Abstract
Benzylsulfanyl-triazolyl-indole scaffold was synthesized through coupling of 4-amino-5-(1H-indol-2-yl)-1,2,4-triazol-3(2H)-thione and benzyl bromide in EtOH under basic conditions (K2CO3). The benzylation direction was deduced from the 13C NMR signal found at 35.09 ppm, assigned for the methylene carbon of the benzyl group, this value indicates that the benzyl group attacks sulfur, not nitrogen. 1H NMR, 13C NMR, COSY, HMQC, HRMS and X-ray single crystal diffraction analysis were used for structure assignment. The desired compound accomplished in good yield. Hirshfeld analysis revealed the importance of the short N...H (1.994–2.595 Ǻ), S…H (2.282 Ǻ) and C…H (2.670 Ǻ) contacts as well as the weak π-π stacking interactions in the molecular packing of benzylthio-triazolyl-indole scaffold. Its electronic and structural aspects were predicted using density functional theory (DFT) calculations and the reactivity descriptors as well. The Uv-Vis spectral bands were assigned based on the time-dependant density functional theory TD-DFT calculations, while the gauge-including atomic orbitals (GIAO) method was used to predict the 1H and 13C NMR chemical shifts.
1. Introduction
The 1,2,4-triazole scaffold is a remarkable ring in the field of medicinal chemistry and synthon for many molecules with high importance in pharmaceutical field [1,2,3,4,5]. Many drugs on the market are decorated with the triazole motif. Letrozole (anti-cancer agent), Fluconazole (anti-fungal agent), and Maraviroc (Anti-HIV agent) are common drugs bearing the triazole motif. The introduction of thiol and amino groups into positions 3 and 4 of 1,2,4-triazole core structure make this scaffold highly desirable, because it can provide a divergent in the molecular complexity with promising medicinal targets [6,7] such as antifungal, [8] antimicrobial, [9], corrosion inhibitors [10], and also as a chelating agent with metals for fluorescent applications [11].
One of the most privileged structures known in the field of chemistry is the indole scaffold, which has been proven to be active for cancer treatment [12]. The cytotoxicity of the substituted indole compounds has been widely explored towards many of the human cancer lines, for example, the MOLT-3 and HepG-2 cancer cell lines [13], breast cancer cell lines (MCF-7) [14], parental (HCT15 and MES-SA) promyelocytic leukemic cells [15], and multidrug-resistant (MDR; HCT15/CL02 and MES-SA/DX5) cell lines [16], and other cancer cell lines as well [17,18]. The combination of the indole moiety with variety of other privilege core structure, for example, triazoles, [19], thiazoles [20], and sulfonamides [21], leads to the enhancement of the anticancer activity [22].
In 2013, the Westwell, A.D. group synthesized a series of compounds by attaching position 3- of the indole ring with 4-amino-3-benzylthio-1,2,4-triazole, which have been exhibited a potential pro-apoptotic Bcl-2-inhibitory anticancer activities [23].
On the other hand, Boraei, A.T.A. and his group focused on linking the substituted 1,2,4-triazoles to position 2- of the indole moiety. The new entities proved to be active towards MCF-7 and HEPG-2 cancer cell with IC50 = 4.53 μg/mL and 3.58 μg/mL, respectively, compared with the positive control doxorubicin (IC50 4.0 μg/mL) which can possibly act as EGFR and Akt inhibitors [24]. The group has been reported a series of compounds having both scaffold indoles and triazole moieties as a representative example, which proved to act as PARP-1 inhibitors [25]. Zhang, X-R. and his co-workers, in 2008, reported the X-ray single crystal of 3-benzyl-sulfanyl-5-(4-phenyl-1H-1,2,3-triazol-1-ylmeth-yl)-4H-1,2,4-triazol-4-amine [26].
Building on the findings mentioned above, and continuing in our research program [27,28,29,30,31], we have reported the synthesis of a new S-benzylated compound based on the indole and 1,2,4-triazole moieties. The new hit structure was assigned based on the nuclear magnetic resonance and X-ray diffraction analyses. Hirshfeld analysis and density functional theory (DFT) study were also explored.
2. Materials and Methods
2.1. General
Melting points are uncorrected and measured using a melting-point apparatus (SMP10) in open capillaries. The progress of the reaction was observed by thin layer chromatography (TLC) using ethyl acetate/n-hexane 1:1 as eluent. 1H NMR and 13C NMR and 2D NMR spectra were recorded using a Brucker 300 MHz spectrometer in DMSO-d6 using TMS as internal standard. Mass spectra were recorded on JMS–600H JEOL spectrometer. λmax was measured using T90 + UV/VIS spectrometer. All software employed in this study, including X-ray diffraction analysis, Hirshfeld surface analysis, and computational methods, are described in the Supplementary Materials. Starting material 1 was synthesized in our laboratory at Suez Canal University (Ismailia, Egypt), benzyl bromide, K2CO3 and ethanol were purchased from Merck (Munich, Germany).
2.2. S-Benzylation Method for the Synthesis of 3-(Benzylsulfanyl)-5-(1H-Indol-2-yl)-4H-1,2,4-Triazol-4-Amine 2
A mixture of 4-amino-5-(1H-indol-2-yl)-1,2,4-triazol-3(2H)-thione 1 (1.0 mmol, 0.23 g) and K2CO3 (1.2 mmol, 0.17 g) in abs. ethanol 10 mL was stirred at room temperature for 1 h, then the benzyl bromide (1.2 mmol, 0.19 g) was added and stirring was continued overnight, the progress of the reaction was monitored using thin layer chromatography until 1 is completely consumed. The solvent was removed under vacuum, cold water was added and the precipitate was collected by filtration, dried and recrystallized from ethanol.
Yield: 87%, m.p. 270–271 °C; 1H NMR (DMSO-d6, 300 MHz) δ 4.45 (s, 2 H, SCH2Ph), 6.21 (s, 2 H, NH2), 7.03 (dd, 1 H, J4,5 7.8, J5,6 7.2 Hz, H-5indole), 7.16 (dd, 1H, J5,6 7.2, J6,7 7.8 Hz, H-6indole), 7.23–7.35 (3, 4 H, H-3indole + 3HPh), 7.45 (d, 3 H, J 7.8 Hz, H-7indole + 2HPh), 7.60 (d, 1H, J4,5 7.8 Hz, H-4indole), 11.74 (s, 1H, NHindole); 13C NMR (DMSO-d6, 75 MHz) δ 35.09 (SCH2Ph), 102.34 (C-3indole), 111.85 (C-7indole), 119.62 (C-5indole), 120.76 (C-4indole), 122.83 (C-6indole), 123.98 (C-2indole), 127.39 (CHp-Ph), 127.60 (C-3aindole), 128.44 (2CHPh), 129.04 (2CHPh), 136.51 (C-7aindole), 137.45 (CPh), 149.31 (Ctriazole-indole), 152.85 (Ctriazol-s-Bn); LREIMS m/z (Int.%): 65.1 (15.2%), 89.1 (11.8%), 90.2 (6.9%), 91.1 (100%), 106.2 (29%), 142.1 (29.6%), 143.2 (26.3%), 321.2 (28.6% M+), 322.2 (6.8% M+1); HRMS (EI) calcd for C17H15N5S (M+): 321.1048. Found: 321.1055.
3. Results and Discussion
3.1. Synthesis of the Target Compound and Structural Elucidation
4-Amino-5-(1H-indol-2-yl)-1,2,4-triazol-3(2H)-thione 1 was synthesized, according to the reported procedures in [32], and reacted with benzyl bromide in the presence of K2CO3 in EtOH and stirring overnight. Coupling was explored that it proceed at sulfur to give 3-(benzylsulfanyl)-5-(1H-indol-2-yl)-4H-1,2,4-triazol-4-amine 2 (Scheme 1). The compound 2 was obtained in a pure form after recrystallization. The chemical feature of the S-benzylated compound has been assigned based on NMR and X-ray diffraction analysis.
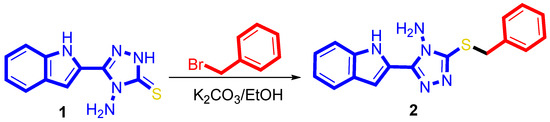
Scheme 1.
Synthesis of the 3-(benzylthio)-5-(1H-indol-2-yl)-4H-1,2,4-triazol-4-amine, 2.
The structure of 3-(benzylsulfanyl)-5-(1H-indol-2-yl)-4H-1,2,4-triazol-4-amine was confirmed based upon its NMR and mass spectral data. 1H NMR displayed the methylene protons of the benzyl group at 4.45 ppm, the amino group protons appeared at 6.21 ppm, the indole and phenyl CH protons appeared between 7.03 and 7.60 ppm, and the indole NH was found at 11.74 ppm. 13C NMR demonstrated the methylene carbon of the benzyl group at 35.09 ppm, the indole and phenyl CH carbons appeared as eight signals between 102.35 and 129.05 ppm. The quaternary carbons appeared at 123.98, 127.60, 136.51, 137.46, 149.91 and 152.85 ppm. Sulfur not nitrogen alkylation was confirmed from the methylene carbon signal of the benzyl group which was found in 13C NMR at 35.09 ppm. 1H-1H correlation spectroscopy (COSY) was used for assigning the correlation between the vicinal protons, and 2D HMQC showed the correlation between the carbons and directly attached hydrogens (all the spectrum are provided in the Supplementary Information, Figures S1–S6)).
3.2. Structural Features of the Target Compound
The structure of 2 crystallized in monoclinic crystal system and space group C2/c with one molecule per asymmetric unit and Z = 8. The structure details and refinement parameters are listed in Table 1. Tables S1 and S2 (Supplementary data) contains the geometric parameters of 2, as obtained from the X-ray structure. The structure comprised three planar rings which are the indole (ring A), triazole (ring B) and phenyl (ring C) moieties (Figure 1). The two rings A and B are slightly not coplanar, where the angle between the mean planes through them is only 8.2º. In contrast, the two rings B and C are strongly twisted from one another. The angle between their mean planes is 69.4º.

Table 1.
Crystal and refinements data of 2.
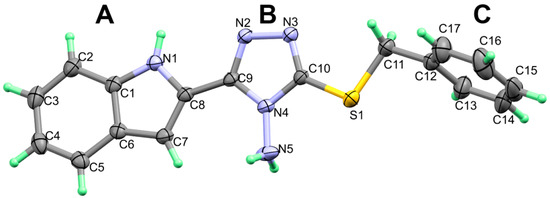
Figure 1.
X-ray structure showing atom numbering and thermal ellipsoids at 30% probability level.
The molecular packing of 2 is controlled mainly by strong N-H…N hydrogen bonds listed in Table 2 and shown in Figure 2A. In addition, there is one N-H…π interaction between one N-H bond of the amino group and carbon atom from phenyl ring in a neighboring molecule with H…C distance of 2.389 Å. The packing of molecular units via strong N-H…N and weak N-H…π interactions is shown in Figure 2B.

Table 2.
Hydrogen-bond geometry (Å).
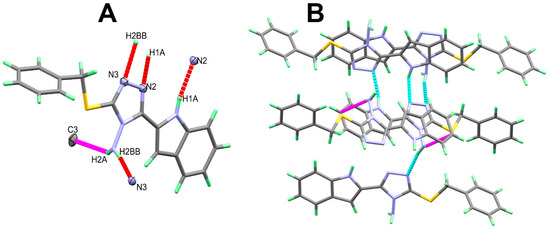
Figure 2.
Intermolecular contacts (A) and packing (B) of molecular units in 2. For clarity, the N-H...π interactions are labelled in magenta dotted lines.
3.3. Hirshfeld Analysis of Molecular Packing
Hirshfeld topology calculations are important to analyze the different intermolecular contacts in the structure of crystalline materials. Additionally, it sheds the light on the significance, strength and percentage of each intermolecular contact. The percentages of different contacts observed in the crystal structure of 2 based on Hirshfeld calculations are shown in Figure 3, while the complete Hirshfeld surfaces are given in Figure S7 (Supplementary Data). The packing of molecules in the crystal is mainly dependant on significant N...H (1.994–2.595 Ǻ) and S…H (2.282 Ǻ) contacts, as well as weak C...H contact with minimum C...H distance of 2.670 Ǻ corresponding to the N-H...π interaction. Presentation of the N…H and S…H hydrogen bonds and the weak C…H interactions mapped over dnorm Hirshfeld surfaces are presented in Figure 4. These interactions contributed by 12.3%, 7.1% and 21.1% from the whole fingerprint area, respectively. Only the N...H, C…H and S...H contacts appeared as red spots in the corresponding dnorm maps, indicating their significance (Figure 4). The contributions of the weaker H...H, C…N and C...C interactions are 48.4%, 4.6% and 6.1%, respectively. These contacts appeared either as white or blue regions, indicating less important interactions.
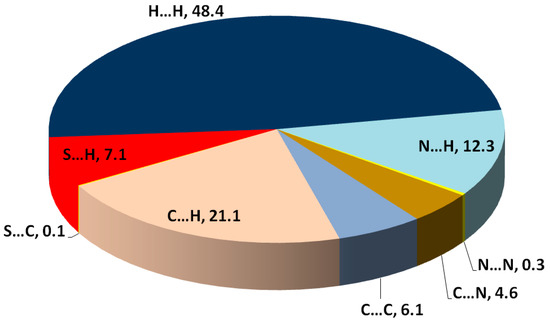
Figure 3.
Summary of the intermolecular interactions contributed in the molecular packing of 2 and their percentages.
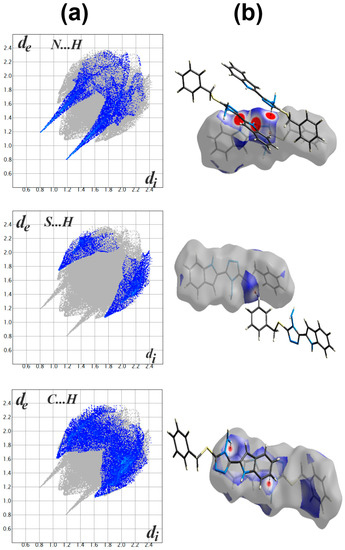
Figure 4.
Fingerprint plot (a) and dnorm surfaces (b) of the N...H, S...H and C…H contacts.
The presence of some C…C (6.1%) and C…N (4.6%) contacts, as well as the blue/red triangles in the shape index Hirshfeld surface (Figure 5), are the main characteristics for the presence of π-π stacking interactions. The shortest C…C and C…N interaction distances are presented in Table 3. Generally, all these contacts are slightly longer than the van der Waals radii sum of the interacting elements, indicating weak π-π contacts.
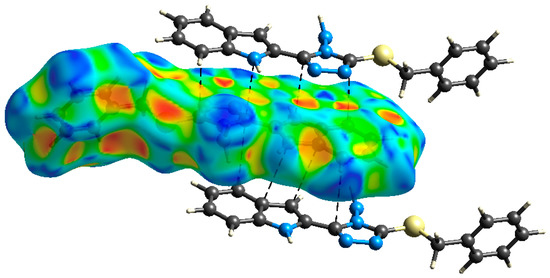
Figure 5.
Shape index map showing the shortest π-π interactions.

Table 3.
Contact distances of the most significant π-π interactions.
DFT studies: the optimized geometry of 2 is presented in Figure 6. Structure matching between the computed molecular geometry with the experimental one is also presented. This structure comparison indicated very well the good agreement between the optimized and X-ray structures. In addition, the very good straight-line relations between the calculated and experimental geometric parameters further confirm this conclusion. The values of correlation coefficients are very close to 1 for both cases (Figure 7). A complete set of bond distances and angles are given in Table S3 (Supplementary Data).
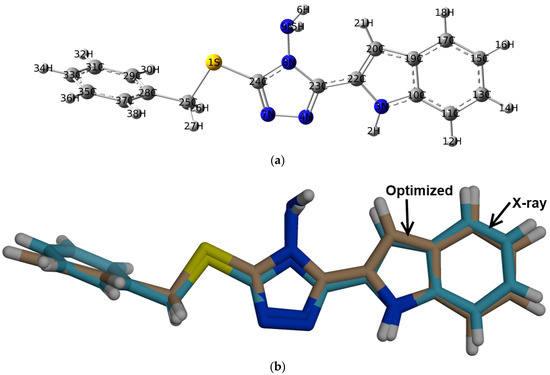
Figure 6.
The optimized geometry (a) and overlay of the optimized (beige) with experimental (blue) structures, (b) for 2.
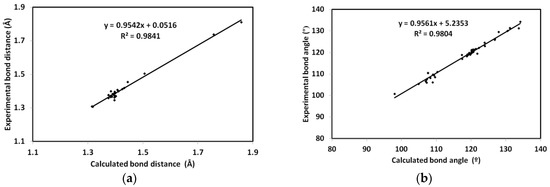
Figure 7.
The straight-line correlations between the calculated and experimental bond distances (a) and angles (b).
The natural charges obtained from the NBO calculations are listed in Table 4. All hydrogen atoms are electropositive with the highest partial charges located at the NH protons. Additionally, the sulphur atom has partial positive charge of 0.3054 e. In contrast, all nitrogen atoms have negative partial charges. The maximum negative charge is located over the amine nitrogen atom. Additionally, all carbon atoms have negative partial charges except those attached directly to the electronegative nitrogen sites. As a result of this charge distribution, the compound is predicted to be polar molecule with calculated dipole moment of 2.2557 Debye. Molecular electrostatic potential (MESP), along with the dipole vector are presented Figure 8.

Table 4.
Natural atomic charges of 2 a.
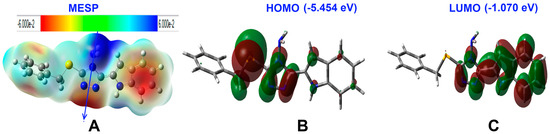
Figure 8.
The molecular electrostatic potential (MESP; (A)), highest occupied (HOMO; (B)) and lowest unoccupied molecular orbitals (LUMO; (C)) of 2.
In addition, the HOMO and LUMO are important for the molecule reactivity [33,34,35,36,37,38,39]. Their energies were calculated to be −5.454 and −1.070 eV, respectively. The energies of these molecular orbitals were used to calculate the reactivity descriptors using Equations (1)–(5).
I = −EHOMO
A = −ELUMO
η = (I − A)/2
μ = −(I + A)/2
ω = μ2/2η
Hence, the calculated ionization potential (I) and electron affinity (A) are 5.454 and 1.070 eV, respectively. Additionally, the hardness (η), electrophilicity index (ω) and chemical potential (μ) are 4.384, 1.213 and −3.262 eV, respectively.
The HOMO is located over the sulphur atom and the triazole π-system. These sites represent the ground state demand for electronic transition to the higher energy level (LUMO). The latter is distributed over the triazole and indole moieties. Hence, the HOMO to LUMO excitation represent mixed n-π* and π-π* transitions. The energy needed for this intermolecular charge transfer is 4.384 eV.
UV-Vis and NMR spectra: The experimental UV-Vis electronic spectra of 2 in ethanol showed absorption bands at 244 and 307 nm, and a shoulder at 295 nm. The assignments of these electronic transitions are presented in Table 5 based on the TD-DFT calculations. The experimental and simulated electronic spectra are shown in Figure 9. The experimentally observed bands were calculated at 232.3 nm (f = 0.248), 305.5 nm (f = 1.020) and 288.8 nm (f = 0.069), respectively, which correspond to HOMO→L+3 (81%), HOMO→LUMO (96%) and H-1→LUMO (92%) transitions, respectively. Presentation of the molecular orbitals involved in these electronic transitions is shown in Figure 10.

Table 5.
Assignment of the electronic spectra of 2.
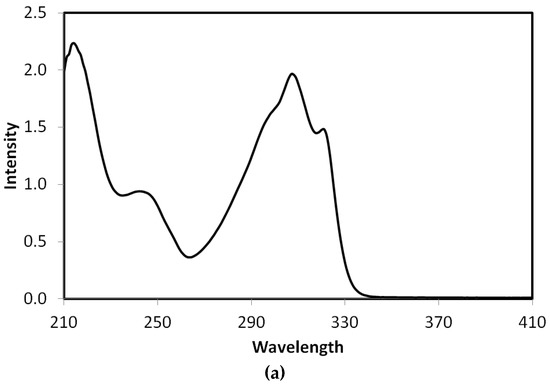
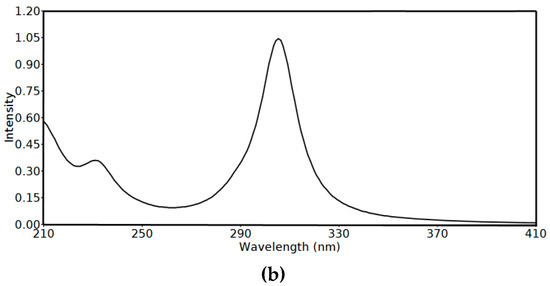
Figure 9.
The experimental (a) and simulated (b) Uv-Vis spectra of 2 using the time-dependant density functional theory (TD-DFT) method.
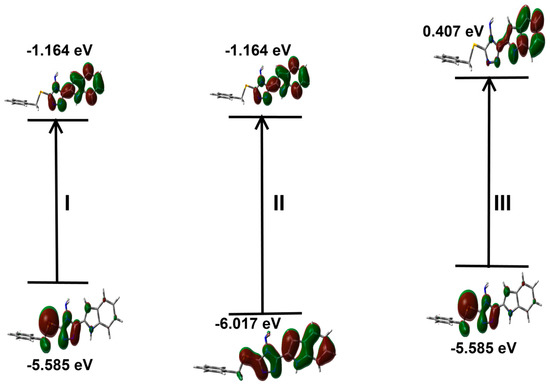
Figure 10.
Presentation of the molecular orbitals included in the electronic transitions of 2 in ethanol as solvent. The definition of I, II and III refer to Table 5.
In addition, the geometry optimization is performed in DMSO as solvent, then the NMR chemical shifts are calculated in the same solvent using TMS as internal standard. The results are summarized in Table S4 (Supplementary Data), in comparison with the experimental data. Moreover, graphical plots of the experimental chemical shifts against the calculated data for protons and carbons are presented in Figure 11. As can be seen from this figure, the straight lines have good correlation coefficients, indicating the good agreement between the calculated and experimental results.
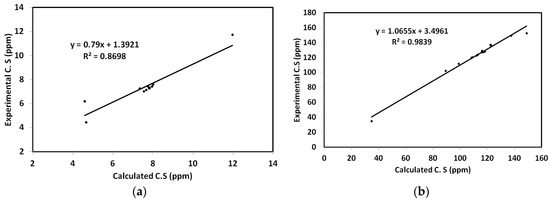
Figure 11.
Correlation graphs between the calculated and experimental 1H (a) and 13C (b) NMR chemical shifts.
NBO analysis: the electron delocalization processes from occupied orbitals to antibonding empty orbitals stabilized the system, due to the conjugation effect [40,41]. The stabilization energies (E(2)) of these electron delocalization processes in 2 are listed in Table 6. The molecule is stabilized by many σ-σ*, π→π*, n→σ* and n→π* IMCT interactions. The σ-σ* and n→σ* IMCT interactions are generally weak, with maximum stabilization of 6.79 and 9.95 kcal/mol for σ(C19-C20)→ σ*C22-C23 and n(N9)→σ*(N8-C23) IMCT interactions, respectively. In contrast, the π→π*, and n→π* are generally stronger, which stabilized the system up to 20.67 and 45.73 kcal/mol for π(C31-C33)→ π*(C28-C29) and n(N8)→ π *(N7-C24) IMCT interactions, respectively.

Table 6.
The E(2) (kcal/mol) values for the intramolecular charge transfer interactions in 2 a.
4. Conclusions
S-Benzylation of 4-amino-5-(1H-indol-2-yl)-1,2,4-triazol-3(2H)-thione was done with benzyl bromide and stirring overnight in the presence of K2CO3 as an acid scavenger in ethanol, which afforded 3-(benzylsulfanyl)-5-(1H-indol-2-yl)-4H-1,2,4-triazol-4-amine in excellent yield. The different interactions affecting the molecular packing in the crystal of 2 were determined using Hirshfeld calculations. The N...H, S…H, C…H and π-π interactions are the most important based on the Hirshfeld analysis. Using TD-DFT calculations, the two Uv-Vis spectral bands at 244 and 307 nm and the shoulder at 295 nm were assigned to be for HOMO→L+3 (81%), HOMO→LUMO (96%) and H-1→ LUMO (92%) transitions, respectively. Additionally, NMR spectra of the compound were matched with the calculated gauge-including atomic orbitals (GIAO) chemical shifts.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/10/8/685/s1. Figure S1: 1H NMR of the target compound; Figure S2: 13C NMR of the target compound; Figure S3: D-COSY of the target compound; Figure S4: DEPT-135 of the target compound; Figure S5: DEPT-90 of the target compound; Figure S6: 2D-HMQC of the target compound; Figure S7: LRMS (EI) of the target compound; Figure S8: Hirshfeld surfaces of 2; Table S1: Bond lengths for 2; Table S2: Bond angles for 2; Table S3: The calculated geometric parameters of the studied compounda; Table S4: The calculated and experimental chemical shifts (ppm) for the studied compound a.
Author Contributions
Conceptualization, A.T.A.B. and A.B.; Data curation, S.M.S. and S.Y.; Formal analysis, S.Y.; Investigation, A.T.A.B.; Methodology, A.T.A.B.; Software, S.M.S.; Visualization, S.M.S. and A.B.; funding acquisition: A.B. Writing—original draft, A.T.A.B., S.M.S. and A.B. All authors approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The research project funded by King Saud University, Researchers Supporting Project Number (RSP-2020/64).
Acknowledgments
The authors would like to extend their sincere appreciation to Researchers Supporting Project Number (RSP-2020/64), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, R.; Dwivedi, A.R.; Kumar, B.; Kumar, V. Recent developments on 1, 2, 4-triazole nucleus in anticancer compounds: A review. Anti-Cancer Agent. Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Foroumadi, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Ayoup, M.S.; Khattab, S.N.; Hassan, S.Y.; Langer, V.; Senior, S.; El Massry, A.M. A regio-and stereo-controlled approach to triazoloquinoxalinyl C-nucleosides. Carbohydr. Res. 2010, 345, 2474–2484. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A Promising Antitubercular Agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Ayoup, M.S.; Ahmed, H.E.A.; El Massry, A.M.; Senior, S.; Khattab, S.N.; Hassan, S.Y.; Amer, A. Synthesis, Docking, and Evaluation of Antimicrob. Activity of a New Series of Acyclo C-Nucleosides of 1,2,4-Triazolo [4, 3-a] quinoxaline Derivatives. J. Heterocycl. Chem. 2016, 53, 153–163. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N. Triazolothiadiazoles and triazolothiadiazines–biologically attractive scaffolds. Eur. J. Med. Chem. 2013, 63, 854–868. [Google Scholar] [CrossRef]
- Kucukguzel, S.G.; Cikla-Suzgun, P. Recent advances bioactive 1, 2, 4-triazole-3-thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; El-Sayed, H.A.; El-Shahat, M.; El-Sayed, A.A.; Darwesh, O.M. Novel triazolothiadiazole and triazolothiadiazine derivatives containing pyridine moiety: Design, synthesis, bactericidal and fungicidal activities. Curr. Bioact. Compd. 2018, 14, 169–179. [Google Scholar] [CrossRef]
- Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Synthesis and biological evaluation of some Schiff bases of 4-amino-5-(4-methylsulfonyl) benzyl-2, 4-dihydro-3H-[1,2,4]-triazole-3-thione. Med. Chem. Res. 2013, 22, 2921–2928. [Google Scholar] [CrossRef]
- Guo, L.; Wu, M.; Leng, S.; Qiang, Y.; Zheng, X. Synergistic effect of purpald with tartaric acid on the corrosion inhibition of mild steel: From electrochemical to theoretical insights. Prot. Met. Phys. Chem. Surf. 2018, 54, 917–925. [Google Scholar] [CrossRef]
- Yan, D.; Xiang, Y.; Li, K.; Chen, Y.; Yang, Z.; Guo, D. Synthesis, characterization and properties of 1, 2, 4-triazolo [3–b][1,3,4] thiadiazole derivatives and their europium complexes. J. Mol. Struct. 2014, 1074, 487–495. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and cytotoxicity of novel 2, 2′-bis-and 2, 2′, 2″-tris-indolylmethanes-based bengacarboline analogs. Arch. Pharm. Res. 2012, 35, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Firestone, G.L.; Bjeldanes, L.F. Bcl-2 family-mediated apoptotic effects of 3, 3′-diindolylmethane (DIM) in human breast cancer cells. Biochem. Pharmacol. 2002, 63, 1085–1097. [Google Scholar] [CrossRef]
- Yoo, M.; Choi, S.-U.; Choi, K.Y.; Yon, G.H.; Chae, J.-C.; Kim, D.; Zylstra, G.; Kim, E. Trisindoline synthesis and anticancer activity. Biochem. Biophys. Res. Commun. 2008, 376, 96–99. [Google Scholar] [CrossRef]
- Rahman, K.W.; Li, Y.; Wang, Z.; Sarkar, S.H.; Sarkar, F.H. Gene expression profiling revealed survivin as a target of 3, 3′-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006, 66, 4952–4960. [Google Scholar] [CrossRef]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis (3′-indolyl) methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef]
- Foderaro, T.A.; Barrows, L.R.; Lassota, P.; Ireland, C.M. Bengacarboline, a New β-Carboline from a Marine Ascidian Didemnum sp. J. Org. Chem. 1997, 62, 6064–6065. [Google Scholar] [CrossRef]
- Nagarsenkar, A.; Prajapti, S.K.; Guggilapu, S.D.; Birineni, S.; Kotapalli, S.S.; Ummanni, R.; Babu, B.N. Investigation of triazole-linked indole and oxindole glycoconjugates as potential anticancer agents: Novel Akt/PKB signaling pathway inhibitors. Med. Chem. Commun. 2016, 7, 646–653. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Bednarczyk-Cwynar, B.; Vasylenko, O.; Kazakova, O.; Zimenkovsky, B.; Zaprutko, L.; Lesyk, R. Synthesis of new potential anticancer agents based on 4-thiazolidinone and oleanane scaffolds. Med. Chem. Res. 2012, 21, 3568–3580. [Google Scholar] [CrossRef]
- Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and structure–activity relationship of mono-indole-, bis-indole-, and tris-indole-based sulfonamides as potential anticancer agents. Mol. Divers. 2013, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.J.; Bao, C.J.; Tao, X.; Wang, J.; Atoyan, R.; Qu, H.; Wang, D.G.; Yin, L.; Samson, M.; Forrester, J.; et al. CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity. Cancer Res. 2010, 70, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Ziedan, N.; Ali, S.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 3-(benzylthio)-5-(1H-indol-3-yl)-1,2,4-triazol-4-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.A.; Gomaa, M.S.; El Sayed, E.S.H.; Duerkop, A. Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur. J. Med. Chem. 2017, 125, 360–371. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Singh, P.K.; Sechi, M.; Satta, S. Discovery of novel functionalized 1, 2, 4-triazoles as PARP-1 inhibitors in breast cancer: Design, synthesis and antitumor activity evaluation. Eur. J. Med. Chem. 2019, 182, 111621. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.-Z.; Zhou, H.-R.; Zhang, X.-R. 3-benzyl-sulfanyl-5-(4-phenyl-1H-1,2,3-triazol-1-ylmeth-yl)-4H-1,2,4-triazol-4-amine. Acta Cryst. 2008, E64, o1611. [Google Scholar]
- Boraei, A.T.A.; Sarhan, A.A.M.; Yousuf, S.; Barakat, A. Synthesis of a New Series of Nitrogen/Sulfur Heterocycles by Linking Four Rings: Indole; 1,2,4-Triazole; Pyridazine; and Quinoxaline. Molecules 2020, 25, 450. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.A.; Boraei, A.T.A.; Barakat, A.; Nafie, M.S. Discovery of hydrazide-based pyridazino [4–b] indole scaffold as a new phosphoinositide 3-kinase (PI3K) inhibitor for breast cancer therapy. RSC Adv. 2020, 10, 19534–19541. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Ghabbour, H.A.; Gomaa, M.S.; El Ashry, E.S.H.; Barakat, A. Synthesis and anti-proliferative assessment of triazolo-thiadiazepine and triazolo-thiadiazine scaffolds. Molecules 2019, 24, 4471. [Google Scholar] [CrossRef]
- Badria, F.A.; Soliman, S.M.; Atef, S.; Islam, M.S.; Al-Majid, A.M.; Dege, N.; Ghabbour, H.A.; Ali, M.; El-Senduny, F.F.; Barakat, A. Anticancer Indole-Based Chalcones: A Structural and Theoretical Analysis. Molecules 2019, 24, 3728. [Google Scholar] [CrossRef]
- Boraei, A.T.; El Ashry, E.S.H.; Barakat, A.; Ghabbour, H.A. Synthesis of new functionalized indoles based on ethyl indol-2-carboxylate. Molecules 2016, 21, 333. [Google Scholar] [CrossRef]
- Boraei, A.T. A new direct synthetic access to 4-Amino-2-N-(glycosyl/propyl)-1,2,4-triazole-3-thiones via hydrazinolysis of 3-N-((Acylated gylcosyl)/allyl)-1,3,4-oxadiazole-2-thiones. Arkivoc 2016, 3, 71–81. [Google Scholar]
- Foresman, J.B.; Frisch, A.E. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Hubert Joe, I.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Pinzaru, S.C. Theoretical and vibrational spectral investigation of sodium salt of acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar]
- Sebastian, S.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).