Formation of Tetranuclear Nickel(II) Complexes with Schiff-Bases: Crystal Structures and Magnetic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Methods
2.3. Synthesis of Ligands and Complexes
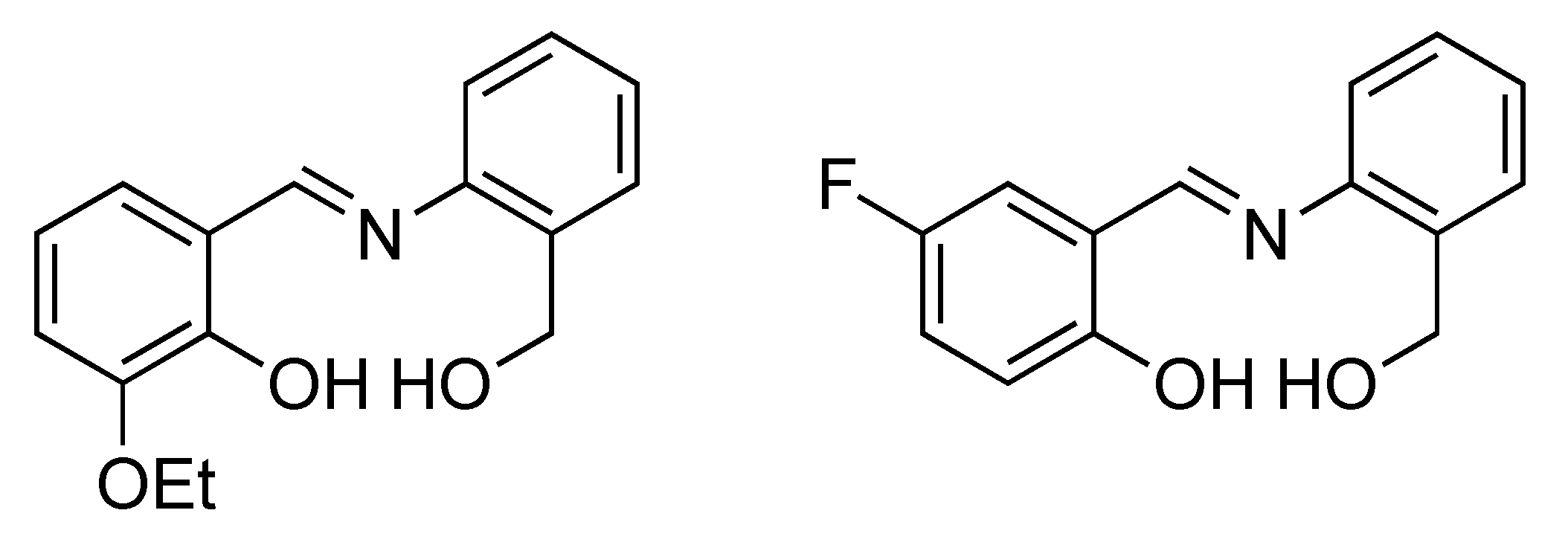
2.3.1. Synthesis of H2L1
2.3.2. Synthesis of H2L2
2.3.3. Synthesis of [Ni4(L1)4(EtOH)4] (1)
2.3.4. Synthesis of [Ni4(L2)4(MeOH)4] (2)
2.4. X-ray Crystallography
3. Results and Discussion
3.1. Synthesis and Characterization
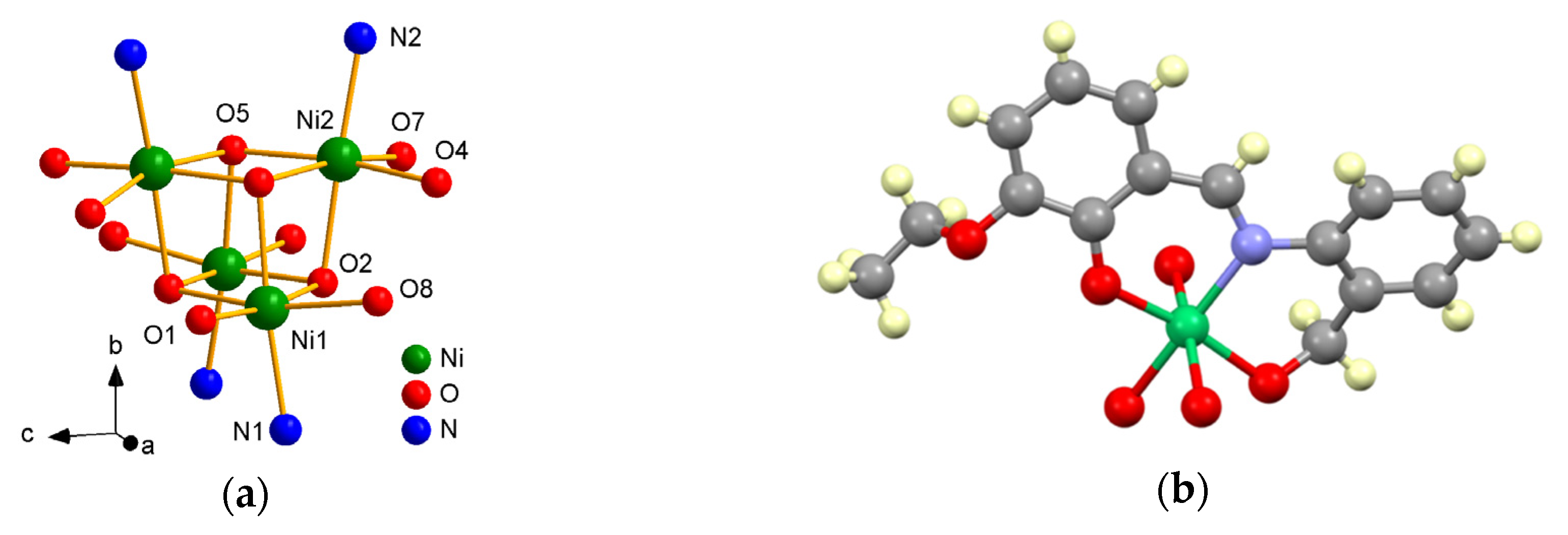
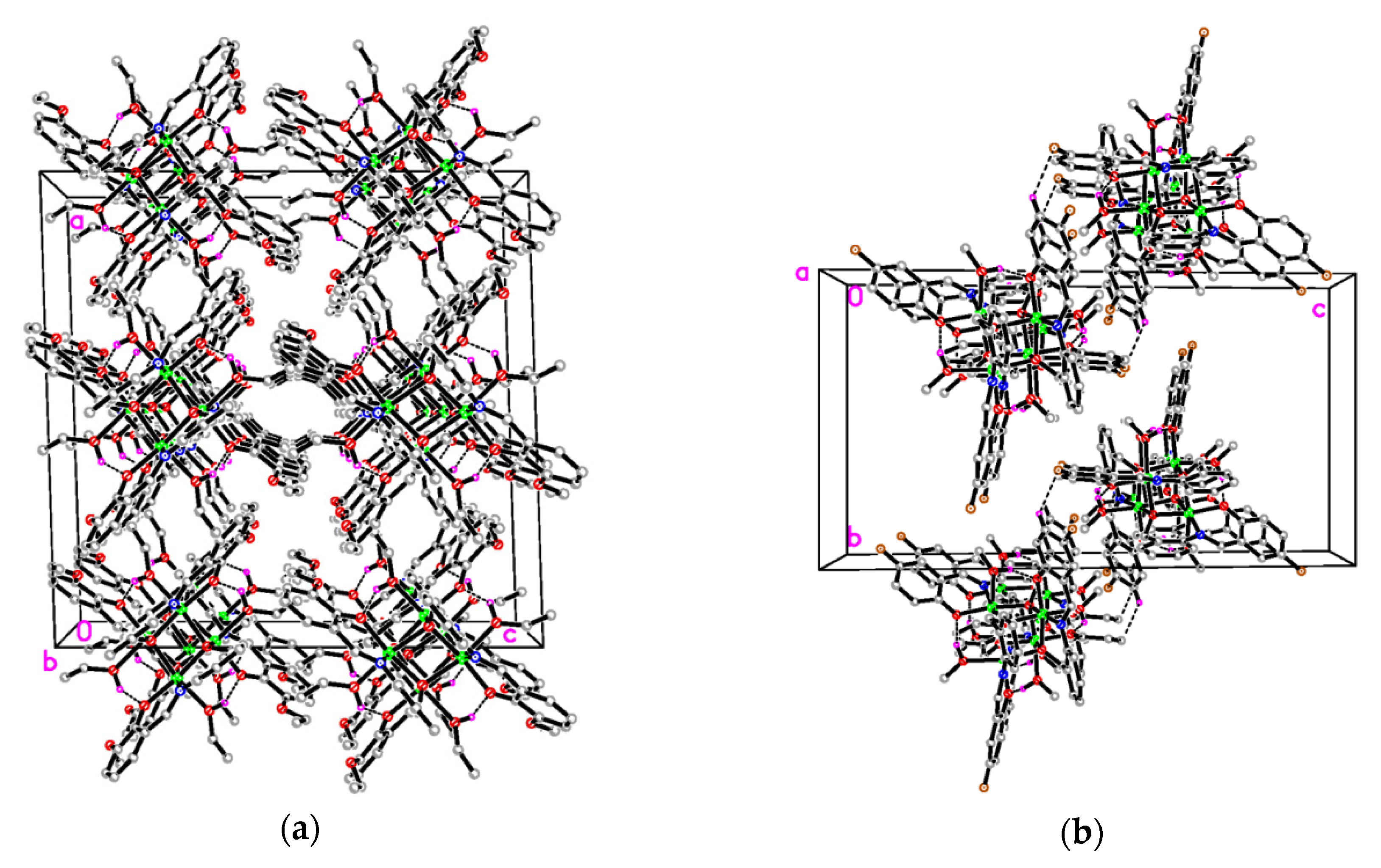
3.2. Structural Description of the Complexes
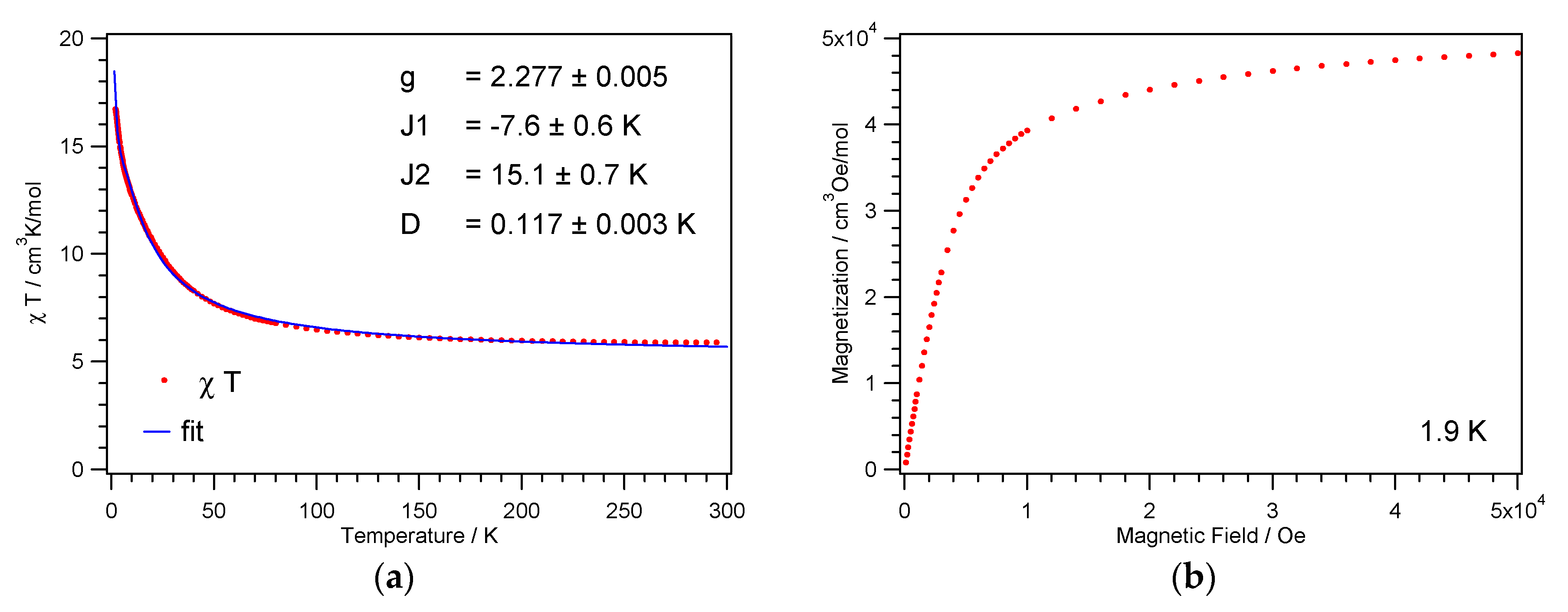
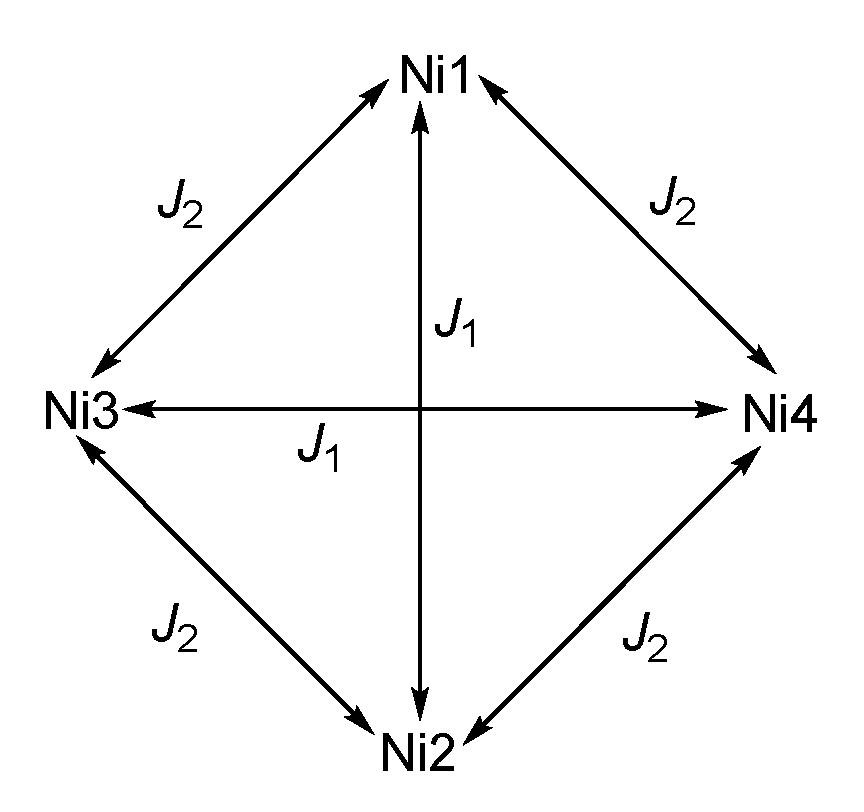
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chaudhuri, P.; Kataev, V.; Büchner, B.; Klauss, H.-H.; Kersting, B.; Meyer, F. Tetranuclear complexes in molecular magnetism: Targeted synthesis, high-field EPR and pulsed-field magnetization. Coord. Chem. Rev. 2009, 253, 2261–2285. [Google Scholar] [CrossRef]
- Thompson, L.K. Polynuclear coordination complexes—From dinuclear to nonanuclear and beyond. Coord. Chem. Rev. 2002, 233–234, 193–206. [Google Scholar] [CrossRef]
- Fielden, J.; Speldrich, M.; Besson, C.; Kögerler, P. Chiral hexanuclear ferric wheels. Inorg. Chem. 2012, 51, 2734–2736. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Ram, S. The role of ligands, polytopic ligands and Metal Organic Ligands (Mols) in coordination chemistry. Chem. Sci. Rev. Lett. 2015, 4, 414–428. [Google Scholar]
- Bonanno, M.N.; Lough, J.A.; Poddutoori, K.P.; Lemaire, T.M. Synthesis, characterization and Copper(2+) coordination chemistry of a polytopic paramagnetic ligand. Magnetochemistry 2017, 3, 15. [Google Scholar] [CrossRef]
- Winpenny, R.E.P. Molecular Cluster Magnets; World Scientific: London, UK, 2012. [Google Scholar]
- Gatteschi, D. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Liu, X.; Hamon, J.-R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Clarke, R.M.; Herasymchuk, K.; Storr, T. Electronic structure elucidation in oxidized metal–salen complexes. Coord. Chem. Rev. 2017, 352, 67–82. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Mondal, K.C.; Kostakis, G.E.; Lan, Y.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. Defect-dicubane Ni2Ln2 (Ln = Dy, Tb) single molecule magnets. Inorg. Chem. 2011, 50, 11604–11611. [Google Scholar] [CrossRef]
- Kühne, I.A.; Griffiths, K.; Hutchings, A.-J.; Townrow, O.P.E.; Eichhöfer, A.; Anson, C.E.; Kostakis, G.E.; Powell, A.K. Stepwise investigation of the influences of steric groups versus counterions to target Cu/Dy complexes. Cryst. Growth Des. 2017, 17, 5178–5190. [Google Scholar] [CrossRef]
- Gheorghe, R.; Andreea Ionita, G.; Maxim, C.; Caneschi, A.; Sorace, L.; Andruh, M. Aggregation of heptanuclear [MII7] (M = Co, Ni, Zn) clusters by a Schiff-base ligand derived from o-vanillin: Synthesis, crystal structures and magnetic properties. Polyhedron 2019, 171, 269–278. [Google Scholar] [CrossRef]
- Wu, J.-C.; Liu, S.-X.; Keene, T.D.; Neels, A.; Mereacre, V.; Powell, A.K.; Decurtins, S. Coordination chemistry of a π-extended, rigid and redox-active tetrathiafulvalene-fused Schiff-base ligand. Inorg. Chem. 2008, 47, 3452–3459. [Google Scholar] [CrossRef] [PubMed]
- Opstal, T.; Verpoort, F. Synthesis of highly active ruthenium indenylidene complexes for atom-transfer radical polymerization and ring-opening-metathesis polymerization. Angew. Chem. Int. Ed. 2003, 42, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, B.; Verpoort, F. Atom transfer radical polymerization of vinyl monomers mediated by Schiff base ruthenium−alkylidene catalysts and the adventitious effect of water in polymerizations with the analogous cationic complexes. Macromolecules 2002, 35, 8943–8947. [Google Scholar] [CrossRef]
- Hazra, S.; Koner, R.; Lemoine, P.; Sañudo, E.C.; Mohanta, S. Syntheses, structures and magnetic properties of heterobridged dinuclear and cubane-type tetranuclear complexes of Nickel(II) derived from a Schiff base ligand. Eur. J. Inorg. Chem. 2009, 23, 3458–3466. [Google Scholar] [CrossRef]
- Mukherjee, P.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. The crucial role of polyatomic anions in molecular architecture: Structural and magnetic versatility of five Nickel(II) complexes derived from A N,N,O-Donor Schiff base ligand. Inorg. Chem. 2009, 48, 5848–5860. [Google Scholar] [CrossRef]
- Bonadio, F.; Senna, M.-C.; Ensling, J.; Sieber, A.; Neels, A.; Stoeckli-Evans, H.; Decurtins, S. Cyano-bridged structures based on [MnII(N3O2−Macrocycle)]2+: A synthetic, structural, and magnetic study. Inorg. Chem. 2005, 44, 969–978. [Google Scholar] [CrossRef]
- Pioquinto-Mendoza, J.R.; Rosas-Ortiz, J.A.; Reyes-Martínez, R.; Conelly-Espinosa, P.; Toscano, R.A.; Germán-Acacio, J.M.; Avila-Sorrosa, A.; Baldovino-Pantaleón, O.; Morales-Morales, D. Synthesis, characterization and molecular structures of Ni(II) complexes derived from Schiff base pyridylimine ligands. Inorg. Chim. Acta 2015, 438, 146–152. [Google Scholar] [CrossRef]
- Pioquinto-Mendoza, J.R.; Conelly-Espinosa, P.; Reyes-Martínez, R.; Toscano, R.A.; Germán-Acacio, J.M.; Avila-Sorrosa, A.; Baldovino-Pantaleón, O.; Morales-Morales, D. A simple and facile to prepare Pd(II) complex containing the pyridyl imine ligand [C5H4N-2−CH3C=N-(CH2)3NH2]. Structural characterization and catalytic evaluation in Suzuki–Miyaura C–C couplings. J. Organomet. Chem. 2015, 797, 153–158. [Google Scholar] [CrossRef]
- Williams, A.F. A structural analysis of {M4O4} cubanes where M = Mn and Fe. Dalton Trans. 2008, 6, 818–821. [Google Scholar] [CrossRef]
- Isele, K.; Gigon, F.; Williams, A.F.; Bernardinelli, G.; Franz, P.; Decurtins, S. Synthesis, structure and properties of {M4O4} cubanes containing nickel(ii) and cobalt(ii). Dalton Trans. 2007, 3, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Prescimone, A.; Mishra, A.; Parsons, S.; Wernsdorfer, W.; Christou, G.; Perlepes, S.P.; Brechin, E.K. A rare ferromagnetic manganese(iii) ‘cube’. Chem. Commun. 2007, 2, 153–155. [Google Scholar] [CrossRef]
- Aronica, C.; Chumakov, Y.; Jeanneau, E.; Luneau, D.; Neugebauer, P.; Barra, A.-L.; Gillon, B.; Goujon, A.; Cousson, A.; Tercero, J.; et al. Structure, magnetic properties, polarized neutron diffraction, and theoretical study of a Copper(II) Cubane. Chem. Eur. J. 2008, 14, 9540–9548. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ding, S.; Xu, X.; Wang, R.; Song, Y.; Wang, Y.; Du, C.-f.; Liu, Z.-l. Synthesis, structure and magnetic properties of a series of cubane-like clusters derived from Schiff base ligands. Polyhedron 2014, 83, 36–43. [Google Scholar] [CrossRef]
- Isele, K.; Franz, P.; Ambrus, C.; Bernardinelli, G.; Decurtins, S.; Williams, A.F. Self-assembly and interconversion of tetranuclear Copper(II) complexes. Inorg. Chem. 2005, 44, 3896–3906. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Escuer, A.; Mautner, F.A.; Raptopoulou, C.; Terzis, A.; Perlepes, S.P.; Vicente, R. Use of the Di-2−pyridyl Ketone/Acetate/Dicyanamide “Blend” in Manganese(II), Cobalt(II) and Nickel(II) Chemistry: Neutral Cubane Complexes. Eur. J. Inorg. Chem. 2005, 5, 879–893. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ohtani, R.; Teraoka, S.; Kosaka, W.; Miyasaka, H.; Zhang, Y.; Lindoy, L.F.; Hayami, S.; Nakamura, M. Syntheses, structures and magnetic properties of tetranuclear cubane-type and heptanuclear wheel-type nickel(ii) complexes with 3-methoxysalicylic acid derivatives. Dalton Trans. 2017, 46, 8555–8561. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Oshio, H. Molecular cubes with high-spin ground states. Sci. Technol. Adv. Mater 2005, 6, 565–570. [Google Scholar] [CrossRef]
- Sartorel, A.; Bonchio, M.; Campagna, S.; Scandola, F. Tetrametallic molecular catalysts for photochemical water oxidation. Chem. Soc. Rev. 2013, 4, 2262–2280. [Google Scholar] [CrossRef]
- Song, F.; Al-Ameed, K.; Schilling, M.; Fox, T.; Luber, S.; Patzke, G.R. Mechanistically Driven Control over Cubane Oxo Cluster Catalysts. J. Am. Chem. Soc. 2019, 141, 8846–8857. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Zhong, C.; Li, S.; Shen, Z.; Pu, J.; Liu, J.; Zhou, Y.; Zhang, H.; Ma, H. (Co/Fe)4O4 cubane-containing nanorings fabricated by phosphorylating cobalt ferrite for highly efficient oxygen evolution reaction. ACS Catal. 2019, 9, 3878–3887. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Tian, J.-W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.-F.; Li, D.-S.; Lan, Y.-Q.; Bu, X. Bi-Microporous metal–organic frameworks with cubane [M4(OH)4] (M=Ni, Co) clusters and pore-space partition for electrocatalytic methanol oxidation reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-C.; Wernsdorfer, W.; Zakharov, L.N.; Karaki, Y.; Yamaguchi, A.; Isidro, R.M.; Lu, G.-D.; Wilson, S.A.; Rheingold, A.L.; Ishimoto, H.; et al. Fast magnetization tunneling in Tetranickel(II) single-molecule magnets. Inorg. Chem. 2006, 45, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Iasco, O.; Chumakov, Y.; Guégan, F.; Gillon, B.; Lenertz, M.; Bataille, A.; Jacquot, J.-F.; Luneau, D. Mapping the magnetic anisotropy inside a Ni4 cubane spin cluster using polarized neutron diffraction. Magnetochemistry 2017, 3, 25. [Google Scholar] [CrossRef]
- Ponomaryov, A.N.; Kim, N.; Hwang, J.; Nojiri, H.; van Tol, J.; Ozarowski, A.; Park, J.; Jang, Z.; Suh, B.; Yoon, S.; et al. Structural tailoring effects on the magnetic behavior of symmetric and asymmetric cubane-type Ni complexes. Chem. Asian J. 2013, 8, 1152–1159. [Google Scholar] [CrossRef]
- Rudbari, H.A.; Lloret, F.; Khorshidifard, M.; Bruno, G.; Julve, M. Effects of electron donating/withdrawing groups in the 5-substituted-2−hydroxybenzaldehyde on the synthesis of neutral cubanes with a NiII4O4 core: Synthesis, crystal structures and magnetic properties. RSC Adv. 2016, 6, 7189–7194. [Google Scholar] [CrossRef]
- Petit, S.; Neugebauer, P.; Pilet, G.; Chastanet, G.; Barra, A.-L.; Antunes, A.B.; Wernsdorfer, W.; Luneau, D. Condensation of a nickel tetranuclear cubane into a heptanuclear single-molecule magnet. Inorg. Chem. 2012, 51, 6645–6654. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Weyhermüller, T.; Bothe, E.; Wieghardt, K.; Chaudhuri, P. Single-atom o-bridged urea in a Dinickel(II) complex together with NiII4, CuII2 and CuII4 complexes of a pentadentate phenol-containing Schiff base with (O,N,O,N,O)-Donor atoms. Eur. J. Inorg. Chem. 2003, 5, 863–875. [Google Scholar] [CrossRef]
- Halcrow, M.A.; Sun, J.-S.; Huffman, J.C.; Christou, G. Structural and magnetic properties of [Ni4(μ3-OMe)4(dbm)4(MeOH)4] and [Ni4(η1,μ3-N3)4(dbm)4(EtOH)4]. Magnetostructural correlations for [Ni4X4]4+ cubane complexes. Inorg. Chem. 1995, 34, 4167–4177. [Google Scholar] [CrossRef]
- Das, A.; Klinke, F.J.; Demeshko, S.; Meyer, S.; Dechert, S.; Meyer, F. Reversible solvatomagnetic effect in novel tetranuclear cubane-type Ni4 complexes and magnetostructural correlations for the [Ni4(μ3-O)4] core. Inorg. Chem. 2012, 51, 8141–8149. [Google Scholar] [CrossRef]
- Karmakar, S.; Khanra, S. Polynuclear coordination compounds: A magnetostructural study of ferromagnetically coupled Ni4O4 cubane core motif. CrystEngComm 2014, 16, 2371–2383. [Google Scholar] [CrossRef]
- Syamal, A.; Kumar, D. New oxozirconium(IV) complexes with the Schiff bases derived from salicylaldehyde or substituted salicylaldehydes and o-aminobenzyl alcohol. Indian J. Chem. Sect. A 1980, 19A, 1018–1020. [Google Scholar]
- Syamal, A.; Singhal, O.P. New dioxouranium(VI) complexes with tridentate dibasic Schiff bases containing ONO donor sets. Transit. Met. Chem. 1979, 4, 179–182. [Google Scholar] [CrossRef]
- Bruker. SMART (Version 5.628) and SAINT (Version 6.02); Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction of Area Detector; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Ray, A.; Sadhukhan, D.; Rosair, G.M.; Gómez-García, C.J.; Mitra, S. An unprecedented CuII–Schiff base complex existing as two different trinuclear units with strong antiferromagnetic couplings. Polyhedron 2009, 28, 3542–3550. [Google Scholar] [CrossRef]
- Marinescu, G.; Madalan, A.M.; Shova, S.; Andruh, M. Tetranuclear Zn(II) complexes with compartmental and dicyanamido ligands: Synthesis, structure, and luminescent properties. J. Coord. Chem. 2012, 65, 1539–1547. [Google Scholar] [CrossRef]
- Majumder, A.; Rosair, G.M.; Mallick, A.; Chattopadhyay, N.; Mitra, S. Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N,N,O-tridentate Schiff base N-2−pyridylmethylidene-2−hydroxy-phenylamine. Polyhedron 2006, 25, 1753–1762. [Google Scholar] [CrossRef]
- El-Sherif, A.A.; Fetoh, A.; Abdulhamed, Y.K.; Abu El-Reash, G.M. Synthesis, structural characterization, DFT studies and biological activity of Cu(II) and Ni(II) complexes of novel hydrazone. Inorg. Chim. Acta 2018, 480, 1–15. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Ray, A.; Pilet, G.; Rizzoli, C.; Rosair, G.M.; Gómez-García, C.J.; Signorella, S.; Bellú, S.; Mitra, S. Weak interactions modulating the dimensionality in supramolecular architectures in three new Nickel(II)-hydrazone complexes, magnetostructural correlation, and catalytic potential for epoxidation of alkenes under phase transfer conditions. Inorg. Chem. 2011, 50, 8326–8339. [Google Scholar] [CrossRef]
- Bessy Raj, B.N.; Prathapachandra Kurup, M.R.; Suresh, E. Synthesis, spectral characterization and crystal structure of N-2−hydroxy-4-methoxybenzaldehyde-N′-4-nitrobenzoyl hydrazone and its square planar Cu(II) complex. Spectrochim. Acta A 2008, 71, 1253–1260. [Google Scholar] [CrossRef]
- Zangrando, E.; Islam, M.T.; Islam, M.A.-A.A.A.; Sheikh, M.C.; Tarafder, M.T.H.; Miyatake, R.; Zahan, R.; Hossain, M.A. Synthesis, characterization and bio-activity of nickel(II) and copper(II) complexes of a bidentate NS Schiff base of S-benzyl dithiocarbazate. Inorg. Chim. Acta 2015, 427, 278–284. [Google Scholar] [CrossRef]
- Escuer, A.; Font-Bardıa, M.; Kumar, S.B.; Solans, X.; Vicente, R. Two new nickel(II) cubane compounds derived from pyridine-2−methoxide (Pym): {Ni4(Pym)4Cl4(CH3OH)4} and {Ni4(Pym)4(N3)4(CH3OH)4}. Crystal structures and magnetic properties. Polyhedron 1999, 18, 909–914. [Google Scholar] [CrossRef]
- Yoshitake, M.; Nishihashi, M.; Ogata, Y.; Yoneda, K.; Yamada, Y.; Sakiyama, H.; Mishima, A.; Ohba, M.; Koikawa, M. Syntheses, structures, and magnetic properties of cubane-based cobalt and nickel complexes with ONO-tridentate ligands. Polyhedron 2017, 136, 136–142. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, T.; Guo, W.; Lu, J.; Fan, C. Synthesis, structure and magnetism of three cubane Cu(II) and Ni(II) complexes based on flexible Schiff-base ligands. Inorg. Chim. Acta 2013, 400, 191–196. [Google Scholar] [CrossRef]
- Wikstrom, J.P.; Nazarenko, A.Y.; Reiff, W.M.; Rybak-Akimova, E.V. Synthesis and characterization of tetrakis(μ-hydroxo)tetrakis(2,2′-dipicolylamine)tetranickel perchlorate, a nickel-hydroxy cubane complex. Inorg. Chim. Acta 2007, 360, 3733–3740. [Google Scholar] [CrossRef]
- Wang, J.; Feng, C.; Ge, C.M.; Zhang, S.; Hai, H. Two new cubane-type tetranuclear compounds of Copper(II), Nickel(II) derived from reduced schiff base ligand: Syntheses, structures and magnetic properties. J. Clust. Sci. 2016, 27, 2001–2011. [Google Scholar] [CrossRef]
- Gungor, E.; Kara, H. Ferromagnetic coupling in two tetranuclear Ni(II) complexes with cubane–like Ni4(μ3–O)4 core: Structure, spectroscopic and luminescence properties. J. Mol. Struct. 2020, 1208, 127859. [Google Scholar] [CrossRef]
- Jana, M.S.; Priego, J.L.; Jiménez-Aparicio, R.; Mondal, T.K. Novel tetranuclear Ni(II) Schiff base complex containing Ni4O4 cubane core: Synthesis, X-ray structure, spectra and magnetic properties. Spectrochim. Acta A 2014, 133, 714–719. [Google Scholar] [CrossRef]
- Torić, F.; Pavlović, G.; Pajić, D.; Cindrić, M.; Zadro, K. Tetranuclear Ni4 cubane complexes with high χT maxima: Magneto-structural analysis. CrystEngComm 2018, 20, 3917–3927. [Google Scholar] [CrossRef]


| 1 | 2 | |
|---|---|---|
| Empirical formula | C72H84N4Ni4O16 | C60H56F4N4Ni4O12 |
| Formula weight/g mol−1 | 1496.27 | 1335.93 |
| Crystal shape/colour | block/green | block/green |
| Crystal size/mm | 0.15 × 0.13 × 0.12 | 0.11 × 0.10 × 0.07 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n |
| a (Å) | 21.4621(12) | 14.8500(13) |
| b (Å) | 14.9753(11) | 15.0271(11) |
| c (Å) | 21.9416(13) | 27.5089(17) |
| β (°) | 91.826(1) | 97.873(1) |
| V (Å3) | 7048.5(8) | 6080.8(8) |
| Z | 4 | 4 |
| μ (MoKα) (cm−1) | 1.121 | 1.295 |
| Tmin | 0.8498 | 0.8707 |
| Tmax | 0.8772 | 0.9148 |
| Reflections/parameters | 20581/454 | 35744/761 |
| Unique reflections | 6554 | 11296 |
| Observed reflections [I ≥ 2σ(I)] | 3916 | 6171 |
| Restraints | 7 | 0 |
| Goodness of fit on F2 | 0.984 | 1.014 |
| R1, wR2 [I ≥ 2σ(I)] | 0.0469, 0.1027 | 0.0658, 0.1734 |
| R1, wR2 (all data) | 0.0970, 0.1282 | 0.1322, 0.2174 |
| 1 | |||
|---|---|---|---|
| Ni1–O1 | 1.972(3) | Ni1–O2 | 2.002(2) |
| Ni1–N1 | 2.049(3) | Ni1–O5 | 2.092(3) |
| Ni1–O2′ | 2.093(3) | Ni1–O8 | 2.171(3) |
| Ni2–O4 | 1.975(3) | Ni2–O5 | 2.003(3) |
| Ni2–N2 | 2.056(4) | Ni2–O5′ | 2.095(2) |
| Ni2–O2′ | 2.100(3) | Ni2–O7 | 2.143(3) |
| Ni1–O2–Ni1′ | 101.56(10) | Ni1′–O5–Ni2 | 95.20(10) |
| Ni1–O2–Ni2 | 97.82(11) | Ni1′–O5–Ni2 | 97.62(11) |
| Ni1–O2′–Ni2′ | 94.63(10) | Ni2–O5–Ni2′ | 101.53(10) |
| 2 | |||
| Ni1–O1 | 1.971(5) | Ni1–O2 | 1.994(4) |
| Ni1–N1 | 2.047(6) | Ni1–O4 | 2.099(4) |
| Ni1–O6 | 2.114(4) | Ni1–O9 | 2.153(5) |
| Ni2–O3 | 1.971(4) | Ni2–O4 | 1.999(4) |
| Ni2–N2 | 2.050(5) | Ni2–O8 | 2.085(4) |
| Ni2–O2 | 2.093(4) | Ni2–O10 | 2.158(5) |
| Ni3–O5 | 1.973(5) | Ni3–O6 | 2.001(4) |
| Ni3–N3 | 2.060(6) | Ni3–O8 | 2.102(4) |
| Ni3–O4 | 2.102(4) | Ni3–O11 | 2.182(5) |
| Ni4–O7 | 1.970(4) | Ni4–O8 | 1.995(4) |
| Ni4–N4 | 2.049(5) | Ni4–O6 | 2.086(4) |
| Ni4–O2 | 2.099(4) | Ni4–O12 | 2.166(5) |
| Ni1–O2–Ni2 | 100.6(2) | Ni1–O2–Ni4 | 98.6(2) |
| Ni1–O4–Ni2 | 100.3(2) | Ni2–O2–Ni4 | 94.5(2) |
| Ni1-O4-Ni3 | 96.0(2) | Ni2–O4–Ni3 | 98.3(2) |
| Ni1–O6–Ni3 | 98.7(2) | Ni3–O6–Ni4 | 100.3(2) |
| Ni1–O6–Ni4 | 95.3(2) | Ni2–O8–Ni3 | 95.7(2) |
| Ni3–O8–Ni4 | 99.9(2) | Ni2–O8–Ni4 | 97.8(2) |
| D–H∙∙∙A | d(D–H) | d(H∙∙∙A) | d(D∙∙∙A) | Angle (D–H∙∙∙A) |
|---|---|---|---|---|
| 1 | ||||
| O8–H8A∙∙∙O6 | 0.93 | 2.66 | 3.462(5) | 146(3) |
| O8–H8A∙∙∙O4 | 0.93 | 2.14 | 2.633(4) | 112(3) |
| O7–H7A∙∙∙O3 | 0.93 | 2.64 | 3.377(5) | 136(3) |
| O7–H7A∙∙∙O1 | 0.93 | 1.77 | 2.628(4) | 152(3) |
| 2 | ||||
| O12–H12∙∙∙O3 | 0.93 | 1.93 | 2.637(6) | 131(4) |
| O11–H11A∙∙∙O1 | 0.93 | 1.72 | 2.594(8) | 156(4) |
| O10–H10∙∙∙O5 | 0.93 | 1.79 | 2.649(7) | 152(4) |
| O9–H9∙∙∙O7 | 0.93 | 1.90 | 2.623(7) | 133(4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Z.; Luo, Y.; Herringer, S.; Li, Y.; Decurtins, S.; Krämer, K.W.; Liu, S.-X. Formation of Tetranuclear Nickel(II) Complexes with Schiff-Bases: Crystal Structures and Magnetic Properties. Crystals 2020, 10, 592. https://doi.org/10.3390/cryst10070592
You Z, Luo Y, Herringer S, Li Y, Decurtins S, Krämer KW, Liu S-X. Formation of Tetranuclear Nickel(II) Complexes with Schiff-Bases: Crystal Structures and Magnetic Properties. Crystals. 2020; 10(7):592. https://doi.org/10.3390/cryst10070592
Chicago/Turabian StyleYou, Zhonglu, Yingying Luo, Susan Herringer, Yanmin Li, Silvio Decurtins, Karl W. Krämer, and Shi-Xia Liu. 2020. "Formation of Tetranuclear Nickel(II) Complexes with Schiff-Bases: Crystal Structures and Magnetic Properties" Crystals 10, no. 7: 592. https://doi.org/10.3390/cryst10070592
APA StyleYou, Z., Luo, Y., Herringer, S., Li, Y., Decurtins, S., Krämer, K. W., & Liu, S.-X. (2020). Formation of Tetranuclear Nickel(II) Complexes with Schiff-Bases: Crystal Structures and Magnetic Properties. Crystals, 10(7), 592. https://doi.org/10.3390/cryst10070592







