Biocrystals in Plants: A Short Review on Biomineralization Processes and the Role of Phototropins into the Uptake of Calcium
Abstract
1. Introduction
2. Calcium Oxalate Crystals Synthesized in Plants
2.1. Soluble Crystals: Oxalic Acid and Sodium, Potassium, and Ammonium Oxalate
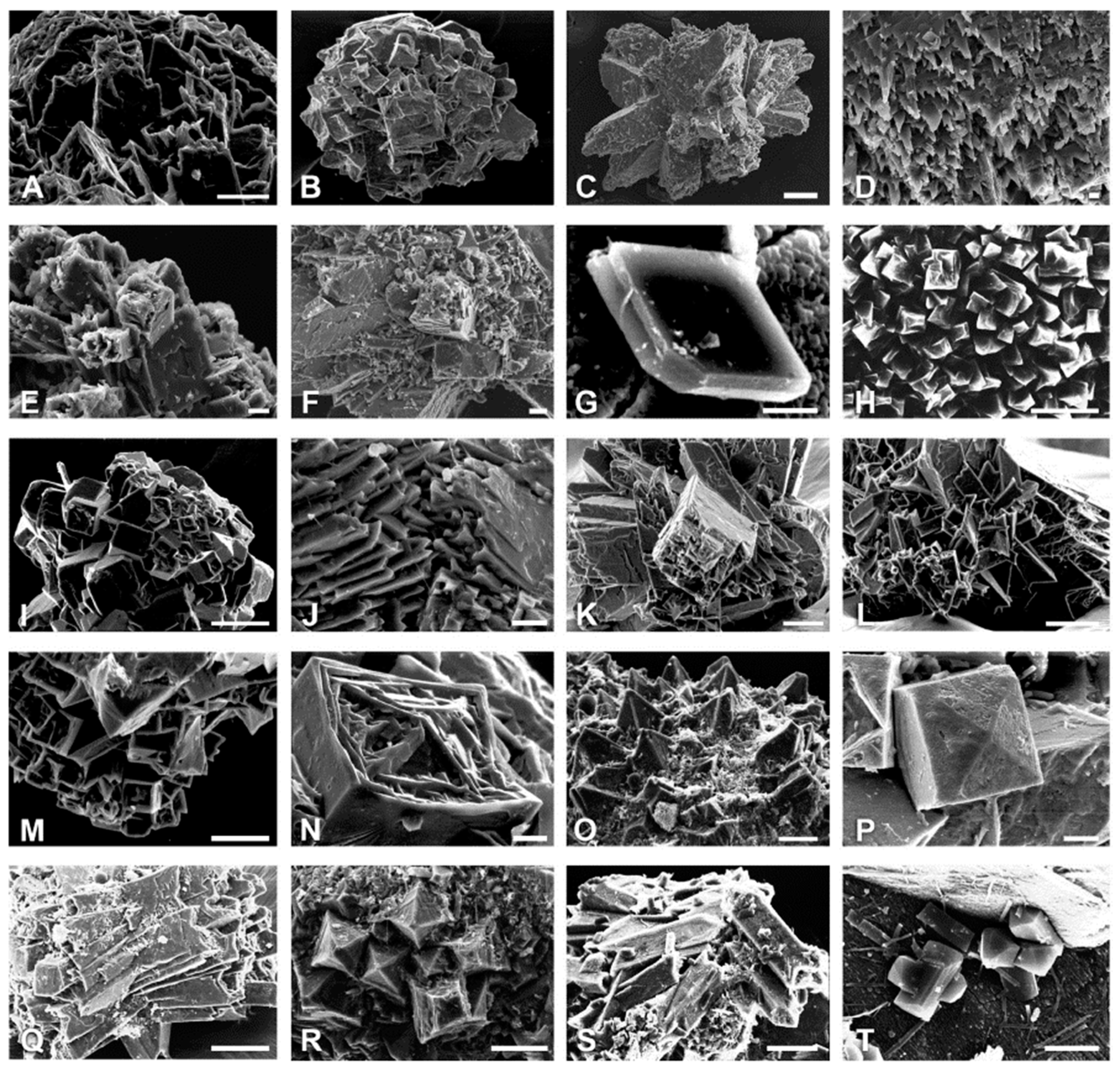
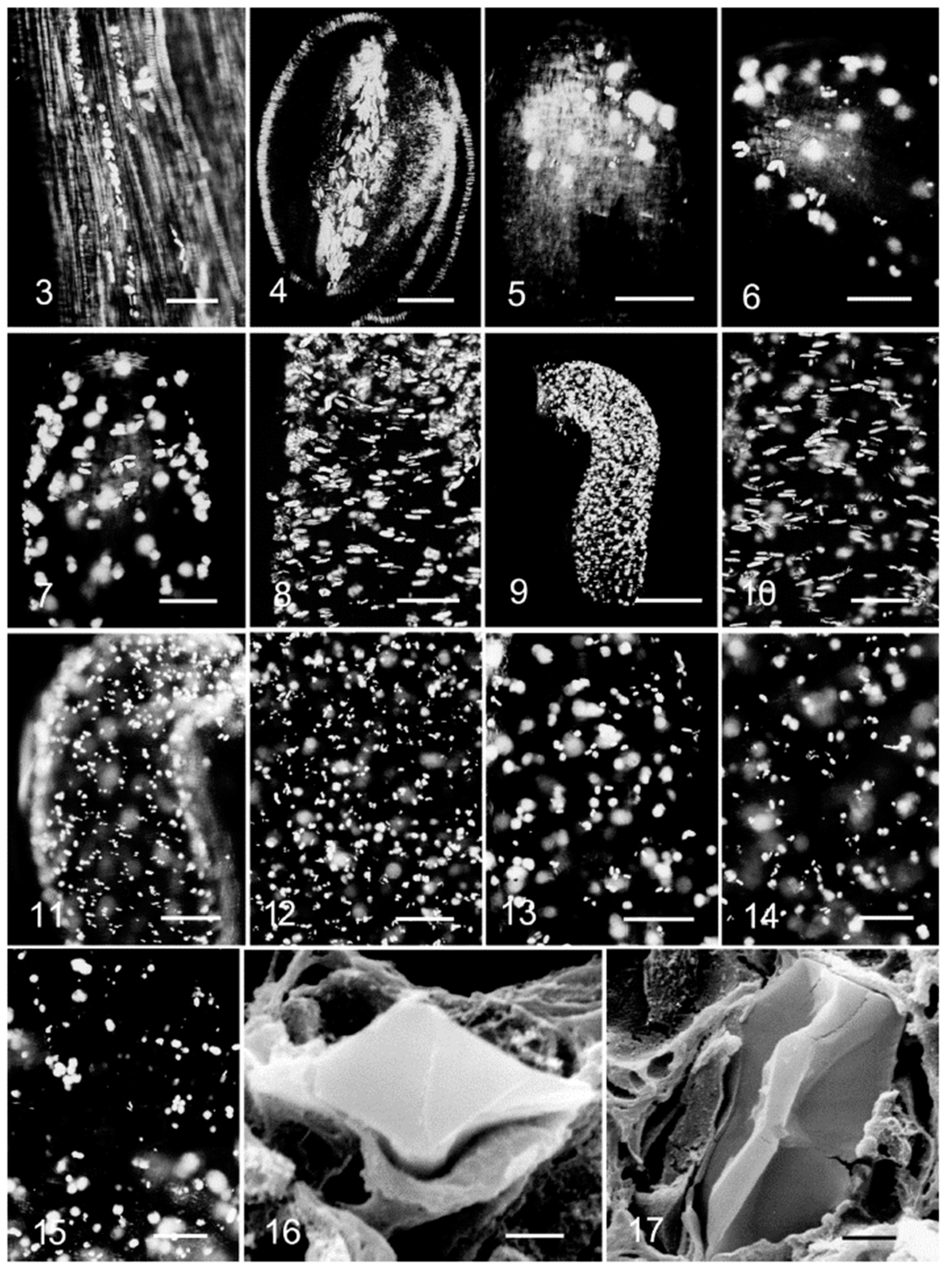
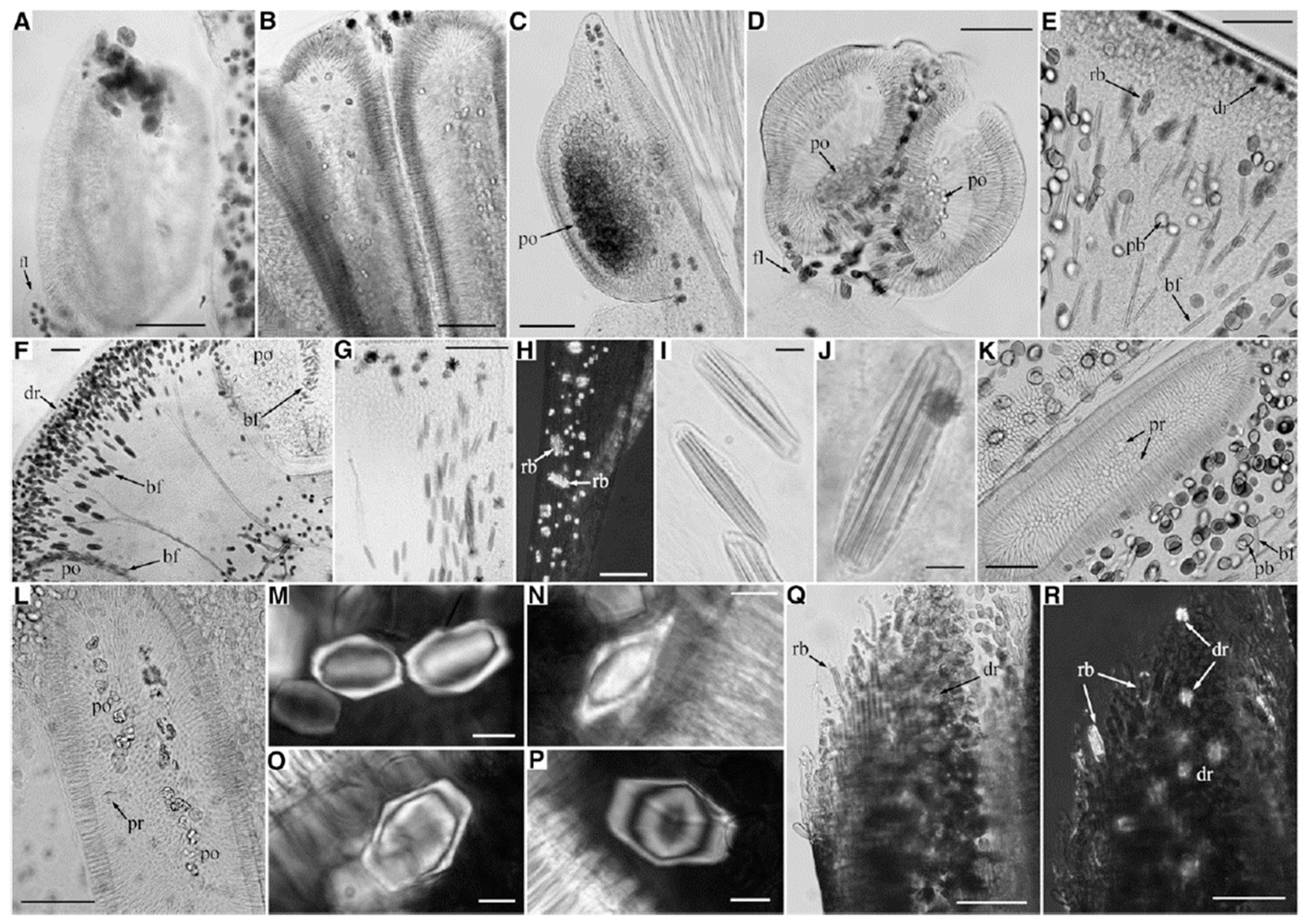
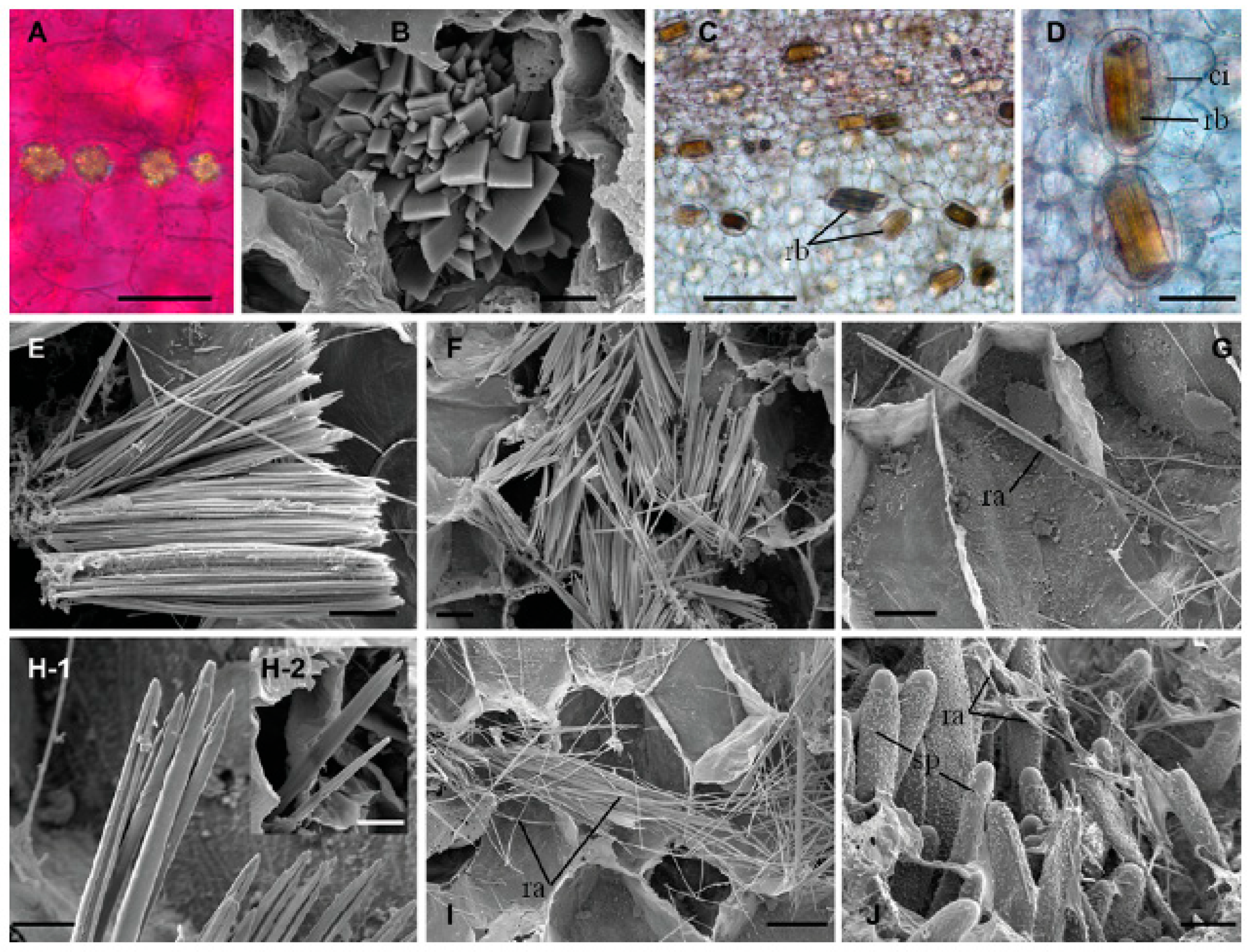
2.2. Insoluble Oxalates: Calcium Oxalate
2.2.1. The Cactaceae Family of Plants
2.2.2. Fabaceae or Leguminosae Family
2.2.3. The Gramineae or Poaceae Family
2.2.4. Plants of Human Consumption That Produce Caox
2.2.5. The Araceae Family
3. Morphological Chirality of Calcium Oxalate Crystals and Crystallographic Data
4. The New Trends in Biomineralization Processes, the Role of Photoreceptors into Calcium Uptake, and Their Influence on Growth of Plants and Form
4.1. Phototropins
4.2. The Light-Oxygen-Voltage (LOVs) Domains
4.3. The Flavin Mononucleotide Molecule and the Photocycle
4.4. The Origin and Evolution of Phototropins
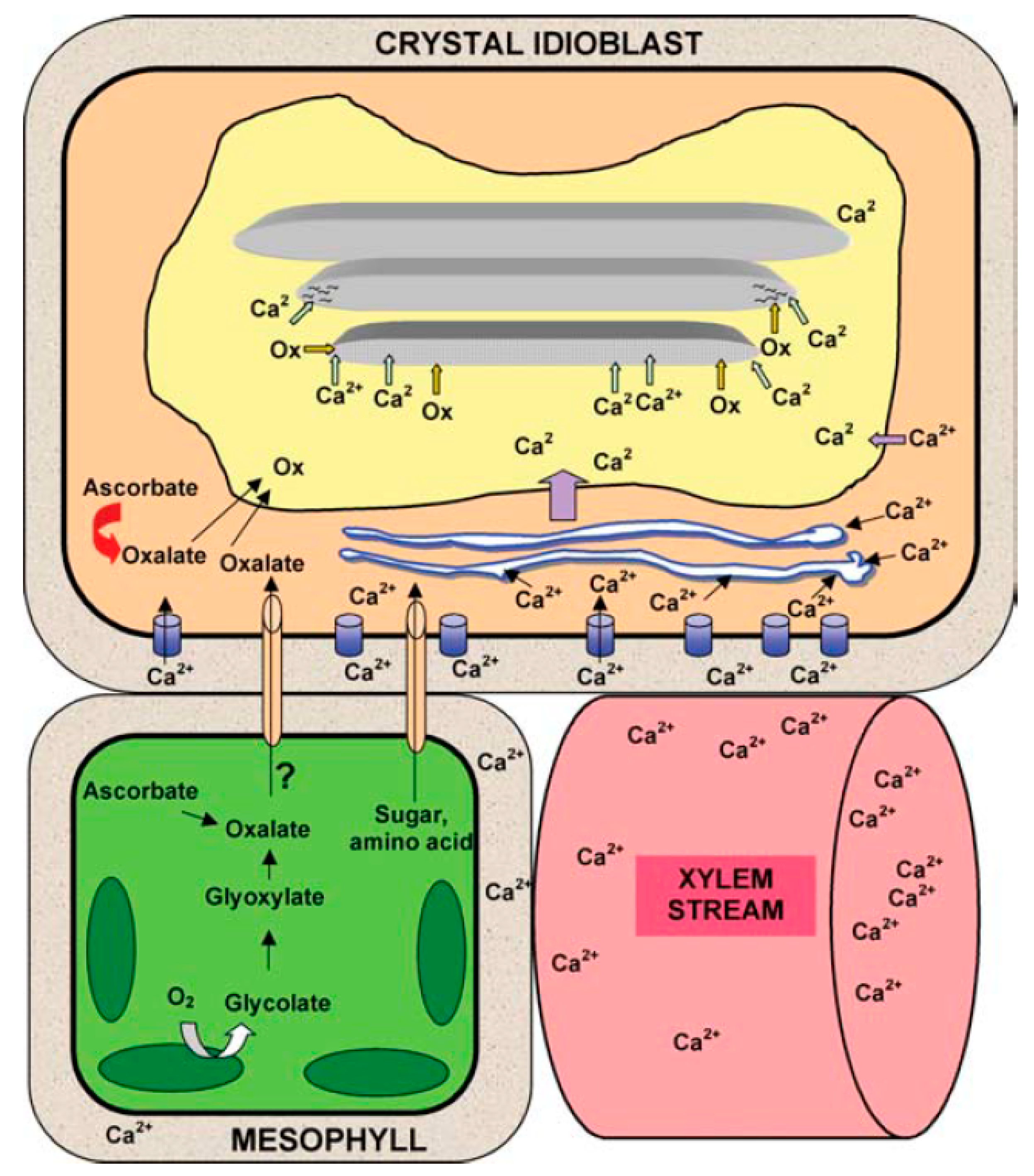
4.5. Calcium Uptake and Its Importance in Plant.Growth and Form
5. Concluding Remark
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernal, J.D. The physical basis of life. Proc. R. Soc. 1949, 257, 635–641. [Google Scholar]
- Wachtershauser, G. Pyrite formation, the first energy source for life: A hypothesis. Syst. Appl. Microbiol. 1988, 10, 207–210. [Google Scholar] [CrossRef]
- Keller, M.; Blochl, E.; Wachtershauser, G.; Stetter, K.O. Formation of amide bonds without a condensation agent: Implications for the origin of life. Nature 1994, 368, 836. [Google Scholar] [CrossRef]
- James, P.F. Mineral catalysis and prebiotic synthesis: Montmorillonite-catalyzed formation of RNA. Elements 2005, 1, 145–149. [Google Scholar]
- Orgel, L.E. Polymerization on the rocks: Theorical introduction. Orig. Life Evol. Biosph. 1998, 28, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gedulin, B.; Arrhenius, G. Sources and geochemical evolution of RNA precursor molecules: The role of phosphate. In Early Life on Earth; Nobel Symposium No. 84; Bengtson, S., Ed.; Columbia U.P.: New York, NY, USA, 1994; pp. 91–106. [Google Scholar]
- Arrhenius, T.; Arrhenius, G.; Paplawsky, W.J. Archean geochemistry of formaldehyde and cyanide and the oligomerization of cyanohydrin. Orig. Life Evol. Biosph. 1994, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pitsch, S.; Eschenmoser, A.; Gedulin, B.; Hui, S.; Arrhenius, G. Mineral induced formation of sugar phosphates. Orig. Life Evol. Biosph. 1995, 25, 294–334. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G. Seven Clues to the Origin of Life; Cambridge University Press: Cambridge, UK, 1985; pp. 74–86. [Google Scholar]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef]
- Dubbels, B.; Dispirito, A.; Morton, J.; Semrau, J.; Neto, J.; Bazylinsky, D. Evidence for a copper-dependent iron transport system in the marine, magnetotactic bacterium strain MV-1. Microbiology 2004, 150, 2931–2945. [Google Scholar] [CrossRef]
- Raschdorf, O.; Müller, F.D.; Pósfai, M.; Plitzko, J.M.; Schüler, D. The magnetosome proteins MamX, MamZ and MamH are involved in redox control of magnetite biomineralization in Magnetospirillum gryphiswaldense. Mol. Microbiol. 2013, 89, 872–886. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.R.; Moreno, A. Obtainment of spherical-shaped calcite crystals induced by intramineral proteins isolated from eggshells of ostrich and emu. Cryst. Growth Des. 2014, 14, 5137–5143. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.R.; Medrano, F.J.; Moreno, A.; Romero, A. Crystal structure of struthiocalcin-1, an intramineral protein from Struthio camelus eggshell, in two crystal forms. Acta Crystallogr. 2015, 71, 809–818. [Google Scholar]
- Reyes-Grajeda, J.P.; Moreno, A.; Romero, A. Crystal structure of ovocleidin-17, a major protein of the calcified Gallus gallus eggshell: Implications in the calcite mineral growth pattern. J. Biol. Chem. 2004, 279, 40876–40881. [Google Scholar] [CrossRef] [PubMed]
- Colfen, H. Biomineralization: A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Histological aspects of the “fixed-particle” model of stone formation: Animal studies. Urolithiasis 2017, 45, 75–87. [Google Scholar] [CrossRef]
- Mann, K. The calcified eggshell matrix proteome of a songbird, the zebra finch (Taeniopygia guttata). Proteome Sci. 2015, 13, 29. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M. Synthesis of inorganic and organic crystals mediated by proteins in different biological organisms. A mechanism of biomineralization conserved throughout evolution in all living species. Prog. Cryst. Growth Charact. Mater. 2017, 63, 94–103. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan shell proteins: Primary structure, origin, and evolution. Curr. Top. Dev. Biol. 2008, 80, 209–276. [Google Scholar]
- Rozycka, M.; Wojtas, M.; Jakob, M.; Stigloher, C.; Grzeszkowiak, M.; Mazur, M.; Ozyhar, A. Intrinsically disordered and pliable starmaker-like protein from medaka (Oryzias latipes) controls the formation of calcium carbonate crystals. PLoS ONE 2014, 9, e114308. [Google Scholar] [CrossRef]
- Xiang, L.; Su, J.; Zheng, G.; Liang, J.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Patterns of expression in the matrix proteins responsible for nucleation and growth of aragonite crystals in flat pearls of Pinctada fucata. PLoS ONE 2013, 8, e66564. [Google Scholar] [CrossRef]
- Gallaher, R.N. The occurrence of calcium in plant tissue as crystals of calcium oxalate. Commun. Soil Sci. Plant Anal. 1975, 6, 315–321. [Google Scholar] [CrossRef]
- Marín-García, L.; Frontana-Uribe, B.A.; Reyes-Grajeda, J.P.; Stojanoff, V.; Serrano-Posada, H.J.; Moreno, A. Chemical Recognition of Carbonate Anions by Proteins Involved in Biomineralization Processes and their Influence on Calcite Growth. Cryst. Growth Des. 2008, 8, 1340–1345. [Google Scholar] [CrossRef]
- Al- Rais, A.H.; Myers, A.; Watson, L. The isolation and properties of oxalate crystals from plants. Ann. Bot. 1971, 35, 1213–1218. [Google Scholar] [CrossRef]
- Fahn, A. Anatomía Vegetal; H. Blume Ediciones: Madrid, Spain, 1978. [Google Scholar]
- Prasad, R.; Singh-Shivay, Y. Oxalic Acid/Oxalates in plants:from self-defence to phytoremediation. Curr. Sci. 2017, 112, 1665–1667. [Google Scholar] [CrossRef]
- Gmelin, L.; Watts, H. Handbook of Chemistry; Cavendish Society: London, UK, 1855; Volume 9, p. 111. [Google Scholar]
- Elejalde-Cadena, N.R.; Cuéllar-Cruz, M.; Moreno, A. The role of silica and alkaline earth metals with biomolecules in the biomineralization processes: The eggshells´s formation and the crystallization in vivo for x-ray crystallography. Prog. Cryst. Growth Charact. Mater. 2020, 66, 100473. [Google Scholar] [CrossRef]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, O. A method for protecting surfaces of stone material or stone-based cultural relics. Chinese Patent No. CN 1330609, 8 August 2007. [Google Scholar]
- Sharma, Y.K.; Gilhotra, U.K. A Review on Urolithiasis. J. Biomed. Pharm. Res. 2019, 8, 50–57. [Google Scholar] [CrossRef]
- Ensikat, H.-J.; Geisler, T.; Weigend, M. A first report of hydroxyapatite as structural biomineral in Loasaceae-plants´teeth against herbovores. Nature Sci. Reports 2016, 6, 26073. [Google Scholar]
- Weigend, M.; Mustafa, A.; Ensikat, H.-J. Calcium Phosphate in Plant Trichomes: The Overlooked Biomineral. Planta 2018, 247, 277–285. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Zakharenko, A.M.; Zemchenko, I.V.; Haider, M.S.; Ali, M.A.; Imtiaz, M.; Chung, G.; Tsatsakis, A.; Sun, S.; Golokhvast, K.S. Phytolith Formation in Plants: From Soil to Cell. Plants 2019, 8, 249. [Google Scholar] [CrossRef]
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and ecological significance of biomineralization in plants. Trends Plant Sci. 2014, 19, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Shimizu, M.H.; Gois, P.H.; Volpini, R.A.; Canale, D.; Luchi, W.M.; Froeder, L.; Heilberg, I.P.; Seguro, A.C. N-acetylcysteine protects against star fruit induced acute kidney injury. Ren. Fail. 2017, 39, 193–202. [Google Scholar] [CrossRef]
- Morton, J.F. Carambola. In Fruits of Warm Climates; Morton, J.F., Ed.; Florida Flair Books: Florida, FL, USA, 1987; pp. 125–128. [Google Scholar]
- Nguimezong, M.B.N.; Foba-Tendo, J.; Yufanyi, D.M.; Etape, E.P.; Eko, J.N.; Ngolui, L.J. Averrhoa carambola: A renewable source of oxalic acid for the facile and green synthesis of divalent metal (Fe, Co, Ni, Zn, and Cu) oxalates and oxide nanoparticles. J. Appl. Chem. 2014, 2014, 767695. [Google Scholar]
- Ananna, M.A.; Hasan, R.; Samad, T.; Rahim, M.A.; Rahman, M.E.; Haque, W.M.; Billah, M.; Hoque, H.F. Acute kidney injury due to star fruit ingestion: A case report. Bangabandhu Sheikh Mujib Med. Univ. J. 2014, 7, 151–153. [Google Scholar] [CrossRef]
- Monje, P.V.; Barán, E.J. Characterization of calcium oxalates generated as biominerals in cacti. Plant Physiology. 2002, 128, 707–713. [Google Scholar] [CrossRef]
- Macnish, A.J.; Irving, D.E.; Joyce, D.C.; Vasanthe, V.A.H.; Wearing, R.I.; Webb, A.; Frost, R.L. Identification of intracellular calcium oxalate crystals in Chamelaucium uncinatum (Myrtaceae). Austral. J. Bot. 2003, 51, 565–572. [Google Scholar] [CrossRef]
- Jáuregui-Zuñiga, D.; Reyes-Grajeda, J.P.; Moreno, A. Modifications on the Morphology of Synthetically grown Calcium Oxalate Crystals by Crystal-associated Proteins Isolated from Bean Seed Coats (Phaseolus vulgaris). Plant Sci. 2005, 168, 1163–1169. [Google Scholar] [CrossRef]
- Hartl, W.P.; Klapper, H.; Barbier, B.; Ensikat, H.J.; Dronskowski, R.; Müller, P.; Ostendorp, G.; Tye, A.; Bauer, R.; Barthlott, W. Diversity of calcium oxalate crystals in Cactaceae. Can. J. Bot. 2007, 85, 501–517. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rivera-Muñoz, E.M.; Gutiérrez-Cortez, E.; Real, A.; Rodríguez-García, M.E. Characterization of crystalline structures in Opuntia ficus-indica. J. Biol. Phys. 2015, 41, 99–112. [Google Scholar] [CrossRef]
- Rojas-Molina, I.; Gutiérrez-Cortez, E.; Bah, M.; Rojas-Molina, A.; Ibarra-Alvarado, C.; Rivera- Muñoz, E.; Del Real, A.; Aguilera-Barreiro, M.A. Characterization of calcium compounds in Opuntia ficus-indica as a source of calcium for human diet. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Webb, M. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell 1999, 11, 751–761. [Google Scholar] [CrossRef][Green Version]
- Franceschi, V.; Horner, H. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Franceschi, V.; Li, L.; Zhang, D.; Okita, T. Calsequestrin-like calcium binding protein is expressed in calcium accumulating cells of Pistia stratiotes. Proc. Nat. Acad. Sci. USA 1993, 90, 6986–6990. [Google Scholar] [CrossRef]
- Volk, G.; Lynch-Holm, V.; Kostman, T.; Goss, L.; Franceschi, R. The Role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biol. 2002, 4, 34–45. [Google Scholar] [CrossRef]
- Keates, S.; Tarlyn, N.; Loewus, F.; Franceschi, V. L-Ascorbic acid and L-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes. Phytochemistry 2000, 53, 433–440. [Google Scholar] [CrossRef]
- Kostman, T.; Tarlyn, N.; Loewus, F.; Franceschi, V. Biosynthesis of L-ascorbic acid and conversion of carbons 1 and 2 of L-ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiol. 2001, 125, 634–640. [Google Scholar] [CrossRef]
- Wattendorff, J. A third type of raphide crystal in the plant kingdom: Six-sided raphides with laminated sheaths in Agave americana L. Planta 1976, 130, 303–311. [Google Scholar] [CrossRef]
- Yang, J.; Loewus, F.A. Metabolic conversion of L-Ascorbic acid in oxalate-accumulating plants. Plant Physiol. 1975, 56, 283–285. [Google Scholar] [CrossRef]
- Bárcenas-Argüello, M.L.; Gutiérrez-Castorena, M.C.; Terrazas, T. The polymorphic weddellite crystals in three species of Cephalocereus (Cactaceae). Micron 2015, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Viñas, M.; Jiménez, V.M. Occurrence and characterisation of calcium oxalate crystals in stems and fruits of Hylocereus costaricensis and Selenicereus megalanthus (Cactaceae: Hylocereeae). Micron 2016, 89, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Frausto-Reyes, C.; Loza-Cornejo, S.; Terrazas, T.; De la Luz Miranda-Beltrán, M.; Aparicio-Fernández, X.; López-Macías, B.M. Morales-Martínez, S.E. Raman spectroscopy study of calcium oxalate extracted from cacti stems. Appl. Spectrosc. 2014, 68, 1260–1265. [Google Scholar] [CrossRef]
- López-Macías, B.M.; Morales-Martínez, S.E.; Loza-Cornejo, S.; Reyes, C.F.; Terrazas, T.; Patakfalvi, R.J. Variability and composition of calcium oxalate crystals in embryos-seedlings-adult plants of the globose cacti Mammillaria uncinata. Micron 2019, 125, 102731. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, N.; Benvenuto, M.L.; Osterrieth, M. Calcium oxalate crystal production and density at different phenological stages of soybean plants (Glycine max L.) from the southeast of the Pampean Plain, Argentina. Plant Biol. (Stuttg.) 2016, 18, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Arnott, H.J.; Webb, M.A. Twin crystals of calcium oxalate in the seed coat of the kidney bean. Protoplasma 1983, 114, 23–34. [Google Scholar] [CrossRef]
- Gulliver, G. On the crystals in the testa and pericarp of several orders of plants, and in other parts of the order Leguminosae. Mon. Micro. J. 1873, 10, 259–266. [Google Scholar] [CrossRef]
- Rodrigues, T.M.; Machado, S.R. The pulvinus endodermal cells and their relation to leaf movement in legumes of the Brazilian cerrado. PlantBiology (Stuttgart, Germany) 2007, 9, 469–477. [Google Scholar] [CrossRef]
- Horner, H.T.; Zindler-Frank, E. Calcium oxalate crystals cells in the leaves of Rhynchosia caribaea (Leguminosae: Papilionoideae. Protoplasma 1982, 111, 10–18. [Google Scholar] [CrossRef]
- Jáuregui-Zuñiga, D.; Moreno-Cárcamo, A. La biomineralización del oxalato de calcio en plantas: Retos y potencial. Rev. Edu. Bioquim. 2004, 23, 18–23. [Google Scholar]
- Jáuregui-Zúñiga, D.; Ferrer, M.A.; Caldero, A.A.; Munoz, R.; Moreno, A. Heavy metal stress reduces the deposition of calcium oxalate crystals in leaves of Phaseolus vulgaris. J. Plant Physiol. 2005, 162, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.R.; Wolf, S. Anatomical characters of the phyllode and stem of Acacia podalyriifolia A. Cunn. ex G. Don (Fabaceae). Rev. Bras. Pharmacog. 2005, 15, 71–76. [Google Scholar] [CrossRef]
- Barnabas, A.D.; Howard, J.A. Calcium oxalate crystal formation in the bean (Phaseolus Vulgaris, L.) seed coat. Bot. Gaz. Int. J. Plant Sci. 1990, 151, 331–341. [Google Scholar] [CrossRef]
- Zindler-Frank, E.; Wichmann, E.; Korneli, M. Cells with crystals of calcium oxalate in the Leaves of Phaseolus vulgaris—A Comparison with those in Canavalia ensiformis. Bot. Acta 1988, 101, 246–253. [Google Scholar] [CrossRef]
- Zindler-Frank, E. Calcium oxalate crystals in legumes. In Advances in Legume Systematic, Part 3; Stirton, E., Ed.; Royal Botanic Gardens: Kew, UK, 1987; pp. 279–316. [Google Scholar]
- Zindler-Frank, E.; Honow, R.; Hesse, A. Calcium and oxalate content of the leaves of Phaseolus vulgaris at different calcium supply in relation to calcium oxalate crystal formation. J. Plant Physiol. 2001, 158, 139–144. [Google Scholar] [CrossRef]
- Borchert, R. Functional anatomy of the calcium excreting system of Gleditsia triacanthos L. Bot. Gaz. 1984, 145, 474–482. [Google Scholar] [CrossRef]
- Ilarsalan, H.; Palmer, R.G.; Horner, H.T. Calcium oxalate crystals in developing seeds of Soybean. Ann. Bot. 2001, 88, 243–257. [Google Scholar] [CrossRef]
- Nakata, P.A.; McConn, M.M. Isolation of Medicago truncatula mutants defective in calcium oxalate crystal formation. Plant Physiol. 2000, 124, 1097–1104. [Google Scholar] [CrossRef]
- Ward, G.; Harbers, L.H.; Blaha, J.J. Calcium containing crystals in Alfalfa: Their fate in cattle. J. Dairy Sci. 1979, 62, 715–722. [Google Scholar] [CrossRef]
- Korth, K.L.; Doege, S.J.; Park, S.H.; Goggin, F.L.; Wang, Q.; Gomez, S.K.; Liu, G.; Jia, L.; Nakata, P.A. Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol. 2006, 141, 188–195. [Google Scholar]
- Benecke, W. Ueber Oxalsaurebildung in grunen Pflanzen. Bot. Ztg. 1903, 61, 79–110. [Google Scholar]
- Lersten, N.R. Crystals of calcium compounds in Gramineae. New Phytol. 1983, 93, 633–637. [Google Scholar] [CrossRef]
- Hernandéz-Valencia, R.E.M.; López-Franco, R.; Ruíz-Ordoñez, J.; Ramirez-Rodriguez, H.; Benavides-Mendoza, A. The stomatal complex and picuticular characteristics of crown rot disease of Agave tequilana weber (Agavaceae). Acta Hortic. 2003, 618, 427–433. [Google Scholar] [CrossRef]
- Ishii, Y. Needle crystal of calcium oxalate monohydrate found in plant. J. Electron Microsc. 1992, 41, 53–56. [Google Scholar]
- Bernardino-Nicanor, A.; Teniente-Martínez, G.; Juarez-Goiz, J.M.S.; Filardo-Kerstupp, S.; Montañez-Soto, J.L.; González-Cruz, L. Changes in the concentration and characteristics of calcium oxalate crystals during development stages of Agave atrovirens. Adv. Biores. 2012, 3, 22–28. [Google Scholar]
- Den Outer, R.W.; Van Veenendaal, W.L.H. Gold speckles and crystals in tomato fruits (Lycopersicon esculentum Mill.). J. Hortic. Sci. 1988, 63, 645–649. [Google Scholar] [CrossRef]
- De Kreij, C.; Janse, J.; Van Goor, B.J.; Van Doesburg, J.D.J. The incidence of calcium oxalate crystals in fruit walls of tomato (Lycopersicon esculentum Mill.) as affected by humidity, phosphate and calcium supply. J. Hortic. Sci. 1992, 67, 45–50. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S.; Walker, G.D. The form, distribution and seasonal accumulation of calcium in kiwifruit leaves. New Phytologist 1987, 105, 477–486. [Google Scholar] [CrossRef]
- Franceschi, V.R. Calcium oxalate formation is a rapid and reversible process in Lemna minor L. Protoplasma 1989, 148, 130–137. [Google Scholar] [CrossRef]
- Barabé, D.; Lacroix, C.; Chouteau, M.; Gibernau, M. On the presence of extracellular calcium oxalate crystals on the inflorescences of Araceae. Bot. J. Linn. Soc. 2004, 146, 181–190. [Google Scholar] [CrossRef]
- Coté, G.G.; Gibernau, M. Distribution of calcium oxalate crystals infloral organs of Araceae in relation to pollination strategy. Am. J. Bot. 2012, 99, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Tabanca, N.; Demirci, B.; Khan, I. Studies on the Floral Anatomy and Scent Chemistry of Titan Arum (Amorphophallus titanum, Araceae). Turk. J. Bot. 2017, 41, 63–74. [Google Scholar] [CrossRef]
- Barthlott, W.; Szarzynski, J.; Vlek, P.; Lobin, W.; Korotkova, N. A torch in the rain forest: Thermogenesis of the Titan arum (Amorphophallus titanum). Plant Biol. (Stuttg.) 2009, 11, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Osuji, J.O. Probable functions of calcium oxalate crystals in different tissues of the edible aroids (Xanthosoma and Colocasia spp.) in Nigeria. Afr. J. Biotechnol. 2013, 12, 3952–3956. [Google Scholar]
- Levy-Lior, A.; Weiner, S.; Addadi, L. Achiral Calcium-Oxalate Crystals with Chiral Morphology from the Leaves of Some Solanacea Plants. Helv. Chim. Acta. 2003, 86, 4007–4017. [Google Scholar] [CrossRef]
- Addadi, L.; Geva, M. Molecular recognition at the interface between crystals and biology: Generation, manifestation and detection of chirality at crystals surfaces. Cryst. Eng. Comm. 2003, 5, 140–146. [Google Scholar] [CrossRef]
- Bouropoulos, N.; Weiner, S.; Addadi, L. Calcium Oxalate Crystals in Tomato and Tobbaco Plants: Morphology and in Vitro Interactions of Crystal-Associated Macromolecules. Chem. Eur. J. 2001, 7, 1881–1888. [Google Scholar] [CrossRef]
- Liu, N.; Xie, H.; Ping, H.; Wang, L.; Liu, Z.; Tao, F.; Guo, J.; Su, B.L. Shape and structure controlling of calcium oxalate crystals by a combination of additives in the process of biomineralization. RSC Adv. 2018, 8, 11014. [Google Scholar] [CrossRef]
- Cho, K.R.; Salter, E.A.; De Yoreo, J.J.; Wierzbicki, A.; Elhadj, S.; Huang, Y.; Qiu, S.R. Impact of Chiral Molecules on the Formation of Biominerals: A Calcium Oxalate Monohydrate Example. Cryst. Growth Des. 2012, 12, 5939–5947. [Google Scholar] [CrossRef]
- Prywer, J. Theoretical analysis of specific evolution of some faces of plant COM crystals. Cryst. Eng. Comm. 2009, 11, 196–202. [Google Scholar] [CrossRef]
- Jáuregui-Zúñiga, D.; Reyes-Grajeda, J.P.; Sepúlveda-Sánchez, J.D.; Whitaker, J.R.; Moreno, A. Crystallochemical characterization of calcium oxalate crystals isolated from seed coats of Phaseolus vulgaris and leaves of Vitis vinifera. J. Plant Physiol. 2003, 160, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kharshiing, E.; Sreelakshmi, Y.; Sharma, R. The Light Awakens! Sensing Light and Darkness. In Sensory Biology of Plants; Sopory, S., Ed.; Springer Nature: Singapore, 2019; pp. 21–57. [Google Scholar]
- Franklin, K.A.; Quail, P.H. Phytochrome Functions in Arabidopsis Development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, G.; Wang, H.; Wang, D.X. Phytochrome Signaling Mechanisms. Arabidopsis Book 2011, 9, e0148. [Google Scholar] [CrossRef]
- Ahmad, M.; Galland, P.; Ritz, T.; Wiltschko, W. Magnetic Intensity Affects Cryptochrome-dependent Responses in Arabidopsis thaliana. Planta 2007, 225, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, L.; Favory, J.; Cloix, C.; Faggionato, D.; O´Hara, A.; Kaiserli, E. Perception of UV-B by the Arabidopsis UVR8. Protein Sci. 2011, 332, 103–106. [Google Scholar]
- Vanhaelewyn, L.; Va Der Streaten, D.; Vandenbussche, F. Determination of Photropism by UV-B Radiation. In Phototropism. Methods and Protocols. Methods in Molecular Biology; Springer Nature: Singapore, 2019; Volume 1924. [Google Scholar]
- Vandenbussche, F.; Tilbrook, K.; Fierro, A.C.; Poelman, D.; Van Der Straeten, D.; Ulm, R. Photoreceptor-mediated Bending towards UV-B in Arabidopsis. Mol. Plant 2014, 7, 1041–1052. [Google Scholar] [CrossRef]
- Christie, J.M. Phototropin Blue-Light Receptors. Ann. Rev. Plant. Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef]
- Huala, E.; Oelle, P.W.; Liscum, E.; Han, I.-S.; LArsen, E.; Briggs, W.R. Arabidopsis NPH1: A Protein Kinase with a Putative Redox-Sensing Domain. Science 1997, 278, 2120–2123. [Google Scholar] [CrossRef]
- Christie, J.M.; Salomon, M.; Nouze, K.; Wada, M.; Briggs, W.R. LOV (Light Oxygen, or Voltage) Domains od the Blue-light Receptor Phototropin (NPH1): Binding Sites for the FMN Chromophore Flavin Mononucleotide. Proc. Natl. Acad. Sci. USA 1999, 96, 8779–8783. [Google Scholar] [CrossRef]
- Briggs, W.R.; Beck, C.F.; Cashmore, A.R.; Christie, J.M.; Hughes, J.; Jarillo, J.A.; Kawaga, T.; Kanegae, H.; Liscum, E.; Nagatami, A.; et al. The Phototropin Family of Receptors. Plant Cell 2001, 13, 993–997. [Google Scholar] [CrossRef]
- Sakai, T.; Kagawa, T.; Kasahara, M.; Swartz, T.E.; Christie, J.M.; Briggs, W.R.; Wada, M.; Okada, K. Arabidopsis NPH1 and NPL1: Blue-light Receptors that Mediate both Phototropism and Chloroplast relocation. Proc. Natl. Acad. Sci. USA 2001, 12, 6969–6974. [Google Scholar] [CrossRef]
- Jarillo, J.A.; Gabrys, H.; Capel, J.; Alonso, J.M.; Ecker, J.R.; Cashmore, A.R. Phototropin-related PNL1 Controls Chloroplast Relocation Induced by Blue Light. Nature 2001, 410, 952–954. [Google Scholar] [CrossRef]
- Sakai, T.; Wada, T.; Ishiguro, S.; Okada, K. RTP2: A Signal Transducer of the Phototropic Response in Arabidopsis. Plant Cell 2000, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Crosson, S.; Moffat, K. Photoexcited Structure of a Plant Photoreceptor Domain Reveals a Light-driven Molecular Switch. Plant Cell 2002, 14, 1067–1075. [Google Scholar] [CrossRef]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguo, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis PNL1: A Phototropin Homolog Controlling High-light Avoidance Response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kugawa, T.; Wada, M.; Shimazaki, K.-I. PHO1, PHO2 Mediate Blue Light Regulation of Stomatal Opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Lin, C. Phototropin Blue Light Receptors and Light-induced Movement Responses in Plants. Sci. STKE 2002, 118, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, B.L.; Zhulin, I.B. PAS Domain: Internal Sensors of Oxygen, Redox Potential, and Light. Microbiol. Mol. Biol. Rev. 1999, 36, 479–506. [Google Scholar] [CrossRef]
- Nakasako, M.; Hirata, M.; Shimizu, N.; Hosokawa, S.; Matsukoa, D.; Oka, T.; Yamamoto, M.; Tokutomi, S. Crystallization and Preliminary X-ray Diffraction Analysis of the LOV1 Domains of Phototropin 1 and 2 from Arabidopsis thaliana. Acta Cryst. 2008, F64, 617–621. [Google Scholar]
- Matsuoka, D.; Tukomi, S. Blue Light-regulated Molecular Switch of Ser/Thr Kinase in Phototropin. Proc. Natl. Acad. Sci. USA 2005, 102, 13337–13342. [Google Scholar] [CrossRef]
- Nakasako, M.; Iwata, T.; Matsuoka, D.; Tukomi, S. Light-Induced Structural Changes of LOV-Domain-Containing Polypeptides from Arabidopsis Phototropin 1 and 2 Studied by Small-Angle X-Ray Scattering. Biochemistry 2004, 43, 14881–14890. [Google Scholar] [CrossRef] [PubMed]
- Oide, M.; Okajima, K.; Nakagami, H.; Kato, T.; Sekigushi, Y.; Oroguchi, T.; Hikima, T.; Yamamoto, M.; Nakasako, M. Blue Light-excited LOV1 and LOV2 Domains Cooperatively Regulate the Kinase Activity of Full Length Phototropin2 from Arabidopsis. J. Biol. Chem. 2018, 293, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Kagawa, T.; Oikara, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast Movement Reduces Photodamage in Plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Iwata, T.; Nozaki, D.; Tokutomi, S.; Kandori, H. Comprarative Investigation of the LOV1 and LOV2 Domains is Adiantum Phytochrom3. Biochemistry 2005, 44, 7427–7434. [Google Scholar] [CrossRef]
- Christie, J.M.; Reymond, P.; Powell, G.K.; Bernasconi, P.; Raibekas, A.A.; Liscum, E.; Briggs, W.R. Arabidopsis NPH1: A Flavoprotein with the Properties of a Photoreceptor for Phototropism. Science 1998, 282, 1698–1701. [Google Scholar] [CrossRef]
- Salomon, M.; Christie, J.M.; Knieb, E.; Lempert, U.; Briggs, W.R. Photochemical and Mutational Analysis of the FMN-binding Domains of the Plant Blue Light Receptor, Phototropin. Biochemistry 2000, 39, 9401–9410. [Google Scholar] [CrossRef] [PubMed]
- Halavaty, A.S.; Moffat, K. Coiled-coil Dimerization of the LOV2 Domain of the Blue-Light Phototropin 1 from Arabidopsis thaliana. Acta Cryst. 2013, F69, 1316–1321. [Google Scholar]
- Christie, J.M.; Hitomi, H.; Arvai, A.S.; Hartfield, K.A.; Mettlen, M.; Pratt, A.J.; Tainer, J.A.; Getzoff, E.D. Structural Tunning of the Fluorescence Protein iLOV for Improved Photostability. J. Biol. Chem. 2012, 287, 22295–22304. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.E.; Corchnoy, S.B.; Chirstie, J.M.; Lewis, J.W.; Szundi, I.; Brigg, W.R.; Bogomolni, R.A. The Photcycle of a Flavin-binding Domain of the Blue Light Photoreceptor Phototropin. J. Biol. Chem. 2001, 276, 35493–36500. [Google Scholar] [CrossRef]
- Komatsu, A.; Terai, M.; Ishisaki, K.; Suetsugu, N.; Tsuboi, H.; Nishihama, R.; Yamato, K.T.; Wada, M.; Kohchi, T. Phototropin Encoded by a single-Copy Gene Mediates Chloroplast Photorelocation Movements in the Liverwort Merchantia polymorpha. Plant Physiol. 2014, 166, 411–427. [Google Scholar] [CrossRef]
- Li, F.-W.; Rothfels, C.J.; Melkonian, M.; Villarreal, J.C.; Stevenson, D.W.; Graham, S.W.; Wong, G.K.-S.; Mathews, S.; Pryer, K.M. The Origin and Evolution of Phototropins. Front. Plant Sci. 2015, 6, 637. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kulshrestha, K. Controlling Light with Light in Blue-light Plant Photoreceptor Phototropin. Curr. Sci. 2007, 92, 9. [Google Scholar]
- Oide, M.; Okijama, K.; Kashojiya, S.; Takayama, K.; Oroguchi, T.; Hikima, T.; Yamamoto, M.; Nakasako, M. Blue Light-excited Light-oxygen-voltage-sensing Domain 2 (LOV2) Triggers a Rearrengement of the Kinase Domain to Induce Phosphorylation Activity in Arabidopsis Phototropin1. J. Biol. Chem. 2016, 291, 19975–19984. [Google Scholar] [CrossRef] [PubMed]
- Schieppers, J.; Vogt, J. Setting the PAS, the Role of Circadian PAS Domain Proteins during Environmental Adaptation in Plants. Front. Plant Sci. 2015, 6, 513. [Google Scholar]
- Pilbeam, D.J.; Morley, P.S. Calcium. In Handbook of Plant Nutrition, 2nd ed.; Barker, A., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 121–140. [Google Scholar]
- Nakata, P.A. An Assessment of Engineered Calcium Oxalate Crystals Formation on Plant Growth and Development as a Step toward Evaluating its Use to Enhance Plant Defense. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Marmé, D. ATP-dependent Ca2+ Uptake into Plant Membrane Vesicles. Proc. Natl. Acad. Sci. USA 1978, 75, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.M.; Gawthorne, J.; Young, G.; Fraser, N.J.; Roe, A.J. LOV to BLUF: Flavoprotein Contributions to the Optogenetic Toolkit. Mol. Plant 2012, 19, 1–12. [Google Scholar] [CrossRef]
- Banerjee, S.; Mitra, D. Structural Basis of the Design and Engineering for Advanced Plant Optogenetics. Trends Plant. Sci. 2020, 25, 35–65. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuéllar-Cruz, M.; Pérez, K.S.; Mendoza, M.E.; Moreno, A. Biocrystals in Plants: A Short Review on Biomineralization Processes and the Role of Phototropins into the Uptake of Calcium. Crystals 2020, 10, 591. https://doi.org/10.3390/cryst10070591
Cuéllar-Cruz M, Pérez KS, Mendoza ME, Moreno A. Biocrystals in Plants: A Short Review on Biomineralization Processes and the Role of Phototropins into the Uptake of Calcium. Crystals. 2020; 10(7):591. https://doi.org/10.3390/cryst10070591
Chicago/Turabian StyleCuéllar-Cruz, Mayra, Karina Sandra Pérez, María Eugenia Mendoza, and Abel Moreno. 2020. "Biocrystals in Plants: A Short Review on Biomineralization Processes and the Role of Phototropins into the Uptake of Calcium" Crystals 10, no. 7: 591. https://doi.org/10.3390/cryst10070591
APA StyleCuéllar-Cruz, M., Pérez, K. S., Mendoza, M. E., & Moreno, A. (2020). Biocrystals in Plants: A Short Review on Biomineralization Processes and the Role of Phototropins into the Uptake of Calcium. Crystals, 10(7), 591. https://doi.org/10.3390/cryst10070591






