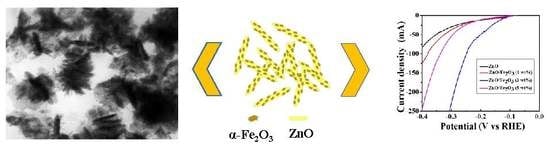
Fabrication of Hybrid Catalyst ZnO Nanorod/α-Fe2O3 Composites for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
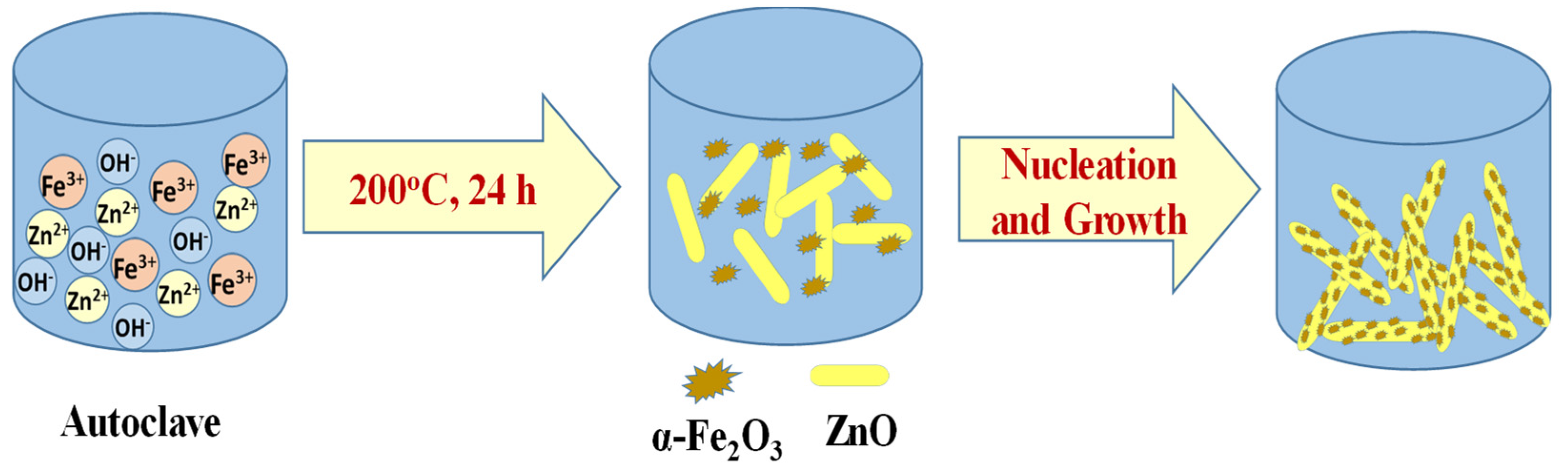
2.1. Preparation of ZnO/α-Fe2O3 Nanorod Composites
2.2. Hydrogen Evolution Reaction (HER)
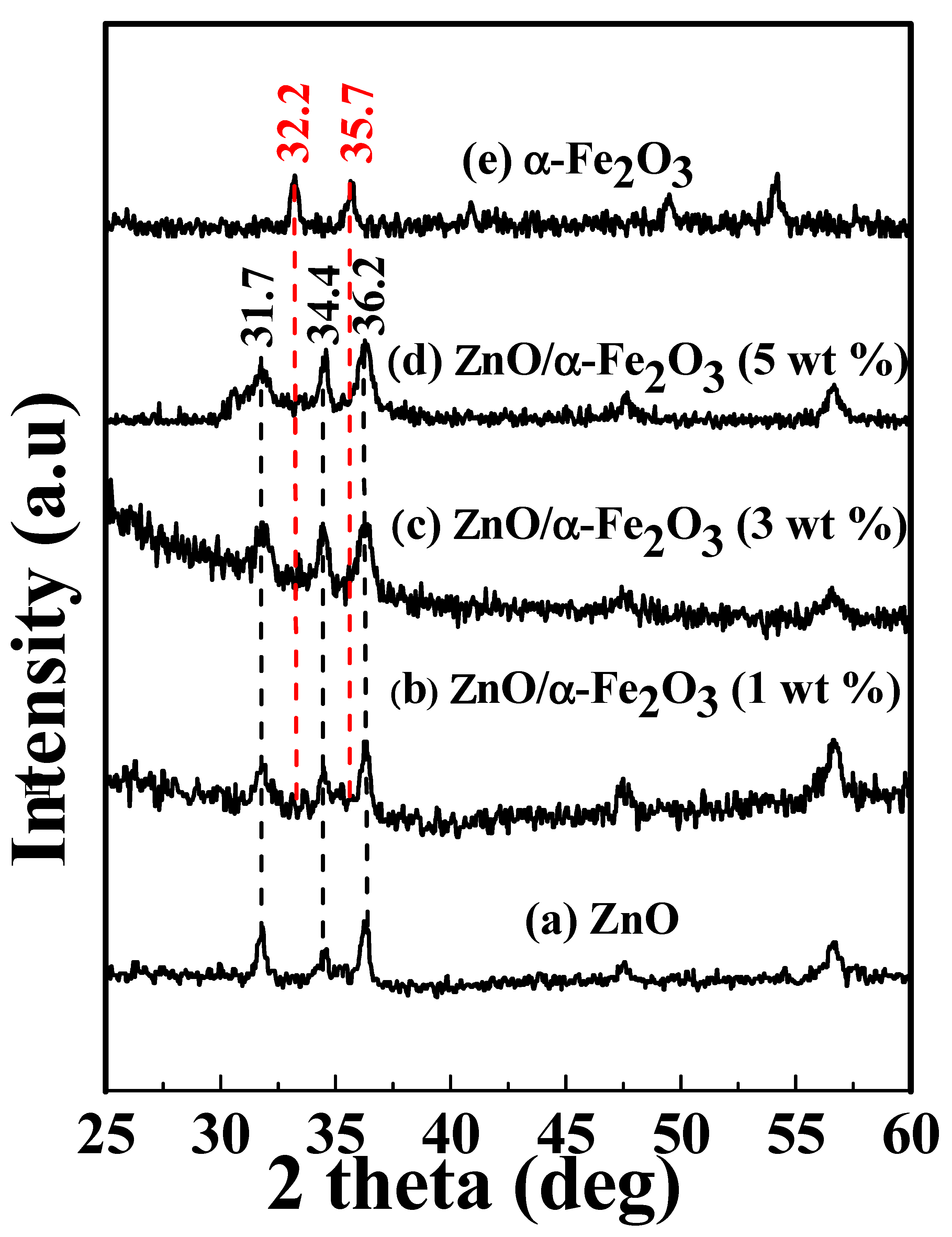
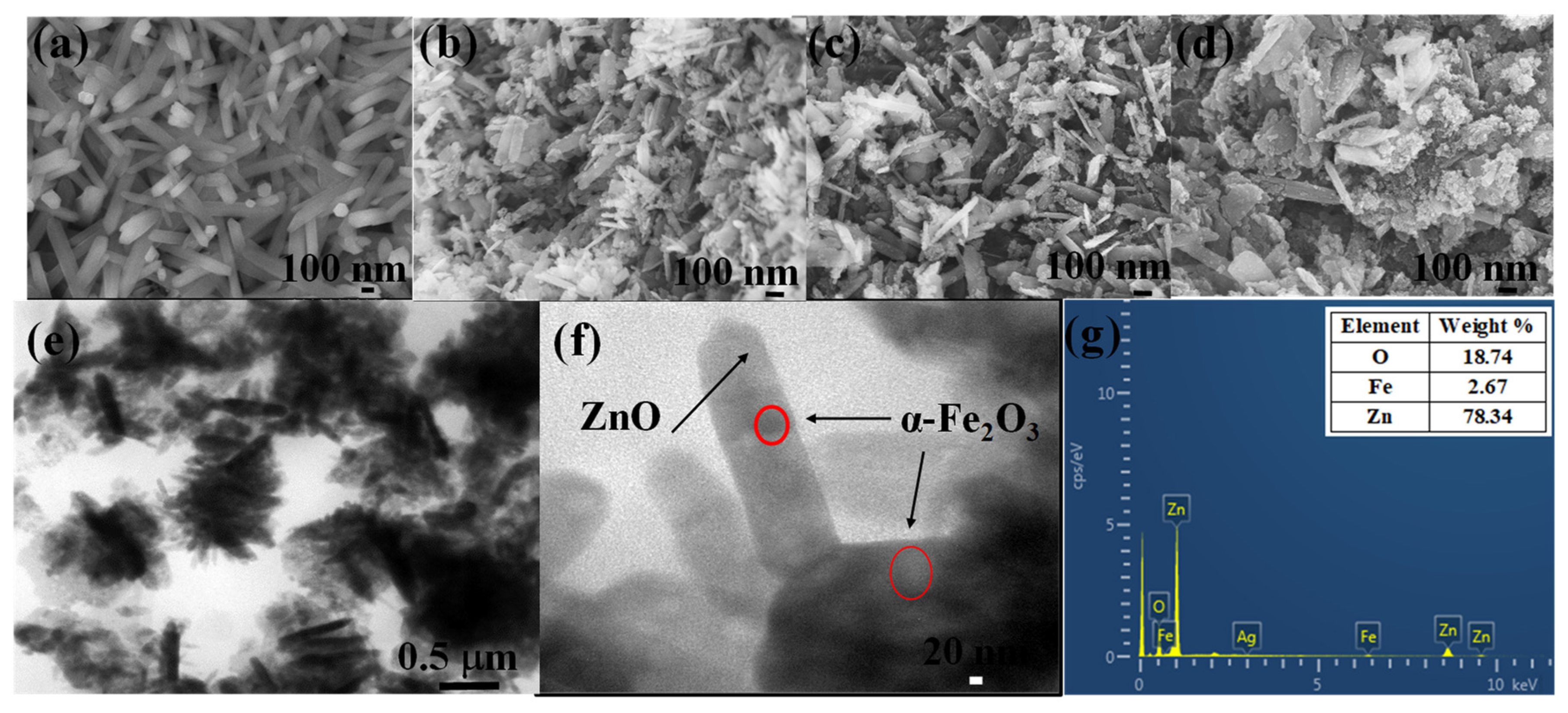
2.3. Characterization
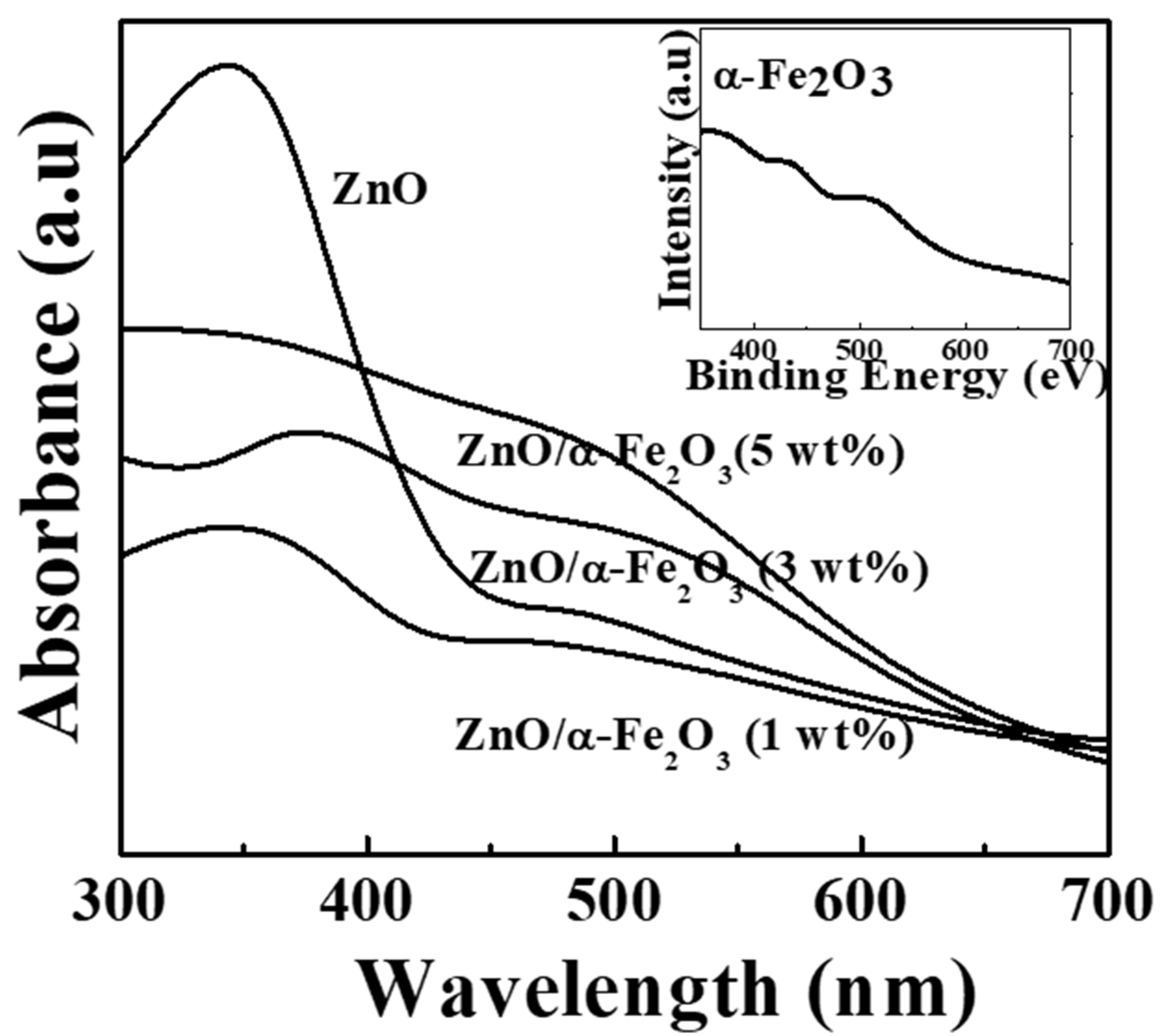
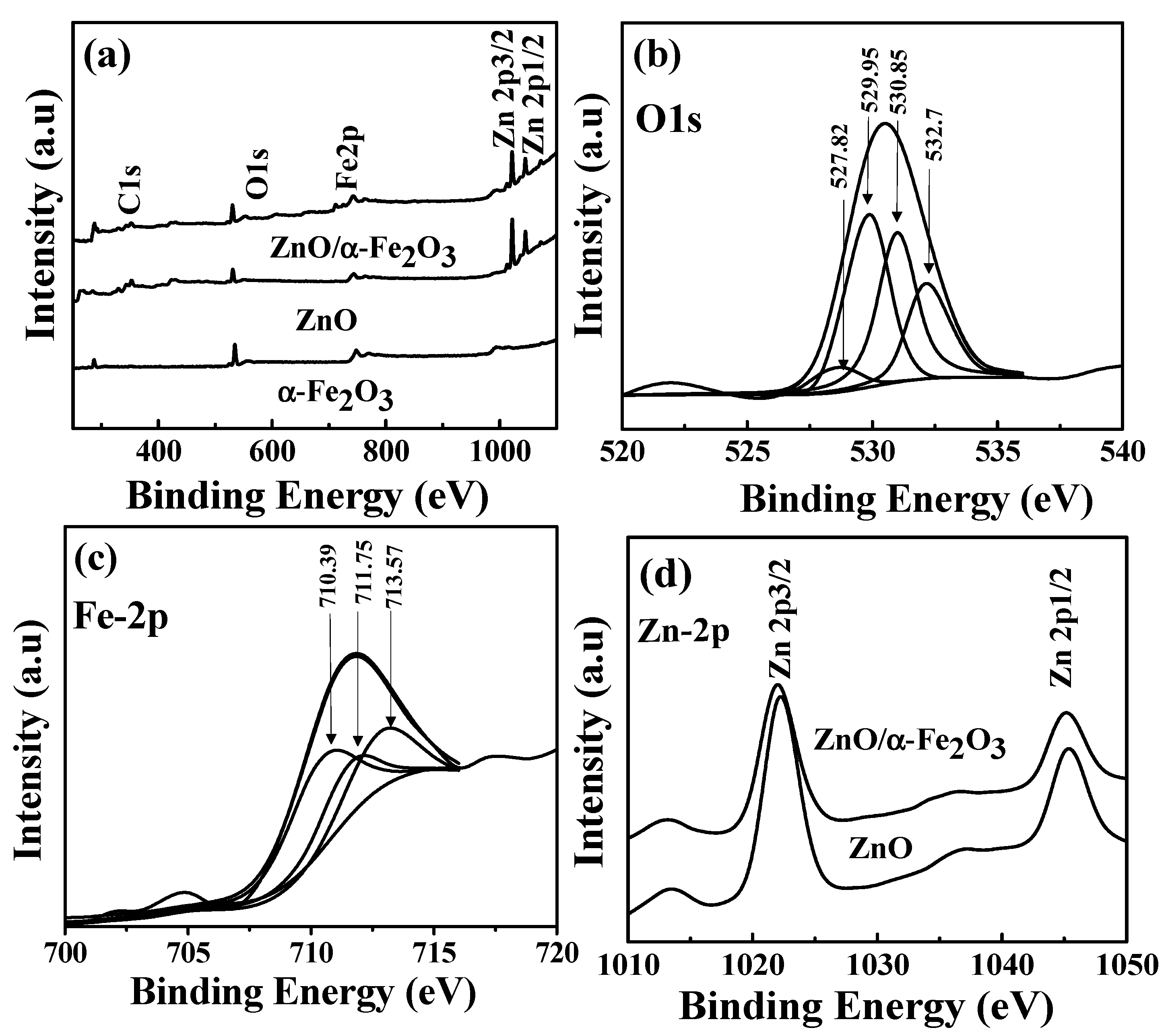
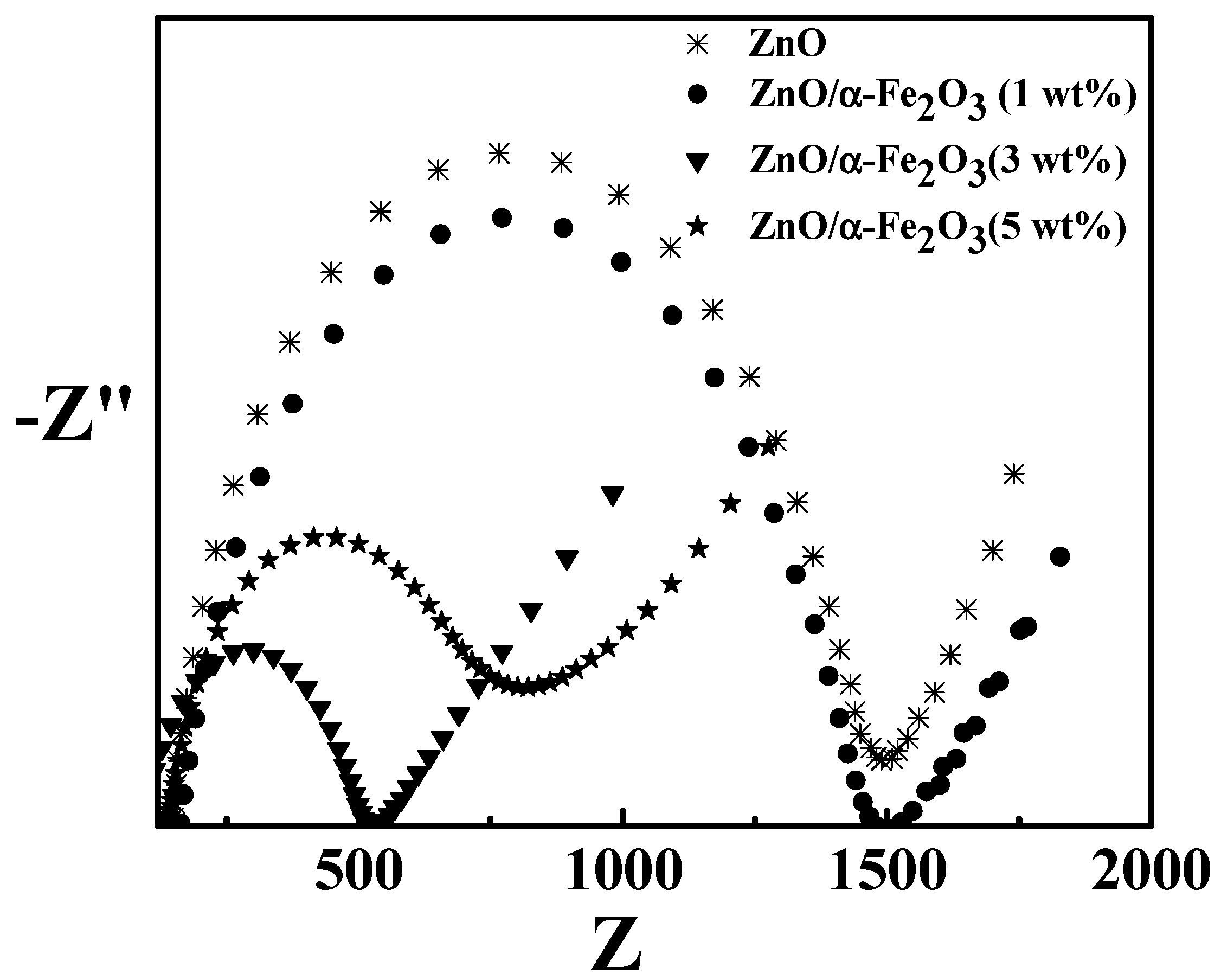
3. Results and Discussion
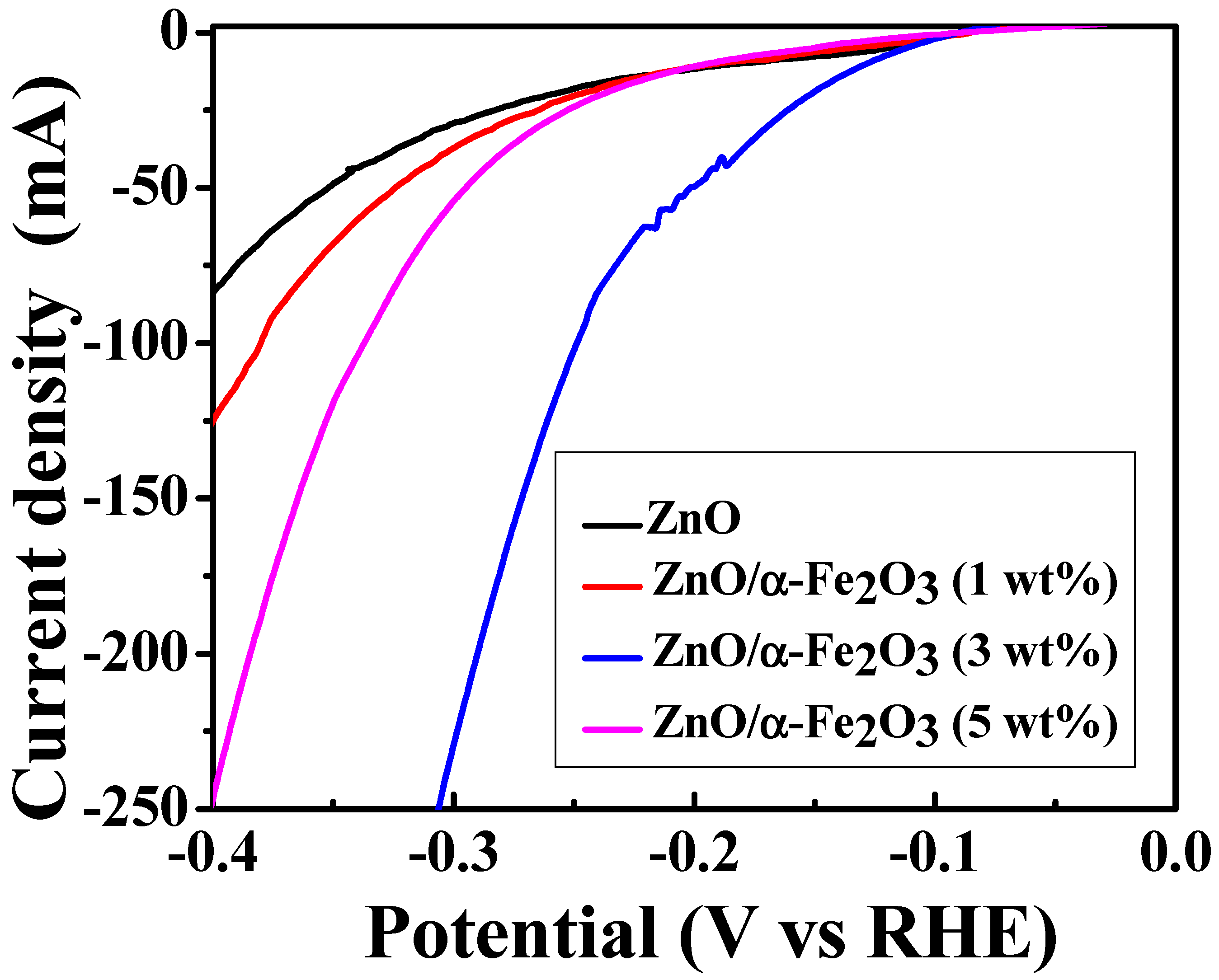
Hydrogen Evolution Reaction (HER)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hashimoto, K.; Kawai, T.; Sakata, T. Photocatalytic reactions of hydrocarbons and fossil fuels with water. Hydrogen production and oxidation. J. Phys. Chem. 1984, 88, 4083–4088. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Wang, L.; Xu, N.; Pan, X.; He, Y.; Wang, X.; Su, W. Cobalt lactate complex as a hole cocatalyst for significantly enhanced photocatalytic H2 production activity over CdS nanorods. Catal. Sci. Technol. 2018, 8, 1599–1605. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li1, F.; Ma, Y.; Du, A.; Qiao, S. Ti3C2 M Xene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, W.; Zhang, P.; Bi, J.; He, Y.; Wang, J.; Su, W. Sulfur-doped covalent triazine-based frameworks for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. A 2016, 4, 12402–12406. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Tan, Z.; Guo, B.; Lei, X.; Fu, H.; Zhu, Z. Charge-Transfer Induced High Efficient Hydrogen Evolution of MoS2/graphene Cocatalyst. Sci. Rep. 2015, 5, 18730. [Google Scholar] [CrossRef]
- Huang, C.-W.; Wu, M.-C.; Nguyen, V.-H.; Nguyen, B.-S. Preparation and Characterization of Nanocrystalline TiO2 on Microsericite for High-Efficiency Photo-Energy Conversion of Methanol to Hydrogen. Crystals 2019, 9, 380. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Merki, D.; Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 2011, 4, 3878–3888. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.; Xu, Z.; Wang, X. Recent Development of Molybdenum Sulfides as Advanced Electrocatalysts for Hydrogen Evolution Reaction. ACS Catal. 2014, 4, 1693–1705. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition Metal Dichalcogenides and Beyond: Synthesis, Properties, and Applications of Single- and Few-Layer Nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, X.; Du, K.; Ma, Y.; Liu, M.; Sun, H.; Ma, X.; Zhang, X. Reduced Graphene Oxide/O-MWCNT Hybrids Functionalized with p-Phenylenediamine as High-Performance MoS2 Electrocatalyst Support for Hydrogen Evolution Reaction. J. Phys. Chem. C 2016, 120, 1478–1487. [Google Scholar] [CrossRef]
- Pellegrino, F.; Sordello, F.; Minella, M.; Minero, C.; Maurino, V. The Role of Surface Texture on the Photocatalytic H2 Production on TiO2. Catalysts 2019, 9, 32. [Google Scholar] [CrossRef]
- Abdulrazzak, F.H.; Hussein, F.H.; Alkaim, A.F.; Ivanova, I.; Emelinee, A.V.; Bahnemannde, D.W. Sonochemical/hydration–dehydration synthesis of Pt–TiO2 NPs/decorated carbon nanotubes with enhanced photocatalytic hydrogen production activity. Photochem. Photobiol. Sci. 2016, 15, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Geng, Z.; Hou, P.; Yang, P.; Cheng, X.; Huang, S. Rhodamine B Removal of TiO2@SiO2 Core-Shell Nanocomposites Coated to Buildings. Crystals 2020, 10, 80. [Google Scholar] [CrossRef]
- Guo, M.; He, Q.; Wang, A.; Wang, W.; Fu, Z. A Novel, Simple and Green Way to Fabricate BiVO4 with Excellent Photocatalytic Activity and Its Methylene Blue Decomposition Mechanism. Crystals 2016, 6, 81. [Google Scholar] [CrossRef]
- Jia, T.; Li, J.; Long, F.; Fu, F.; Zhao, J.; Deng, Z.; Wang, X.; Zhang, Y. Ultrathin g-C3N4 Nanosheet-Modified BiOCl Hierarchical Flower-Like Plate Heterostructure with Enhanced Photostability and Photocatalytic Performance. Crystals 2017, 7, 266. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, Z.; Yang, D.; Sun, T.; Yu, H.; Hoster, H.E.; Hng, H.H.; Zhang, H.; Yan, Q. Hierarchical hollow spheres composed of ultrathin Fe2O3 nanosheets for lithium storage and photocatalytic water oxidation. Energy Environ. Sci. 2013, 6, 987–993. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Chen, Y.; Tse, W.H.; Chen, L.; Zhang, J. Ag nanoparticles-decorated ZnO nanorod array on a mechanical flexible substrate with enhanced optical and antimicrobial properties. Nanoscale Res. Lett. 2015, 10, 106. [Google Scholar] [CrossRef]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.-J. Controlled Growth of ZnO Nanowires and Their Optical Properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, Z.; Li, S.; Liua, F.; Zhang, Z. Rational design of a novel quaternary ZnO@ZnS/Ag@Ag2S nanojunction system for enhanced photocatalytic H2 production. Inorg. Chem. Front. 2018, 5, 3074–3081. [Google Scholar] [CrossRef]
- Mao, N. Investigating the Heteronjunction between ZnO/Fe2O3 and g-C3N4 for an Enhanced Photocatalytic H2 production under visible-light irradiation. Sci. Rep. 2019, 9, 12383. [Google Scholar] [CrossRef] [PubMed]
- Tama, A.M.; Das, S.; Dutta, S.; Bhuyan, M.D.I.; Islamb, M.N.; Basith, M.A. MoS2 nanosheet incorporated α-Fe2O3/ZnO nanocomposite with enhanced photocatalytic dye degradation and hydrogen production ability. RSC Adv. 2019, 9, 40357–40367. [Google Scholar] [CrossRef]
- Sankaran, S.; Panigrahi, S. Development and Evaluation of ZnO-Fe2O3 Based Nanocomposite Sensors for Butanol Detection. J. Nanosci. Nanotechnol. 2012, 12, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, L.; Chen, X.; Xu, Q.; Song, B.; Pan, Z.; Liu, Y.; Juan, F.; Xu, F.; Cao, B. Enhanced triethylamine sensing performance of α-Fe2O3 nanoparticle/ZnO nanorod heterostructures. Sens. Actuators B Chem. 2019, 298, 126917. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Wu, J.; Gu, L.; Lu, Z.; Wang, X.; Cao, X. Synthesis of highly permeable Fe2O3/ZnO hollow spheres for printable photocatalysis. RSC Adv. 2015, 5, 88277–88286. [Google Scholar] [CrossRef]
- Wolcott, A.; Wolcott, B.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical Study of Nanostructured ZnO Thin Films for Hydrogen Generation from Water Splitting. Adv. Funct. Mater. 2009, 19, 1849–1856. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Graya, H.B.; Muller, A.M. Effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-W.; Xu, C.-L.; Li, H.-L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun. 2008, 6537–6539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Bogoev, L.; Popova, M.; Gul, S.; Yano, J.; Sun, Y. Electrodeposited nickel-sulfide films as competent hydrogen evolution catalysts in neutral water. J. Mater. Chem. A 2014, 2, 19407–19414. [Google Scholar] [CrossRef]
- Ledendecker, M.; Calderon, S.K.; Papp, C.; Steinrück, H.P.; Antonietti, M.; Shalom, M. The Synthesis of Nanostructured Ni5P4 Films and their Use as a Non-Noble Bifunctional Electrocatalyst for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef] [PubMed]
- Hjiri, M. Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol–gel technique. J. Mater. Sci. Mater. Electron. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Ivanovskaya, M.I.; Tolstik, A.I.; Kotsikau, D.A.; Pankov, V.V. The structural characteristics of Zn-Mn ferrite synthesized by spray pyrolysis. Russ. J. Phys. Chem. A 2009, 83, 2081–2086. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Cho, M.H. Facile and single-step route towards ZnO@C core–shell nanoparticles as an oxygen vacancy induced visible light active photocatalyst using the thermal decomposition of Zn(an)2(NO3)2. RSC Adv. 2016, 6, 70644–70652. [Google Scholar] [CrossRef]
- Gayathri, S.; Nirmal Ghosh, O.S.; Sathishkumar, S.; Sudhakara, P.; Jayaramudu, J.; Ray, S.S.; Kasi Viswanath, A. Investigation of physicochemical properties of Ag doped ZnO nanoparticles prepared by chemical route. Appl. Sci. Lett. 2015, 1, 8. [Google Scholar]
- Znaidi, L.; Soler Illia, G.J.A.A.; Benyahia, S.; Sanchez, C.; Kanaev, A.V. Oriented ZnO Thin Films Synthesis by Sol–Gel Process for Laser Application. Thin Solid Film 2003, 428, 257–262. [Google Scholar] [CrossRef]
- Mitra, P.; Dutta, D.; Das, S.; Basu, T.; Pramanik, A.; Patra, A. Antibacterial and Photocatalytic Properties of ZnO–9-Aminoacridine Hydrochloride Hydrate Drug Nanoconjugates. ACS Omega 2018, 3, 7962–7970. [Google Scholar] [CrossRef] [PubMed]
- Rajab, F.H.; Korshed, P.; Liu, Z.; Wang, T.; Li, L. How did the structural ZnO nanowire as antibacterial coatings control the switchable wettability. Appl. Surf. Sci. 2019, 469, 593–606. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Zhang, Z.; Chen, L.; Cheng, J.; Nil, W.; Wang, B.; Xie, E. Light Illuminated α−Fe2O3/Pt Nanoparticles as Water Activation Agent for Photoelectrochemical Water Splitting. Sci. Rep. 2015, 5, 9130. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Im, B.; Kim, J.Y.; Im, Y.-O.; Jang, J.-Y.; Kim, E.S.; Kim, J.Y.; Kang, H.J.; Hong, S.J.; Lee, J.S. Photoelectrochemical water splitting over ordered honeycomb hematite electrodes stabilized by alumina shielding. Energy Environ. Sci. 2012, 5, 6375–6382. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Caglar, Y. Sol–gel derived nanostructure undoped and cobalt doped ZnO: Structural, optical and electrical studies. J. Alloy. Compd. 2013, 560, 181–188. [Google Scholar] [CrossRef]
- Aksoy, S.; Ilican, S.; Caglar, M. Sol–gel derived Li–Mg co-doped ZnO films: Preparation and characterization via XRD, XPS, FESEM. J. Alloy. Compd. 2012, 512, 171–178. [Google Scholar] [CrossRef]
- Karamat, S.; Rawata, R.S.; Lee, P.; Tan, T.L.; Ramanujan, R.V. Structural, elemental, optical and magnetic study of Fe doped ZnO and impurity phase formation. Prog. Nat. Sci. Mater. Int. 2014, 24, 142–149. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, Q.; Wen, G.; Wang, Q.; Hao, J.; Wang, S.; Zhang, J. XPS and Raman scattering studies of room temperature ferromagnetic ZnO:Cu. J. Phys. D Appl. Phys. 2009, 42, 215007. [Google Scholar] [CrossRef]
- Ye, S.; Ding, C.; Chen, R.; Fan, F.; Fu, P.; Yin, H.; Wang, X.; Wang, Z.; Du, P.; Li, C. Mimicking the Key Functions of Photosystem II in Artificial Photosynthesis for Photoelectrocatalytic Water Splitting. J. Am. Chem. Soc. 2018, 140, 3250–3256. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Kang, Z.; Li, Y.; Shen, Y.; Sun, Y.; Wang, L.; Zhang, Y. Synergistic Effect of Surface Plasmonic particles and Surface Passivation layer on ZnO Nanorods Array for Improved Photoelectrochemical Water Splitting. Sci. Rep. 2016, 6, 29907. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Halla, P.B.; Jiang, J.; Hurley, P.T.; Chen, J. Multilayered Si Nanoparticle/Reduced Graphene Oxide Hybrid as a High-Performance Lithium-Ion Battery Anode. Adv. Mater. 2014, 26, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.-Y.; Tang, X. Cycling degradation of an automotive LiFePO4 lithium-ion battery. J. Power Sources 2011, 196, 1513–1520. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uma, K.; Muniranthinam, E.; Chong, S.; Yang, T.C.-K.; Lin, J.-H. Fabrication of Hybrid Catalyst ZnO Nanorod/α-Fe2O3 Composites for Hydrogen Evolution Reaction. Crystals 2020, 10, 356. https://doi.org/10.3390/cryst10050356
Uma K, Muniranthinam E, Chong S, Yang TC-K, Lin J-H. Fabrication of Hybrid Catalyst ZnO Nanorod/α-Fe2O3 Composites for Hydrogen Evolution Reaction. Crystals. 2020; 10(5):356. https://doi.org/10.3390/cryst10050356
Chicago/Turabian StyleUma, Kasimayan, Elavarasan Muniranthinam, Siewhui Chong, Thomas C.-K Yang, and Ja-Hon Lin. 2020. "Fabrication of Hybrid Catalyst ZnO Nanorod/α-Fe2O3 Composites for Hydrogen Evolution Reaction" Crystals 10, no. 5: 356. https://doi.org/10.3390/cryst10050356
APA StyleUma, K., Muniranthinam, E., Chong, S., Yang, T. C.-K., & Lin, J.-H. (2020). Fabrication of Hybrid Catalyst ZnO Nanorod/α-Fe2O3 Composites for Hydrogen Evolution Reaction. Crystals, 10(5), 356. https://doi.org/10.3390/cryst10050356