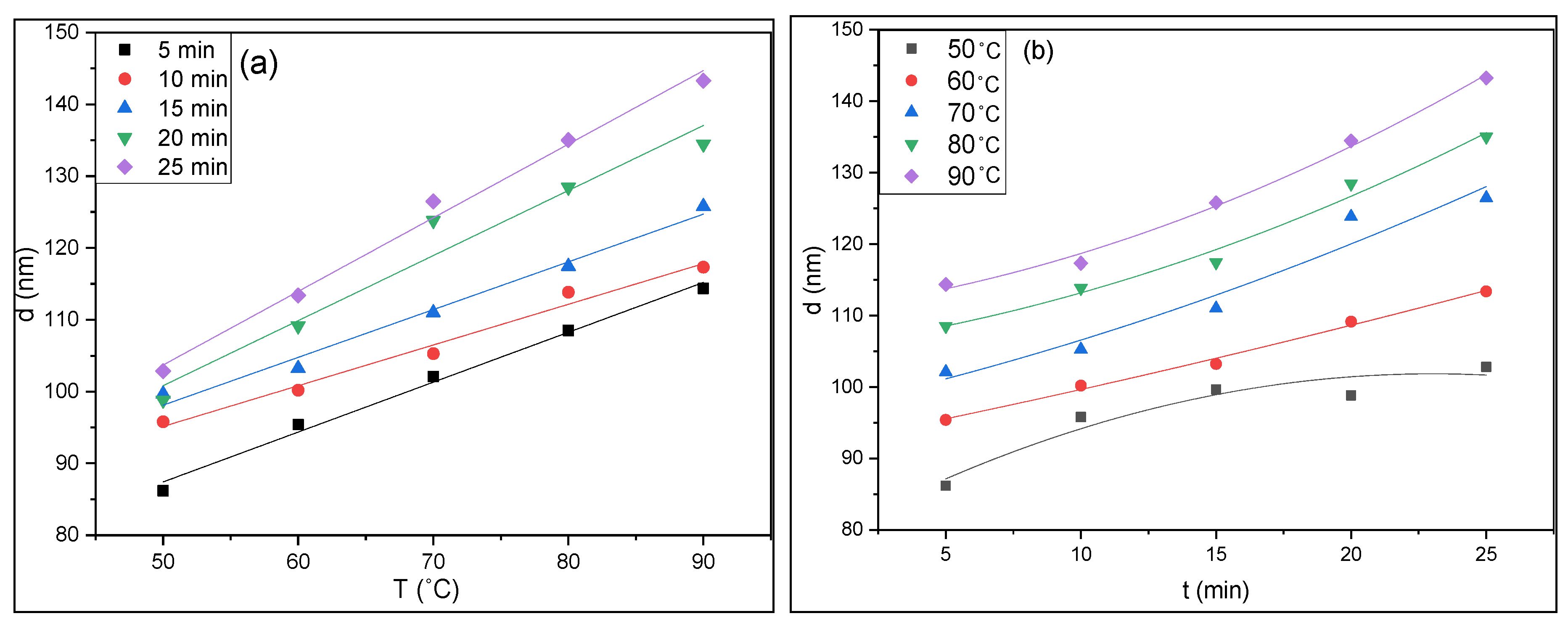
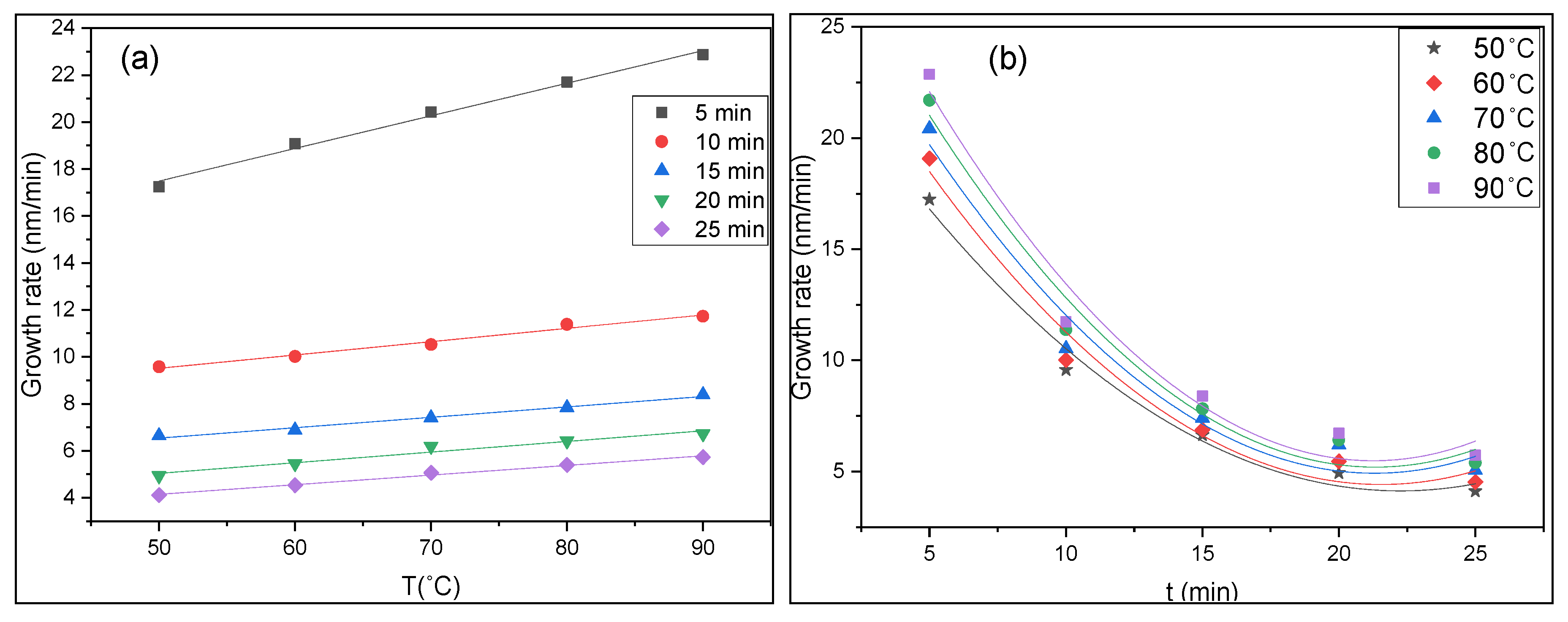
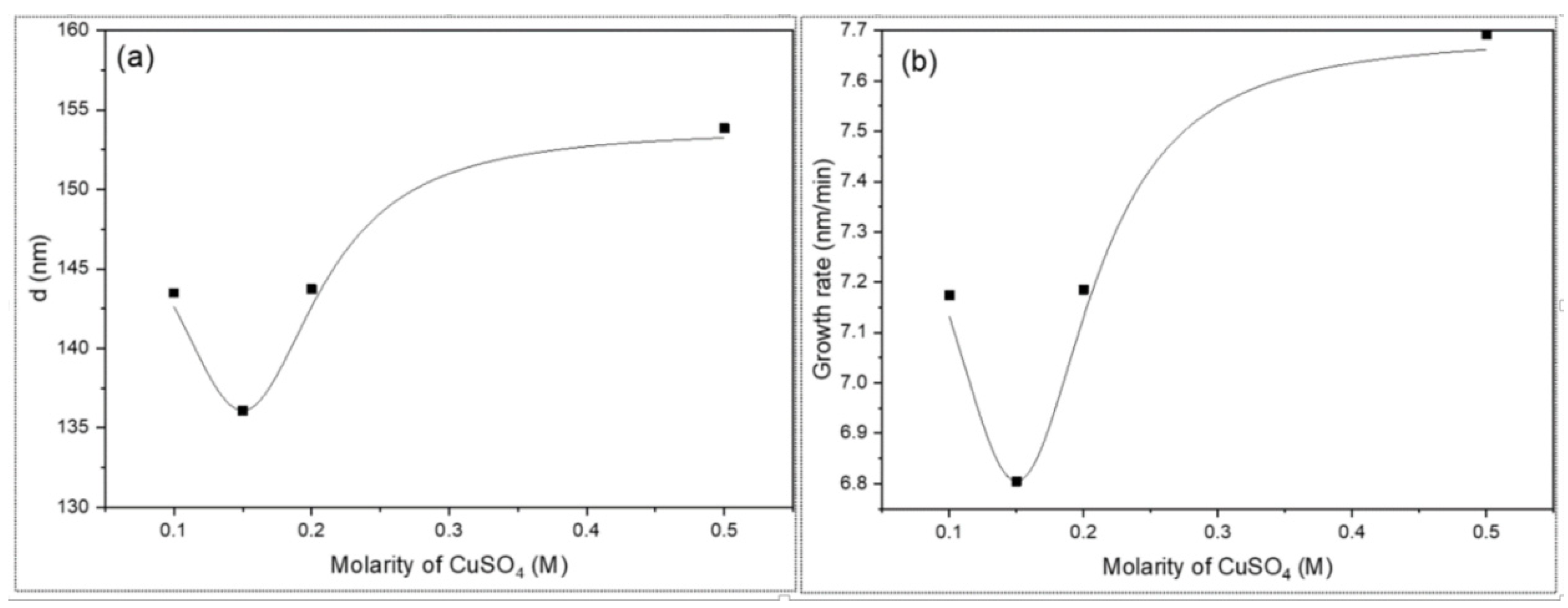
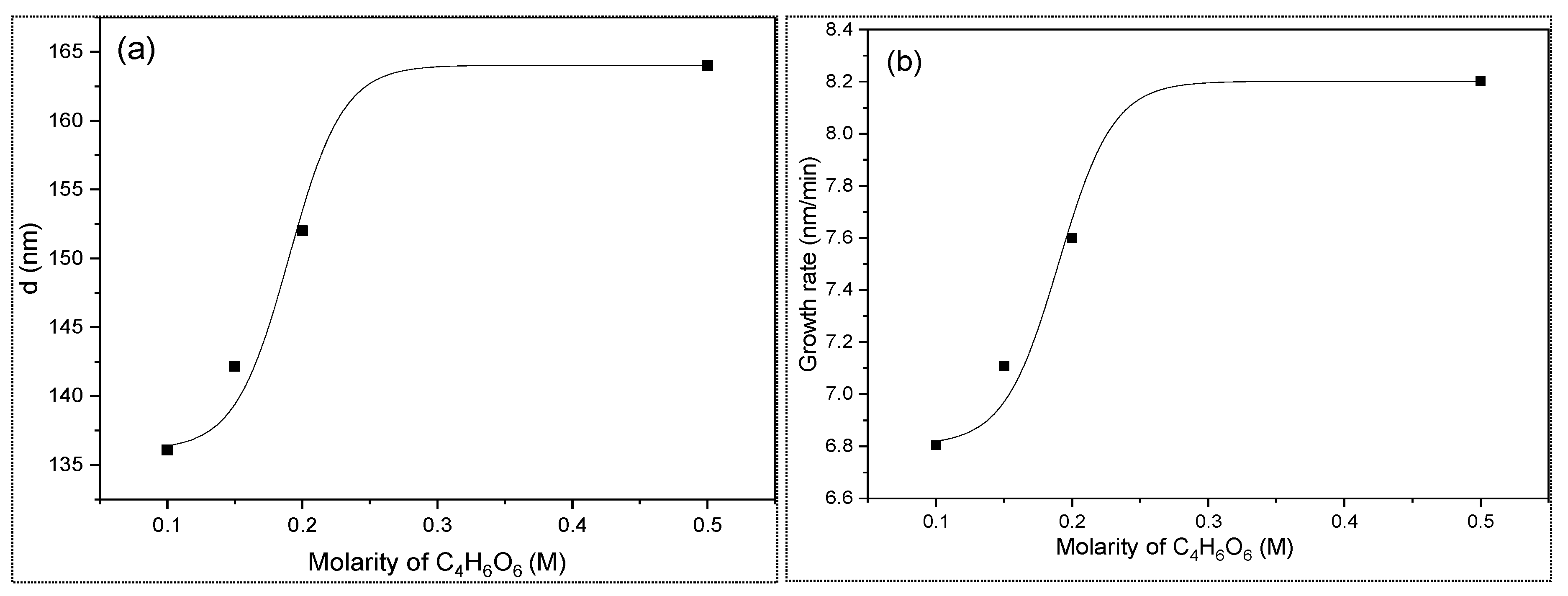
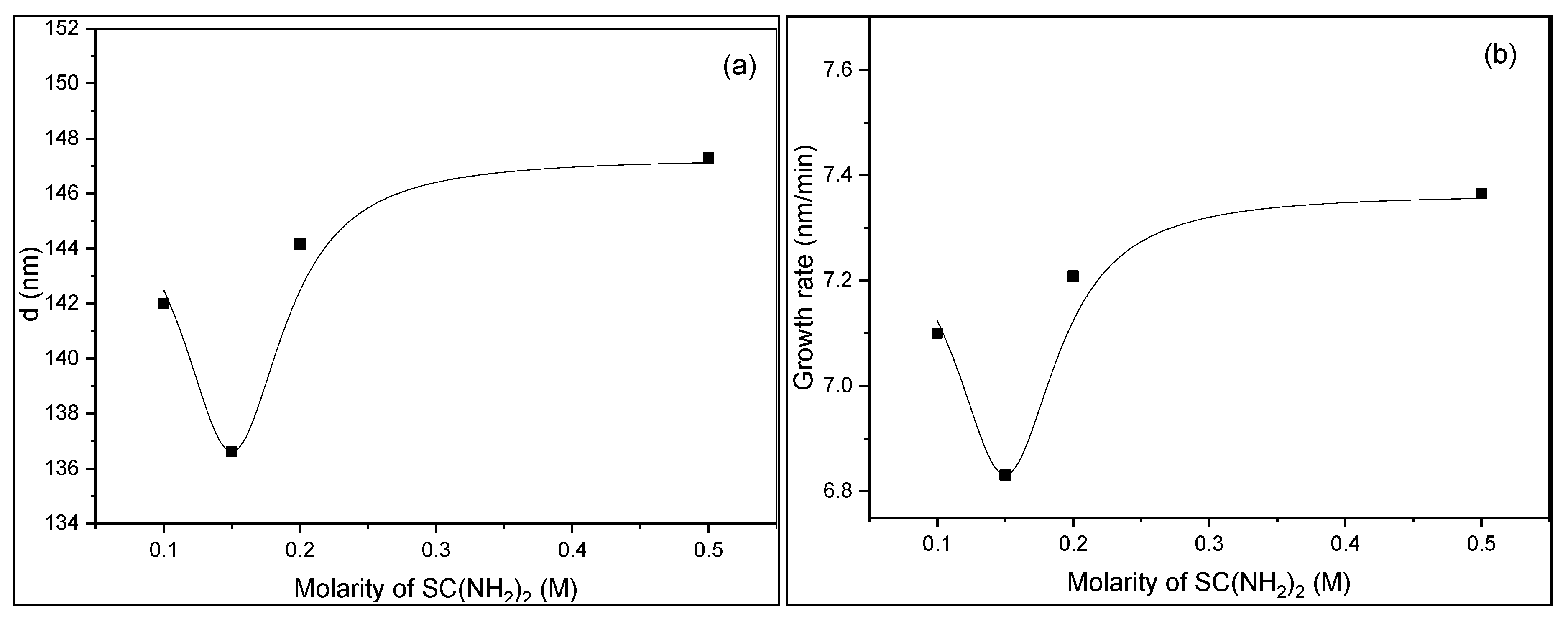
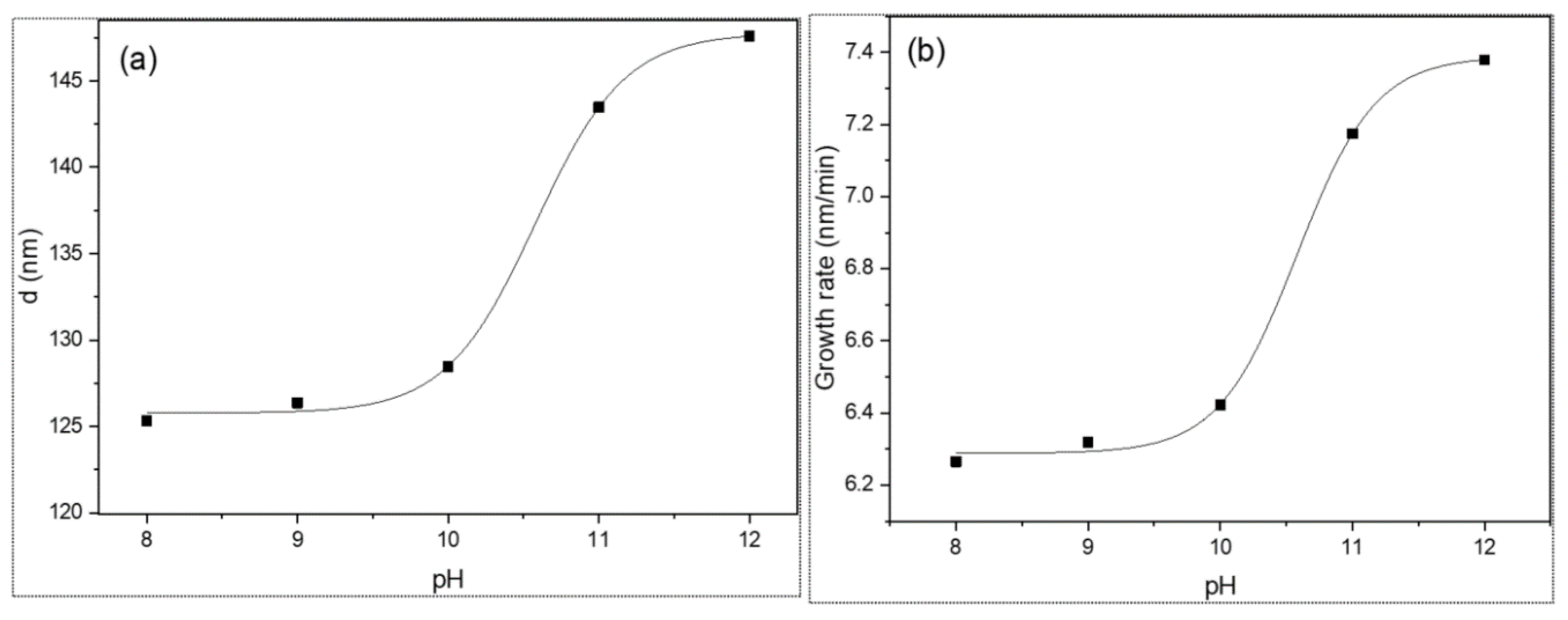
3.1. Thickness and Growth Rate
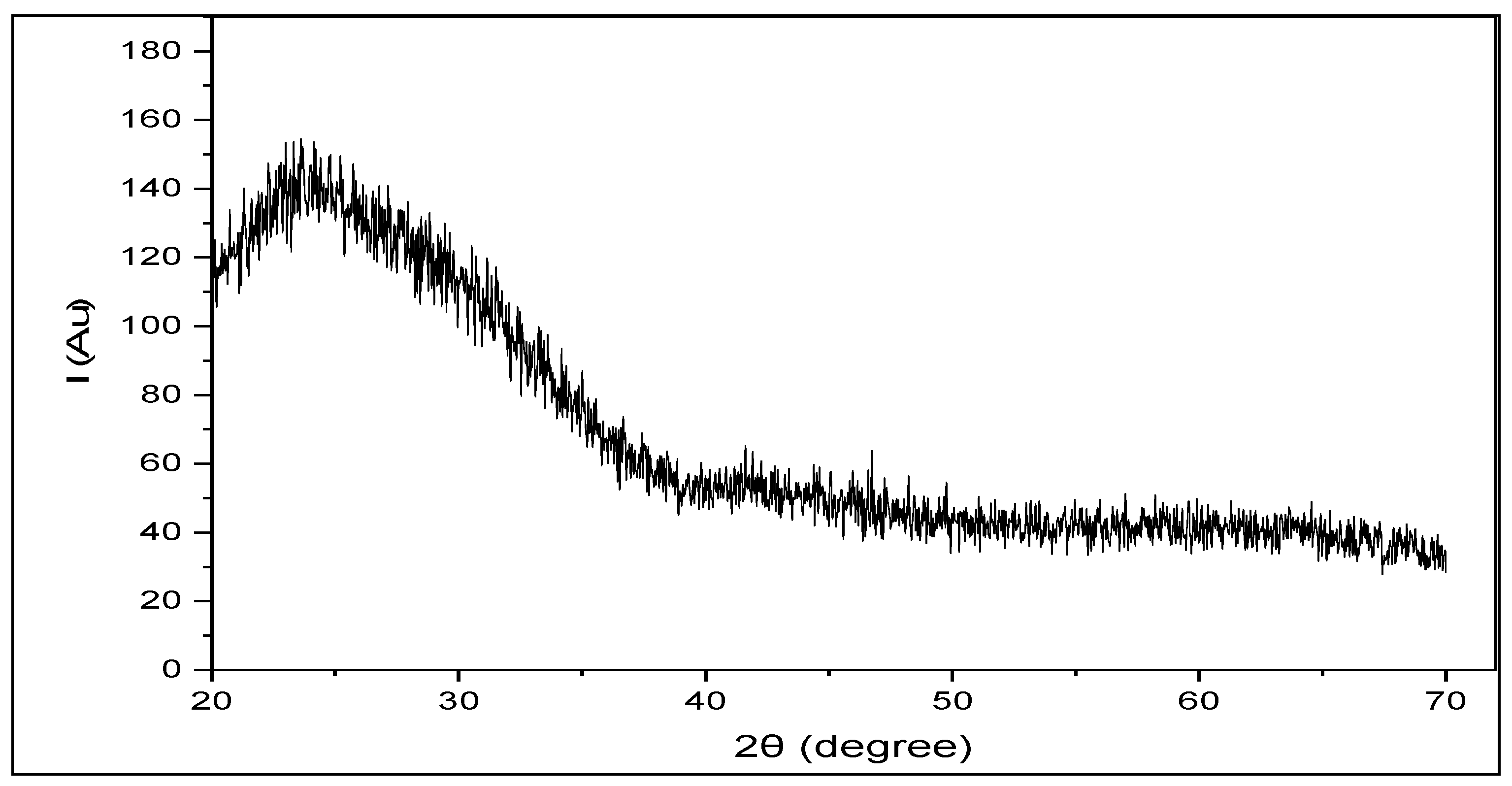
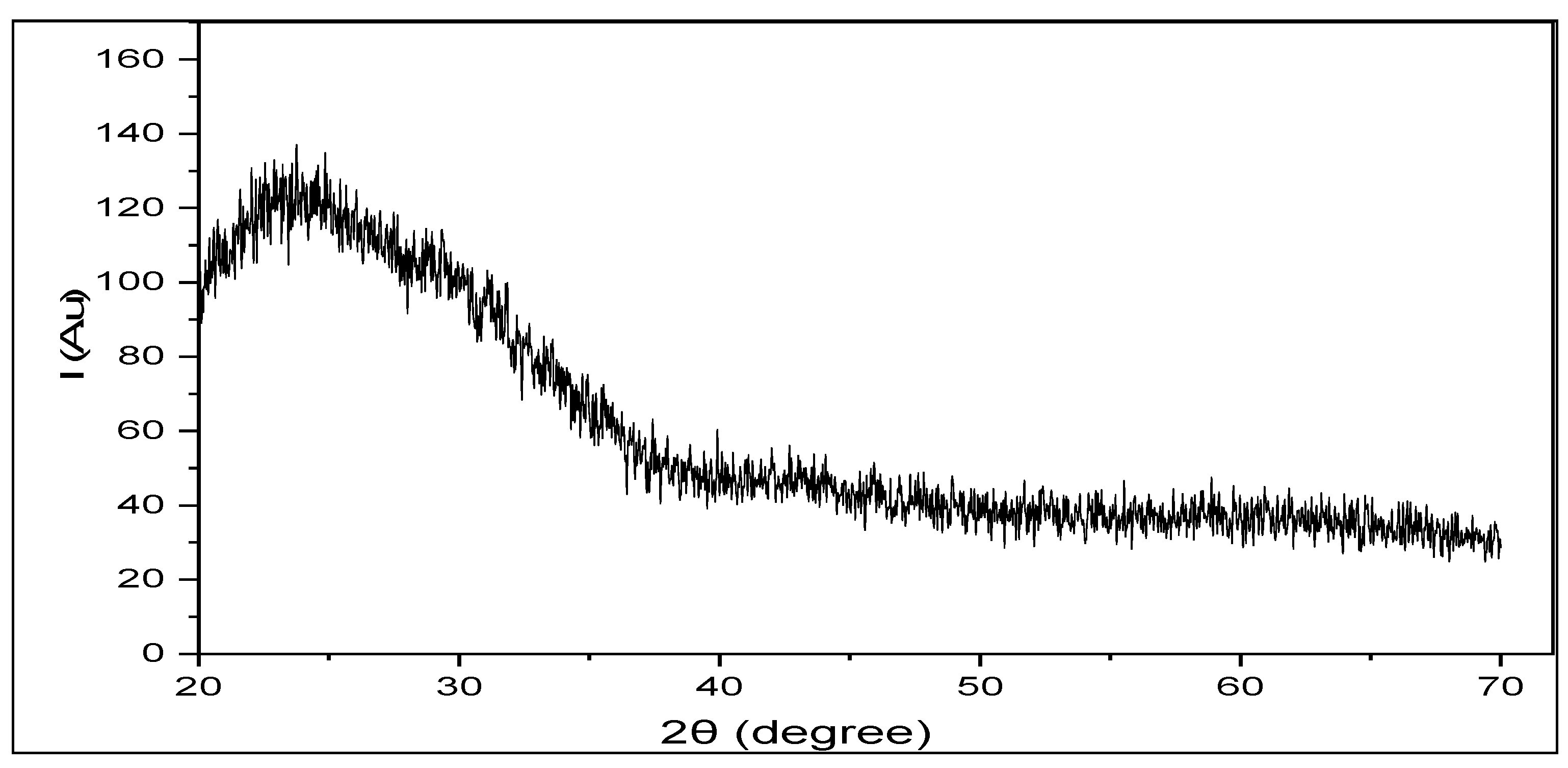
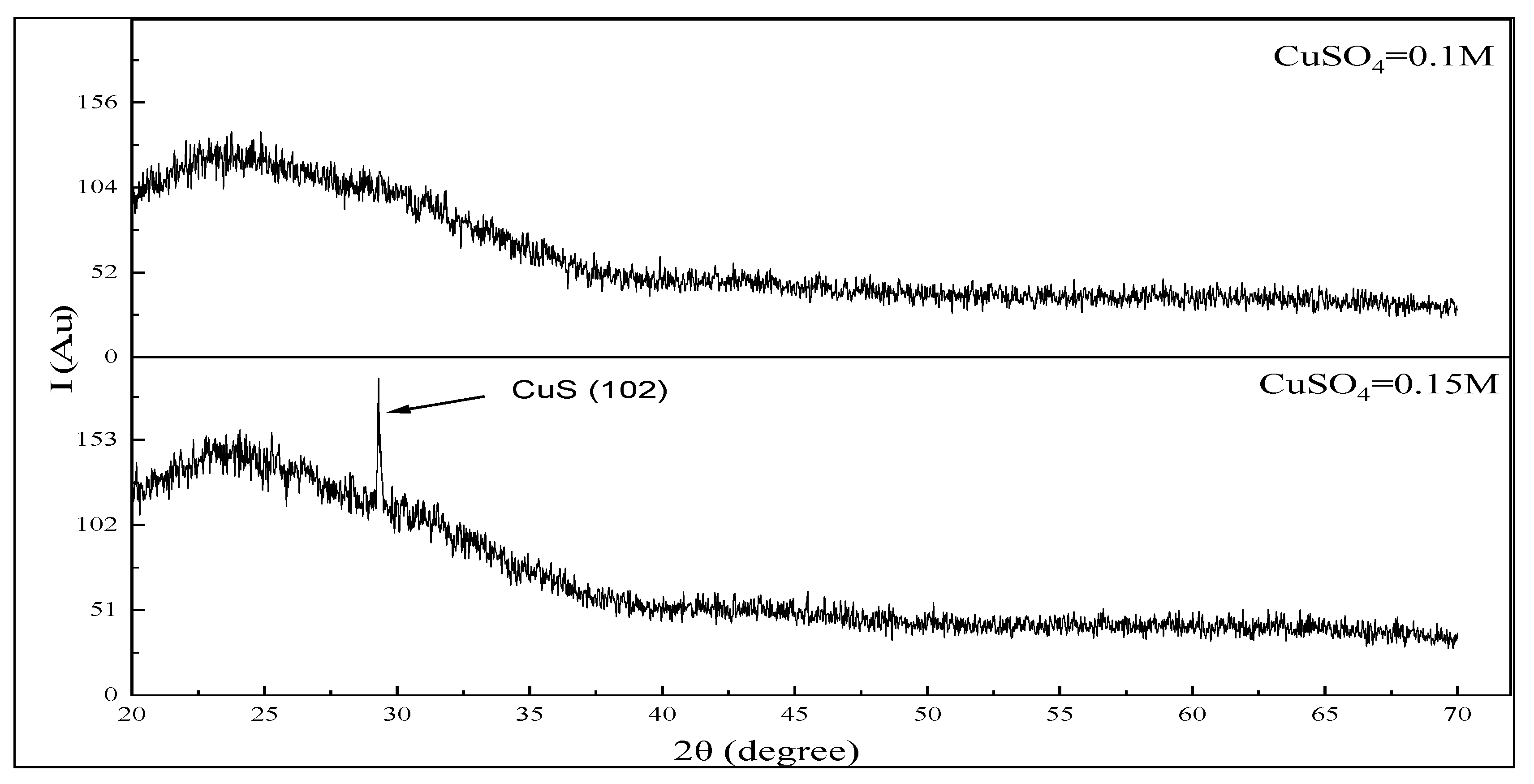
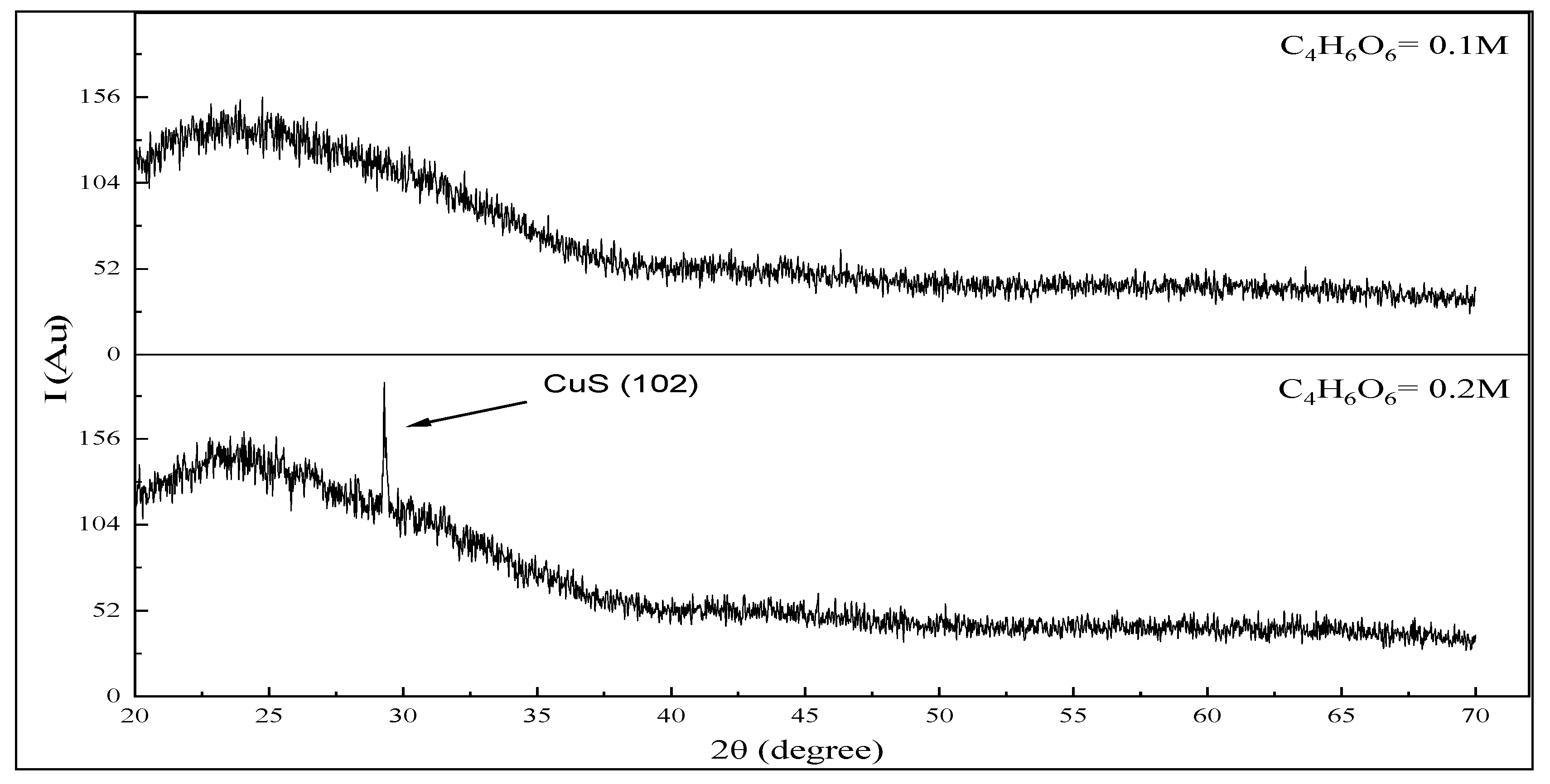
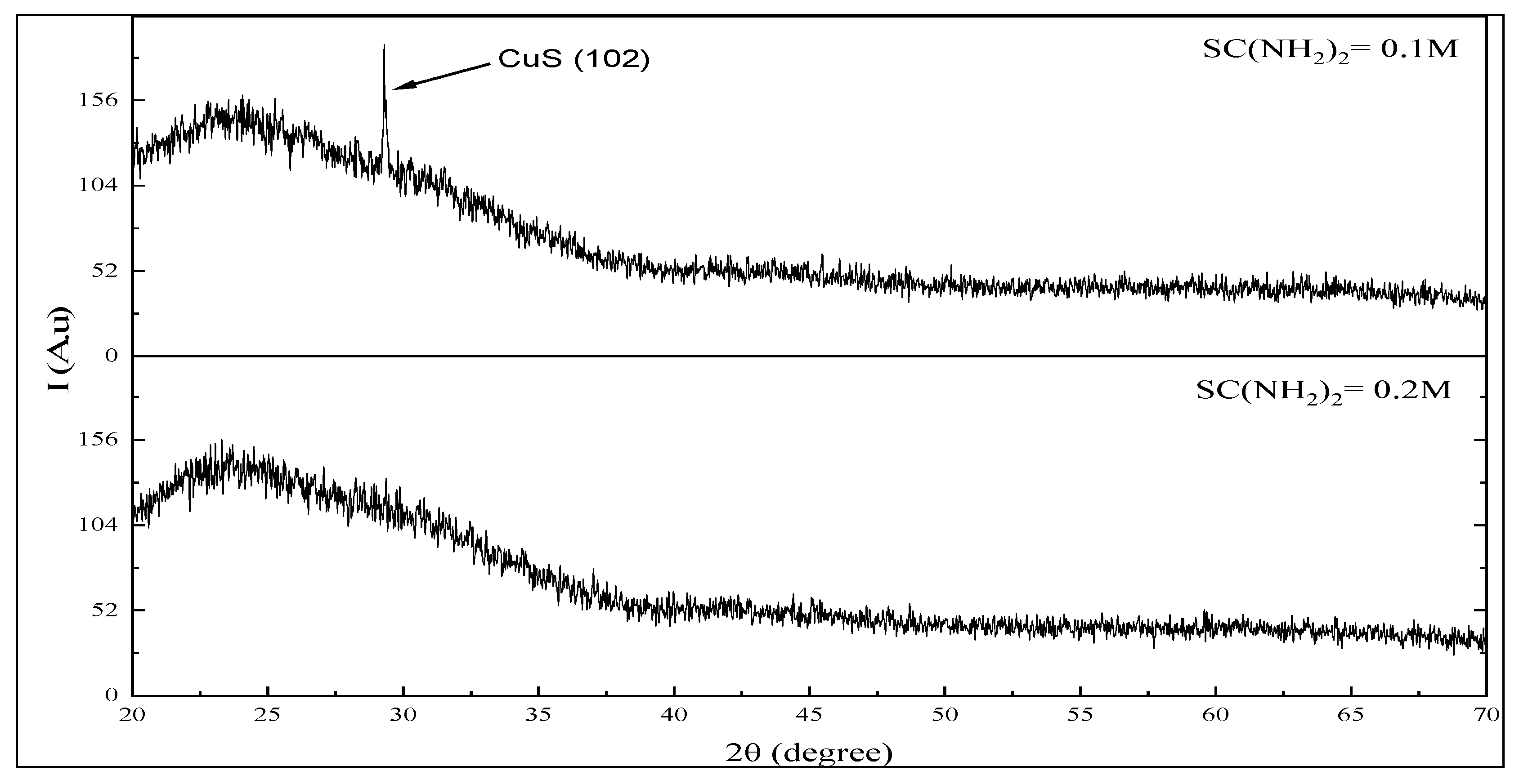
3.2. Structure Properties of CuS Thin Films
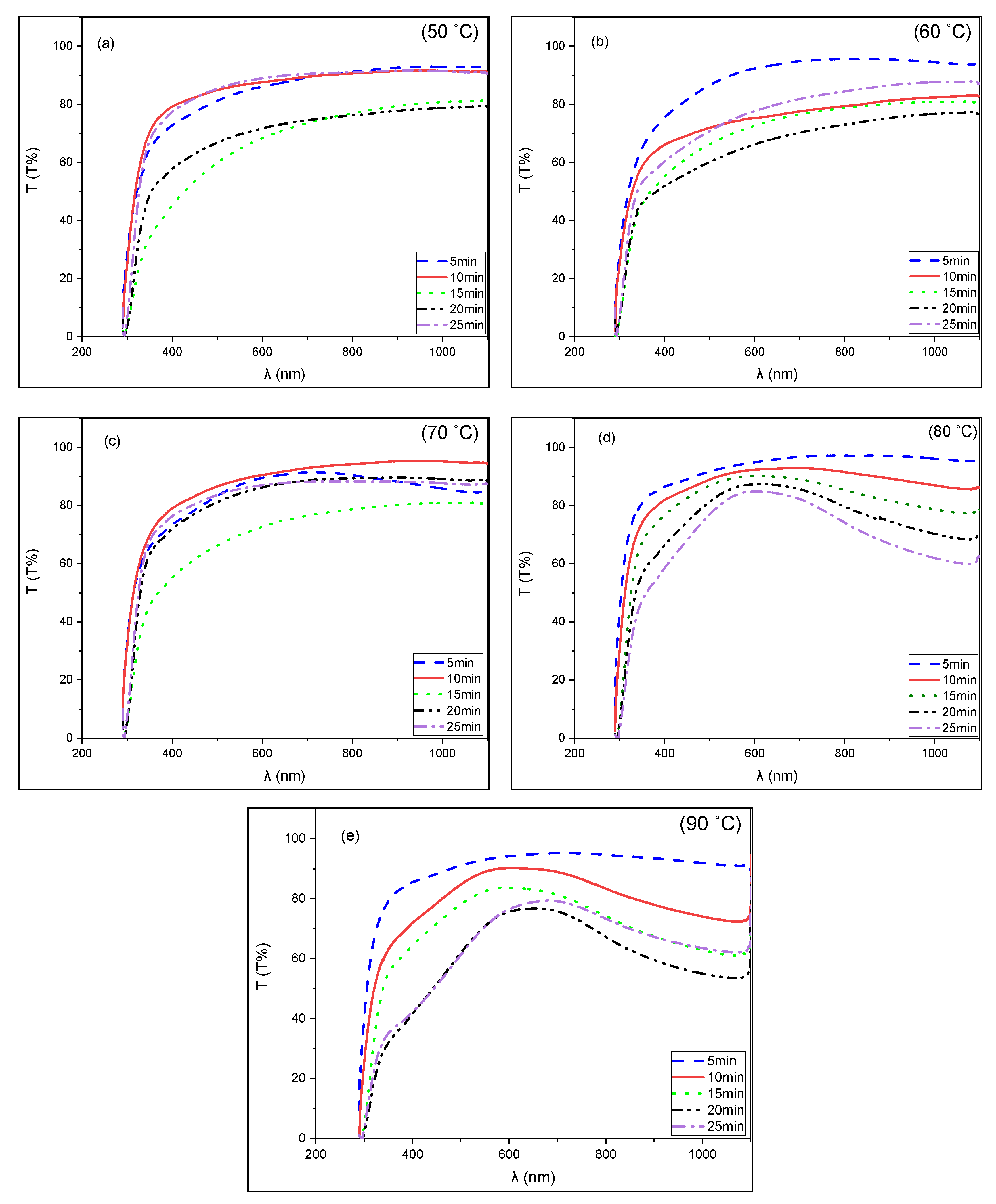
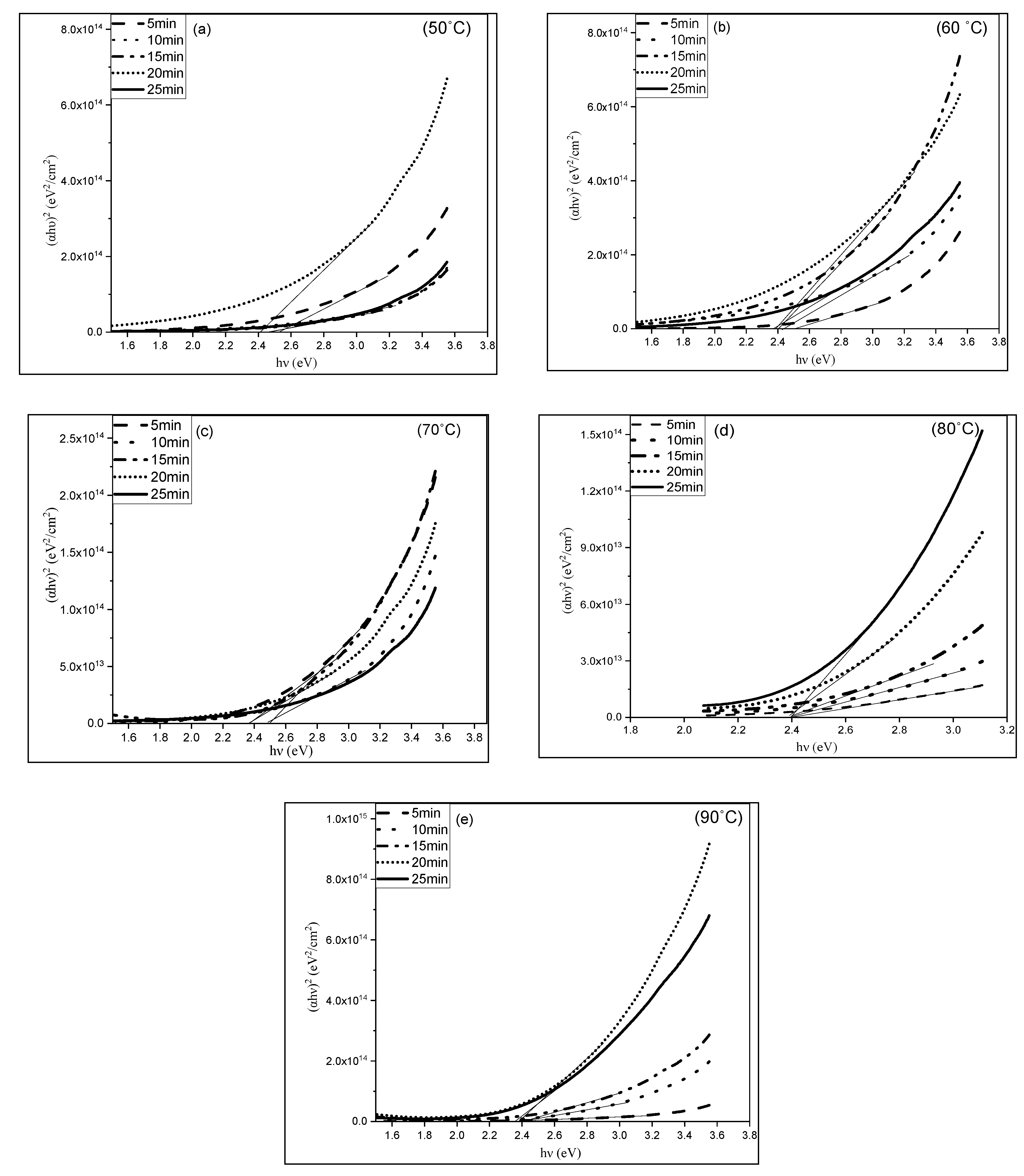
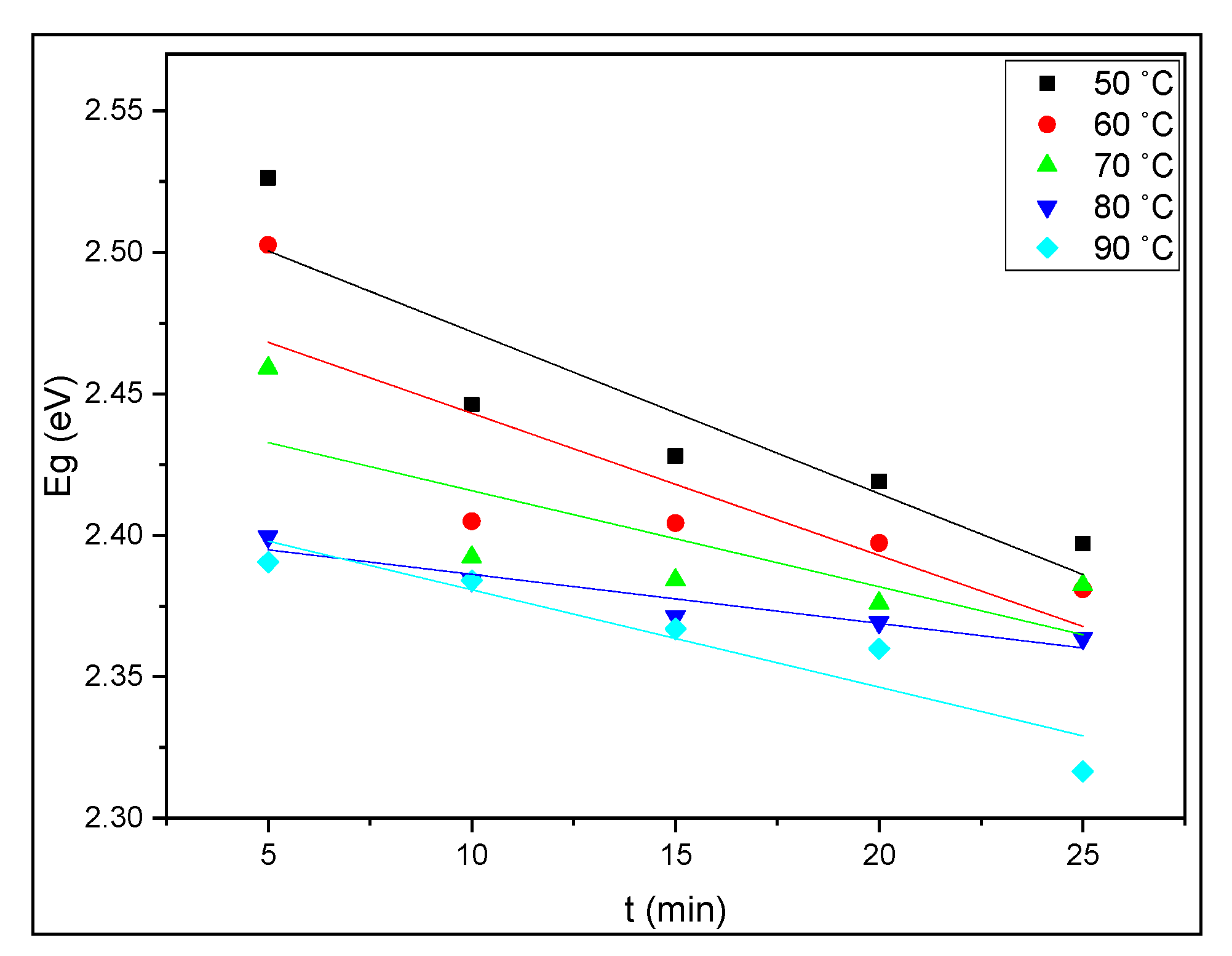
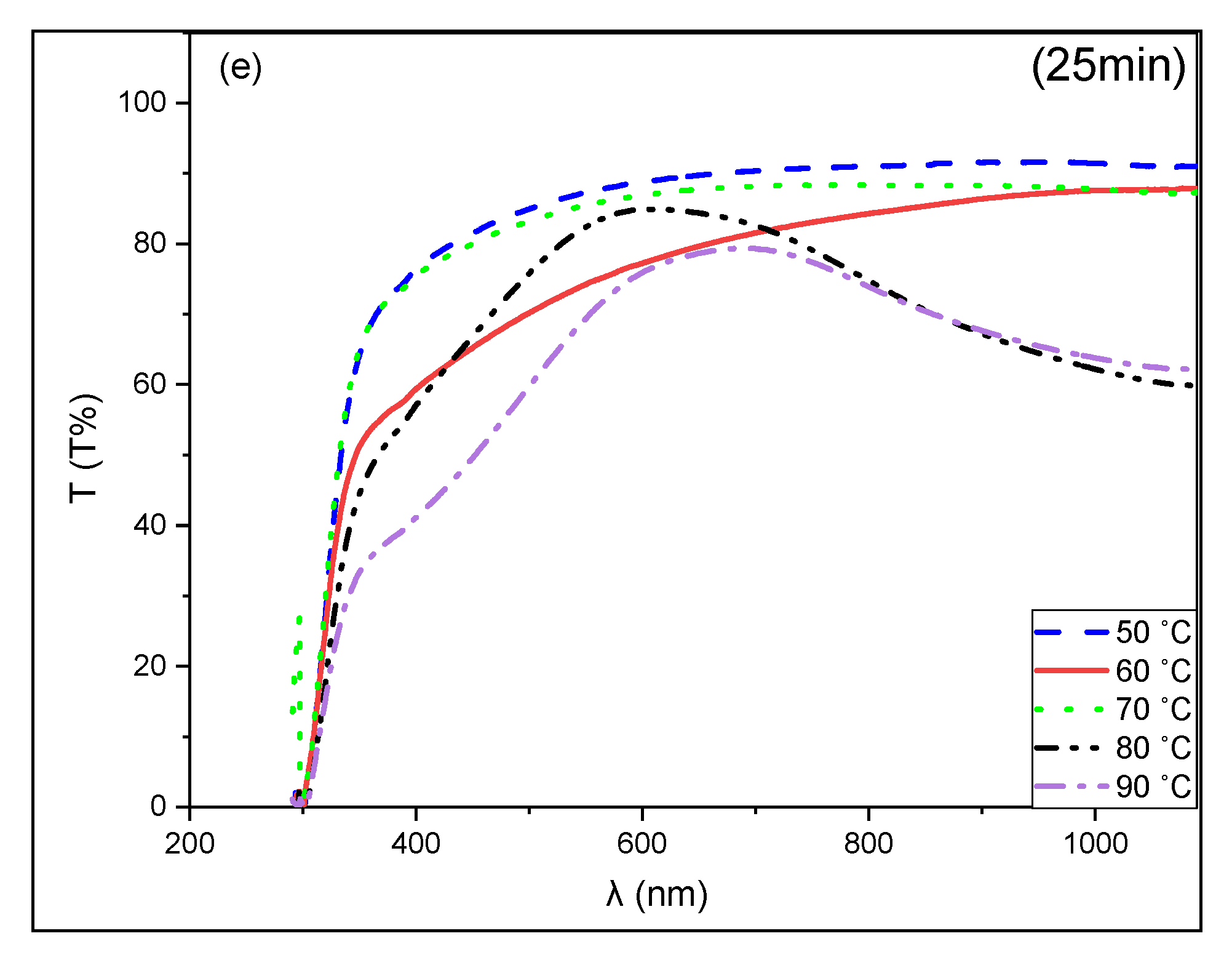
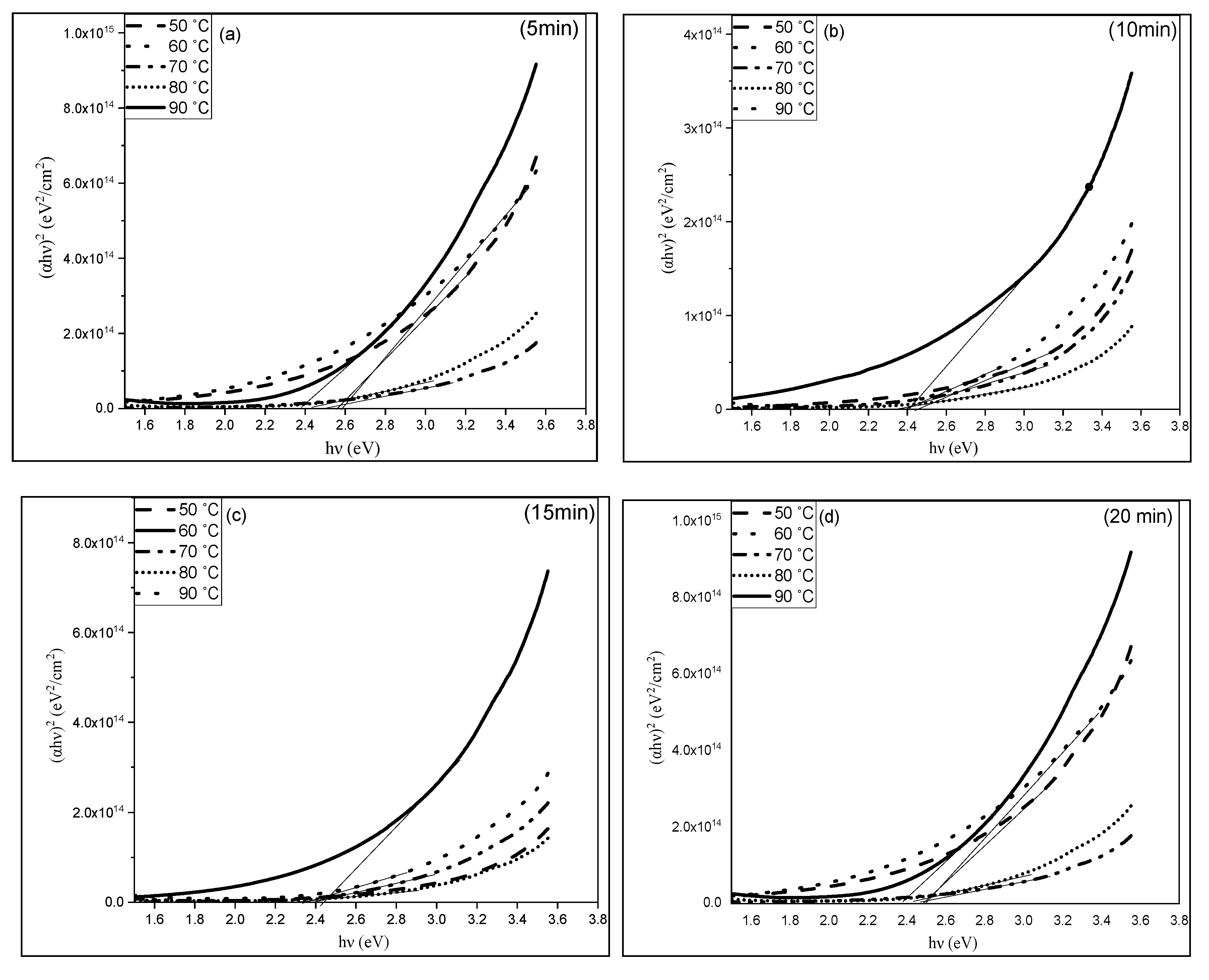
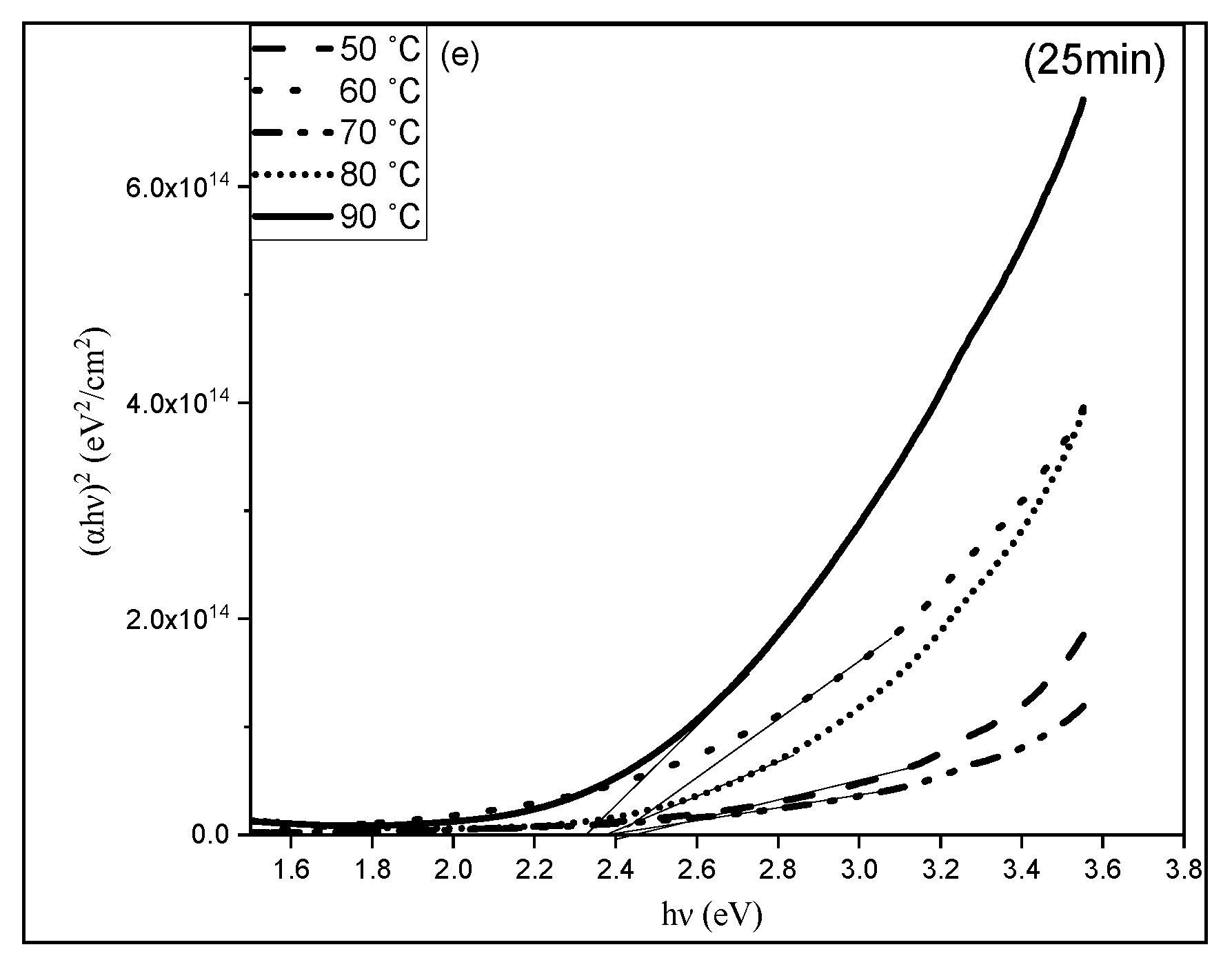
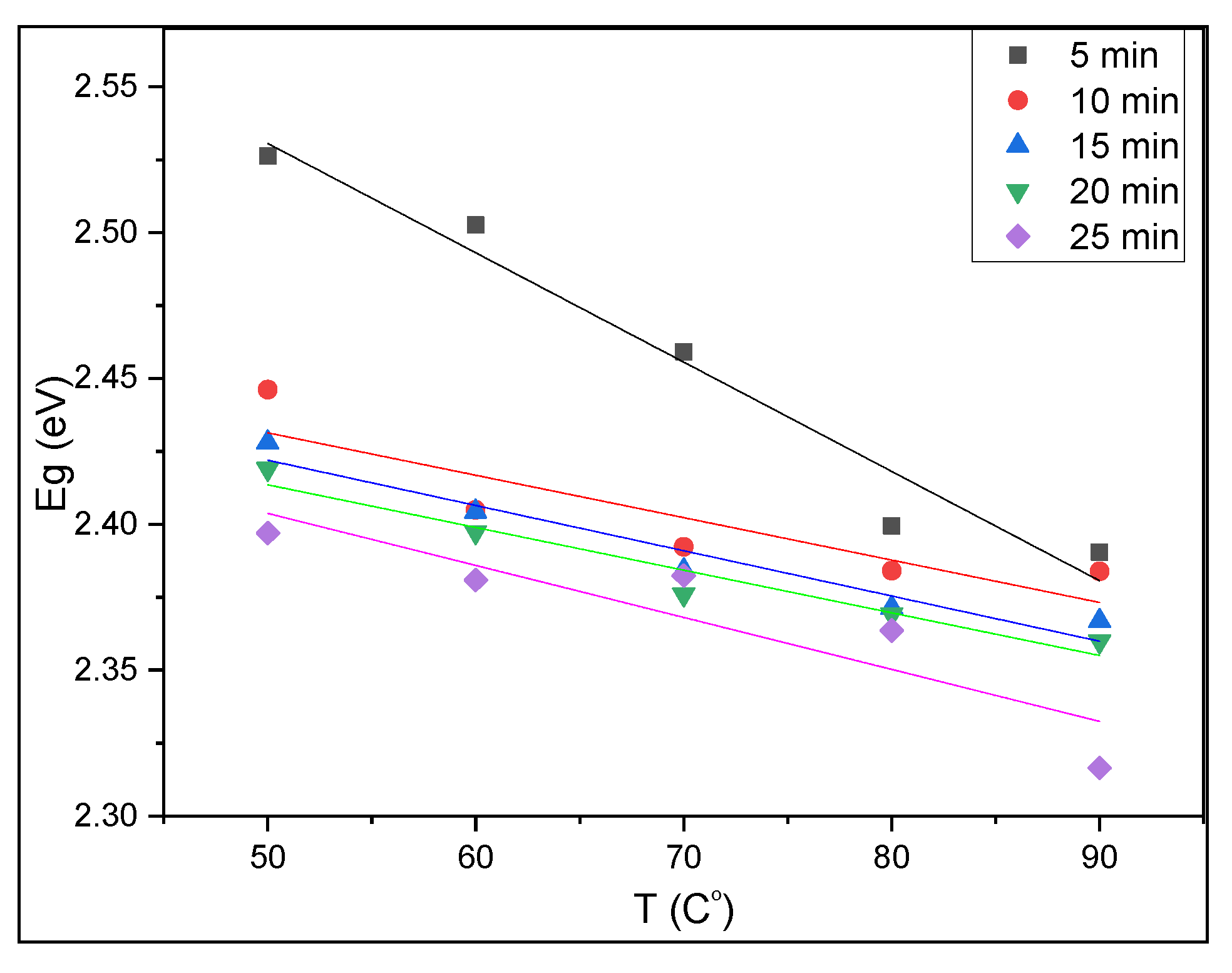
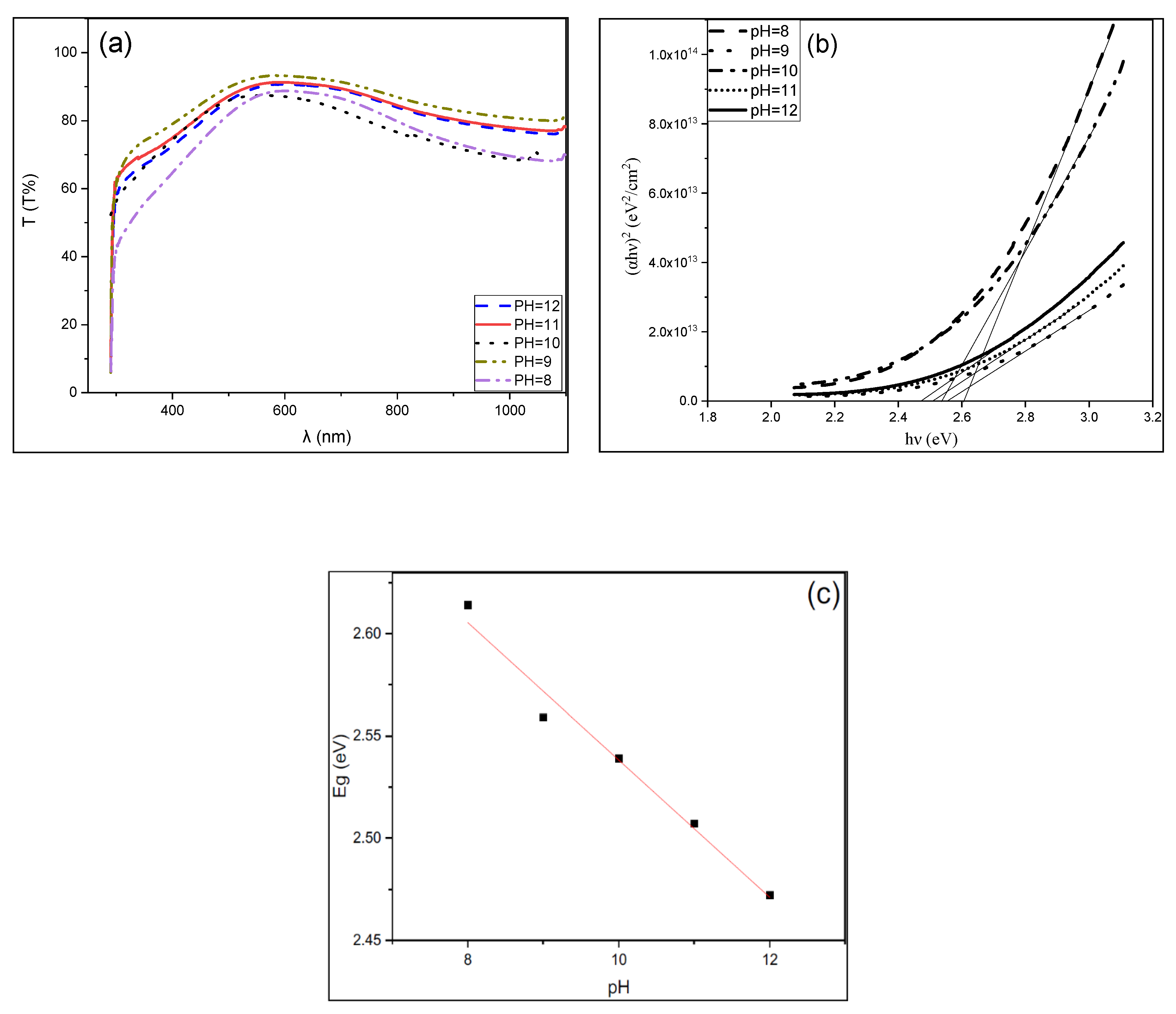
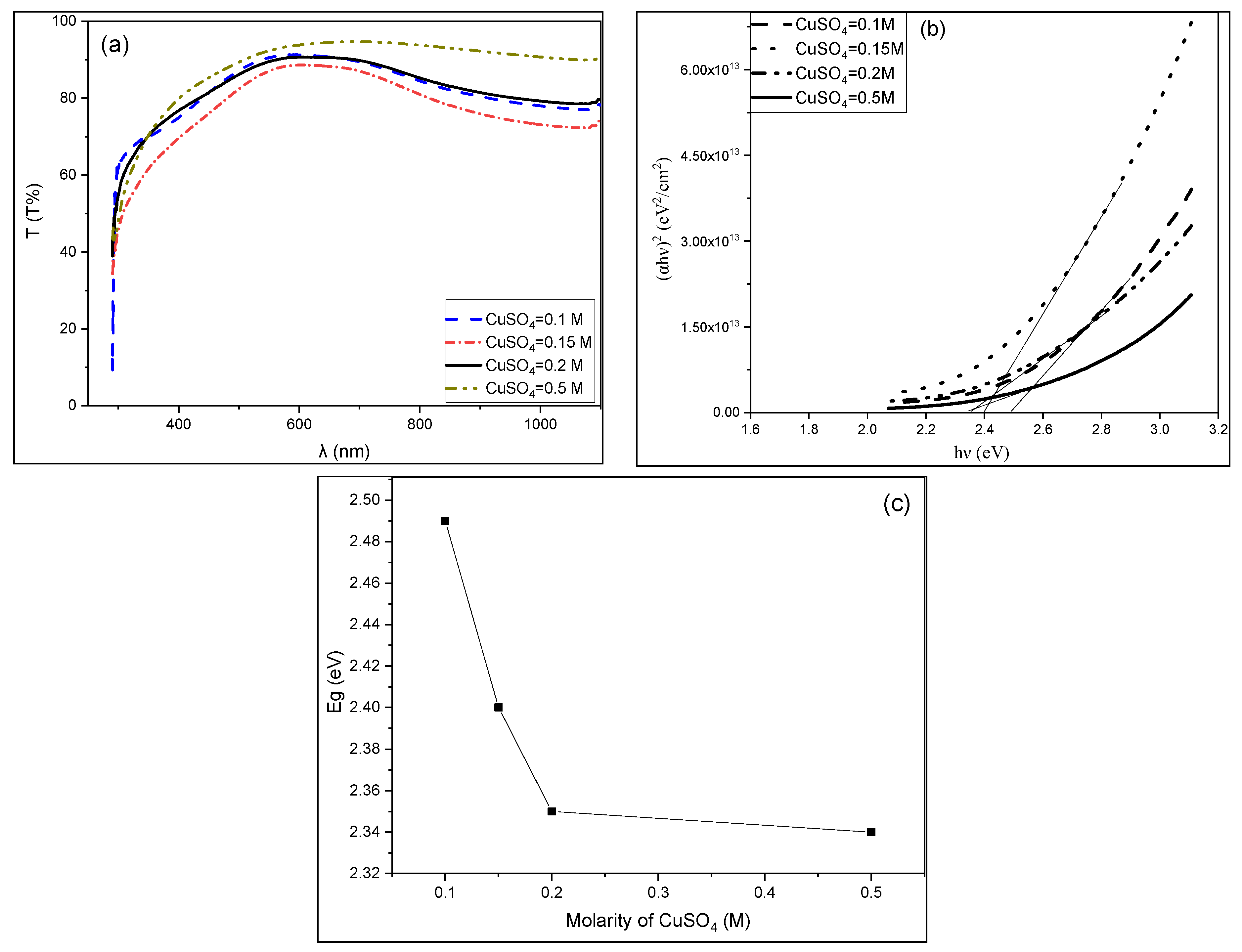
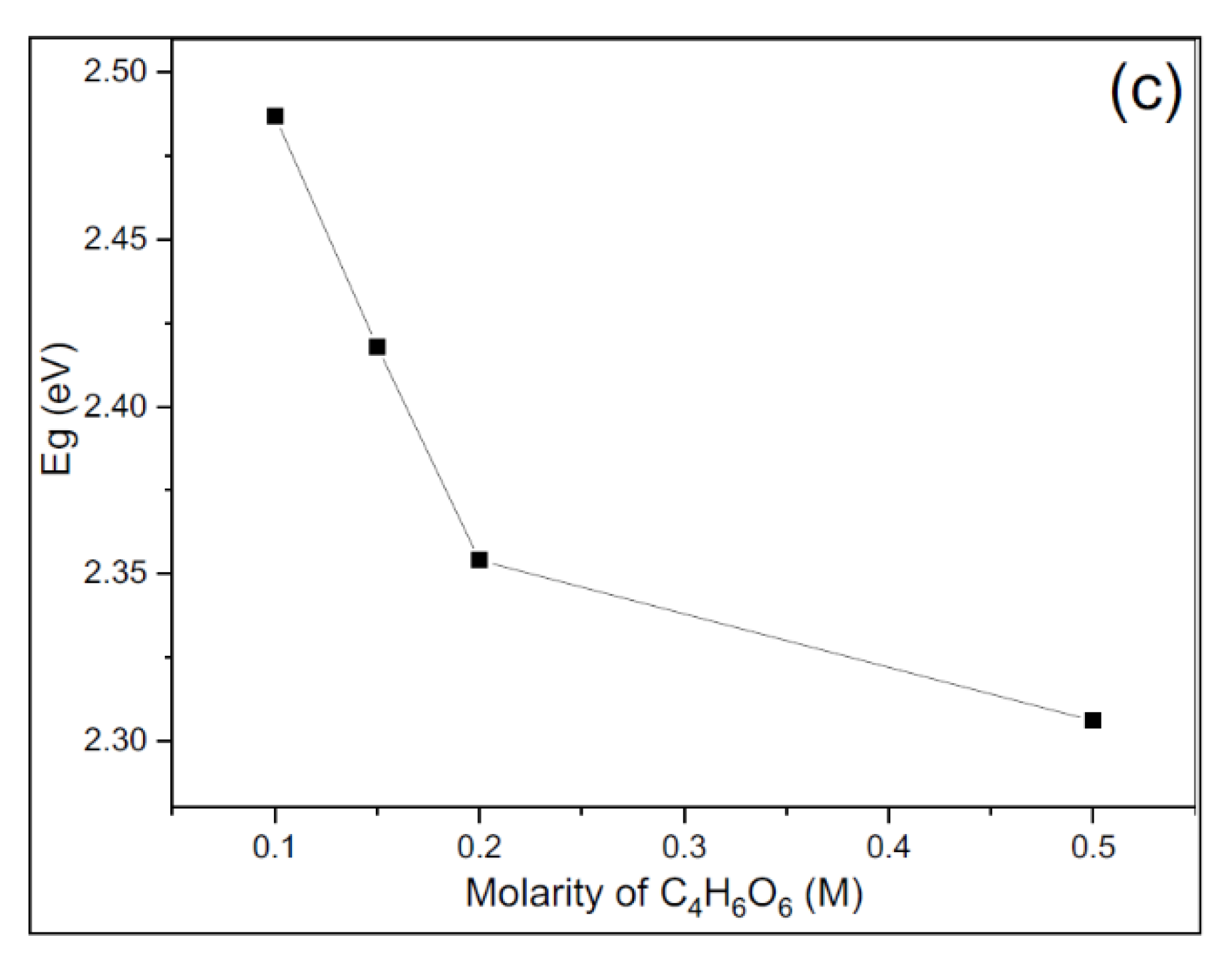
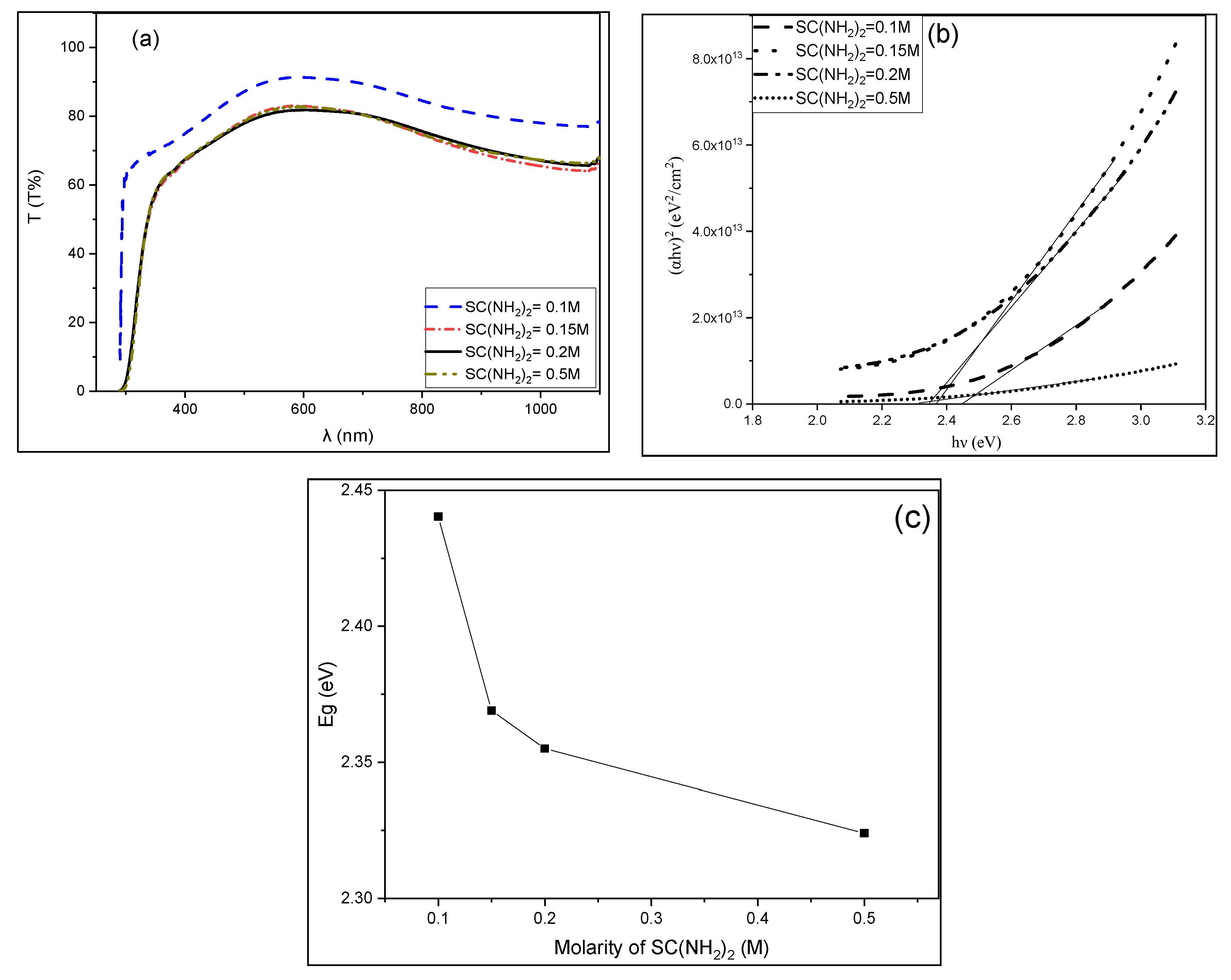
3.3. Optical Properties of CuS Thin Films
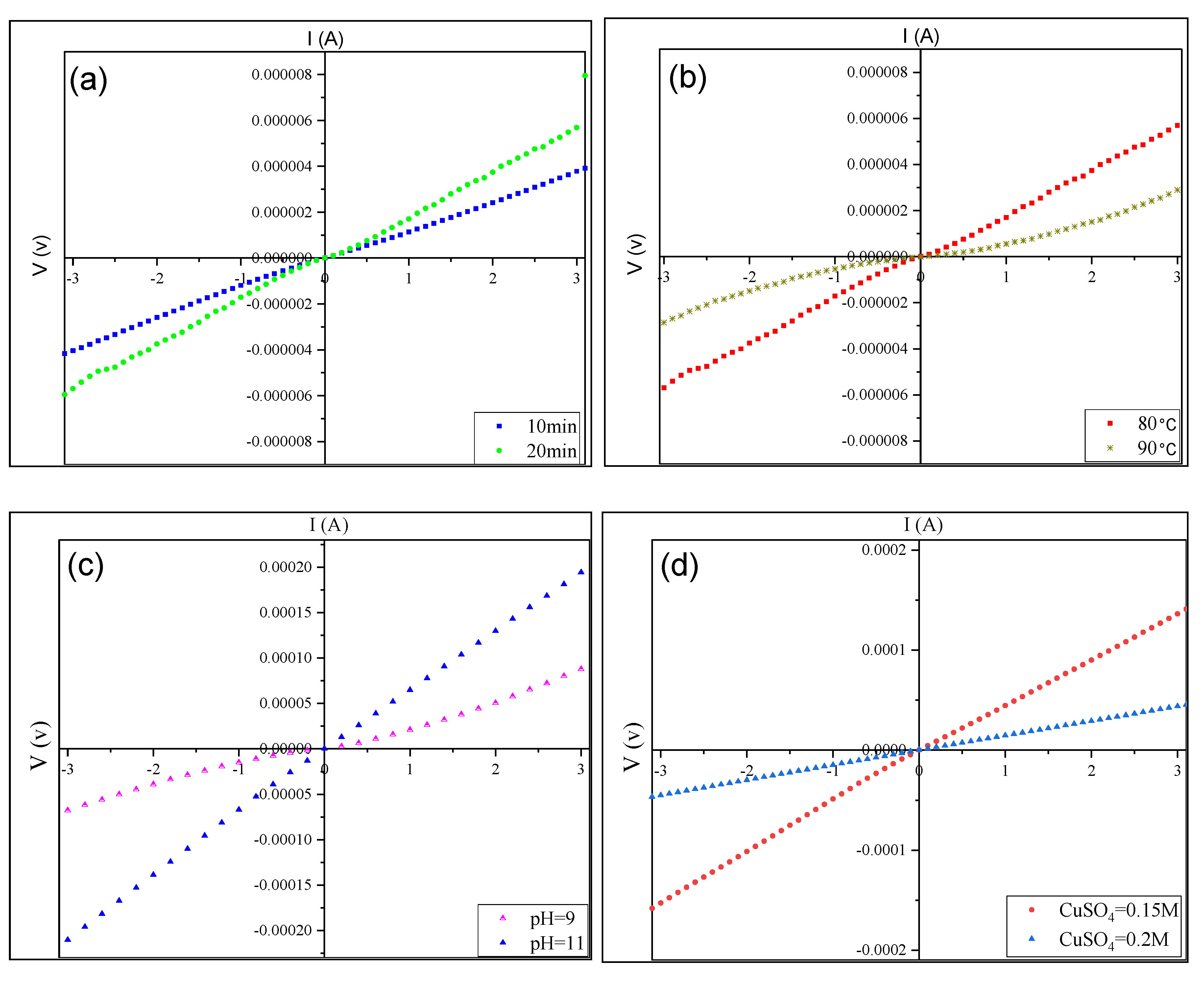
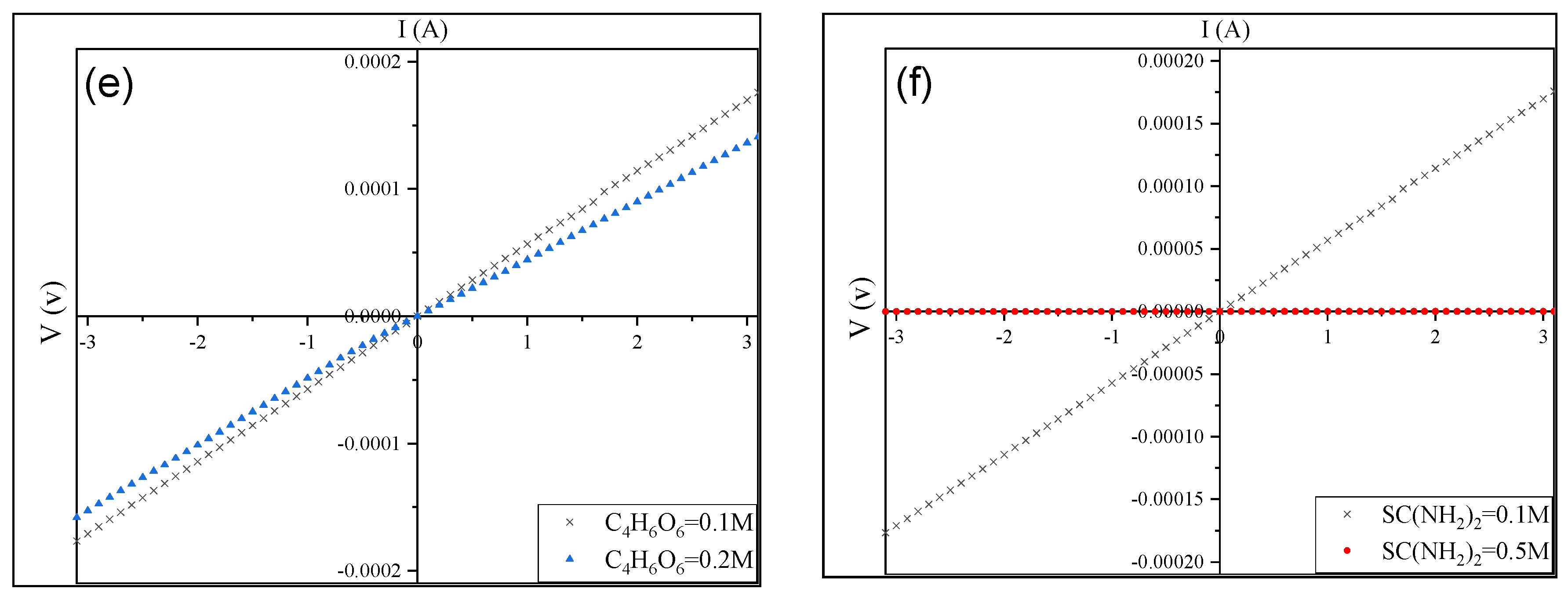
3.4. Electrical Properties of CuS Thin Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Xu, N.; Hu, W.; Xu, X.; Sun, J.; Ying, Z.; Wu, J. Bismuth doped ZnSe Films fabricated on selecone substrate by pulsed laser deposition. Solid State Electron. 2008, 52, 1833–1836. [Google Scholar] [CrossRef]
- Thanikaikarasan, S.; Mahalingam, T.; Adaikalam, K. Characterization of electrodeposited Copper Sulphide thin fil. New Mater. Electrochem. Syst. 2009, 13, 29–33. [Google Scholar]
- Aperathitis, E.; Bryant, F.; Scott, C. Evaporated Copper Sulphide Layers for All-Vacuum Evaporated CuxS/CdS Solar Cell; Elsevier: Amsterdam, The Netherlands, 1990; Volume 20, pp. 15–28. [Google Scholar]
- Chisomam, U.; Charity, O.; Israel, O. Analysis of Chemically deposited Copper Sulfide thin film. Chmeistry Mater. Res. 2014, 6, 1–8. [Google Scholar]
- Luminita, I.; Anca, D.; Angela, K.; Simona, M.; Marian, N. Copper Sulfides obtained by Spray Pyrolysis-Possible absorbers in solid state solar cell. Thin Solid Film. 2007, 515, 5755–5758. [Google Scholar]
- Guizeldir, B.; Saglam, M.; Ates, A. Deposition and characterization of CdS, CuS and ZnS thin films deposition by SILAR Metho. In Proceedings of the Internaional Congress and Advanced in Applied Physics and Material Science, Antalya, Turkey, 12–15 May 2011; Volume 2, pp. 33–35. [Google Scholar]
- Ahamed, M.; Basheer, S.; Bale, A.; Nagarethiam, V.; Thayumanavan, A.; Sanjeeveraja, C.; Jayachandran, M. Structural, optical and electrical properities of electron beam evaporated CdSe thin film. Cryst. Res. Technol. 2010, 45, 387–392. [Google Scholar] [CrossRef]
- Ghribi, F.; Alyamani, A.; Ben Ayadi, Z.; Djessas, K.; EL Mir, L. Study of CuS Thin Films for Solar Cell Applications Sputtered from Nanoparticles Synthesised by Hydrothermal Rout; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 84, pp. 197–203. [Google Scholar]
- Goel, S.; Chen, F.; Cai, W. Synthesis of Biomedical Applications of Copper Sulfide Nanoparticles, from Sensors to Theranostic; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 10, pp. 31–45. [Google Scholar]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Govind, T. Evolution of Hierarchial Hexagonal Stacked plates of CuS from liquid-liquid interface and its Photocatalytic Application for Oxidative Degradation of Different Dyes under Indoor Lightin. ACS Publ. 2010, 6, 6313–6318. [Google Scholar]
- Tian, X.; Qian, L.; Mao, J.; Yuan, H. In Situ Synthesis of CuS nanotubes on Cu electrode for Sensitive nonenzymatic Glucose Senso. Sens. Actuators B Chem. 2013, 176, 952–959. [Google Scholar]
- Chung, J.; Sohn, H. Electrochemical behavior of CuS as a CCathode material for lithium Secondery Batterie. Power Sour. 2002, 108, 226–231. [Google Scholar] [CrossRef]
- Shamraiz, U.; Azadar, R.; Badshah, A. Fabrication and applications of copper sulfide (CuS) nanostructure. J. Solid State Chem. 2016, 238, 25–40. [Google Scholar] [CrossRef]
- McPeak, K.M. Chemical Bath Deposition of Semiconductor Thin Films & Nanostructures in Novel Microreactors. Ph.D. Thesis, Drexel University, Drexel, PA, USA, 2010. [Google Scholar]
- Singh, A.; Mehra, S.; Thool, G. Synthesis of copper Sulfide (CuS) thin film by chemical bath deposition method and its chracterizatio. Eur. Chem. Bull. 2013, 2, 518–523. [Google Scholar]
- Schwartz, B. Ohmic Contacts to Semiconductors; The Electrochemical Society: Pennington, NY, USA, 1969. [Google Scholar]
- Chopra, K.L. Thin Film Phenomena; Mcgraw-Hill Book Company: Boston, MA, USA, 1979. [Google Scholar]
- Elttayaf, A.-H.K.; Ajeel, A.E. Kudair, preparation and study the structural and optical properities of CuS nano fil. Int. J. Thin Films Sci. Technol. 2013, 2, 223–231. [Google Scholar] [CrossRef][Green Version]
- Maria, P.; Gutiérrez, H.L.; Patton, J. Thin Film Surface Resistivity. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.528.6542&rep=rep1&type=pdf (accessed on 6 August 2020).
- Mott, N.; Davis, E. Electronic Processes in Non-Crystalline Materials; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Zeyada, H.; El-Nahass, M.; Makhlouf, M. Electronic transport mechanisms in tetraphenyleprophyrin thin film. Curr. Appl. Phys. 2011, 11, 1326–1331. [Google Scholar] [CrossRef]
- Sudha, L.K.; Sukumar, R. Evaluation of Activation Energy (Ea) Profiles of Nanostructured Alumina Polycarbonate Composite Insulation Material. Int. J. Mater. Mech. Manuf. 2014, 2, 96–100. [Google Scholar]
- Munce, C.G. Chemical Bath Deposition of Copper Sulfide Thin Films. Ph.D. Thesis, Griffith University, Queensland, Austraila, July 2008. [Google Scholar]
- Vas-Umnuay, P.; Chih-hung, C. Growth Kinetics of Copper Sulfide Thin Films by Chemical Bath Depositio. ECS J. Solid State Sci. Technol. 2013, 2, 120–129. [Google Scholar] [CrossRef]
- Sangamesha, M.; Pushapalatha, K.; Shekar, G. Effect of concentration on structural and optical properties of CuS thin film. Int. J. Res. Eng. Technol. 2013, 2, 227–234. [Google Scholar]
- Thornton, J.A. The microstructure of sputter-deposited coating. J. Vac. Sci. Technol. 1988, 4, 3059. [Google Scholar] [CrossRef]
- Ali, M.; Samir, H.; Jean, P. Influence of depositionparameters on optical andelectrical properties of CuxS thin films prepared using chemical bath deposition metho. Iraqi J. Appl. Phys. 2008, 4, 1813–2065. [Google Scholar]
- Ilenikhena, P.K. Comparitive Studies of Improved Chemical Bath Deposited Copper Sulphide (CuS) and Zinc Sulphide (ZnS) Thin Films at 320K and Possible Applications. Afr. Phys. Rev. 2008, 2, 59–67. [Google Scholar]
- Kassim, A.; Nagalingam, S.; Tee, T.W.; Koon, K.L. Effect of PH value and electrolyte concentration on the Copper Sulfide thin films prepared by chemical bath deposition method. GUJS 2010, 23, 435–443. [Google Scholar]
- Al-Khayatt, A.H.O.; Jaafer, M.D. Annealing Effect on The Structuraland Optical Properties of CuS Thin Film Prepared by Chemical Bath Deposition (CBD). J. Kufa Phys. 2013, 5, 79–90. [Google Scholar]
- Carrillo Castillo, A.; Ambrosio Lazaro, R.; Lira Ojeda, E.; De la mota Gonzalez, M.; Quevedo Lopez, M.; Moreno Moreno, M.; Gonzalez Diaz, V.; Guerrero Castellanos, J. charactirization of amourphous CuS thin films obtained from fast time and low temperature of deposition. Chalcogenide Lett. 2016, 13, 217–224. [Google Scholar]
- Nemade, K.; Waghuley, S. Band gap engineering of CuS nanoparticles for artificial photosynthesis. Elsevier Mater. Sci. Semicond. Process. 2015, 39, 781–785. [Google Scholar] [CrossRef]
- Ezima, F.; Nnabuchi, M.; Osuji, R. Optical properities of CuS thin films deposited by chemical bath deposition technique and their applications. Trends Appl. Sci. Res. 2006, 1, 467–476. [Google Scholar]
- Min, H.S.; Kassim, A.T.; Weetee, T.; Nagalingham, S. The Effect of Bath Temperature on the Chemical Bath Deposition of Copper Sulphide Thin Films. Jordan J. Chem. 2010, 5, 165–173. [Google Scholar]
- Mamiyev, Z.Q.; Balayeva, N.O. CuS Nanoparticles Synthesized by a Facile Chemical Route Under Different pH Conditions; Elsevier: Amsterdam, The Netherlands, 2016; Volume 26, pp. 235–237. [Google Scholar]
- Ali, M.; Abbas, F. Influence of post-annealing on the properties of CuxS:Al, Fe films deposited by CBD. J. Eng. Technol. 2009, 27, 1112–1150. [Google Scholar]
- Ngahu, K.M. Optical and Electrical Analysis of Cd1-XFeXS/CuS P-N Junction for Solar Cell Application. Master’s Thesis, School of Pure and Applied Sciences of Kenyatta University, Nairobi City, Kenya, November 2016. [Google Scholar]
- Reda, S.; El-Sherbieny, S. Dye-sensitized nanocrystalline CdS and ZnS solar cells with different organic dyes. J. Mater. Res. 2010, 25, 522–528. [Google Scholar] [CrossRef]
- Popovici, I.; Isac, A. Electrical Conductivity Incopper Sulfides—Influence of Thedeposition Parameters Andprecursor’s Concentration; Bulletin of the Transilvania University: Brasov, Romania, 2009; Volume 2. [Google Scholar]
- Shinde, M.; Ahirrao, P.; Patil, I.; Patil, R. Thickness dependent electrical and optical properties of nanocrystalline copper Sulphide thin films grown by simple chemical route. Indian J. Pure Appl. Phys. 2012, 50, 657–660. [Google Scholar]
- Sabah, F.A.; Ahmed, N.M.; Hassan, Z.; Rasheed, H.S. Effect of Annealing on the Electrical Properties of CuxS Thin Films. Procedia Chem. Elsevier 2016, 19, 15–20. [Google Scholar] [CrossRef]
- Santos, J.; Cruz, S.; Mayeb Hernandez, F.; Paraguay Delgado, O.; Zelaya Angel, R.; Castanedo, P.; Torres Delgado, G. Optical and Electrical Properties of Thin Films of CuS Nanodisks Ensembles Annealed in a Vacuum and Their Photocatalytic Activity. Int. J. Photoenergy 2013, 4, 1–9. [Google Scholar] [CrossRef]





| Bath No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| CuSO4 (M) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1, 0.15, 0.2, 0.5 | 0.15 | 0.15 | 0.15 |
| C4H6O6 (M) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1, 0.15, 0.2, 0.5 | 0.2 | 0.2 |
| CS(NH2)2 (M) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1, 0.15, 0.2, 0.5 | 0.1 |
| pH | 10 | 10 | 10 | 10 | 10 | 8–12 step 1 | 11 | 11 | 11 | 11 |
| t (min) | 5 | 10 | 15 | 20 | 25 | 20 | 20 | 20 | 20 | 20 |
| T (°C) | 50–90 step 10 | 50–90 step 10 | 50–90 step 10 | 50–90 step 10 | 50–90 step 10 | 80° | 80° | 80° | 80° | 80° |
| T (°C) | ρ (Ω·cm) | t (min) | ρ (Ω·cm) |
|---|---|---|---|
| 80 | 15.61 | 10 | 21.74 |
| 90 | 30.81 | 20 | 15.6 |
| pH | ρ (Ω·cm) | Molarity of CuSO4 (M) | ρ (Ω·cm) | Molarity of SC(NH2)2 (M) | ρ (Ω·cm) | Molarity of C4H6O6 (M) | ρ (Ω·cm) |
|---|---|---|---|---|---|---|---|
| 9 | 1.33 | 0.15 | 0.70 | 0.15 | 2189.79 | 0.1 | 0.78 |
| 10 | 13.96 | 0.2 | 2.41 | 0.2 | 0.78 | 0.5 | 1795.19 |
| T (°C) | Ea (eV) | Time (min) | Ea (eV) |
|---|---|---|---|
| 80 | 0.0091 | 10 | 0.012 |
| 90 | 0.0032 | 20 | 0.0091 |
| pH | Ea (eV) | Molarity of CuSO4 (M) | Ea (eV) | Molarity of SC(NH2)2 (M) | Ea (eV) | Molarity of C4H6O6 (M) | Ea (eV) |
|---|---|---|---|---|---|---|---|
| 9 | 0.018 | 0.15 | 0.015 | 0.15 | 0.058 | 0.1 | 0.016 |
| 10 | 0.0091 | 0.2 | 0.006 | 0.2 | 0.016 | 0.5 | 0.078 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, K.A.; Ahmed, S.M.; Mohammed, R.Y. Investigation of Structure, Optical, and Electrical Properties of CuS Thin Films by CBD Technique. Crystals 2020, 10, 684. https://doi.org/10.3390/cryst10080684
Mohammed KA, Ahmed SM, Mohammed RY. Investigation of Structure, Optical, and Electrical Properties of CuS Thin Films by CBD Technique. Crystals. 2020; 10(8):684. https://doi.org/10.3390/cryst10080684
Chicago/Turabian StyleMohammed, Khozik Ahmed, Sabah M. Ahmed, and Raghad Y. Mohammed. 2020. "Investigation of Structure, Optical, and Electrical Properties of CuS Thin Films by CBD Technique" Crystals 10, no. 8: 684. https://doi.org/10.3390/cryst10080684
APA StyleMohammed, K. A., Ahmed, S. M., & Mohammed, R. Y. (2020). Investigation of Structure, Optical, and Electrical Properties of CuS Thin Films by CBD Technique. Crystals, 10(8), 684. https://doi.org/10.3390/cryst10080684







