Synthesis of a Novel In2O3-InN Bottle Nanotube Using In-Situ Partial Oxidation with Enhanced Gas Sensing Platform to Detect NO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Fabrication and Measurement
3. Results
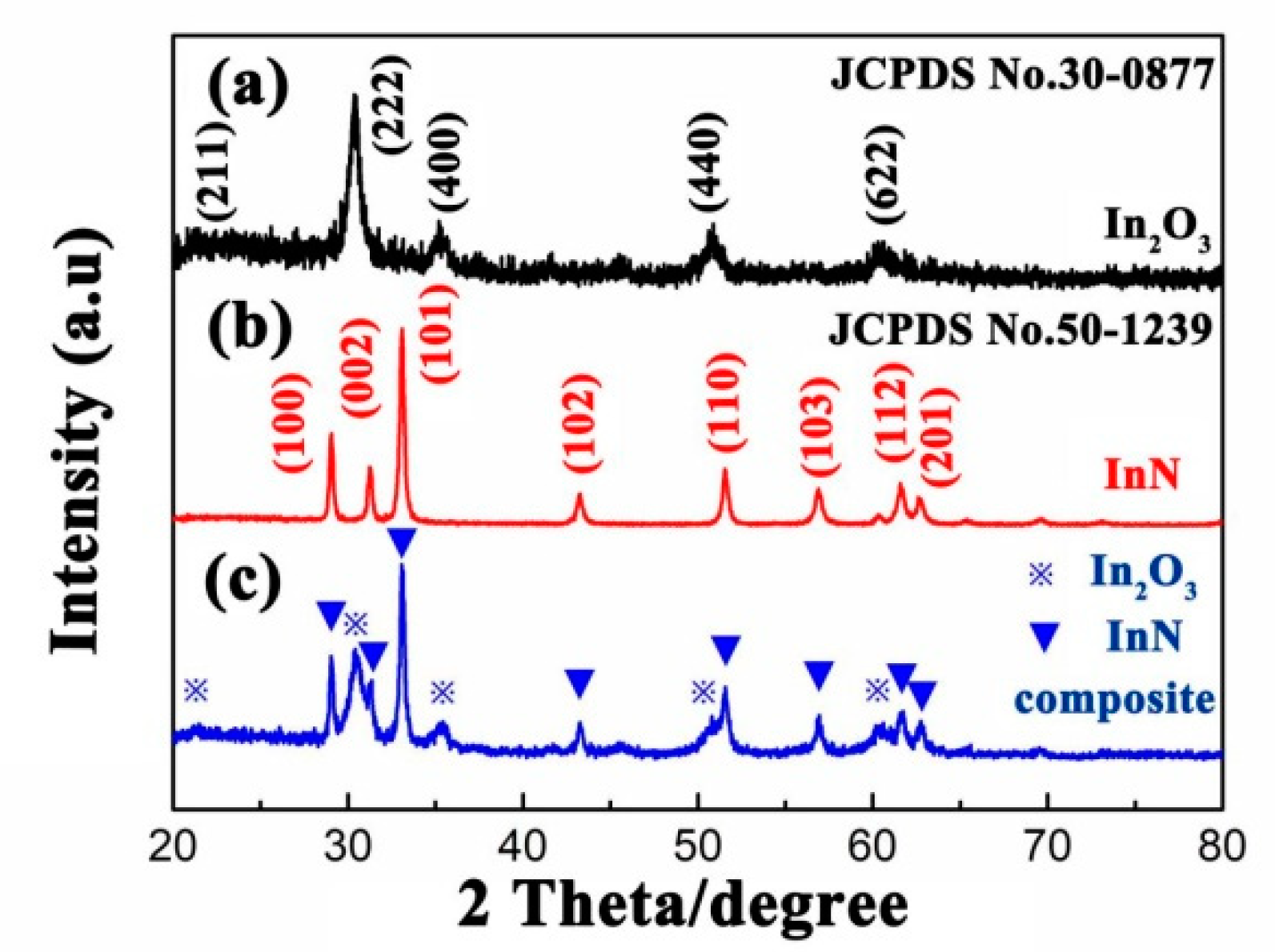
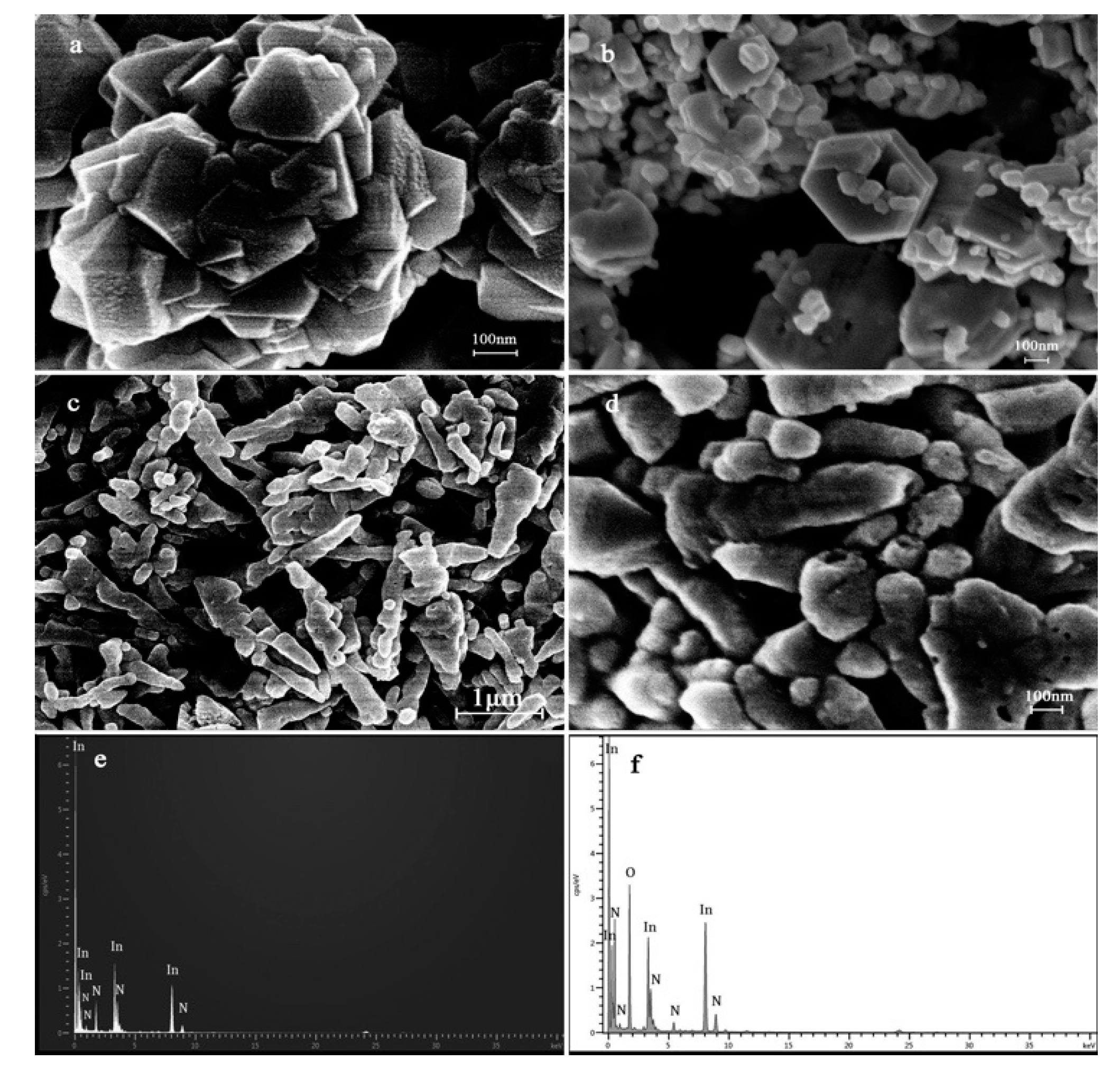
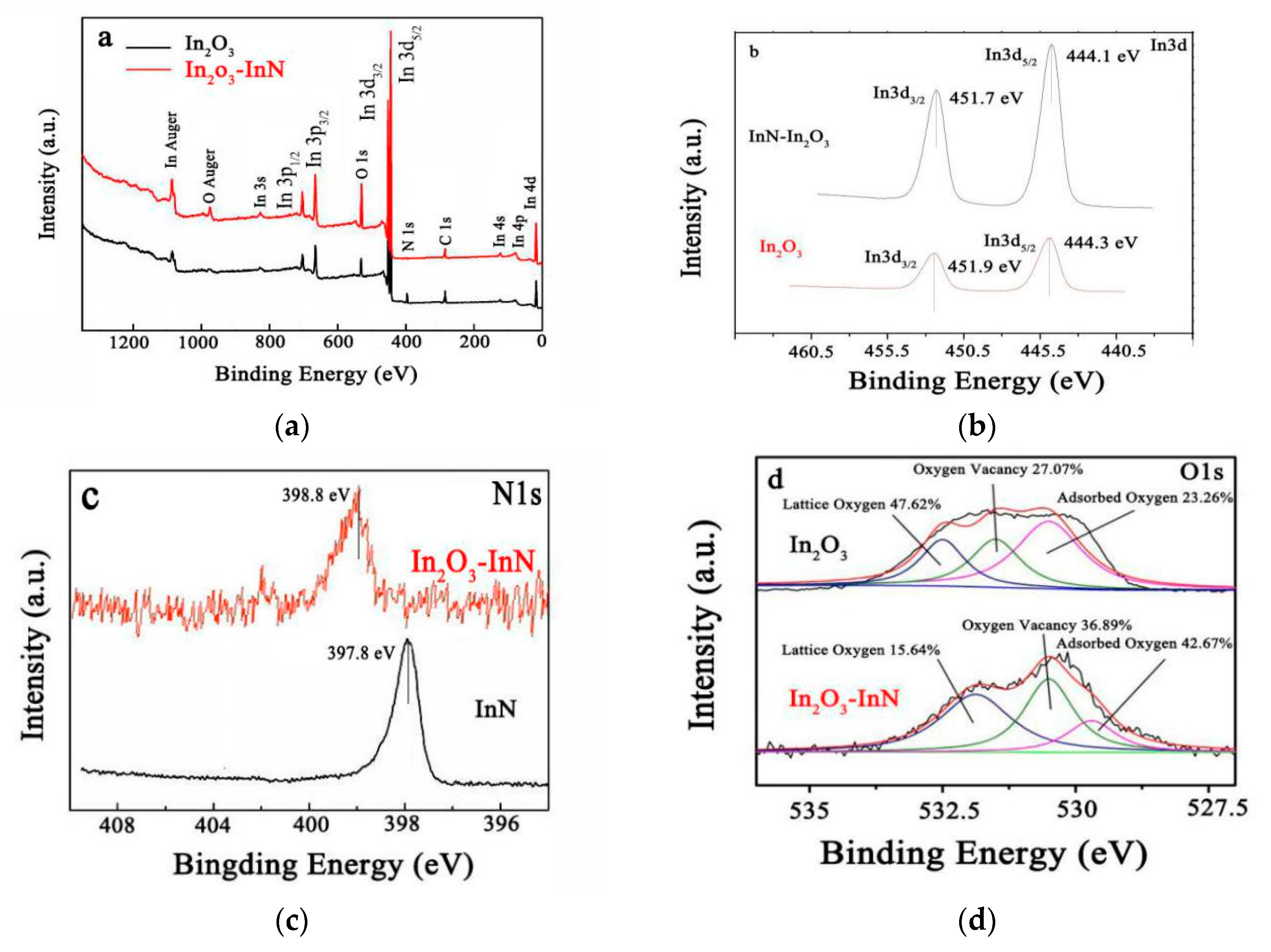
3.1. Materials Characterization
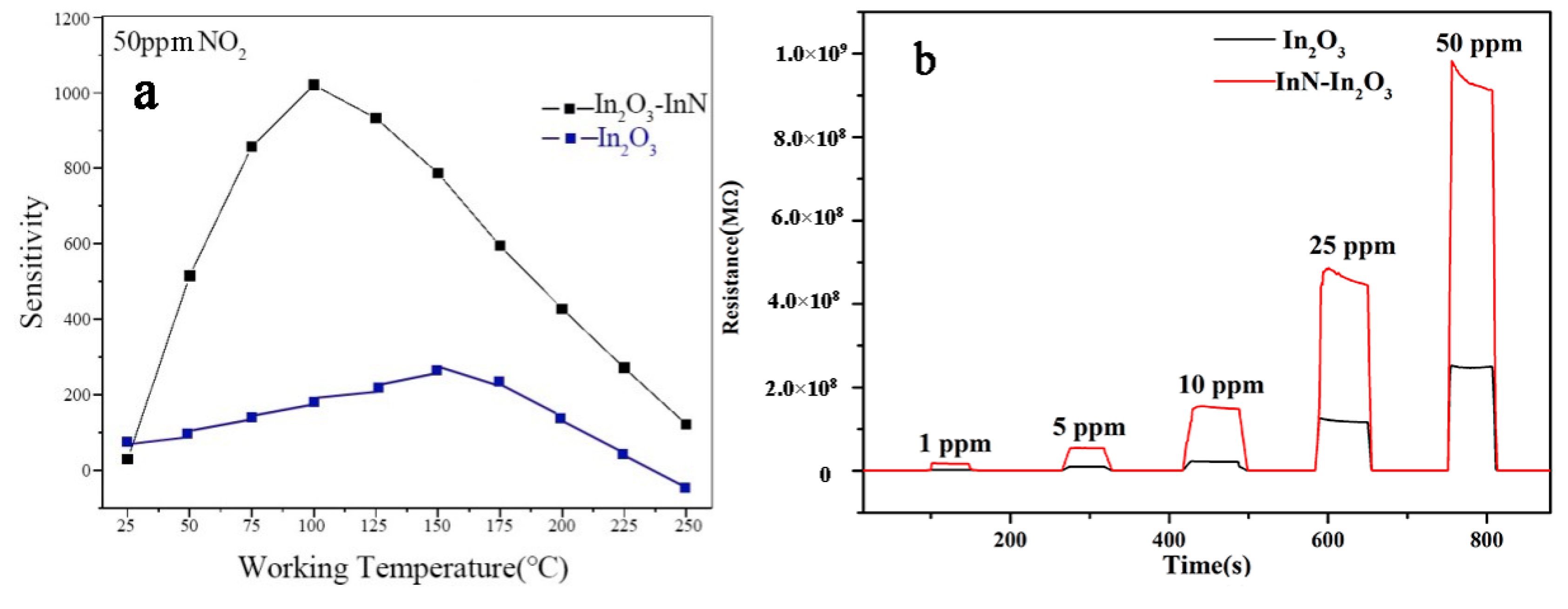
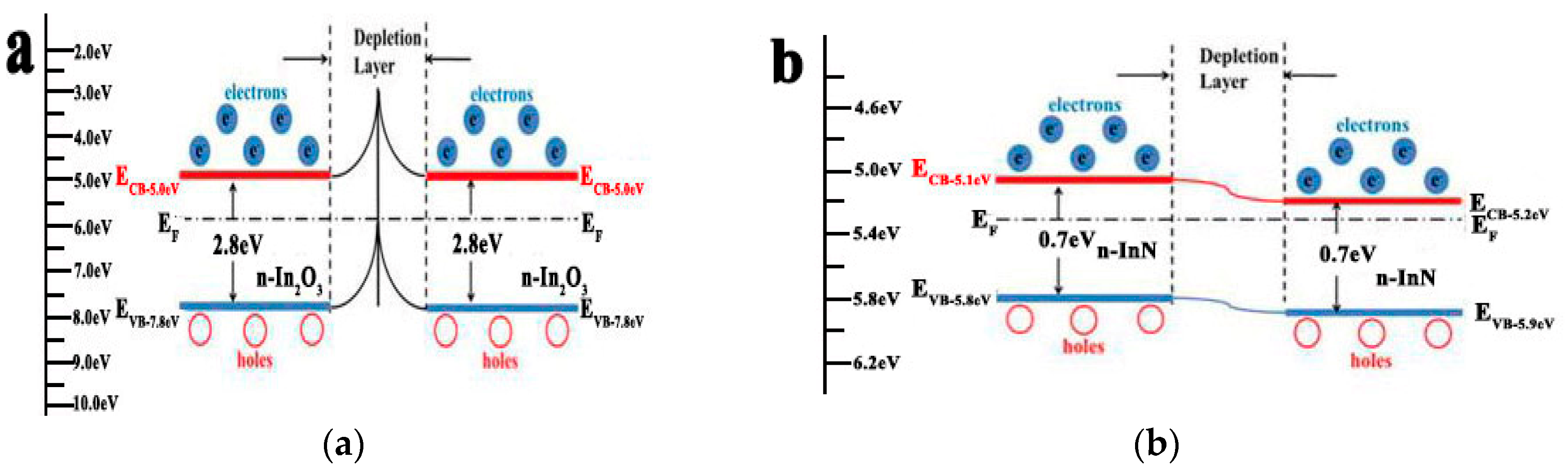
3.2. Gas Sensing Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khiabani, P.S.; Hosseinmardi, A.; Marzbanrad, E.; Ghashghaie, S.; Zamani, C.; Keyanpour-Rad, M.; Raissi, B. NO2 gas sensor fabrication through AC electrophoretic deposition from electrospun In2O3 nanoribbons. Sens. Actuators B Chem. 2012, 162, 102–107. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, R.K.; Lee, P.S. Gold-nanoparticle-functionalized InO nanowires as CO gas sensors with a significant enhancement in response. Acs Appl. Mater. Interfaces 2011, 3, 2246. [Google Scholar]
- Liu, L.; Song, P.; Yang, Z.; Wang, Q. Ultra-fast responding C2H5OH sensors based on hierarchical assembly of SnO2 nanorods on cube-like alpha-Fe2O3. Sens. Actuators B Chem. 2018, 29, 5446–5453. [Google Scholar]
- Piot, D.; Smagghe, G.; Montheillet, F.J.M.S.F. Modeling of Grain-Boundary Mobility and Nucleation Rate During Discontinuous Dynamic Recrystallization in Ni-Nb Alloys and a High-Purity Alloy Derived from SAE 304L. Mater. Sci. Forum 2017, 879, 1501–1506. [Google Scholar] [CrossRef]
- Brigante, D.J.N. New composite materials. Nature 2014, 442, 282–286. [Google Scholar]
- Sharma, A.; Tomar, M.; Gupta, V. Enhanced response characteristics of SnO2 thin film based NO2 gas sensor integrated with nanoscaled metal oxide clusters. Sens. Actuators B Chem. 2013, 181, 735–742. [Google Scholar] [CrossRef]
- Capra, P.P.; Galliana, F.L.; Latino, M.; Bonavita, A.; Donato, N.; Neri, G. A High-Resistance Measurement Setup for MOX Sensing Materials Characterization. Sens. Microsyst. 2014, 268, 149–154. [Google Scholar]
- Shlimak, I.; Ginodman, V.; Gerber, A.B.; Milner, A.; Friedland, K.J.; Paul, D.J. Transverse “resistance overshoot” in a Si/SiGe two-dimensional electron gas in the quantum Hall effect regime. EPL 2005, 69, 997. [Google Scholar] [CrossRef]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.D.; Tiemann, M. Ordered Mesoporous In2O3: Synthesis by Structure Replication and Application as a Methane Gas Sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar] [CrossRef]
- Pearton, S.J.; Ren, F.; Wang, Y.L.; Chu, B.H.; Chen, K.H.; Chang, C.Y.; Lim, W.; Lin, J.; Norton, D.P. Recent advances in wide bandgap semiconductor biological and gas sensors. Progess Mater. Sci. 2010, 55, 1–59. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Z.; Wang, L.; Chuai, X.; Zhang, S.; Lu, G. Hydrothermal synthesis of Ce-doped hierarchical flower-like In2O3 microspheres and their excellent gas-sensing properties. Sens. Actuators B Chem. 2018, 255, 1211–1219. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, D.; Jiao, X.; Liu, S. Research on preparation of In2O3 ceramic nanofiber mats by electrospinning and its gas sensing properties. J. Chem. Ind. Eng. 2011, 32, 1–4. [Google Scholar]
- Chen, C.; Chen, D.; Jiao, X.; Wang, C. Ultrathin corundum-type In2O3 nanotubes derived from orthorhombic InOOH: Synthesis and formation mechanism. Chem. Commun. 2006, 2006, 4632–4634. [Google Scholar] [CrossRef]
- Jahangir, I.; Wilson, A.; Uddin, M.A.; Chandrashekhar, M.V.; Koley, G. Oxygen plasma treated graphene/InN nanowire heterojunction based sensors for toxic gas detection. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Schwenzer, B.; Loeffler, L.; Seshadri, R.; Keller, S.; Lange, F.F.; DenBaars, S.P.; Mishra, U.K. Preparation of indium nitride micro- and nanostructures by ammonolysis of indium oxide. J. Mater. Chem. 2004, 14, 637. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Zhang, Z.; Dou, X.; Liu, L.; Zhao, X.; Liu, D.; Song, L.; Xiang, Y.; Zhou, J.; et al. Bulk-quantity synthesis of single-crystalline indium nitride nanobelts. Chem. Phys. Lett. 2005, 411, 361–365. [Google Scholar] [CrossRef]
- Wang, F.; Xue, C.; Zhuang, H.; Zhang, X.; Ai, Y.; Sun, L.; Yang, Z.; Li, H. Growth and characterization of the InN film ammonification technique. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 664–667. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, Q.; Chen, S.; Yue, T.; Han, B.; Feng, W.; Yang, D. Preparation and Gas Sensing Performance of Hierarchical Porous ZnO-based Materials with Sunflower Rods as a Biological Template. Chem. Res. Chin. Univ. 2019, 35, 755–761. [Google Scholar] [CrossRef]
- Itagaki, Y.; Mori, M.; Hosoya, Y.; Aono, H.; Sadaoka, Y. O3 and NO2 sensing properties of SmFe1-xCoxO3 perovskite oxides. Sens. Actuators B 2007, 122, 315–320. [Google Scholar] [CrossRef]
- Su, X.; Gao, L.; Zhou, F.; Duan, G. A substrate-independent fabrication of hollow sphere arrays via template-assisted hydrothermal approach and their application in gas sensing. Sens. Actuators B Chem. 2017, 251, 74–85. [Google Scholar] [CrossRef]
- Wu, X.; Coutts, T.J.; Mulligan, W.P. Properties of Transparent Conducting Oxides Formed from CdO and ZnO Alloyed with SnO2 and In2O3. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 1057–1062. [Google Scholar] [CrossRef]
- Papageorgiou, P.; Zervos, M.; Othonos, A.J.N.R.L. An investigation into the conversion of In2O3 into InN nanowire. Nanoscale Res. Lett. 2011, 6, 311. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shen, X.; Zhu, G.; Chen, K. Preparation and gas-sensing performance of In2O3 porous nanoplatelets. Sens. Actuators B Chem. 2011, 155, 752–758. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Q.; Wu, G.; Wang, Y.; Sun, Y.; Feng, W. Synthesis of a Novel In2O3-InN Bottle Nanotube Using In-Situ Partial Oxidation with Enhanced Gas Sensing Platform to Detect NO2. Crystals 2020, 10, 570. https://doi.org/10.3390/cryst10070570
Ning Q, Wu G, Wang Y, Sun Y, Feng W. Synthesis of a Novel In2O3-InN Bottle Nanotube Using In-Situ Partial Oxidation with Enhanced Gas Sensing Platform to Detect NO2. Crystals. 2020; 10(7):570. https://doi.org/10.3390/cryst10070570
Chicago/Turabian StyleNing, Qiuyang, Guoguang Wu, Yihui Wang, Yuanbo Sun, and Wei Feng. 2020. "Synthesis of a Novel In2O3-InN Bottle Nanotube Using In-Situ Partial Oxidation with Enhanced Gas Sensing Platform to Detect NO2" Crystals 10, no. 7: 570. https://doi.org/10.3390/cryst10070570
APA StyleNing, Q., Wu, G., Wang, Y., Sun, Y., & Feng, W. (2020). Synthesis of a Novel In2O3-InN Bottle Nanotube Using In-Situ Partial Oxidation with Enhanced Gas Sensing Platform to Detect NO2. Crystals, 10(7), 570. https://doi.org/10.3390/cryst10070570



