Quantum-Sized Zinc Oxide Nanoparticles Synthesised within Mesoporous Silica (SBA-11) by Humid Thermal Decomposition of Zinc Acetate
Abstract
1. Introduction
180–400 °C)
2. Materials and Methods
2.1. Preparing SBA-11
2.2. Synthesis of ZnO Particles inside SBA-11
2.3. Control SBA-11
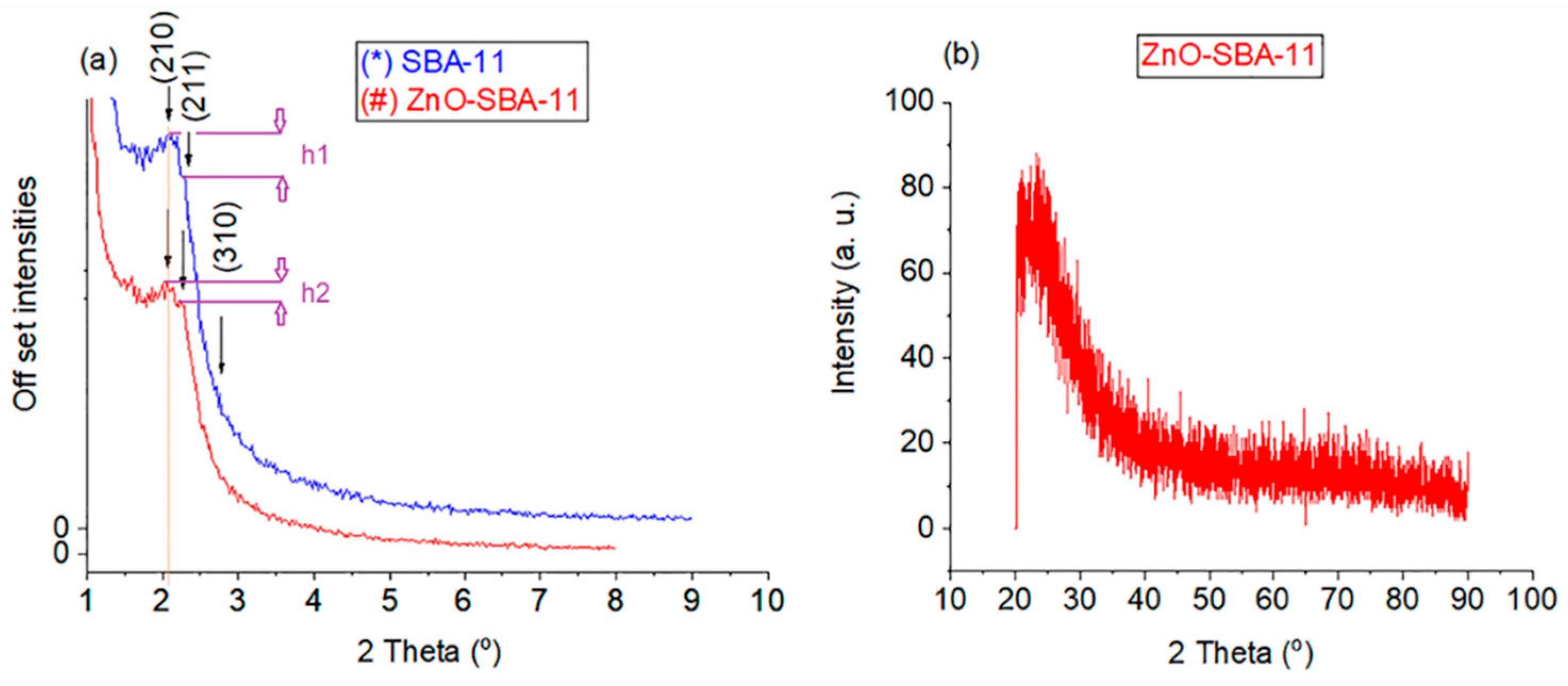
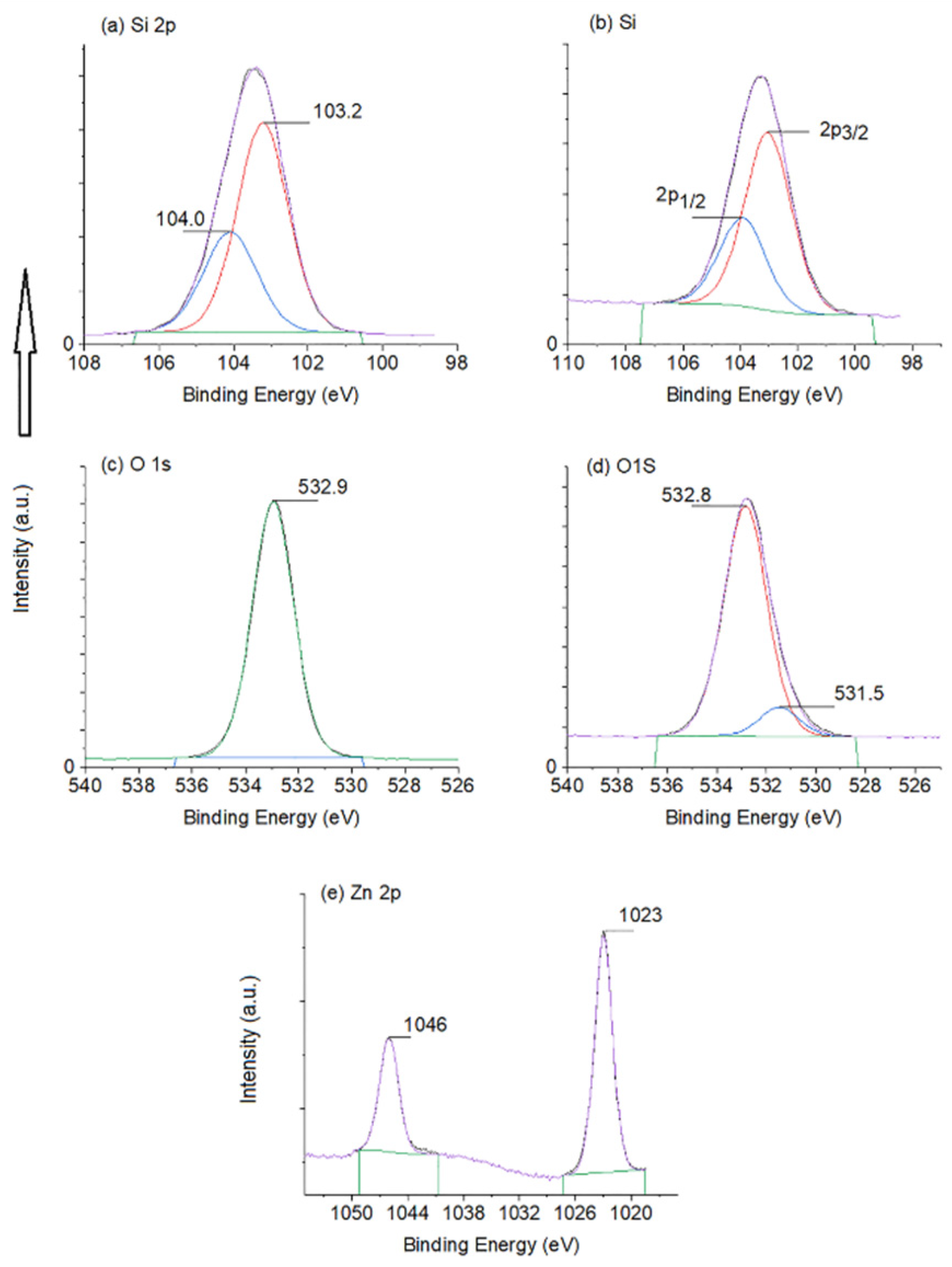
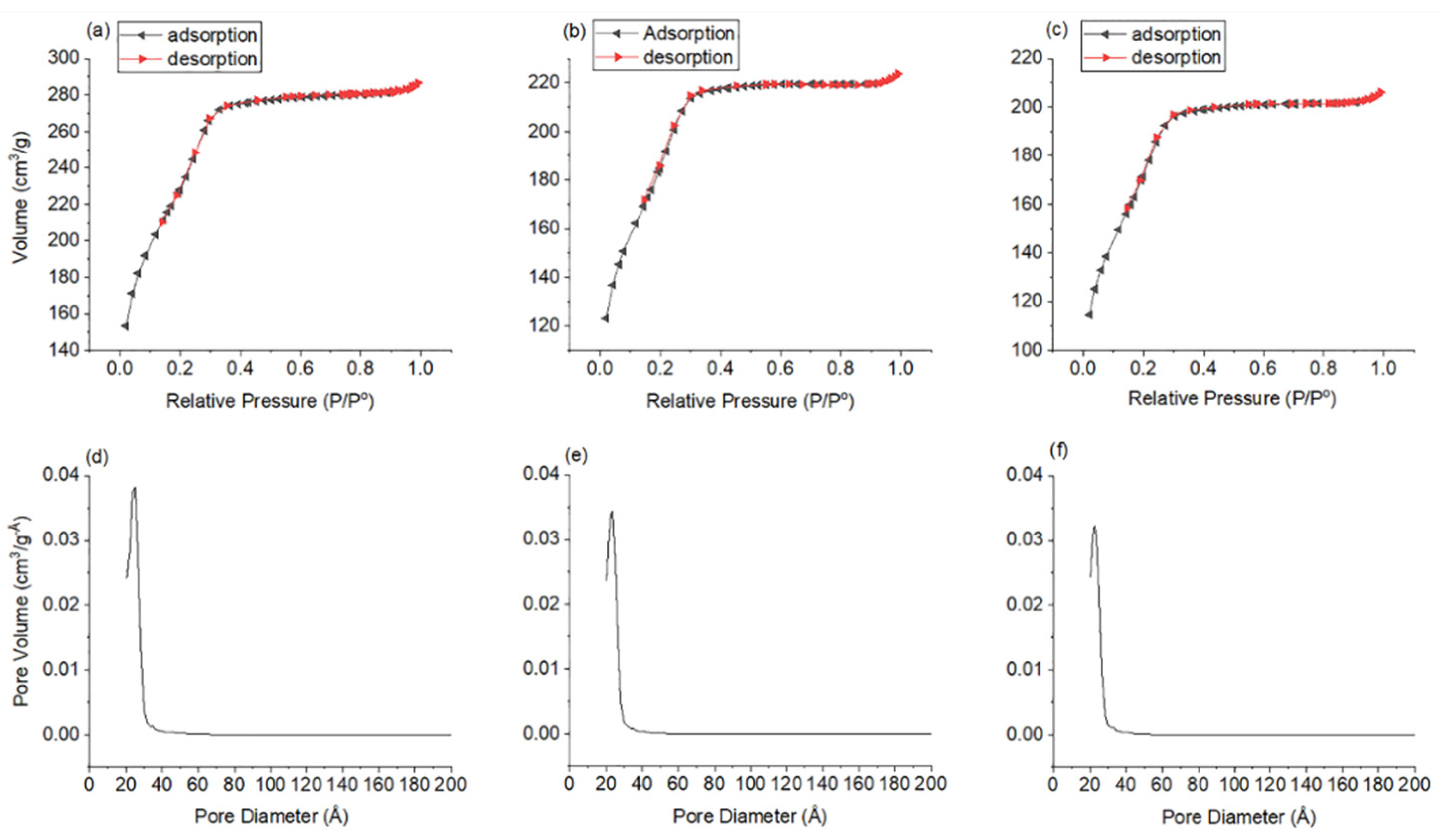
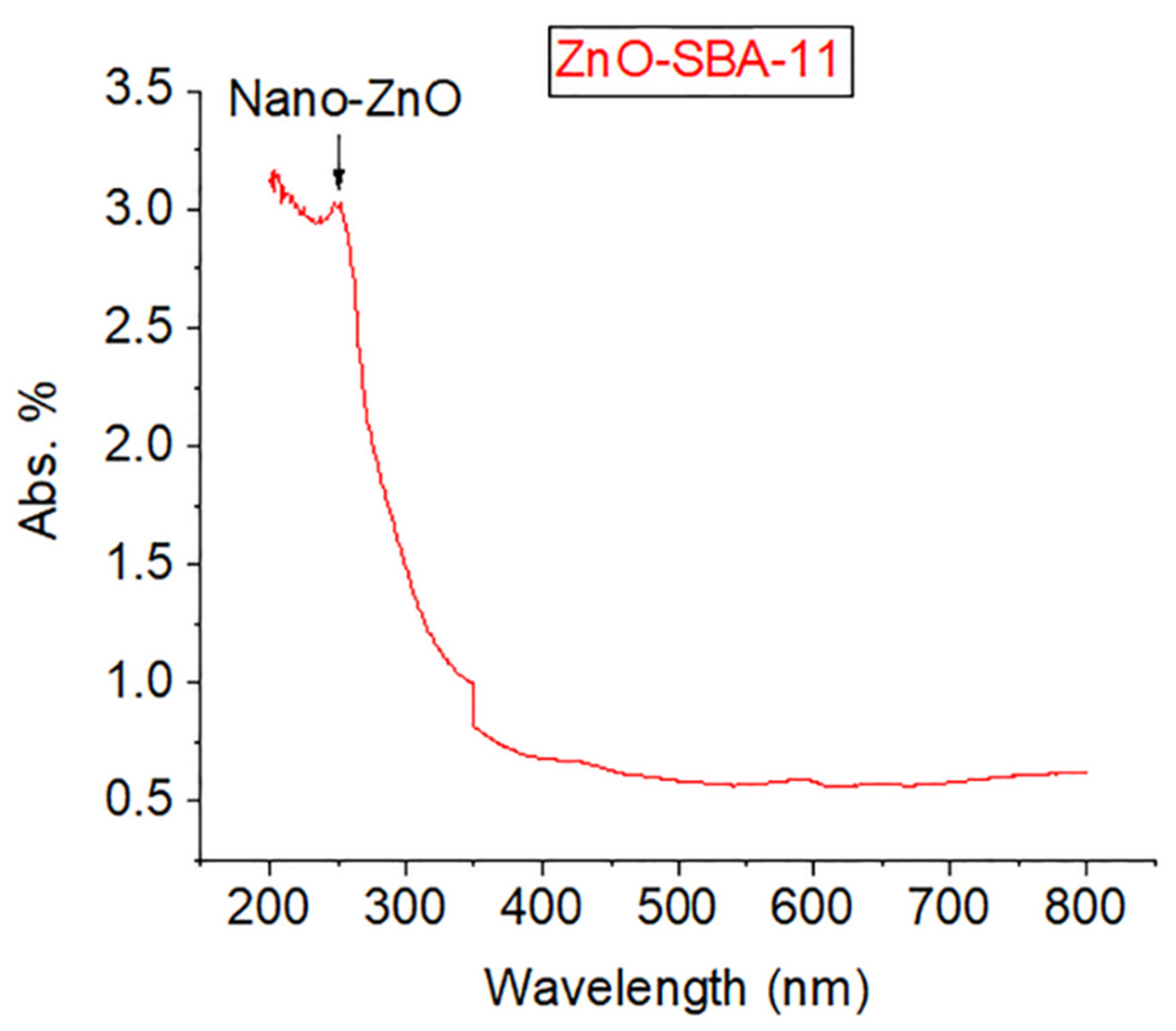
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munirah; Khan, Z.R.; Aziz, A.; Khan, M.S.; Khandaker, M.U. Influence of zinc concentration on band gap and sub-band gap absorption on ZnO nanocrystalline thin films sol-gel grown. Mater. Sci. Pol. 2017, 35, 246–253. [Google Scholar] [CrossRef]
- Arii, T.; Kishi, A. The effect of humidity on thermal process of zinc acetate. Thermochim. Acta 2003, 400, 175–185. [Google Scholar] [CrossRef]
- Schmitt, M. Synthesis and testing of ZnO nanoparticles for photo-initiation: Experimental observation of two different non-migration initiators for bulk polymerization. Nanoscale 2015, 7, 9532–9544. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qiu, W.; Wang, F.; Zhai, T.; Fang, P.; Lu, X.; Tong, Y. Three dimensional architectures: Design, assembly and application in electrochemical capacitors. J. Mater. Chem. A 2015, 3, 15792–15823. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Li, B.; Gu, P.; Xue, H.; Pang, H. Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors. J. Mater. Chem. A 2017, 5, 8155–8186. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Kimura, T. Synthesis, structure and photoelectrochemical performance of micro/nano-textured ZnO/eosin Y electrodes. Electrochim. Acta 2004, 49, 2287–2293. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar light harvesting with multinary metal chalcogenide nanocrystals. Chem. Soc. Rev. 2018, 47, 5354–5422. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, Z.Y.; Wang, Y.M.; Cao, Y.; Zhou, C.F.; Zhu, J.H. Fabrication of photoluminescent ZnO/SBA-15 through directly dispersing zinc nitrate into the as-prepared mesoporous silica occluded with template. J. Mater. Chem. 2006, 16, 1536–1542. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horizons 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, J.; Zhao, L.; Wang, W.; Gong, W.; Zheng, F. Zinc-iron silicate for heterogeneous catalytic ozonation of acrylic acid: Efficiency and mechanism. RSC Adv. 2020, 10, 9146–9154. [Google Scholar] [CrossRef]
- Doghri, H.; Baranova, E.A.; Albela, B.; Saïd-Zina, M.; Bonneviot, L. A bio-inspired zinc finger analogue anchored in 2D hexagonal mesoporous silica for room temperature CO2 activation: Via a hydrogenocarbonate route. New J. Chem. 2017, 41, 6795–6809. [Google Scholar] [CrossRef]
- Marković, S.; Stojković Simatović, I.; Ahmetović, S.; Veselinović, L.; Stojadinović, S.; Rac, V.; Škapin, S.D.; Bajuk Bogdanović, D.; Janković Častvan, I.; Uskoković, D. Surfactant-assisted microwave processing of ZnO particles: A simple way for designing the surface-to-bulk defect ratio and improving photo(electro)catalytic properties. RSC Adv. 2019, 9, 17165–17178. [Google Scholar] [CrossRef]
- Du, Z.; Artemyev, M.; Wang, J.; Tang, J. Performance improvement strategies for quantum dot-sensitized solar cells: A review. J. Mater. Chem. A 2019, 7, 2464–2489. [Google Scholar] [CrossRef]
- Tokumoto, M.S.; Briois, V.; Santilli, C.V.; Pulcinelli, S.H. Preparation of ZnO Nanoparticles: Structural Study. J. Sol-Gel Sci. Technol. 2003, 26, 547–551. [Google Scholar] [CrossRef]
- Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano 2009, 3, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Mahamuni, P.P.; Patil, P.M.; Dhanavade, M.J.; Badiger, M.V.; Shadija, P.G.; Lokhande, A.C.; Bohara, R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019, 17, 71–80. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ito, A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem. Soc. Rev. 2018, 47, 4954–4980. [Google Scholar] [CrossRef]
- Cai, Q.; Xu, J.; Yang, D.; Dai, Y.; Yang, G.; Zhong, C.; Gai, S.; He, F.; Yang, P. Polypyrrole-coated UCNPs@mSiO 2 @ZnO nanocomposite for combined photodynamic and photothermal therapy. J. Mater. Chem. B 2018, 6, 8148–8162. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Ghosh, H.N. Effect of particle size on the reactivity of quantum size ZnO nanoparticles and charge-transfer dynamics with adsorbed catechols. Langmuir 2003, 19, 3006–3012. [Google Scholar] [CrossRef]
- Aqeel, T.; Bumajdad, A. facile and direct preparation of ultrastable mesoporous silica with silver nanoclusters: High surface area. ChemistryOpen 2020, 9, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Raevskaya, A.E.; Panasiuk, Y.V.; Stroyuk, O.L.; Kuchmiy, S.Y.; Dzhagan, V.M.; Milekhin, A.G.; Yeryukov, N.A.; Sveshnikova, L.A.; Rodyakina, E.E.; Plyusnin, V.F.; et al. Spectral and luminescent properties of ZnO-SiO2 core-shell nanoparticles with size-selected ZnO cores. RSC Adv. 2014, 4, 63393–63401. [Google Scholar] [CrossRef]
- Tokumoto, M.S.; Pulcinelli, S.H.; Santilli, C.V.; Craievich, A.F. SAXS study of the kinetics of formation of ZnO colloidal suspensions. J. Non. Cryst. Solids 1999, 247, 176–182. [Google Scholar] [CrossRef]
- Mikrajuddin; Iskandar, F.; Okuyama, K.; Shi, F.G. Stable photoluminescence of zinc oxide quantum dots in silica nanoparticles matrix prepared by the combined sol-gel and spray drying method. J. Appl. Phys. 2001, 89, 6431–6434. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, K.; Wei, X.; Hao, T.; Xu, Y.; Lv, X.; Zhang, Y. A mesoporous fluorescent sensor based on ZnO nanorods for the fluorescent detection and selective recognition of tetracycline. RSC Adv. 2016, 6, 71061–71069. [Google Scholar] [CrossRef]
- He, J.H.; Ho, S.T.; Wu, T.B.; Chen, L.J.; Wang, Z.L. Electrical and photoelectrical performances of nano-photodiode based on ZnO nanowires. Chem. Phys. Lett. 2007, 435, 119–122. [Google Scholar] [CrossRef]
- Zhou, K.; Ding, Y.; Zhang, L.; Wu, H.; Guo, J. Synthesis of mesoporous ZnO/TiO2-SiO2 composite material and its application in photocatalytic adsorption desulfurization without the addition of an extra oxidant. Dalt. Trans. 2020, 49, 1600–1612. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 2019, 11, 6315–6333. [Google Scholar] [CrossRef]
- Qian, G.; Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Ye, J. An immuno-potentiating vehicle made of mesoporous silica-zinc oxide micro-rosettes with enhanced doxorubicin loading for combined chemoimmunotherapy. Chem. Commun. 2019, 55, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef]
- Wu, S.; Huang, X.; Du, X. pH- and redox-triggered synergistic controlled release of a ZnO-gated hollow mesoporous silica drug delivery system. J. Mater. Chem. B 2015, 3, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, H.; Shen, Z.; Hao, L.; Chen, H.; Zhou, X. Synthesis, characterization, and comparison of antibacterial effects and elucidating the mechanism of ZnO, CuO and CuZnO nanoparticles supported on mesoporous silica SBA-3. RSC Adv. 2020, 10, 2767–2785. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Y.; Zhang, J.; Wang, H.; Wang, M.; Wu, C.; Cheng, D.; Chi, Y.; Zhao, Z. The synergetic effect of a structure-engineered mesoporous SiO2-ZnO composite for doxycycline adsorption. RSC Adv. 2019, 9, 38772–38782. [Google Scholar] [CrossRef]
- Govender, K.; Boyle, D.S.; Kenway, P.B.; O’Brien, P. Understanding the factors that govern the deposition and morphology of thin films of ZnO from aqueous solution. J. Mater. Chem. 2004, 14, 2575–2591. [Google Scholar] [CrossRef]
- Spanhel, L.; Anderson, M.A. Semiconductor clusters in the sol-gel process: Quantized aggregation, gelation, and crystal growth in concentrated ZnO colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Synthesis and growth of ZnO nanowires. J. Phys. Chem. B 1998, 102, 5566–5572. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Wei, X.Q.; Man, B.Y.; Liu, M.; Xue, C.S.; Zhuang, H.Z.; Yang, C. Blue luminescent centers and microstructural evaluation by XPS and Raman in ZnO thin films annealed in vacuum, N2 and O2. Phys. B Condens. Matter 2007, 388, 145–152. [Google Scholar] [CrossRef]
- Tkachenko, O.P.; Klementiev, K.V.; Löffler, E.; Ritzkopf, I.; Schüth, F.; Bandyopadhyay, M.; Grabowski, S.; Gies, H.; Hagen, V.; Muhler, M.; et al. The structure of zinc and copper oxide species hosted in porous siliceous matrices. Phys. Chem. Chem. Phys. 2003, 5, 4325–4334. [Google Scholar] [CrossRef]
- Mokaya, R. Improving the stability of mesoporous MCM-41 silica via thicker more highly condensed pore walls. J. Phys. Chem. B 1999, 103, 10204–10208. [Google Scholar] [CrossRef]
- Cassiers, K.; Linssen, T.; Mathieu, M.; Benjelloun, M.; Schrijnemakers, K.; Van Der Voort, P.; Cool, P.; Vansant, E.F. A detailed study of thermal, hydrothermal, and mechanical stabilities of a wide range of surfactant assembled mesoporous silicas. Chem. Mater. 2002, 14, 2317–2324. [Google Scholar] [CrossRef]
- Linssen, T.; Cassiers, K.; Cool, P.; Vansant, E.F. Mesoporous templated silicates: An overview of their synthesis, catalytic activation and evaluation of the stability. Adv. Colloid Interface Sci. 2003, 103, 121–147. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, W.H.; Shi, J.L.; Wang, L.Z.; Yan, D.S. Preparation and characterization of ZnO clusters inside mesoporous silica. Chem. Mater. 2000, 12, 1408–1413. [Google Scholar] [CrossRef]
- Pesika, N.S.; Stebe, K.J.; Searson, P.C. Relationship between absorbance spectra and particle size distributions for quantum-sized nanocrystals. J. Phys. Chem. B 2003, 107, 10412–10415. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aqeel, T.; Greer, H.F. Quantum-Sized Zinc Oxide Nanoparticles Synthesised within Mesoporous Silica (SBA-11) by Humid Thermal Decomposition of Zinc Acetate. Crystals 2020, 10, 549. https://doi.org/10.3390/cryst10060549
Aqeel T, Greer HF. Quantum-Sized Zinc Oxide Nanoparticles Synthesised within Mesoporous Silica (SBA-11) by Humid Thermal Decomposition of Zinc Acetate. Crystals. 2020; 10(6):549. https://doi.org/10.3390/cryst10060549
Chicago/Turabian StyleAqeel, Tariq, and Heather F. Greer. 2020. "Quantum-Sized Zinc Oxide Nanoparticles Synthesised within Mesoporous Silica (SBA-11) by Humid Thermal Decomposition of Zinc Acetate" Crystals 10, no. 6: 549. https://doi.org/10.3390/cryst10060549
APA StyleAqeel, T., & Greer, H. F. (2020). Quantum-Sized Zinc Oxide Nanoparticles Synthesised within Mesoporous Silica (SBA-11) by Humid Thermal Decomposition of Zinc Acetate. Crystals, 10(6), 549. https://doi.org/10.3390/cryst10060549





