Mechanochemical Syntheses of Isostructural Luminescent Cocrystals of 9-Anthracenecarboxylic Acid with two Dipyridines Coformers
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Cocrystals via Ball Mill
2.3. Preparation of Single Crystals of the Cocrystals
2.4. Analytical Methods
2.4.1. Single-Crystal X-ray Diffraction (SCXRD)
2.4.2. Powder X-ray Diffraction (PXRD)
2.4.3. Elemental Analyses
2.4.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.5. Raman
2.4.6. Thermal Analysis (Differential Thermal Analysis-Thermogravimetric (DTA-TG))
2.4.7. Photoluminescence Spectroscopy
2.4.8. Computational Methods
3. Result and Discussion
3.1. Crystal Structures of ACA–BPEE (1) and ACA–BPE (2) Cocrystals
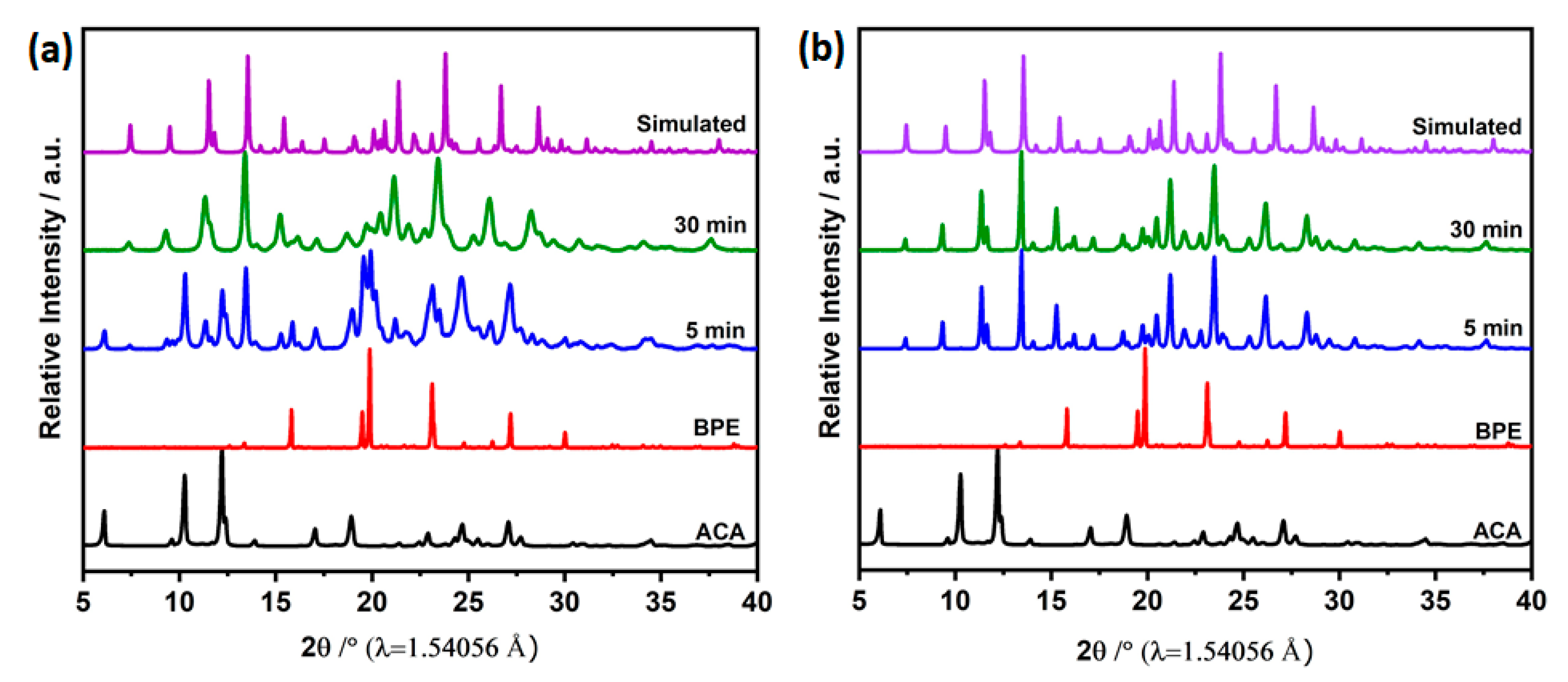
3.2. Mechanochemical Synthesis
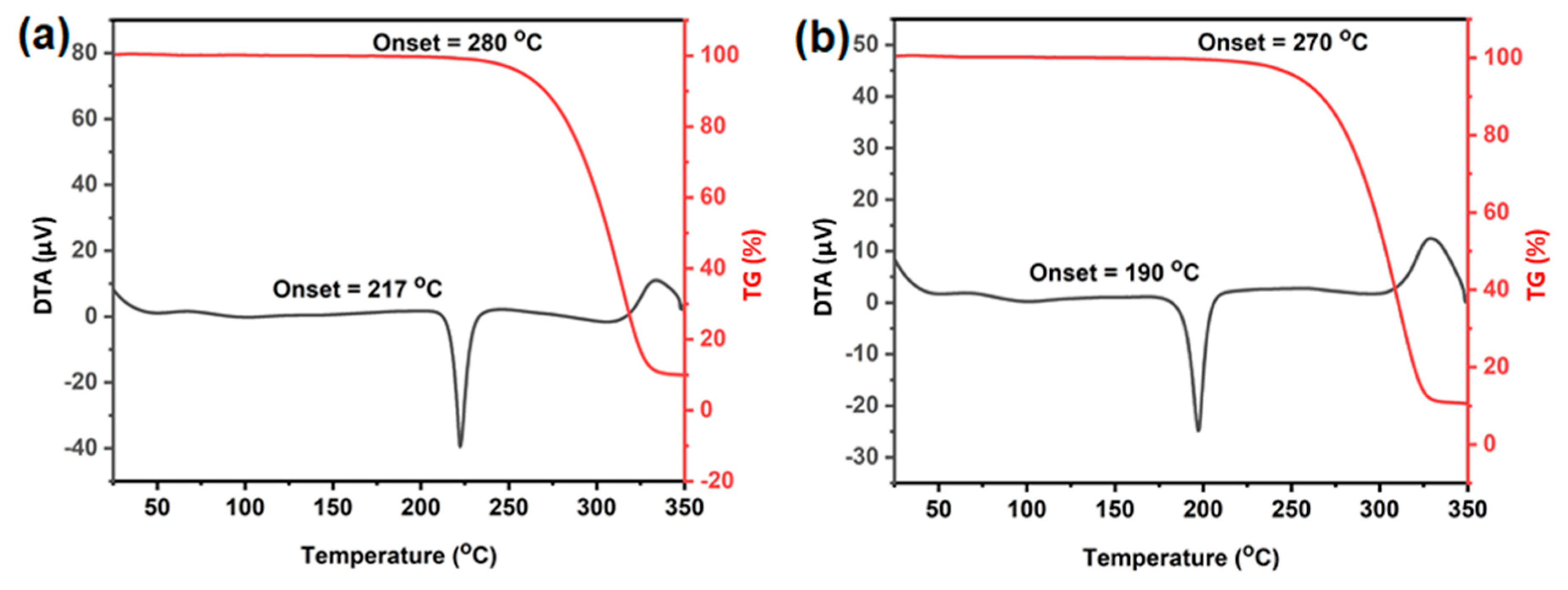
3.3. Thermal Analysis
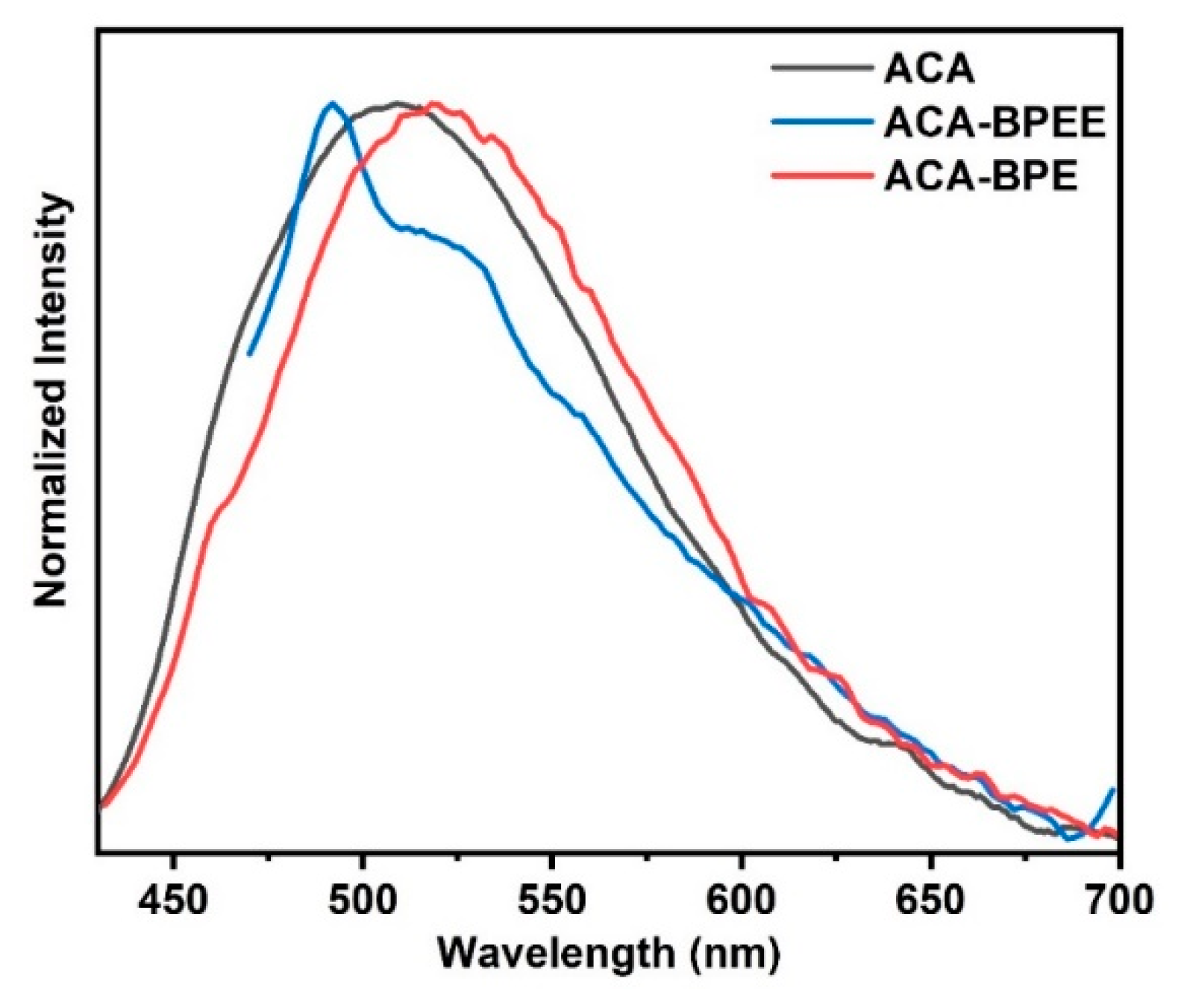
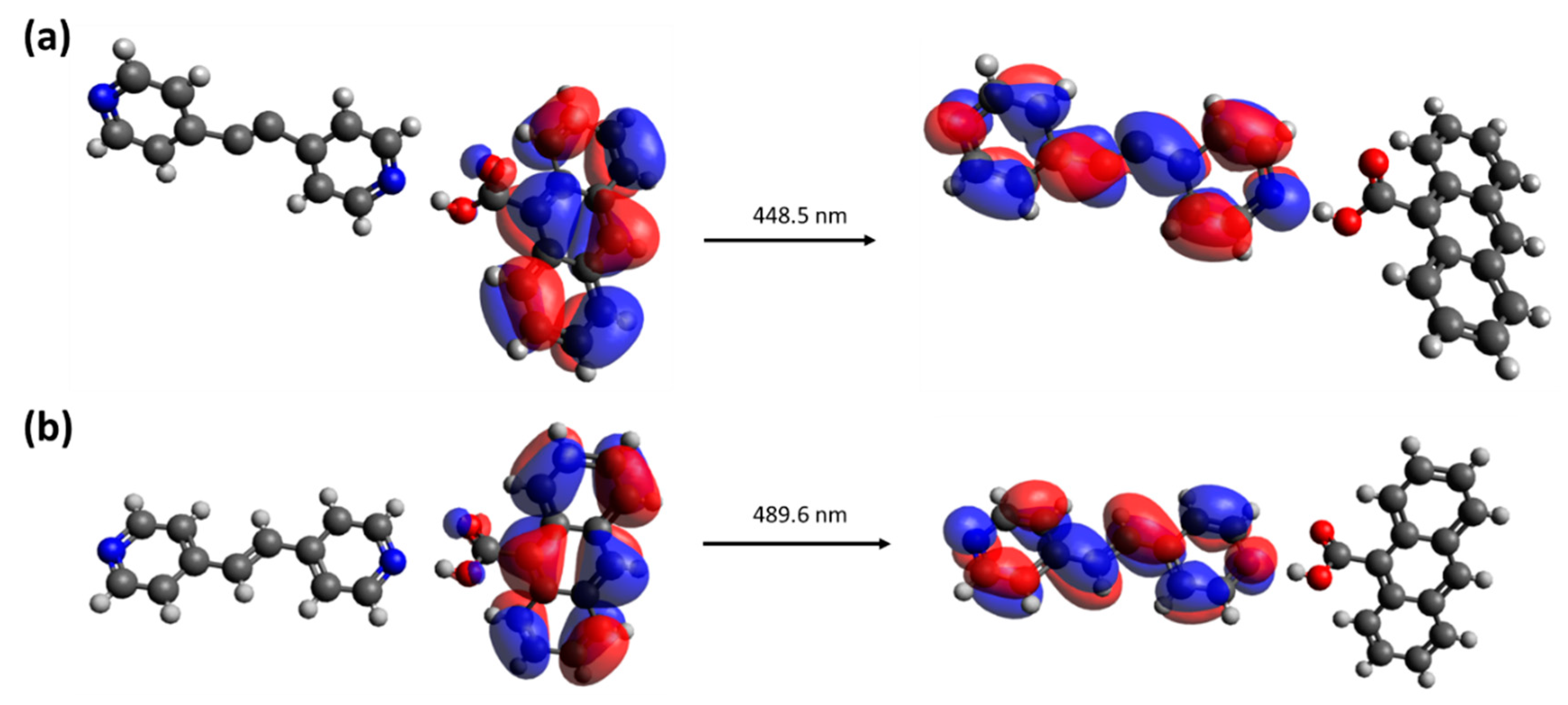
3.4. Luminescent Property
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef] [PubMed]
- Praveen, V.K.; Vedhanarayanan, B.; Mal, A.; Mishra, R.K.; Ajayaghosh, A. Self-Assembled Extended π-Systems for Sensing and Security Applications. Acc. Chem. Res. 2020, 53, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abadía, M.; Giménez, R.; Ros, M.B. Self-Assembled α-Cyanostilbenes for Advanced Functional Materials. Adv. Mater. 2018, 30, 1704161. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-Q.; Wang, X.-D.; Liao, L.-S. Recent Advances in 1D Organic Solid-State Lasers. Adv. Funct. Mater. 2019, 29, 1902981. [Google Scholar] [CrossRef]
- Shimizu, M.; Tamagawa, T.; Nishimura, K. 4,5-Diaminophthalimides: Highly efficient solid-state fluorophores and turn-on type fluorescent probes for hydrazine. Mater. Chem. Front. 2019, 3, 563–570. [Google Scholar] [CrossRef]
- Butkute, R.; Lygaitis, R.; Mimaite, V.; Gudeika, D.; Volyniuk, D.; Sini, G.; Grazulevicius, J.V. Bipolar highly solid-state luminescent phenanthroimidazole derivatives as materials for blue and white organic light emitting diodes exploiting either monomer, exciplex or electroplex emission. Dye. Pigment. 2017, 146, 425–437. [Google Scholar] [CrossRef]
- Anthony, S.P. Polymorph-Dependent Solid-State Fluorescence and Selective Metal-Ion-Sensor Properties of 2-(2-Hydroxyphenyl)-4(3H)-quinazolinone. Chem. Asian J. 2012, 7, 374–379. [Google Scholar] [CrossRef]
- Maini, L.; Gallino, F.; Zambianchi, M.; Durso, M.; Gazzano, M.; Rubini, K.; Gentili, D.; Manet, I.; Muccini, M.; Toffanin, S.; et al. Chemical design enables the control of conformational polymorphism in functional 2,3-thieno(bis)imide-ended materials. Chem. Commun. 2015, 51, 2033–2035. [Google Scholar] [CrossRef]
- Shi, J.; Yoon, S.-J.; Viani, L.; Park, S.Y.; Milián-Medina, B.; Gierschner, J. Twist-Elasticity-Controlled Crystal Emission in Highly Luminescent Polymorphs of Cyano-Substituted Distyrylbenzene (βDCS). Adv. Opt. Mater. 2017, 5, 1700340. [Google Scholar] [CrossRef]
- Anthony, S.P. Organic Solid-State Fluorescence: Strategies for Generating Switchable and Tunable Fluorescent Materials. ChemPlusChem 2012, 77, 518–531. [Google Scholar] [CrossRef]
- Li, E.; Jie, K.; Liu, M.; Sheng, X.; Zhu, W.; Huang, F. Vapochromic crystals: Understanding vapochromism from the perspective of crystal engineering. Chem. Soc. Rev. 2020, 49, 1517–1544. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Tiekink, E. Organic Crystal Engineering: Frontiers in Crystal Engineering; WILEY: New York, NY, USA, 2010. [Google Scholar]
- Bond, A.D. What is a co-crystal? CrystEngComm 2007, 9, 833–834. [Google Scholar] [CrossRef]
- Harmsen, B.; Leyssens, T. Enabling Enantiopurity: Combining Racemization and Dual-Drug Co-crystal Resolution. Cryst. Growth Des. 2018, 18, 3654–3660. [Google Scholar] [CrossRef]
- Martins, I.C.B.; Sardo, M.; Alig, E.; Fink, L.; Schmidt, M.U.; Mafra, L.; Duarte, M.T. Enhancing Adamantylamine Solubility through Salt Formation: Novel Products Studied by X-ray Diffraction and Solid-State NMR. Cryst. Growth Des. 2019, 19, 1860–1873. [Google Scholar] [CrossRef]
- Mazzeo, P.P.; Carraro, C.; Monica, A.; Capucci, D.; Pelagatti, P.; Bianchi, F.; Agazzi, S.; Careri, M.; Raio, A.; Carta, M.; et al. Designing a Palette of Cocrystals Based on Essential Oil Constituents for Agricultural Applications. Acs Sustain. Chem. Eng. 2019, 7, 17929–17940. [Google Scholar] [CrossRef]
- Sarmah, K.K.; Boro, K.; Arhangelskis, M.; Thakuria, R. Crystal structure landscape of ethenzamide: A physicochemical property study. CrystEngComm 2017, 19, 826–833. [Google Scholar] [CrossRef]
- Kumari, N.; Bhattacharya, B.; Roy, P.; Michalchuk, A.A.L.; Emmerling, F.; Ghosh, A. Enhancing the Pharmaceutical Properties of Pirfenidone by Mechanochemical Cocrystallization. Cryst. Growth Des. 2019, 19, 6482–6492. [Google Scholar] [CrossRef]
- Yan, D.; Delori, A.; Lloyd, G.O.; Friščić, T.; Day, G.M.; Jones, W.; Lu, J.; Wei, M.; Evans, D.G.; Duan, X. A Cocrystal Strategy to Tune the Luminescent Properties of Stilbene-Type Organic Solid-State Materials. Angew. Chem. Int. Ed. 2011, 50, 12483–12486. [Google Scholar] [CrossRef]
- Li, J.; Takaishi, S.; Fujinuma, N.; Endo, K.; Yamashita, M.; Matsuzaki, H.; Okamoto, H.; Sawabe, K.; Takenobu, T.; Iwasa, Y. Enhancement of luminescence intensity in TMPY/perylene co-single crystals. J. Mater. Chem. 2011, 21, 17662–17666. [Google Scholar] [CrossRef]
- Zhou, T.; Jia, T.; Zhao, S.; Guo, J.; Zhang, H.; Wang, Y. Acid-Stimuli-Luminescence and Carbonyl-Proton Interaction Dependent Emission Properties of 2,6-Biphenyl-4-pyrone Crystals. Cryst. Growth Des. 2012, 12, 179–184. [Google Scholar] [CrossRef]
- Fischer, F.; Lubjuhn, D.; Greiser, S.; Rademann, K.; Emmerling, F. Supply and Demand in the Ball Mill: Competitive Cocrystal Reactions. Cryst. Growth Des. 2016, 16, 5843–5851. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Friščić, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Fischer, F.; Heidrich, A.; Greiser, S.; Benemann, S.; Rademann, K.; Emmerling, F. Polymorphism of Mechanochemically Synthesized Cocrystals: A Case Study. Cryst. Growth Des. 2016, 16, 1701–1707. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Li, F.; Wang, G.-W. Mechanochemistry of fullerenes and related materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef]
- Gomollón-Bel, F. Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable. Chem. Int. 2019, 41, 12. [Google Scholar] [CrossRef]
- Tan, D.; Loots, L.; Friščić, T. Towards medicinal mechanochemistry: Evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef]
- Han, L.-L.; Li, Z.-H.; Chen, J.-S.; Wang, X.-P.; Sun, D. Solution and Mechanochemical Syntheses of Two Novel Cocrystals: Ligand Length Modulated Interpenetration of Hydrogen-Bonded 2D 63-hcb Networks Based on a Robust Trimeric Heterosynthon. Cryst. Growth Des. 2014, 14, 1221–1226. [Google Scholar] [CrossRef]
- Morelli Frin, K.P.; da Rocha, D.C.; Mamud, J.F.; Polo, A.S. Photoisomerization of di-nuclear rhenium(i) bpe-based compounds. Photochem. Photobiol. Sci. 2018, 17, 1443–1449. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Li, Y.-Q.; Tang, H.-H.; Lin, L.-R.; Ma, L.-H. Near-Infrared Photoluminescence and Reversible Trans-to-Cis Photoisomerization of Mononuclear and Binuclear Ytterbium(III) Complexes Functionalized by Azobenzene Groups. ACS Omega 2018, 3, 5480–5490. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R. Selective anionic dye adsorption, topology and luminescence study of structurally diverse cadmium(ii) coordination polymers. Inorg. Chem. Front. 2019, 6, 278–286. [Google Scholar] [CrossRef]
- Dannenbauer, N.; Matthes, P.R.; Scheller, T.P.; Nitsch, J.; Zottnick, S.H.; Gernert, M.S.; Steffen, A.; Lambert, C.; Müller-Buschbaum, K. Near-Infrared Luminescence and Inner Filter Effects of Lanthanide Coordination Polymers with 1,2-Di(4-pyridyl)ethylene. Inorg. Chem. 2016, 55, 7396–7406. [Google Scholar] [CrossRef] [PubMed]
- Podgajny, R.; Chorazy, S.; Nitek, W.; Rams, M.; Bałanda, M.; Sieklucka, B. {MnII9WV6}n Nanowires Organized into Three-Dimensional Hybrid Network of I1O2 Topology. Cryst. Growth Des. 2010, 10, 4693–4696. [Google Scholar] [CrossRef]
- Muñoz-Lara, F.J.; Gaspar, A.B.; Muñoz, M.C.; Arai, M.; Kitagawa, S.; Ohba, M.; Real, J.A. Sequestering Aromatic Molecules with a Spin-Crossover FeII Microporous Coordination Polymer. Chem. Eur. J. 2012, 18, 8013–8018. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G. SADABS 2.03; University of Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.; Bruno, I.; Chisholm, J.; Edgington, P.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. Mercury CSD 2.0-New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens Matter 2017, 29, 465901. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, E.R. Exchange-hole dipole moment and the dispersion interaction. J. Chem. Phys. 2005, 122, 154104. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wires Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Vahtras, O.; Almlöf, J.; Feyereisen, M.W. Integral approximations for LCAO-SCF calculations. Chem. Phys. Lett. 1993, 213, 514–518. [Google Scholar] [CrossRef]
- Parrish, R.M.; Burns, L.A.; Smith, D.G.A.; Simmonett, A.C.; DePrince, A.E.; Hohenstein, E.G.; Bozkaya, U.; Sokolov, A.Y.; Di Remigio, R.; Richard, R.M.; et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. [Google Scholar] [CrossRef]
- Parker, T.M.; Burns, L.A.; Parrish, R.M.; Ryno, A.G.; Sherrill, C.D. Levels of symmetry adapted perturbation theory (SAPT). I. Efficiency and performance for interaction energies. J. Chem. Phys. 2014, 140, 094106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Al-Kaysi, R.O.; Dillon, R.J.; Tham, F.S.; Bardeen, C.J. Crystal Structures and Photophysical Properties of 9-Anthracene Carboxylic Acid Derivatives for Photomechanical Applications. Cryst. Growth Des. 2011, 11, 4975–4983. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, F.; Salinas, C.; Al-Muhanna, M.K.; Tham, F.S.; Kisailus, D.; Al-Kaysi, R.O.; Bardeen, C.J. Improved Solid-State Photomechanical Materials by Fluorine Substitution of 9-Anthracene Carboxylic Acid. Chem. Mater. 2014, 26, 6007–6015. [Google Scholar] [CrossRef]
- Garza, A.J.; Scuseria, G.E. Predicting Band Gaps with Hybrid Density Functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Bredas, J.-L. Mind the gap! Mater. Horiz. 2014, 1, 17–19. [Google Scholar] [CrossRef]



| Compound Name | ACA-BPEE (1) | ACA-BPE (2) |
|---|---|---|
| Temperature/K | 150 | 150 |
| Formula | C21H15O2N | C21H16O2N |
| Formula Weight | 313.34 | 314.35 |
| Crystal System | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c |
| a/Å | 23.9052(11) | 23.7683(10) |
| b/Å | 9.9355(4) | 10.1119(5) |
| c/Å | 13.0565(5) | 13.0879(6) |
| α/° | 90 | 90 |
| β/° | 93.704(2) | 93.773(2) |
| γ/° | 90 | 90 |
| V/Å3 | 3094.6(2) | 3138.8(2) |
| Z | 8 | 8 |
| Dc/g cm−3 | 1.345 | 1.322 |
| μ/mm−1 | 0.087 | 0.085 |
| F(000) | 1312 | 1304 |
| θ range/° | 2.2–26.8 | 2.2–26.8 |
| Reflections collected | 40,599 | 29,294 |
| Unique reflections | 3310 | 3357 |
| Reflections I > 2σ(I) | 2845 | 2331 |
| Rint | 0.039 | 0.094 |
| Data/restraints/parameters | 3310/0/218 | 3357/0/226 |
| Goodness of fit (F2) | 1.09 | 1.05 |
| R1 (I > 2σ(I)) | 0.0415 | 0.0597 |
| wR2 (I > 2σ(I)) | 0.1293 | 0.1572 |
| CCDC No. | 1971303 | 1971304 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feiler, T.; Bhattacharya, B.; A. L. Michalchuk, A.; Schröder, V.; List-Kratochvil, E.; Emmerling, F. Mechanochemical Syntheses of Isostructural Luminescent Cocrystals of 9-Anthracenecarboxylic Acid with two Dipyridines Coformers. Crystals 2020, 10, 889. https://doi.org/10.3390/cryst10100889
Feiler T, Bhattacharya B, A. L. Michalchuk A, Schröder V, List-Kratochvil E, Emmerling F. Mechanochemical Syntheses of Isostructural Luminescent Cocrystals of 9-Anthracenecarboxylic Acid with two Dipyridines Coformers. Crystals. 2020; 10(10):889. https://doi.org/10.3390/cryst10100889
Chicago/Turabian StyleFeiler, Torvid, Biswajit Bhattacharya, Adam A. L. Michalchuk, Vincent Schröder, Emil List-Kratochvil, and Franziska Emmerling. 2020. "Mechanochemical Syntheses of Isostructural Luminescent Cocrystals of 9-Anthracenecarboxylic Acid with two Dipyridines Coformers" Crystals 10, no. 10: 889. https://doi.org/10.3390/cryst10100889
APA StyleFeiler, T., Bhattacharya, B., A. L. Michalchuk, A., Schröder, V., List-Kratochvil, E., & Emmerling, F. (2020). Mechanochemical Syntheses of Isostructural Luminescent Cocrystals of 9-Anthracenecarboxylic Acid with two Dipyridines Coformers. Crystals, 10(10), 889. https://doi.org/10.3390/cryst10100889








