Effect of Various Nanoparticles (GaF3, ZnF2, Zn(BF4)2 and Ga2O3) Additions on the Activity of CsF-RbF-AlF3 Flux and Mechanical Behavior of Al/Steel Brazed Joints
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
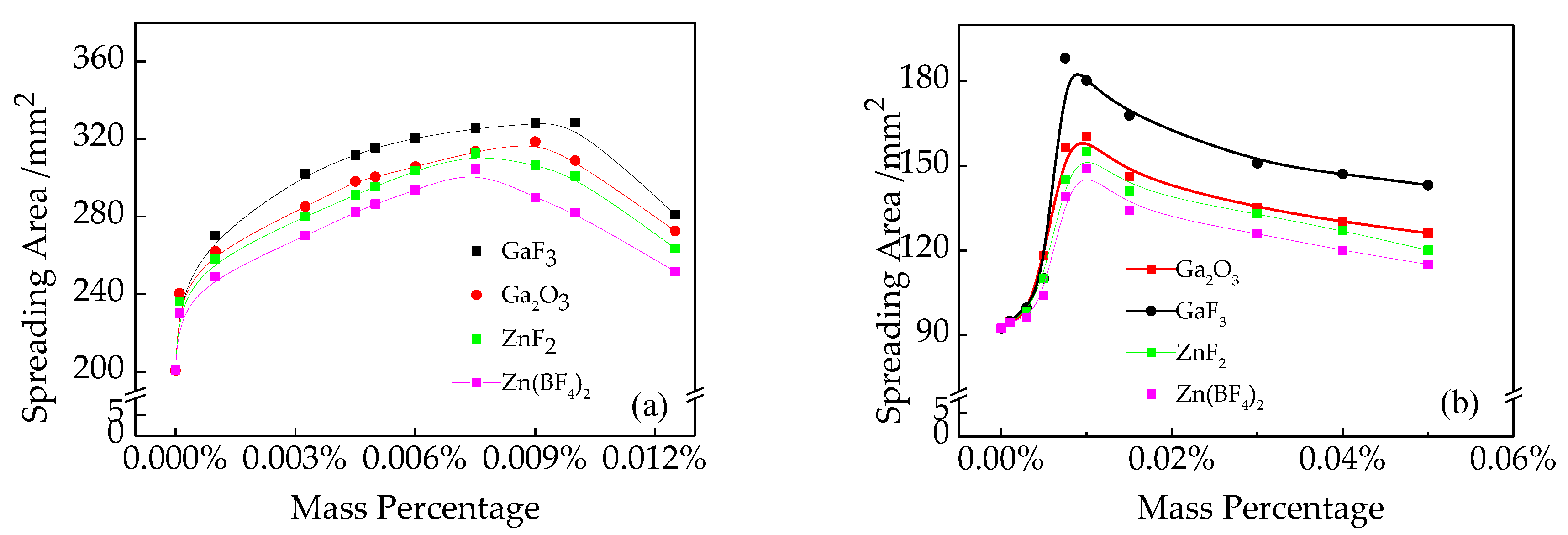
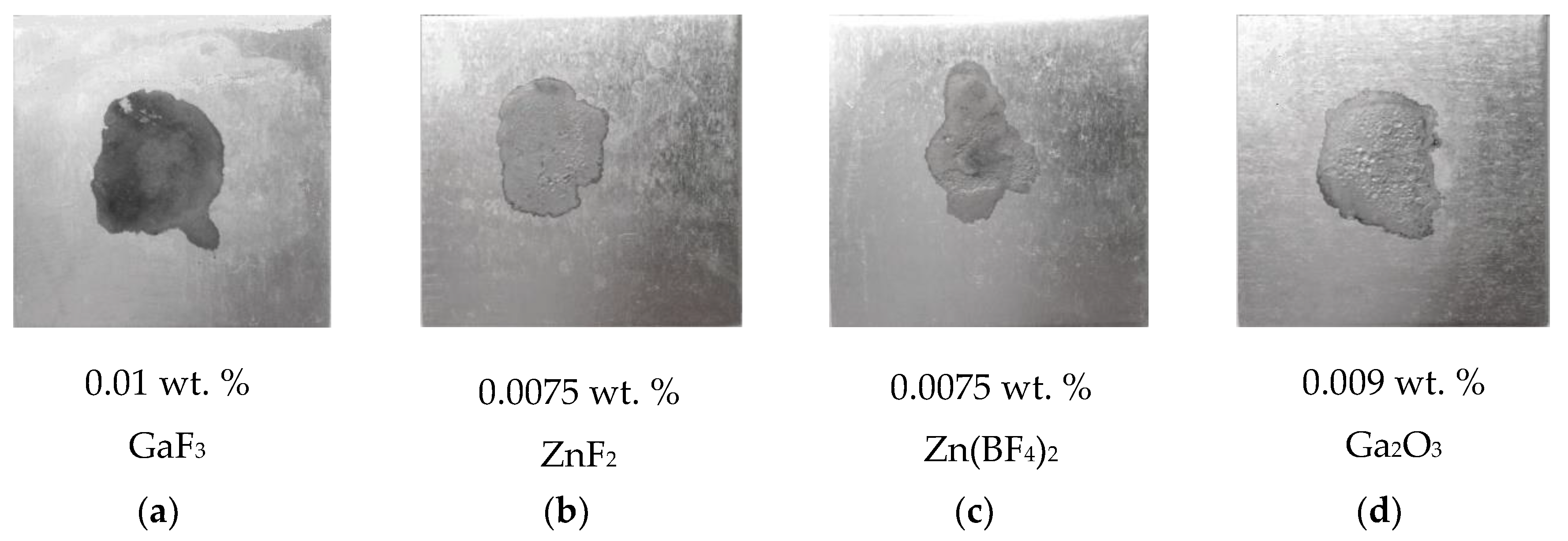
3.1. The Spreadability and Wettability of Zn-15Al Filler Metal
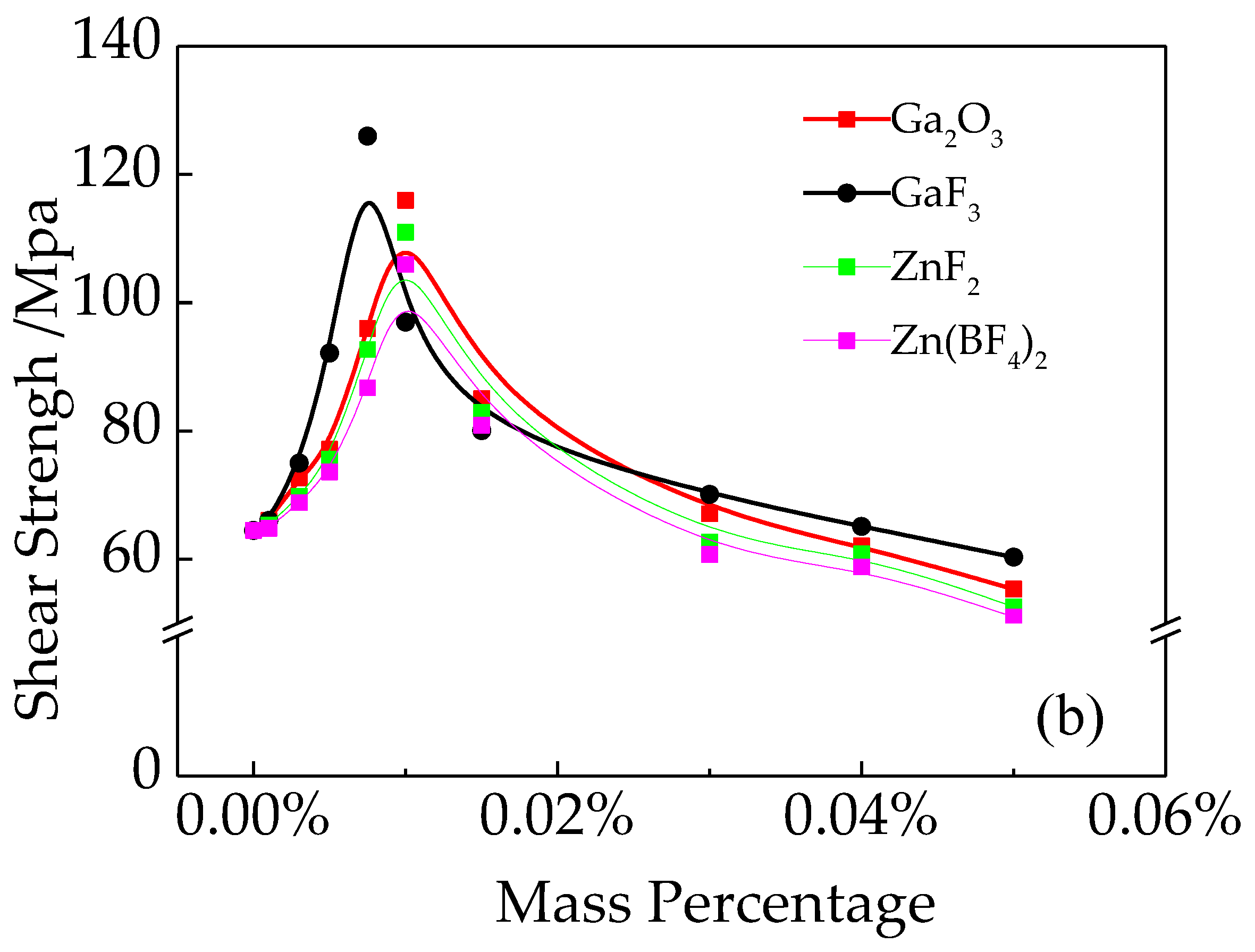
3.2. The Mechanical Properties of Brazed Joints
3.3. Interfacial Effect of Different Particles
3.3.1. Effect of Zn(BF4)2
3.3.2. Effect of ZnF2
3.3.3. Effect of Ga2O3
3.3.4. Effect of GaF3
4. Conclusions
- (1)
- The spreading tests indicated that the spreadability of the Zn-15Al filler metal both on Q235 steel and AA6061 alloy was promoted with a CsF-RbF-AlF3 flux doped with GaF3, ZnF2, Zn(BF4)2 and Ga2O3, and the performance of GaF3 was the best. The suitable ranges of GaF3, ZnF2, Zn(BF4)2 and Ga2O3 respectively were 0.0075–0.01 wt.%, 0.0075–0.01 wt.%, 0.0075–0.01 wt.% and 0.009–0.01 wt.%.
- (2)
- The shear strength of the brazed joints reached its peak at 126 MPa, when 0.075 wt.% GaF3 was added. The second-best performance was Ga2O3. At the same time, Zn(BF4)2 was inferior to others.
- (3)
- The fluxes doped with GaF3 were competent in removing oxides of the base metal and decreasing the interfacial tension, in virtue of the activity of Ga3+, which has a “skin effect”, as well as F−. In consequence, the activity of the CsF-RbF-AlF3 doped with GaF3 was better than that of others.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, P.; Lei, Z.L.; Zhang, X.R.; Liu, J.G.; Chen, Y.B. Effects of laser power on the interfacial intermetallic compounds and mechanical properties of dual-spot laser welded–brazed Ti/Al butt joint. Opt. Laser Technol. 2020, 124, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.S.; Zhang, Y.S.; Senkara, J.; Li, G.Y.; Ma, M.T. Current research and challenges in innovative technology of joining dissimilar materials for electric vehicles. Mater. Rep. 2019, 33, 3853–3861. [Google Scholar]
- Xue, S.B.; Wang, B.; Zhang, L.; Long, W.M. Development of green welding technology in China during the past decade. Mater. Rep. 2019, 33, 2813–2830. [Google Scholar]
- He, P.; Wang, H.Q.; Feng, J.C.; Qian, Y.Y. Optimization of induction coil of high frequency brazing broad plane aluminium to stainless steel. Weld. Join. 2005, 5, 20–23. [Google Scholar]
- Matsuda, T.; Sano, T.; Munekane, M.; Ohata, M.; Hirose, S. Microscale tensile test of galvannealed steel/aluminum dissimilar joint for estimation of intrinsic interfacial strength. Scr. Mater. 2020, 186, 196–201. [Google Scholar] [CrossRef]
- He, P.; Lin, P.P. The systematic project involving brazes development and intelligent brazing technology innovation: A materials genome perspective. Mater. Rep. 2019, 01, 156–161. [Google Scholar]
- El-Sayed, M.H.; Naka, M. Structure and properties of carbon steel–aluminium dissimilar joints. Sci. Technol. Weld. Join. 2005, 10, 27–31. [Google Scholar] [CrossRef]
- Yang, J.L.; Xue, S.B.; Zhang, J.X. Development of novel CsF–RbF–AlF3 flux for brazing aluminum to stainless steel with Zn–Al filler metal. Mater. Des. 2014, 64, 110–115. [Google Scholar] [CrossRef]
- Dong, H.G.; Yang, L.Q.; Zhai, N.; Dong, C. Analysis on microstructure and mechanical properties of aluminum alloy/stainless steel joint made with flux-cored filler metal. Trans. China Weld. Inst. 2011, 32, 1–4. [Google Scholar]
- He, P.; Jiao, Z.; Wang, J.; Lin, T.S. Research and application of joining technology at nanometer scale. Trans. China Weld. Inst. 2013, 34, 109–112. [Google Scholar]
- Yao, Z.; Xue, S.B.; Yang, J.L.; Zhang, J.X. Inducing the effect of a Ga2O3 nano-particle on the CsF-RbF-AlF3 flux for brazing aluminum to carbon steels. Crystals 2020, 10, 183. [Google Scholar] [CrossRef]
- Yao, Z.; Xue, S.B.; Zhang, J.X. Comparative study on the activity of GaF3 and Ga2O3 nanoparticle-doped CsF-AlF3 flux for brazing 6061 Al/Q235 steel joints. Crystals 2020, 10, 498. [Google Scholar] [CrossRef]
- Zhang, L.J. Investigation to fluoride brazing flux used for aluminium brazing. Weld. Join. 1996, 6, 7–8. [Google Scholar]
- Cheng, F.J.; Qi, S.M.; Yang, Z.W.; Yao, J.F.; Zhao, H. Self-brazing mechanism of aluminum alloy at medium temperature. J. Mater. Eng. 2018, 46, 31–36. [Google Scholar]
- Zhang, B.F.; Chen, Q.; Chen, X.W.; Zhang, H.L. Effect of KBF4 addition on the microstructure of Mg-6Zn-1Si alloy. Adv. Mater. Res. 2012, 535, 996–999. [Google Scholar] [CrossRef]
- Xiao, D.F.; Chen, D.H.; Qian, Y.Y.; Mao, J.Y.; Fan, F.H.; Yan, J. Study on corrosion resistance of aluminum soldering joint. Weld. Join. 1985, 09, 3–6. [Google Scholar]
- Zhang, Q.Y.; Liu, S.Q.; Lan, T.; Fu, Y.C. Interficial activity behavior of flux during aluminum brazing. Acta Metall. Sin. 1989, 4, 137–141. [Google Scholar]
- Zhang, J.X.; Xue, S.B.; Xue, P.; Yang, J.L.; Lv, Z.P. Effect of Ga2O3 on the wettability and spreadability of CsF-AlF3 flux/Zn-Al filler metal on the aluminum and steel. Rare Met. Mater. Eng. 2017, 7, 156–160. [Google Scholar]





| Alloy | Mg | Si | Cu | Cr | Mn | Zn | Al |
|---|---|---|---|---|---|---|---|
| 6061 | 1.10 | 0.61 | 0.25 | 0.12 | 0.01 | 0.01 | Bal. |
| Alloy | C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|---|
| Q235 | 0.18 | 0.48 | 0.30 | 0.04 | 0.04 | Bal. |
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | 0 | 0.0001 | 0.001 | 0.00325 | 0.0045 | 0.005 | 0.006 | 0.0075 | 0.009 | 0.01 | 0.0125 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.; Xue, S.; Zhang, J. Effect of Various Nanoparticles (GaF3, ZnF2, Zn(BF4)2 and Ga2O3) Additions on the Activity of CsF-RbF-AlF3 Flux and Mechanical Behavior of Al/Steel Brazed Joints. Crystals 2020, 10, 683. https://doi.org/10.3390/cryst10080683
Yao Z, Xue S, Zhang J. Effect of Various Nanoparticles (GaF3, ZnF2, Zn(BF4)2 and Ga2O3) Additions on the Activity of CsF-RbF-AlF3 Flux and Mechanical Behavior of Al/Steel Brazed Joints. Crystals. 2020; 10(8):683. https://doi.org/10.3390/cryst10080683
Chicago/Turabian StyleYao, Zhen, Songbai Xue, and Junxiong Zhang. 2020. "Effect of Various Nanoparticles (GaF3, ZnF2, Zn(BF4)2 and Ga2O3) Additions on the Activity of CsF-RbF-AlF3 Flux and Mechanical Behavior of Al/Steel Brazed Joints" Crystals 10, no. 8: 683. https://doi.org/10.3390/cryst10080683
APA StyleYao, Z., Xue, S., & Zhang, J. (2020). Effect of Various Nanoparticles (GaF3, ZnF2, Zn(BF4)2 and Ga2O3) Additions on the Activity of CsF-RbF-AlF3 Flux and Mechanical Behavior of Al/Steel Brazed Joints. Crystals, 10(8), 683. https://doi.org/10.3390/cryst10080683






