Nuclear Quadrupole Resonance (NQR)—A Useful Spectroscopic Tool in Pharmacy for the Study of Polymorphism
Abstract
1. Introduction
2. Examples of 14N NQR Studies of Polymorphism
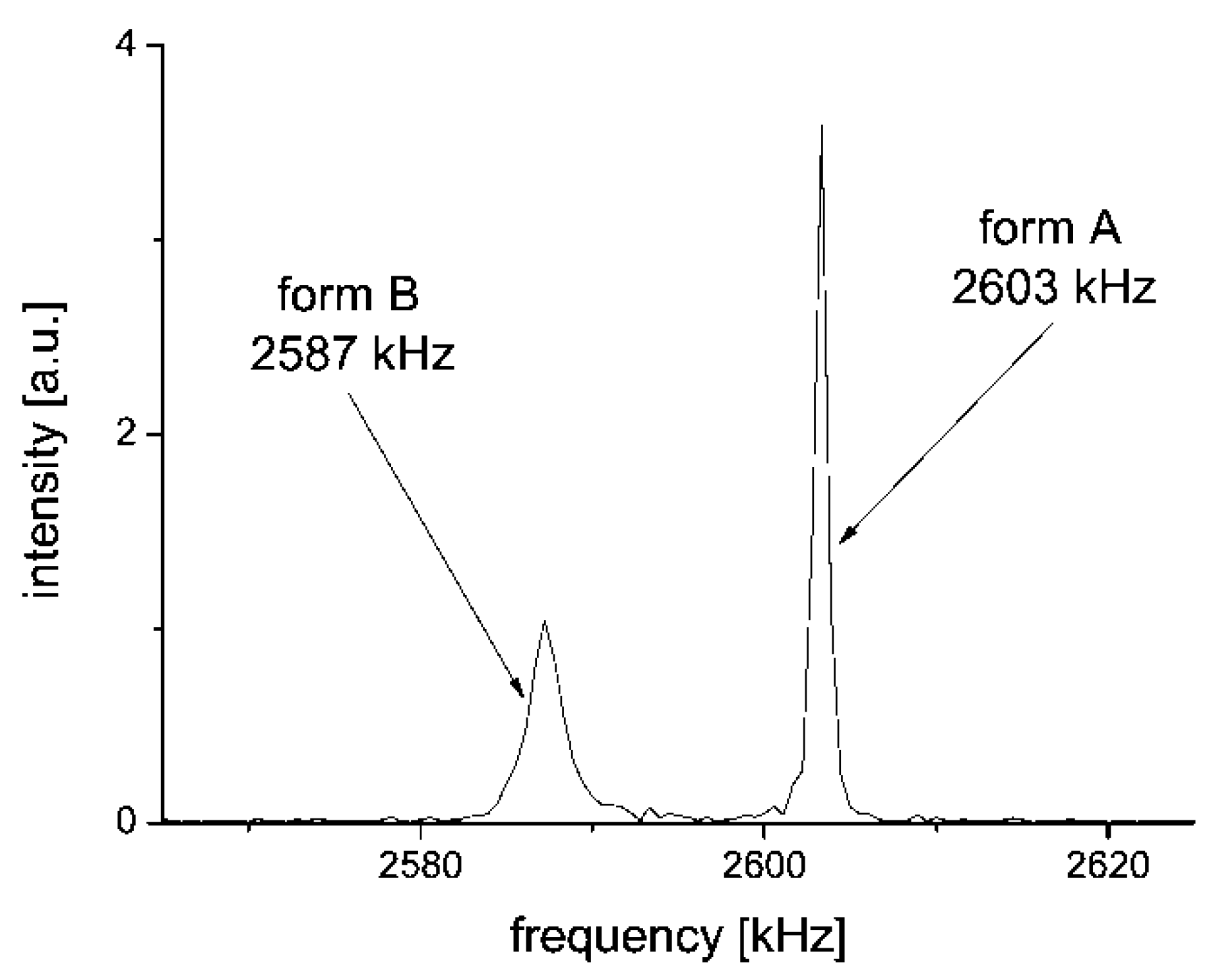
2.1. 14N NQR in Sulfanilamide
2.2. 14N NQR in Famotidine
- (i)
- (ii)
- the correlation between the two pairs ν+/ν− of the two tentatively equivalent nitrogen sites of the polymorphs A and B, should agree with the same empirically confirmed ν+/ν− correlation, characteristic for many other molecules, containing the relevant structurally equivalent nitrogen surroundings [43,44,58,59,60,61] (cf. Figure 8)”.
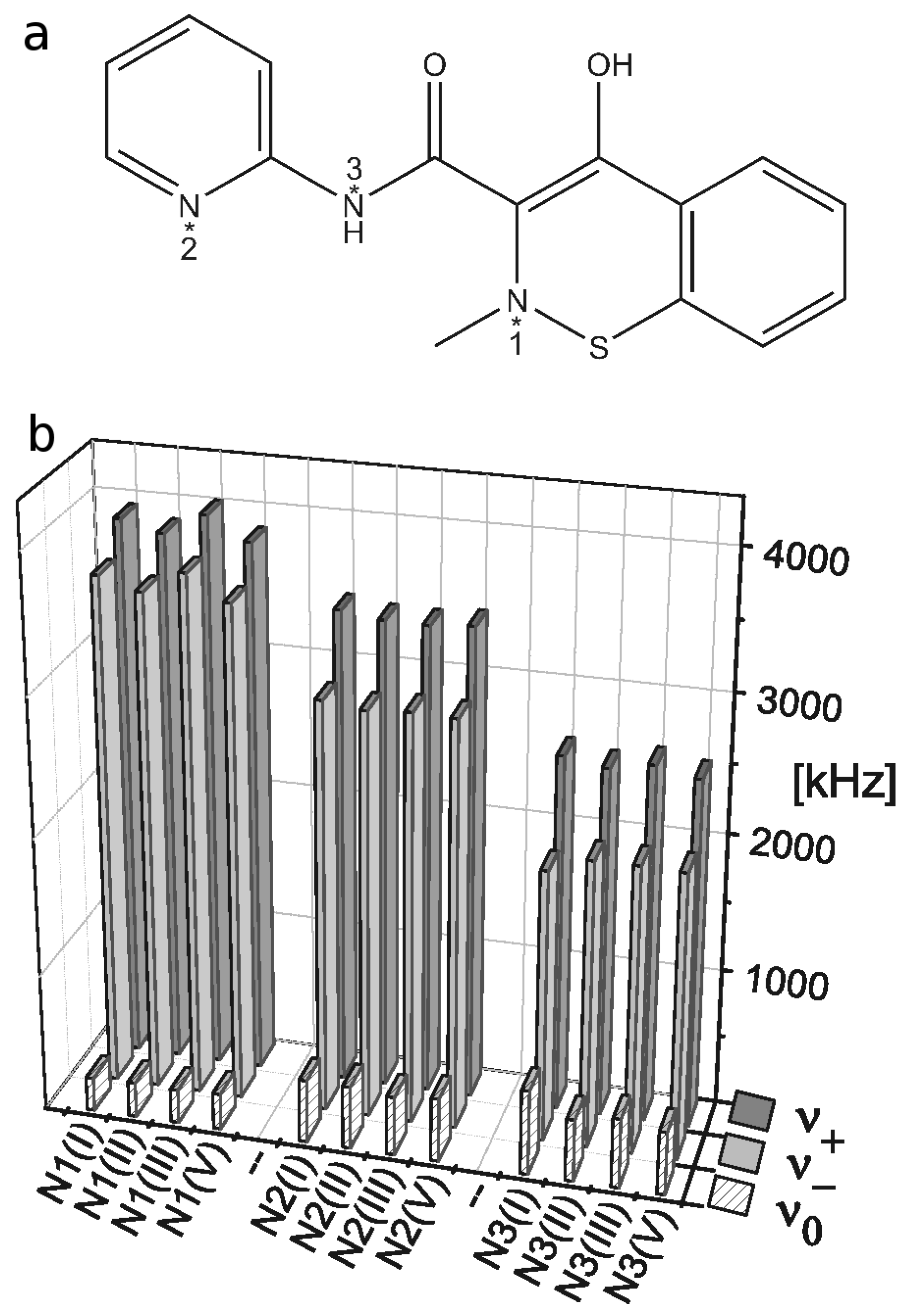
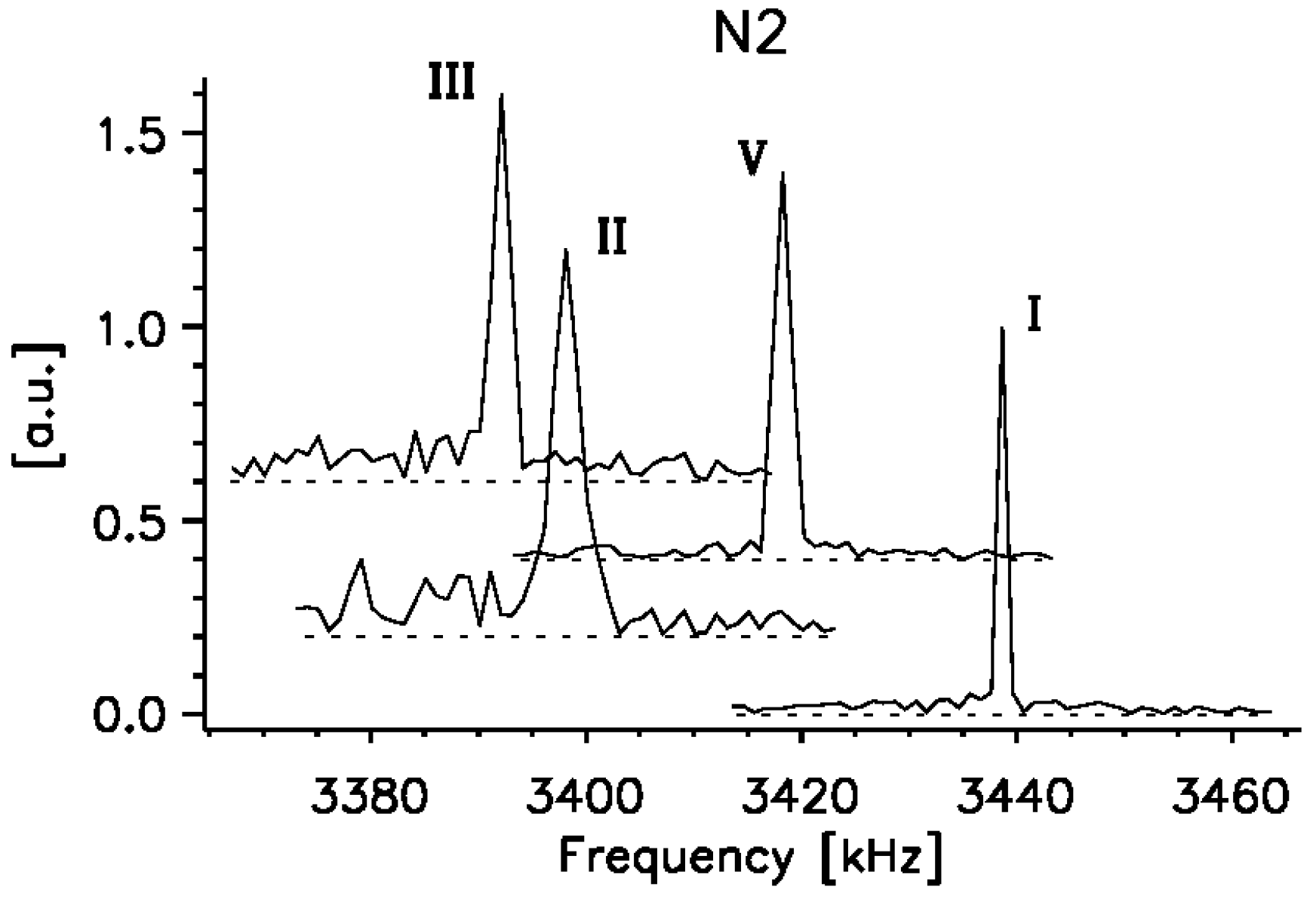
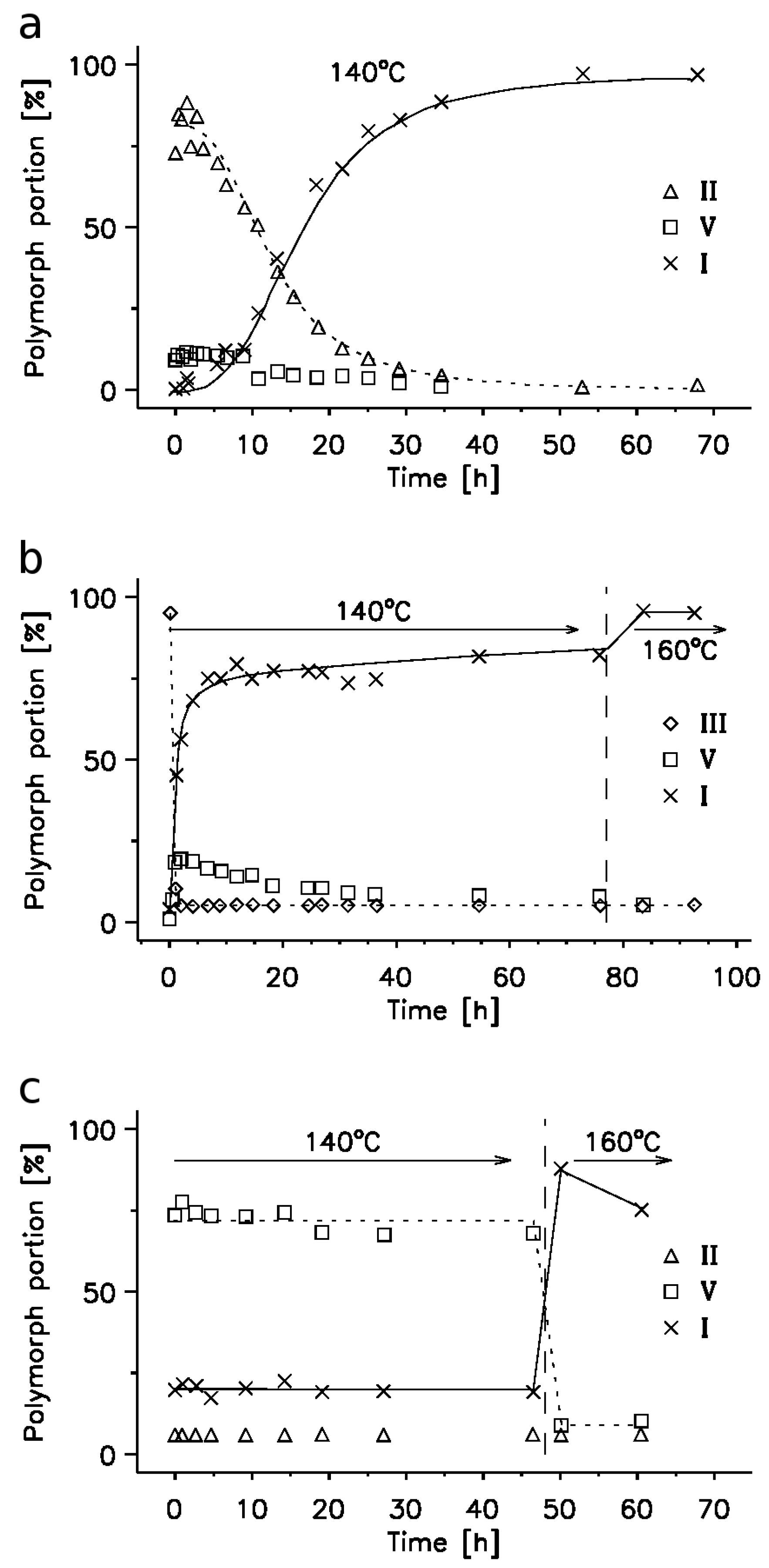
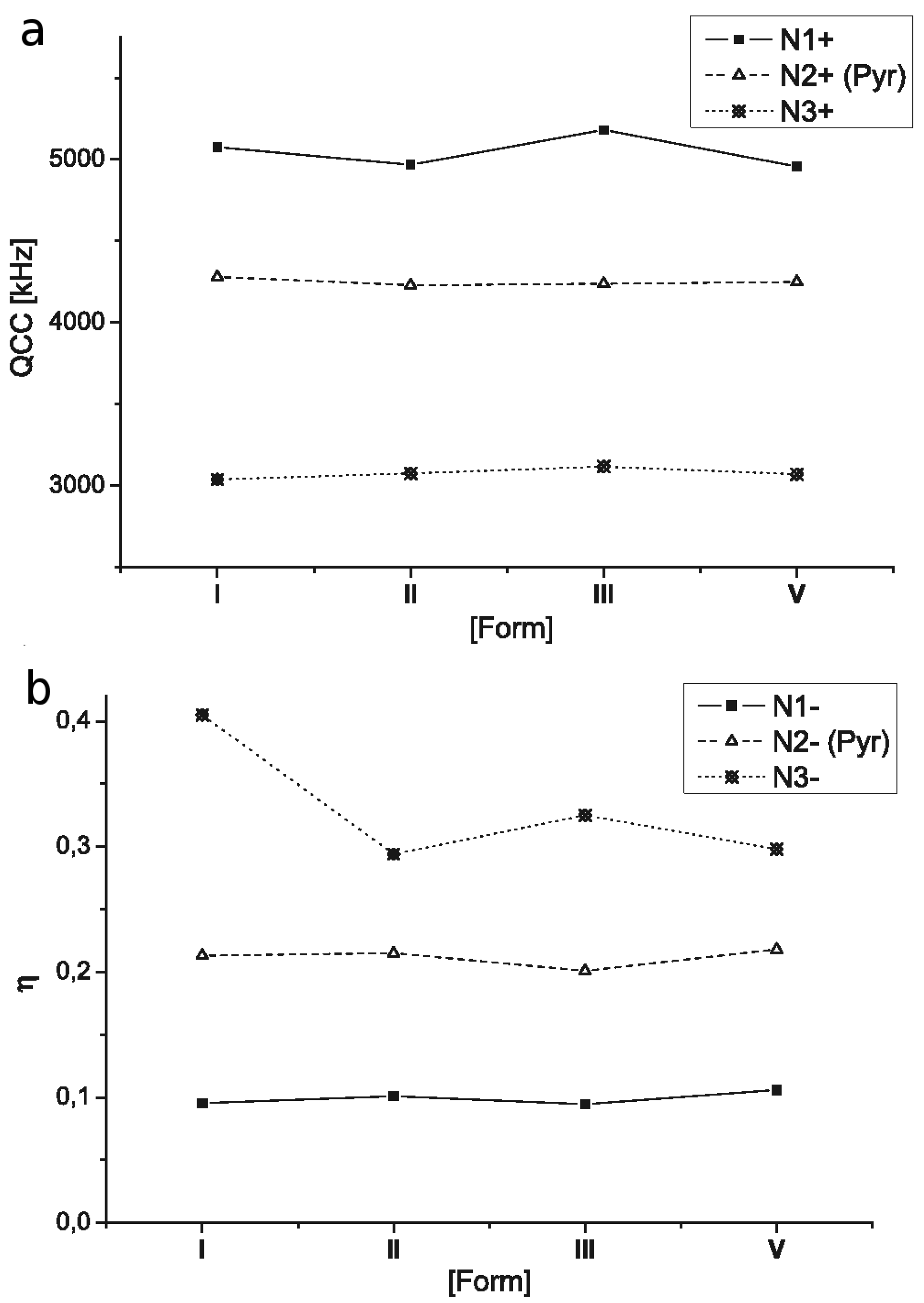
2.3. 14N NQR Study of Piroxicam
- (i)
- (ii)
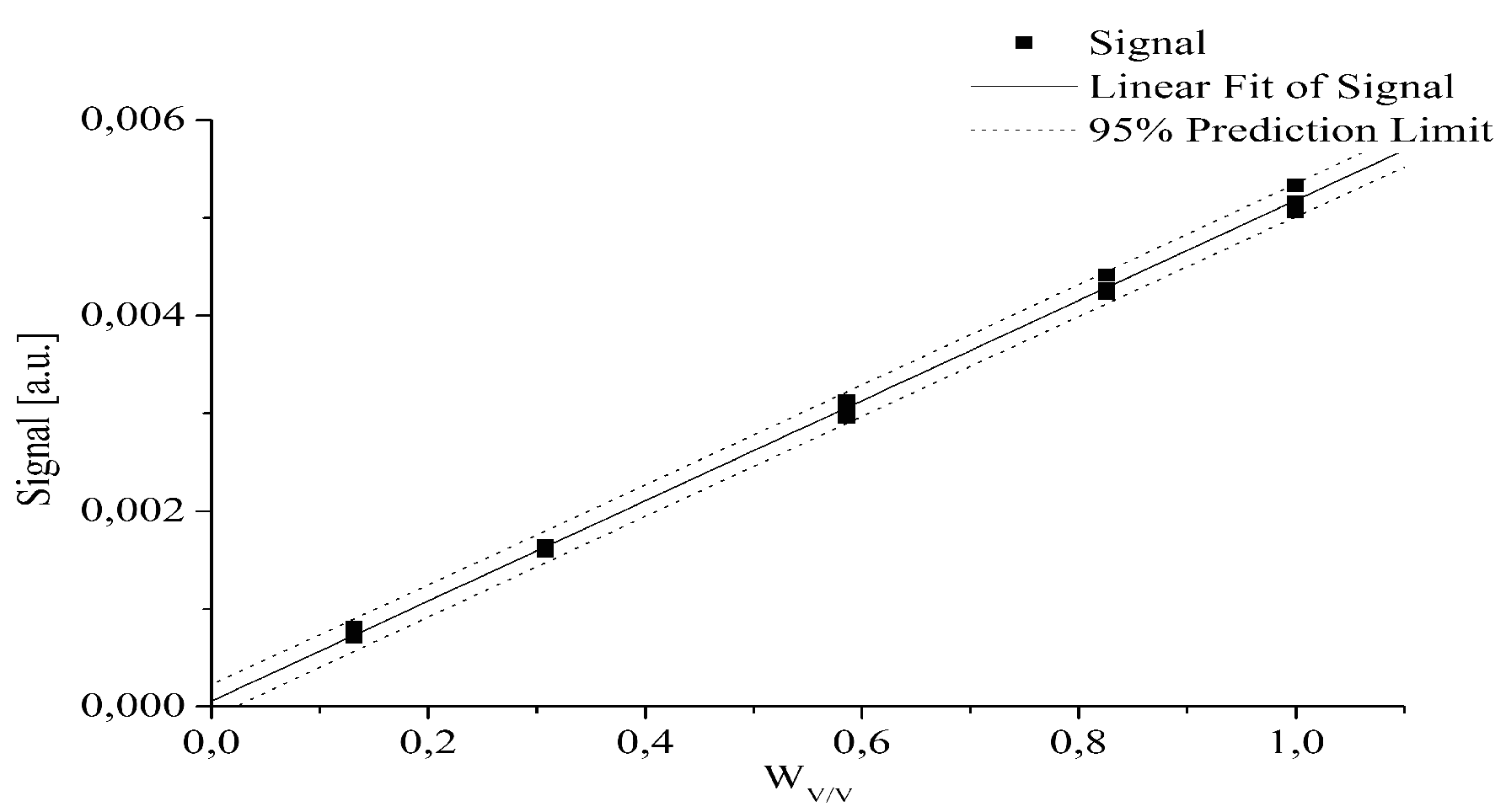
- The quantitative determination of a particular piroxicam polymorph.
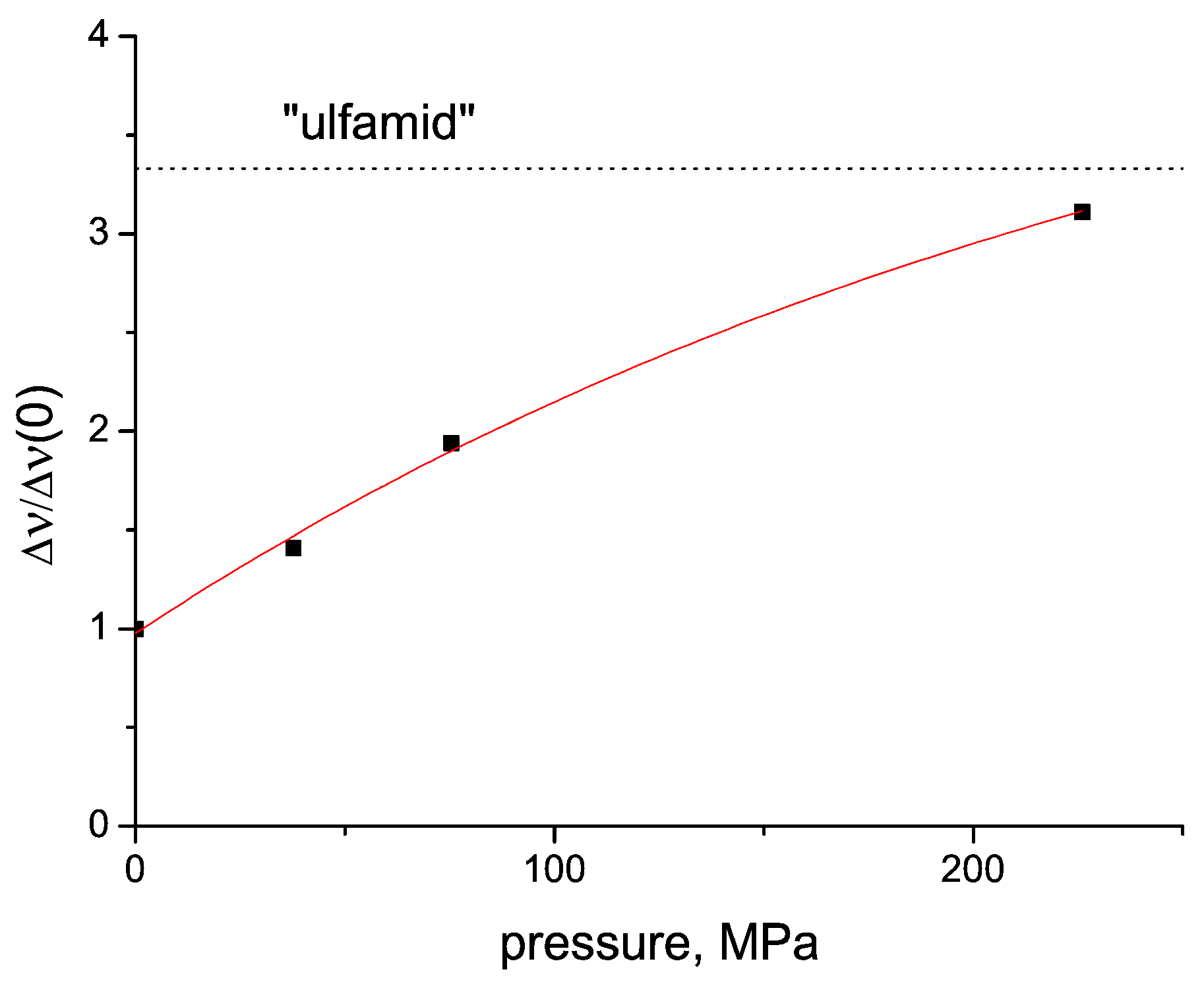
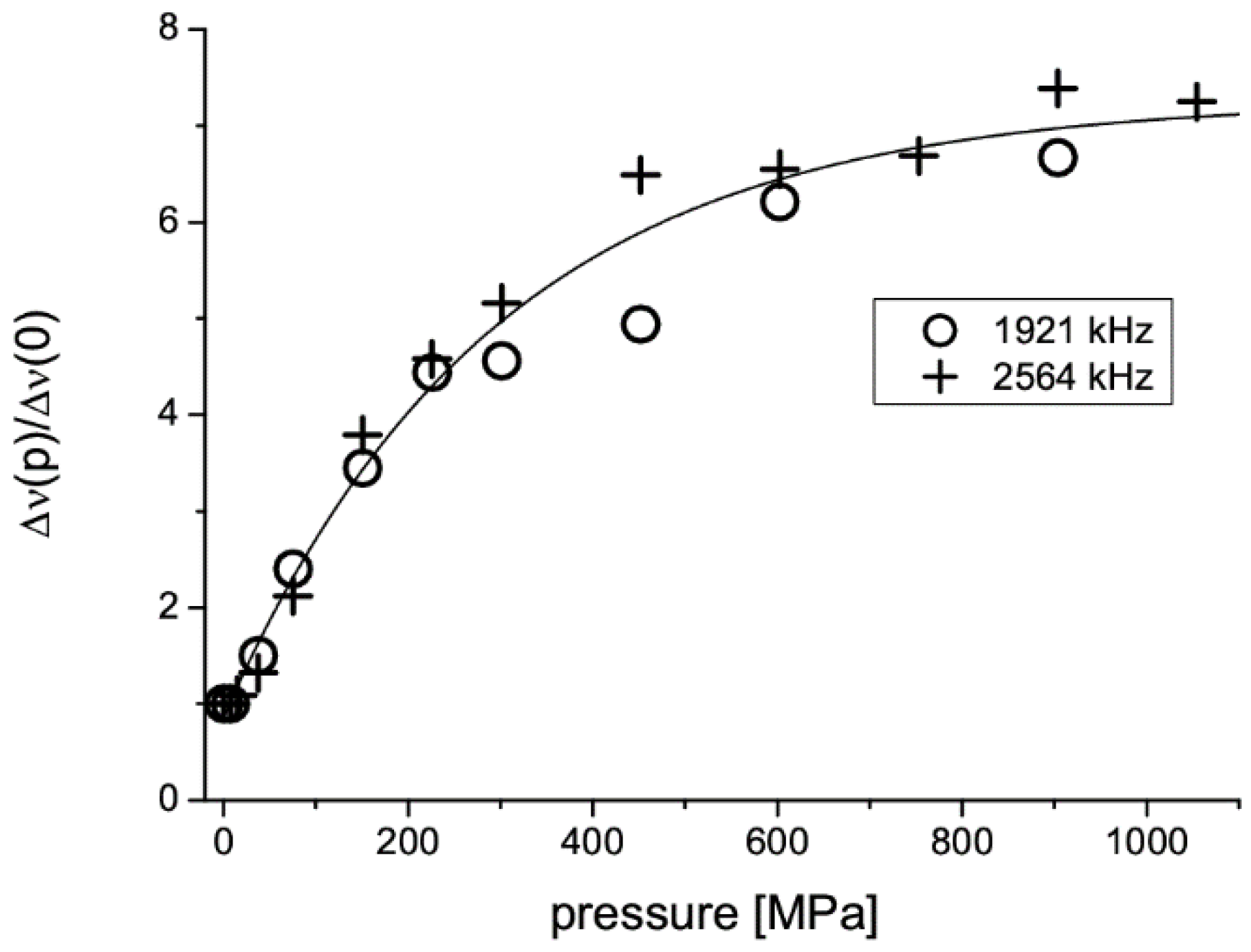
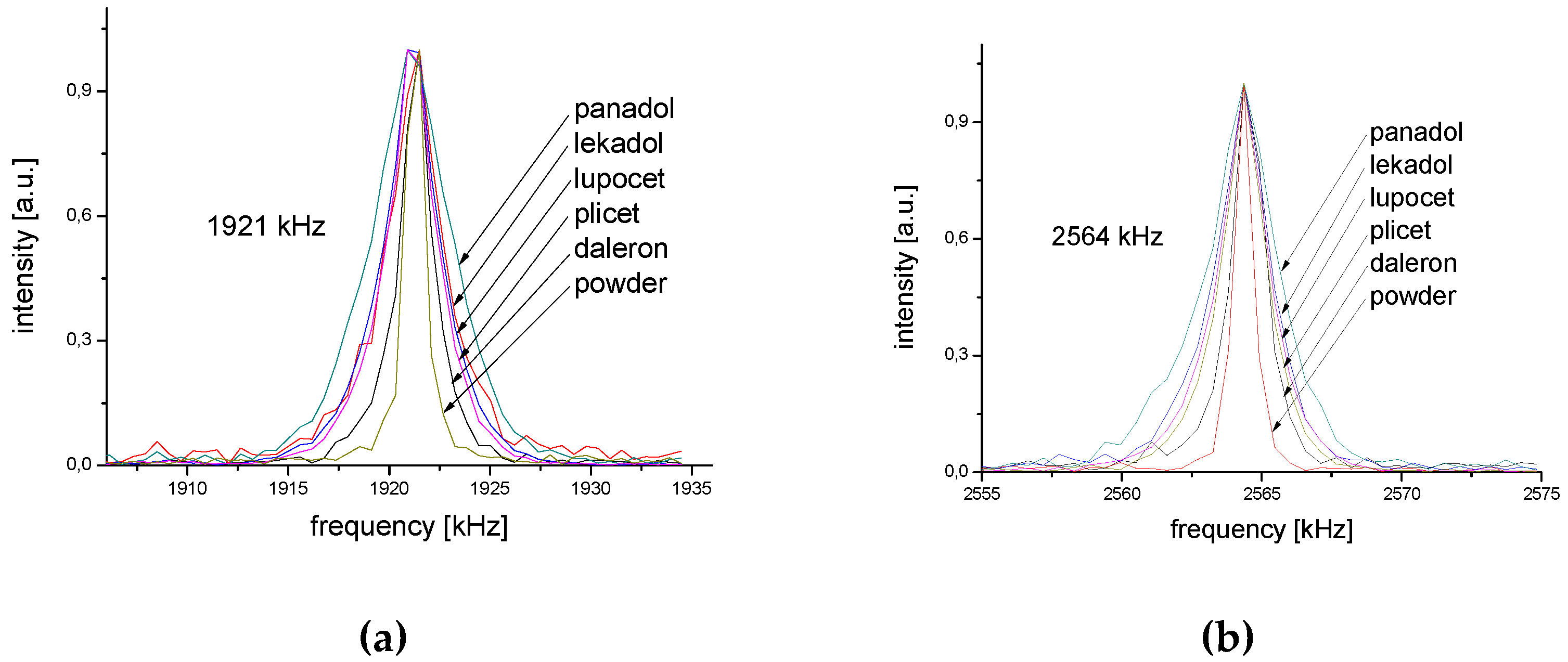
2.4. Tablet Compaction Pressure vs. Linewidth
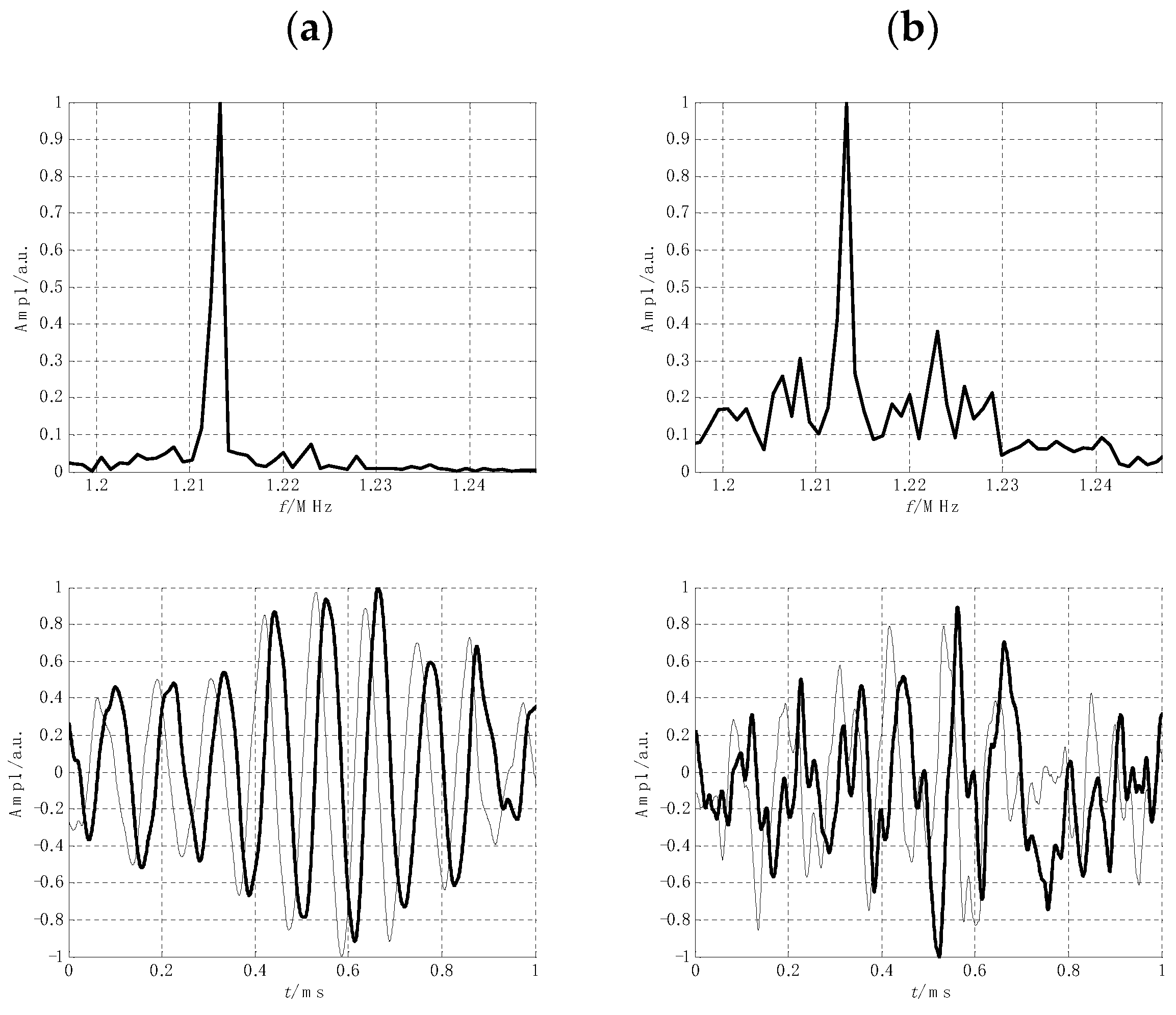
2.5. Instrumentation Improvements
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brittain, H.G. Polymorphism in Pharmaceutical Solids (Drugs and the Pharmaceutical Sciences), 2nd ed.; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Hilfiker, R.; von Ramer, M. Polymorphism in the Pharmaceutical Industry; Solid Form and Drug Development; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Das, T.P.; Hahn, E.L. Nuclear Quadrupole Resonance Spectroscopy; Academic Press Inc.: New York, NY, USA, 1958. [Google Scholar]
- Abragam, A. The Principles of Nuclear Magnetism; Clarendon Press: Oxford, UK, 1961. [Google Scholar]
- Lucken, E.A.C. Nuclear Quadrupole Coupling Constants; Academic Press Inc.: London, UK; New York, NY, USA, 1969. [Google Scholar]
- Seliger, J. Nuclear quadrupole resonance: Theory. In Encyclopedia of Spectroscopy and Spectrometry; Lindon, J.C., Tranter, G.E., Holmes, J.L., Eds.; Academic Press: San Diego, CA, USA, 2000; pp. 1672–1680. [Google Scholar]
- Rigamonti, A. NMR-NQR—studies of structural phase transitions. Adv. Phys. 1984, 33, 115. [Google Scholar] [CrossRef]
- Grechishkin, V.S.; Sinyavskii, N.Y. New technologies: Nuclear quadrupole resonance as an explosive and narcotic detection technique. Phys-Uspekhi. 1997, 40, 393–406. [Google Scholar] [CrossRef]
- Balchin, E.; Malcolme-Lawes, D.J.; Poplett, I.J.F.; Rowe, M.D.; Smith, J.A.S.; Pearce, G.E.S.; Wren, S.A.C. Potential of Nuclear Quadrupole Resonance in Pharmaceutical Analysis. Anal. Chem. 2005, 77, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Chihara, H.; Nakamura, N. Nuclear quadrupole resonance spectroscopy data. Supplement to III/20, III/31. In Landolt-Bornstein, Numerical Data and in Science and Technology, Group III; Condensed Matter; Springer: Berlin, Germany, 1997. [Google Scholar]
- Bailey, W.C. DFT and HF–DFT calculations of 14N quadrupole coupling constants in molecules. Chem. Phys. 2000, 252, 57–66. [Google Scholar] [CrossRef]
- Limandri, S.; Visñovezky, C.; Pérez, S.C.; Schurrer, C.A.; Wolfenson, A.E.; Ferro, M.; Cuffini, S.L.; Gonçalves de Souza, J.; Aguiar, F.A.; Masetto de Gaitani, C. Nuclear Quadrupole Resonance: A Technique to Control Hydration Processes in the Pharmaceutical Industry. Anal. Chem. 2011, 83, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Gregorovič, A. Quantitative Analysis of Hydration Using Nitrogen−14 Nuclear Quadrupole Resonance. Anal. Chem. 2015, 87, 6912–6918. [Google Scholar] [CrossRef]
- Lužnik, J.; Pirnat, J.; Jazbinšek, V.; Lavrič, Z.; Srčič, S.; Trontelj, Z. The influence of pressure in paracetamol tablet compaction on 14N nuclear quadrupole resonance. Appl. Magn. Reson. 2013, 44, 735–743. [Google Scholar] [CrossRef]
- Kyriakidou, G.; Jakobsson, A.; Althoefer, K.; Barras, J. Batch-Specific Discrimination Using Nuclear Quadrupole Resonance Spectroscopy. Anal. Chem. 2015, 87, 3806–3811. [Google Scholar] [CrossRef]
- Slusher, R.E.; Hahn, E.L. Sensitive Detection of Nuclear Quadrupole Interactions in Solids. Phys. Rev. 1968, 166, 332–347. [Google Scholar] [CrossRef]
- Edmonds, D.T. Nuclear quadrupole double resonance. Phys. Rep. 1977, 29, 233–290. [Google Scholar] [CrossRef]
- Stephenson, D.; Smith, J.A.S. Nitrogen−14 Quadrupole Cross-Relaxation Spectroscopy. Proc. R. Soc. Lond. A 1988, 416, 149–178. [Google Scholar] [CrossRef]
- Seliger, J.; Žagar, V. New Methods for Detection of 14N NQR Frequencies. Appl. Magn. Reson. 2012, 43, 469–484. [Google Scholar] [CrossRef]
- Veinberg, S.L.; Friedl, Z.W.; Harris, K.J.; O’Dell, L.A.; Schurko, R.W. Ultra-wideline 14N solid-state NMR as a method for differentiating polymorphs: Glycine as a case study. CrystEngComm 2015, 17, 5225–5236. [Google Scholar] [CrossRef]
- Pérez, S.C.; Cerioni, L.; Wolfenson, A.E.; Faudone, S.; Cuffini, S.L. Utilization of pure nuclear quadrupole resonance spectroscopy for the study of pharmaceutical crystal forms. Int. J. Pharm. 2005, 298, 143–152. [Google Scholar] [CrossRef]
- Wolfenson, A.; Pérez, S.C.; Zuriaga, M.J.; Garnero, C.; Miranda, J.A.; Longhi, M.; Faudone, S.N. Structural and dynamic characterization of solid furosemide polymorphs by NQR and NMR methods. Chem. Phys. Lett. 2005, 641, 163–168. [Google Scholar] [CrossRef]
- Bavin, P.M.G.; Stephenson, D.; Smith, J.A.S. 14N Quadrupole Cross-Relaxation Spectroscopy of a Compound of Pharmacological Interest. Zeitschrift für Naturforschung A 1986, 41, 195–199. [Google Scholar] [CrossRef]
- Trontelj, Z.; Lužnik, J.; Pirnat, J.; Jazbinšek, V.; Lavrič, Z.; Srčič, S. Polymorphism in Sulfanilamide: 14N NQR Study. J. Pharm. Sci. 2019, 108, 2865–2870. [Google Scholar] [CrossRef]
- Lužnik, J.; Pirnat, J.; Jazbinšek, V.; Lavrič, Z.; Žagar, V.; Srčič, S.; Seliger, J.; Trontelj, Z. 14N Nuclear Quadrupole Resonance Study of Polymorphism in Famotidine. J. Pharm. Sci. 2014, 103, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Lavrič, Z.; Pirnat, J.; Lužnik, J.; Seliger, J.; Žagar, V.; Trontelj, Z.; Srčič, S. Application of 14N NQR to the study of piroxicam polymorphism. J. Pharm. Sci. 2010, 99, 4857–4865. [Google Scholar] [CrossRef]
- Lavrič, Z.; Pirnat, J.; Lužnik, J.; Trontelj, Z.; Srčič, S. 14N NQR study of piroxicam: Confirmation of new polymorphic form V. J. Pharm. Sci. 2015, 104, 1909–1918. [Google Scholar] [CrossRef]
- Blinc, R.; Mali, M.; Osredkar, R.; Prelesnik, A.; Seliger, J.; Zupančič, I.; Ehrenberg, L. 14N NQR Spectroscopy of Some Amino Acids and Nucleic Bases via Double Resonance in the Laboratory Frame. J. Chem. Phys. 1972, 57, 5087. [Google Scholar] [CrossRef]
- Hartmann, S.R.; Hahn, E.L. Nuclear double resonance in the rotating frame. Phys. Rev. 1962, 128, 2042–2053. [Google Scholar] [CrossRef]
- Lužnik, J.; Pirnat, J.; Jazbinšek, V.; Apih, T.; Gregorovič, A.; Blinc, R.; Seliger, J.; Trontelj, Z. Polarization enhanced “single shot” N14 nuclear quadrupole resonance detection of trinitrotoluene at room temperature. Appl. Phys. Lett. 2006, 89, 123509. [Google Scholar] [CrossRef]
- Lee, S.-K.; Sauer, K.L.; Seltzer, S.J.; Alem, O.; Romalis, M.V. Subfemtotesla radio-frequency atomic magnetometer for detection of nuclear quadrupole resonance. Appl. Phys. Lett. 2006, 89, 214106. [Google Scholar] [CrossRef]
- Hürlimann, M.D.; Pennington, C.H.; Fan, N.Q.; Clarke, J.; Pines, A.; Hahn, E.L. Pulsed Fourier-transform NQR ofN14with a dc SQUID. Phys. Rev. Lett. 1992, 69, 684–687. [Google Scholar] [CrossRef]
- Alléaume, M.; Decap, J. Affinement tridimensionnel du sulfanilamide β. Acta Crystallogr. 1965, 18, 731–736. [Google Scholar] [CrossRef]
- Alléaume, M.; Decap, J. Affinement tridimensionnel du sulfanilamide χ. Acta Crystallogr. 1965, 19, 934–938. [Google Scholar] [CrossRef]
- O’Connell, A.M.; Maslen, E.N. X-ray and neutron diffraction studies of beta-sulfanilamide. Acta Crystallogr. 1967, 22, 134–145. [Google Scholar] [CrossRef]
- Van Zyp, C. Microscopische identificatie van sulfanilamide. Pharm. Weekbl. 1938, 75, 585–591. [Google Scholar]
- Toscani, S.; Dzyabchenko, A.; Agafonov, V.; Dugue, J.; Céolin, R. Polymorphism of Sulfanilamide: (II) Stability Hierarchy of α-, β- and γ- Forms from Energy Calculations by the Atom-Atom Potential Method and from the Construction of the p, T Phase Diagram. Pharm. Res. 1996, 13, 151–154. [Google Scholar] [CrossRef]
- Lin, H.O.; Guillory, J.K. Polymorphism in Sulfanilamide-d4. J. Pharm. Sci. 1970, 59, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Portieri, A.; Harris, R.K.; Fletton, R.A.; Lancaster, R.W.; Threlfall, T.L. Effects of polymorphic differences for sulfanilamide, as seen through13C and15N solid-state NMR, together with shielding calculations. Magn. Reson. Chem. 2004, 42, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ildiz, G.O.; Akyüz, S. Conformational analysis and vibrational study of sulfanilamide. Vib. Spectrosc. 2012, 58, 12–18. [Google Scholar] [CrossRef]
- Toscani, S.; Thorén, S.; Agafonov, V.; Céolin, R.; Dugue, J. Thermodynamic Study of Sulfanilamide Polymorphism: (I) Monotropy of the α-Variety. Pharm. Res. 1995, 12, 1453–1456. [Google Scholar] [CrossRef]
- Subbarao, S.N.; Bray, P.J. Correlation of carbonic anhydrase inhibitor activities of benzenesulfonamides with the data obtained by use of nitrogen−14 nuclear quadrupole resonance. J. Med. Chem. 1978, 22, 111–114. [Google Scholar] [CrossRef]
- Blinc, R.; Seliger, J.; Zidanšek, A.; Žagar, V.; Milia, F.; Robert, H. 14N nuclear quadrupole resonance of some sulfa drugs. Solid State Nucl. Magn. Reson. 2006, 30, 61–68. [Google Scholar] [CrossRef]
- Barras, J.; Althoefer, K.; Rowe, M.D.; Poplett, I.J.; Smith, J.A.S. The Emerging Field of Medicines Authentication by Nuclear Quadrupole Resonance Spectroscopy. Appl. Magn. Reson. 2012, 43, 511–529. [Google Scholar] [CrossRef]
- Marino, R.A.; Klainer, S.M. Multiple spin echoes in pure quadrupole resonance. J. Chem. Phys. 1977, 67, 3388. [Google Scholar] [CrossRef]
- Hegedüs, B.; Bod, P.; Harsányi, K.; Péter, I.; Kalman, A.; Parkanyi, L. Comparison of the polymorphic modifications of famotidine. J. Pharm. Biomed. Anal. 1989, 7, 563–569. [Google Scholar] [CrossRef]
- Ferenczy, G.G.; Párkányi, L.; Ángyán, J.; Kálmán, A.; Hegedűs, B. Crystal and electronic structure of two polymorphic modifications of famotidine. An experimental and theoretical study. J. Mol. Struct. THEOCHEM 2000, 503, 73–79. [Google Scholar] [CrossRef]
- Overgaard, J.; Hibbs, D.E. The experimental electron density in polymorphs A and B of the anti-ulcer drug famotidine. Acta Crystallogr. Sect. A Found. Crystallogr. 2004, 60, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.-J.; Yang, X.; Ching, C.-B. Polymorphism and Crystallization of Famotidine. Cryst. Growth Des. 2007, 7, 1590–1598. [Google Scholar] [CrossRef]
- Hassan, M.A.; Salem, M.S.; Sueliman, M.S.; Najib, N.M. Characterization of famotidine polymorphic forms. Int. J. Pharm. 1997, 149, 227–232. [Google Scholar] [CrossRef]
- Rudakov, T.; Mikhaltsevich, V. Multiple NQR spin echoes in phase cycled pulse experiments. Phys. Lett. A 2003, 309, 465–469. [Google Scholar] [CrossRef]
- Seliger, J.; Blinc, R.; Arend, H.; Kind, R. Proton-14N double resonance study of the structural phase transitions in the perovskite type layer compound (CH3NH3)2CdCl4. Eur. Phys. J. B 1976, 25, 189–195. [Google Scholar] [CrossRef]
- Grechishkin, V.S.; Shpilevoi, A.A. Indirect methods for studying nuclear quadrupole interactions in solids. Physics-Uspekhi 1996, 39, 713–725. [Google Scholar] [CrossRef]
- Seliger, J.; Žagar, V. Measurement of the 14N nuclear quadrupole resonance frequencies by the solid effect. J. Magn. Reson. 2008, 193, 54–62. [Google Scholar] [CrossRef]
- Luznik, J.; Jazbinšek, V.; Pirnat, J.; Seliger, J.; Trontelj, Z. Zeeman shift – A tool for assignment of 14N NQR lines of nonequivalent 14N atoms in powder samples. J. Magn. Reson. 2011, 212, 149–153. [Google Scholar] [CrossRef]
- Seliger, J.; Žagar, V.; Latosińska, M.; Latosińska, J.N. Electron Configuration and Hydrogen-Bonding Pattern in Several Thymine and Uracil Analogues Studied by 1H–14N NQDR and DFT/QTAIM. J. Phys. Chem. B 2012, 116, 8793–8804. [Google Scholar] [CrossRef]
- Oja, T. Nitrogen-14 nuclear quadrupole resonance study of the guanidinium ion. J. Chem. Phys. 1973, 59, 2668–2675. [Google Scholar] [CrossRef]
- Negita, H.; Kubo, T.; Shibata, K. 14N Nuclear Quadrupole Resonances of Sulfuric Diamide and Its Derivatives. Bull. Chem. Soc. Jpn. 1975, 48, 675–677. [Google Scholar] [CrossRef]
- Seliger, J.; Žagar, V. Nuclear Quadrupole Resonance Study of Hydrogen Bonds in Solid 2-Methylbenzimidazole and 5,6-Dimethylbenzimidazole. J. Phys. Chem. C 2013, 117, 20193–20200. [Google Scholar] [CrossRef]
- Seliger, J.; Žagar, V. Hydrogen Bonds in Cocrystals and Salts of 2-Amino-4,6-dimethylpyrimidine and Carboxylic Acids Studied by Nuclear Quadrupole Resonance. J. Phys. Chem. B 2013, 117, 6946–6956. [Google Scholar] [CrossRef] [PubMed]
- Lavrič, Z. Utilization of Nuclear Quadrupole Resonance for Qualitative and Quantitative Study of Nifedipine and Piroxicam Polymorphism (Uporaba Jedrske Kvadrupolne Resonance za Kvalitativno in Kvantitativno Proučevanje Polimorfizma Nifedipina in Piroksikama). BS Thesis, University of Ljubljana, Ljubljana, Slovenia, 2009. [Google Scholar]
- Kojic-Prodic, B.; Ruzic-Toros, Z. Structure of the antiinflammatory drug 4hydroxy2methylN2pyridyl2H1[lambda]6,2benzothiazine3carboxamide 1,1dioxide (piroxicam). Acta Crystallogr. Sect B. 1982, 38, 2948–2951. [Google Scholar] [CrossRef]
- Sheth, A.R.; Bates, S.; Muller, F.X.; Grant, D.J.W. Polymorphism in Piroxicam. Cryst. Growth Des. 2004, 4, 1091–1098. [Google Scholar] [CrossRef]
- Mihalić, M.; Hofman, H.; Kajfež, F.; Kuftinec, J.; Blažević, N.; Žinić, M. Physicochemical and analytical characteristics of piroxicam. Acta Pharm. Jugosl. 1982, 32, 13–20. [Google Scholar]
- Mihalić, M.; Hofman, H.; Uftinec, J.; Krile, B.; Caplar, V.; Kajfež, F.; Blažević, N. Piroxicam. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Orlando, FL, USA, 1986; pp. 509–531. [Google Scholar]
- Reck, G.; Dietz, G.; Laban, G.; Günther, W.; Bannier, G.; Höhne, E. X-ray studies on piroxicam modifications. Die Pharm. 1988, 43, 477–481. [Google Scholar]
- Reck, G.; Laban, G. Prediction and establishment of a new crystalline piroxicam modification. Die Pharm. 1990, 45, 257–259. [Google Scholar]
- Vrečer, F.; Srčič, S.; Šmid-Korbar, J. Investigation of piroxicam polymorphism. Int. J. Pharm. 1991, 68, 35–41. [Google Scholar] [CrossRef]
- Vrecer, F.; Vrbinc, M.; Meden, A. Characterization of piroxicam crystal modifications. Int. J. Pharm. 2003, 256, 3–15. [Google Scholar] [CrossRef]
- Naelapää, K.; Van De Streek, J.; Rantanen, J.; Bond, A.D. Complementing High-Throughput X-ray Powder Diffraction Data with Quantum–Chemical Calculations: Application to Piroxicam Form III. J. Pharm. Sci. 2012, 101, 4214–4219. [Google Scholar] [CrossRef] [PubMed]
- Puc, U. Jožef Stefan International Postgraduate School. Personal communication: Ljubljana, Slovenia, 2014. [Google Scholar]
- Budker, D.; Romalis, M. Optical magnetometry. Nat. Phys. 2007, 3, 227–234. [Google Scholar] [CrossRef]
- Beguš, S.; Pirnat, J.; Jazbinšek, V.; Trontelj, Z. Optical detection of low frequency NQR signals: A step forward from conventional NQR. J. Phys. D Appl. Phys. 2017, 50, 1–10. [Google Scholar] [CrossRef]
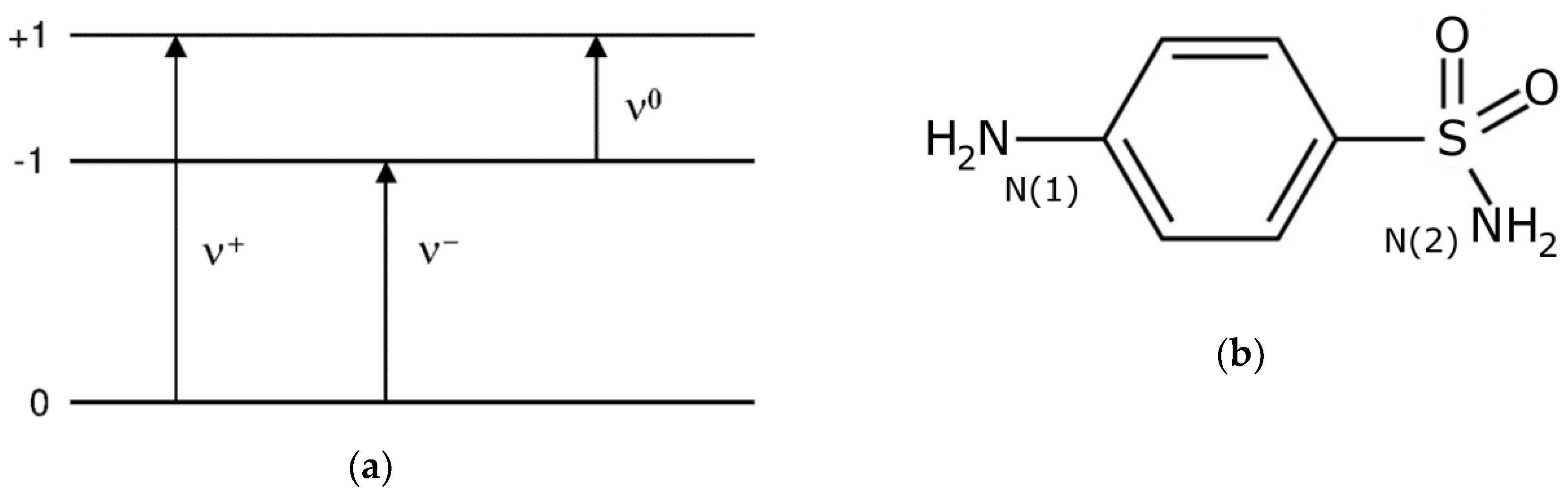
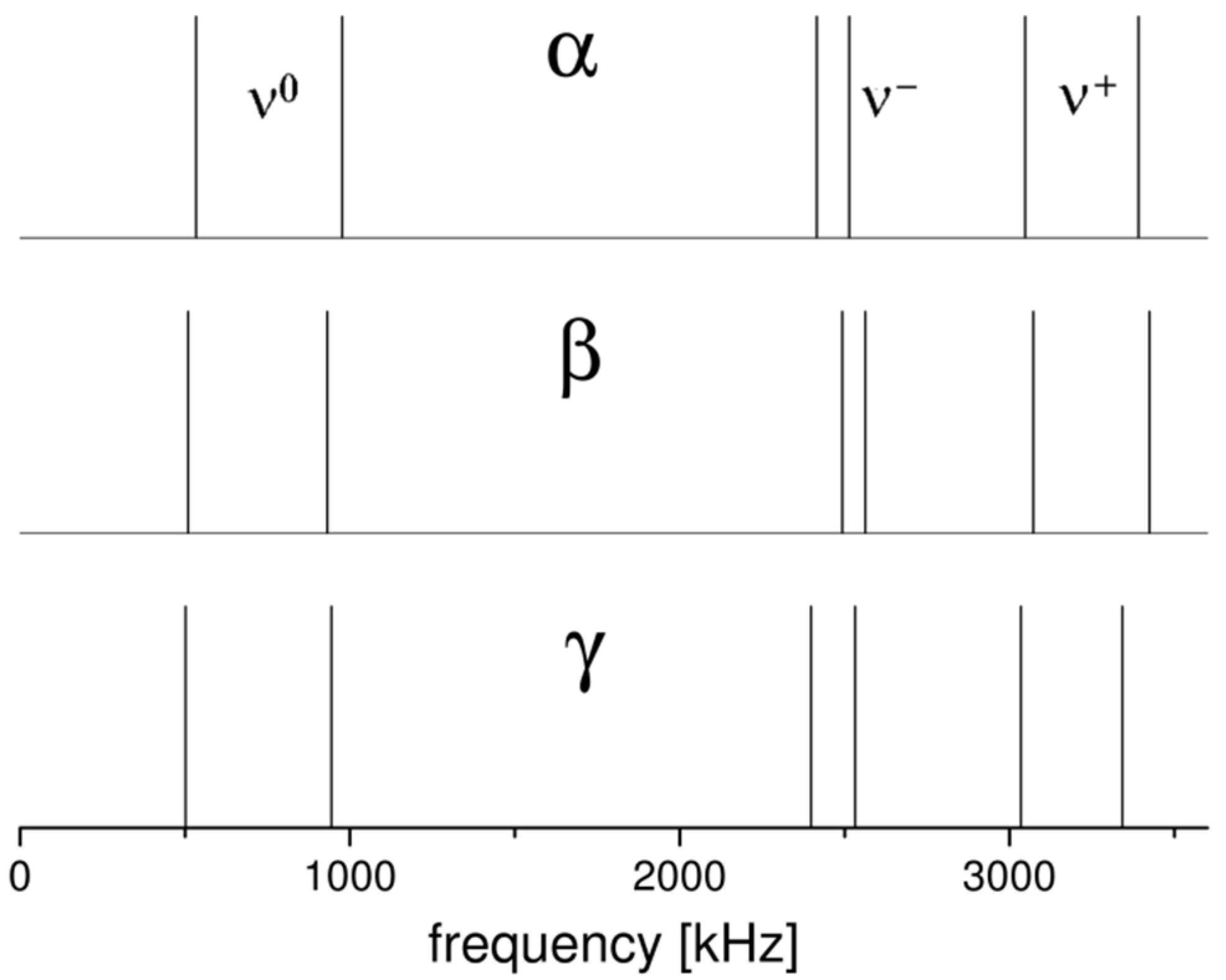
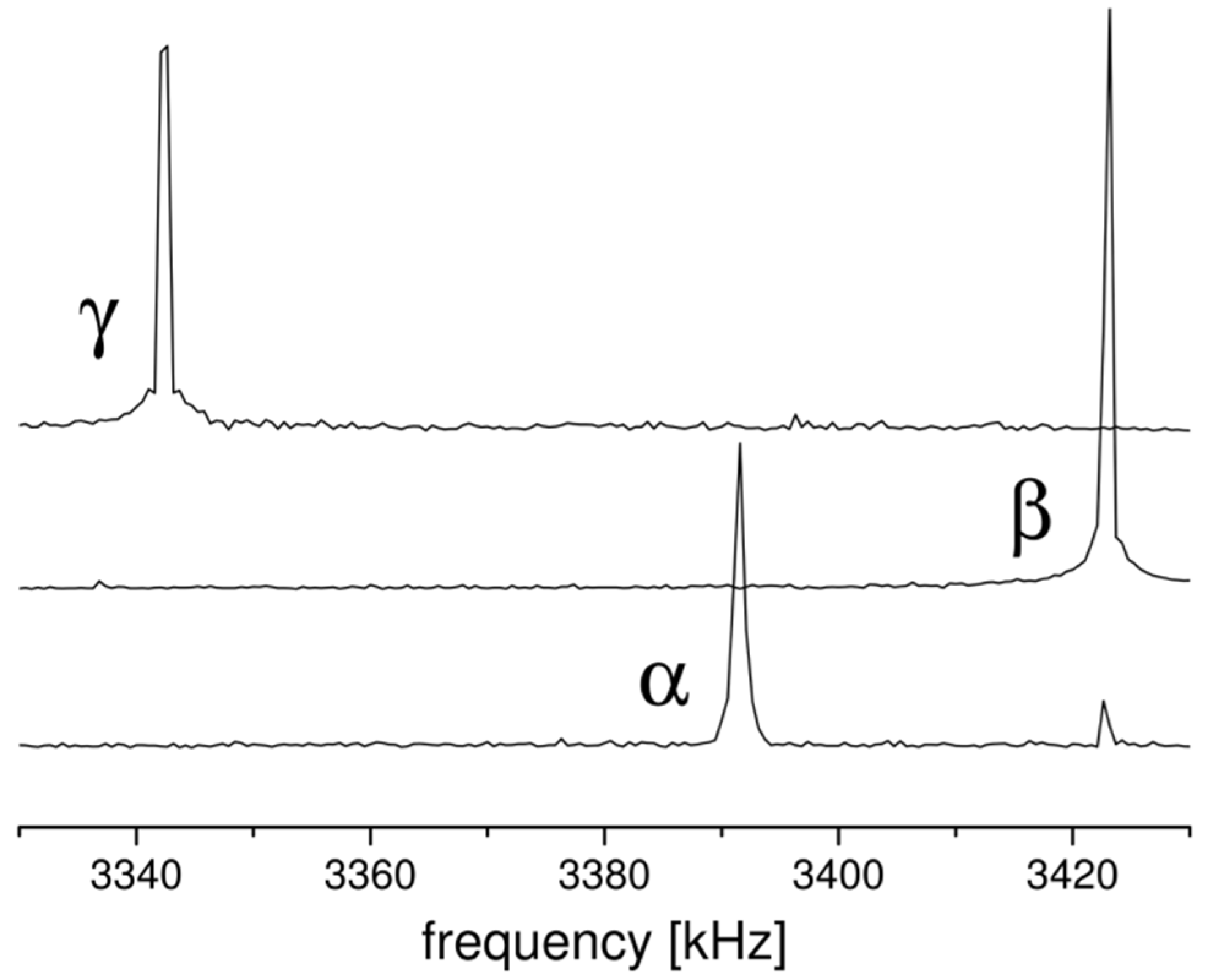
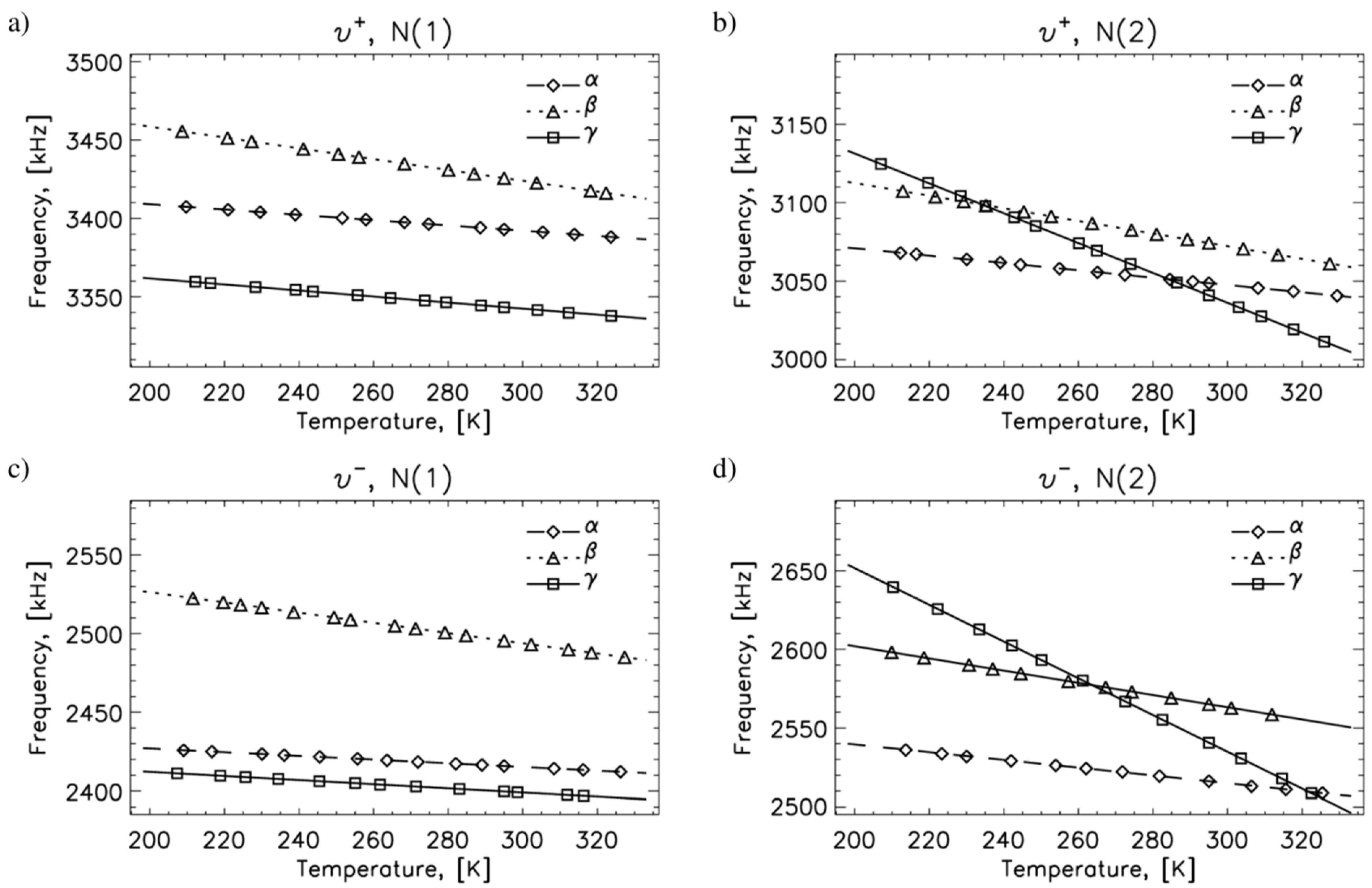
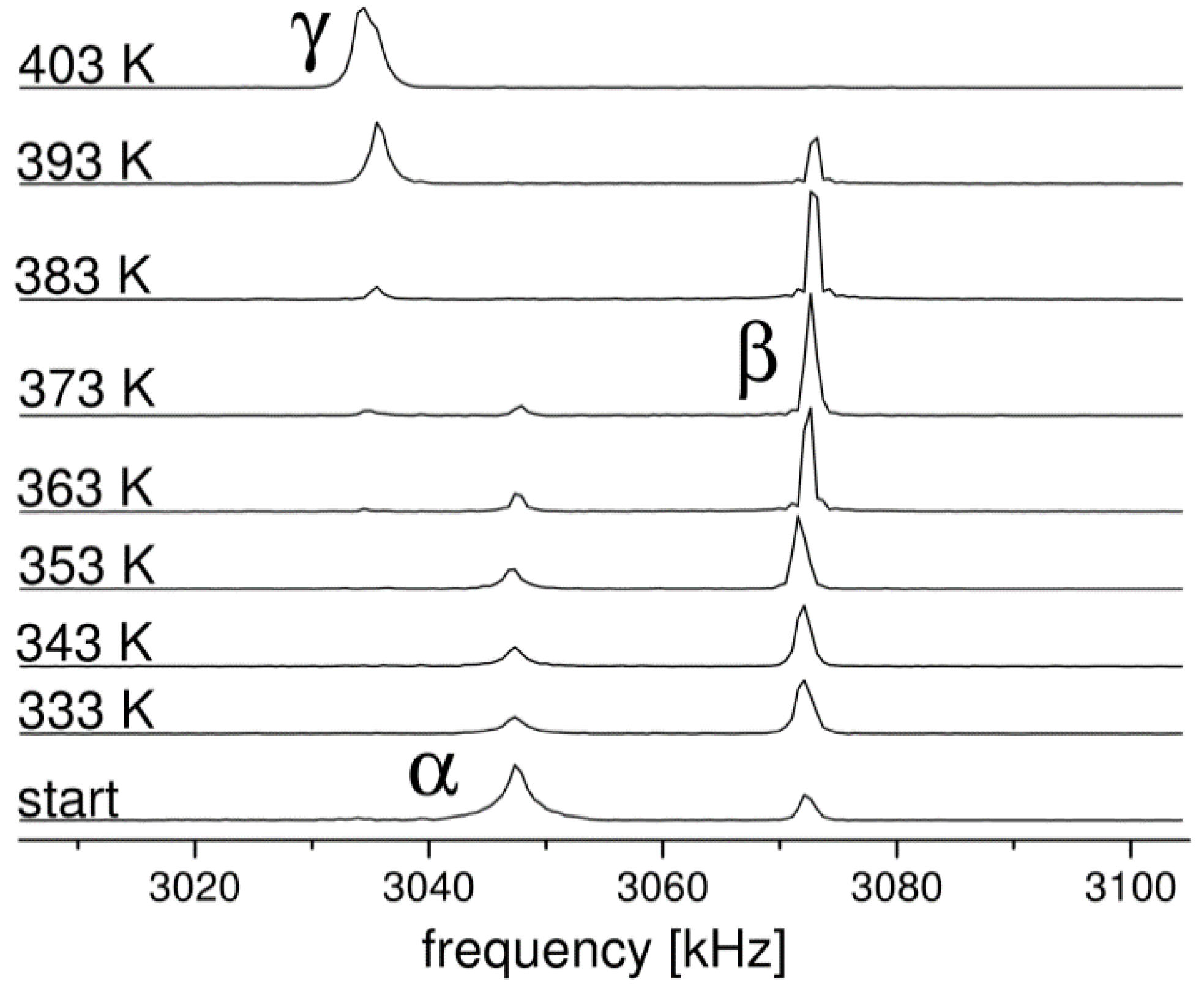
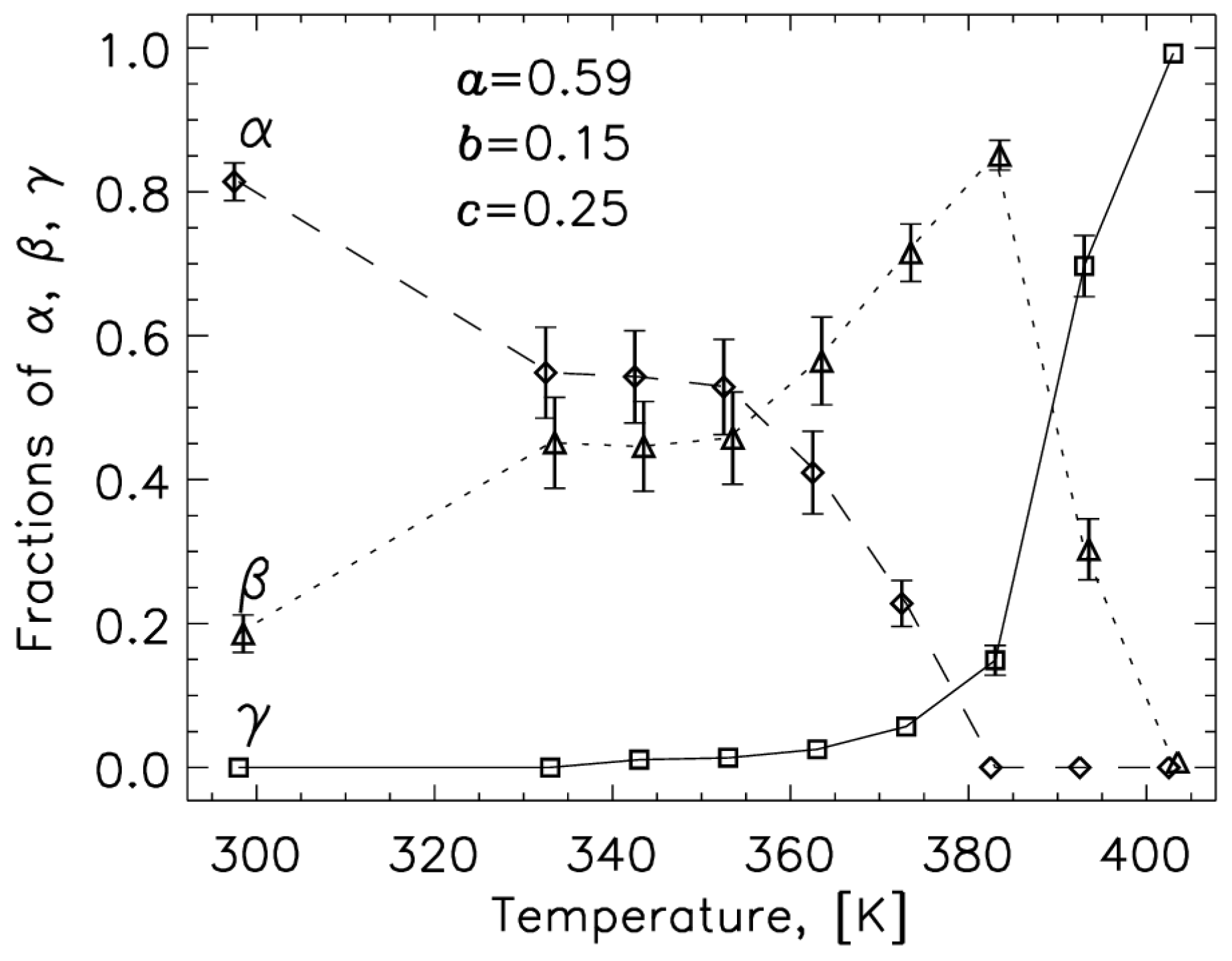
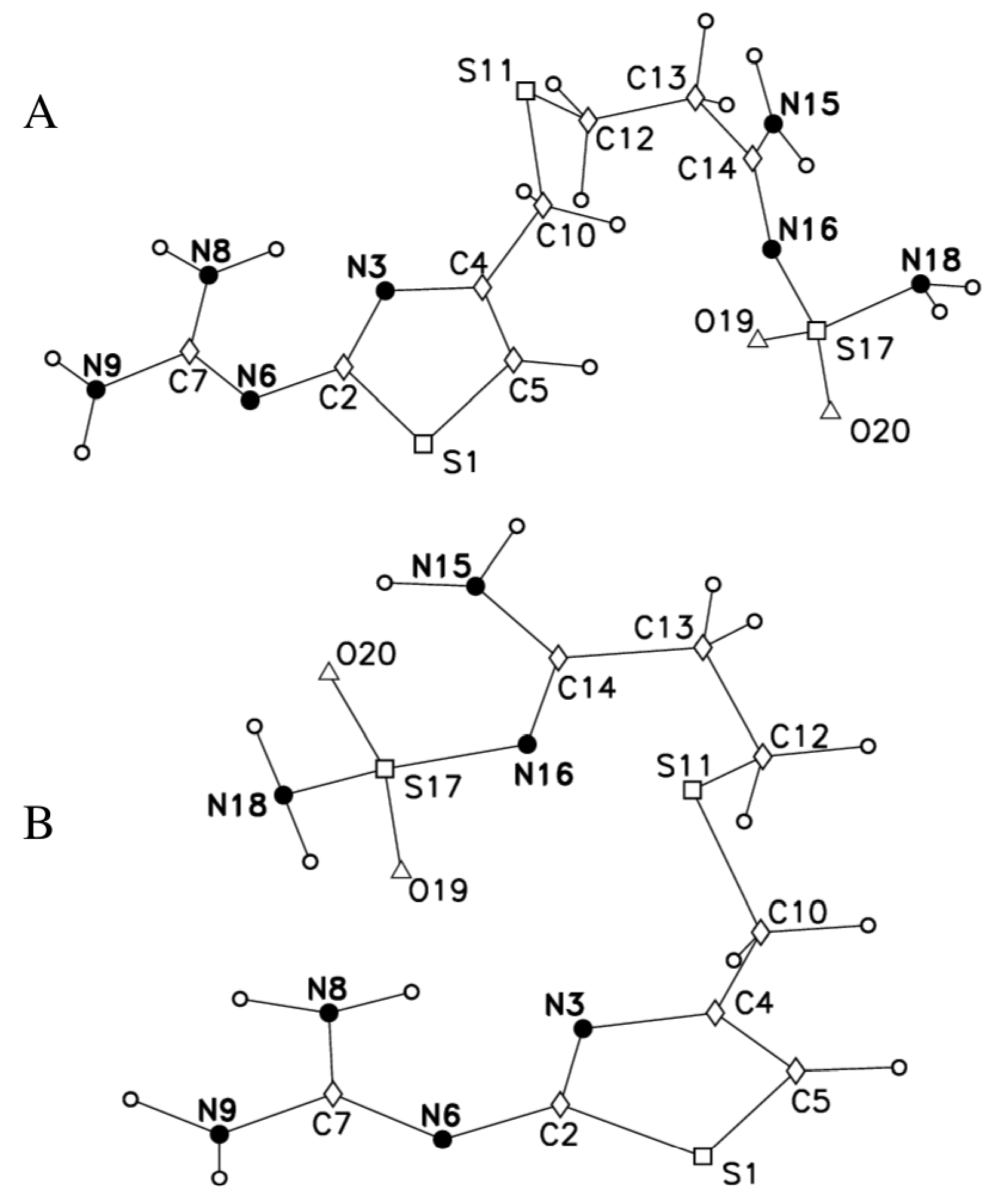
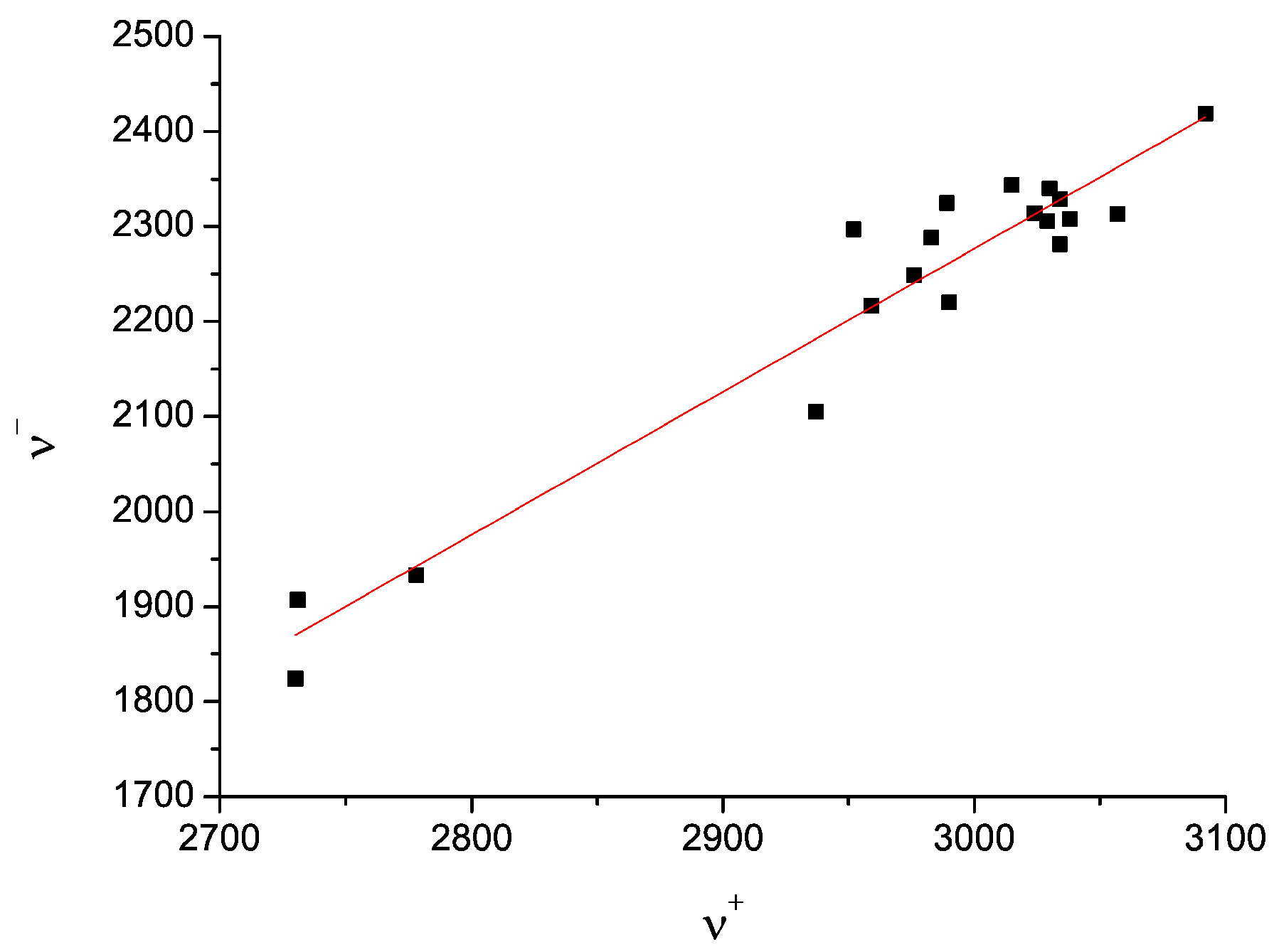
| Polymorph | Atom | ν+ (kHz) | dν+/dT (kHz/K) | ν− (kHz) | dν−/dT (kHz/K) | ν0 (kHz) | QCC (kHz) | η | T1 (ms) |
| A | N(1) | 3393 | −0.17 | 2416 | −0.12 | 977 | 3873 | 0.50 | 25 |
| N(2) | 3049 | −0.23 | 2516 | −0.25 | 533 | 3710 | 0.29 | 400 | |
| Β | N(1) | 3426 | −0.35 | 2496 | −0.32 | 930 | 3947 | 0.47 | 25 |
| N(2) | 3074 | −0.40 | 2565 | −0.39 | 509 | 3743 | 0.29 | 400 | |
| Γ | N(1) | 3343 | −0.19 | 2400 | −0.13 | 944 | 3829 | 0.49 | 25 |
| N(2) | 3041 | −0.95 | 2541 | −1.17 | 500 | 3721 | 0.27 | 25 |
| Form A* | ν+ (kHz) | ν− (kHz) | ν0 (kHz) | QCC (kHz) | η |
| Na | 3455 | 2443 | 1012 | 3932 | 0.51 |
| Nb | 2862 | 2065 | 797 | 3285 | 0.49 |
| Nc | 2819 | 2080 | 739 | 3266 | 0.45 |
| Nd | 2738 | 2274 | 464 | 3341 | 0.28 |
| Ne | 2735 | 2030 | 705 | 3177 | 0.44 |
| Nf | 2603 | 1971 | 632 | 3049 | 0.41 |
| Ng | 1979 | 1457 | 522 | 2291 | 0.46 |
| Form B* | ν+ (kHz) | ν− (kHz) | ν0 (kHz) | QCC (kHz) | η |
| Nh | 3462 | 2472 | 990 | 3956 | 0.50 |
| Ni | 2887 | 2364 | 523 | 3501 | 0.30 |
| Nj | 2848 | 2234 | 614 | 3388 | 0.36 |
| Nk | 2787 | 2043 | 744 | 3220 | 0.46 |
| Nl | 2649 | 2133 | 516 | 3188 | 0.32 |
| Nm | 2587 | 1765 | 822 | 2901 | 0.57 |
| Nn | 1982 | 1339 | 643 | 2214 | 0.58 |
| v+ [kHz] | v− [kHz] | v0 [kHz] | QCC [kHz] | η | ||
|---|---|---|---|---|---|---|
| I | N1 | 3928 | 3686 | 242 | 5076 | 0.0954 |
| N2 | 3439 | 2983 | 456 | 4281 | 0.213 | |
| N3 | 2587 | 1972 | 615 | 3039 | 0.405 | |
| II | N1 | 3852 | 3600 | 252 | 4968 | 0.101 |
| N2 | 3399 | 2946 | 455 | 4230 | 0.215 | |
| N3 | 2533 | 2081 | 452 | 3076 | 0.294 | |
| III | N1 | 4008 | 3763 | 245 | 5181 | 0.0946 |
| N2 | 3392 | 2967 | 425 | 4239 | 0.201 | |
| N3 | 2592 | 2085 | 507 | 3118 | 0.325 | |
| V | N1 | 3849 | 3587 | 262 | 4957 | 0.106 |
| N2 | 3419 | 2956 | 463 | 4250 | 0.218 | |
| N3 | 2532 | 2075 | 457 | 3071 | 0.298 |
| Monoclinic | Orthorhombic | ||||
|---|---|---|---|---|---|
| ν | Δν | T1 | ν | Δν | T1 |
| [kHz] | [Hz] | [s] | [kHz] | [Hz] | [s] |
| 2564 | 1400 | 11 | 2570 | 2300 | 6 |
| 1921 | 1800 | 5 | 1955 | 3000 | 3 |
| 643 | 615 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trontelj, Z.; Pirnat, J.; Jazbinšek, V.; Lužnik, J.; Srčič, S.; Lavrič, Z.; Beguš, S.; Apih, T.; Žagar, V.; Seliger, J. Nuclear Quadrupole Resonance (NQR)—A Useful Spectroscopic Tool in Pharmacy for the Study of Polymorphism. Crystals 2020, 10, 450. https://doi.org/10.3390/cryst10060450
Trontelj Z, Pirnat J, Jazbinšek V, Lužnik J, Srčič S, Lavrič Z, Beguš S, Apih T, Žagar V, Seliger J. Nuclear Quadrupole Resonance (NQR)—A Useful Spectroscopic Tool in Pharmacy for the Study of Polymorphism. Crystals. 2020; 10(6):450. https://doi.org/10.3390/cryst10060450
Chicago/Turabian StyleTrontelj, Zvonko, Janez Pirnat, Vojko Jazbinšek, Janko Lužnik, Stane Srčič, Zoran Lavrič, Samo Beguš, Tomaž Apih, Veselko Žagar, and Janez Seliger. 2020. "Nuclear Quadrupole Resonance (NQR)—A Useful Spectroscopic Tool in Pharmacy for the Study of Polymorphism" Crystals 10, no. 6: 450. https://doi.org/10.3390/cryst10060450
APA StyleTrontelj, Z., Pirnat, J., Jazbinšek, V., Lužnik, J., Srčič, S., Lavrič, Z., Beguš, S., Apih, T., Žagar, V., & Seliger, J. (2020). Nuclear Quadrupole Resonance (NQR)—A Useful Spectroscopic Tool in Pharmacy for the Study of Polymorphism. Crystals, 10(6), 450. https://doi.org/10.3390/cryst10060450




