Synthesis of a New Ruthenate Ba26Ru12O57
Abstract
1. Introduction
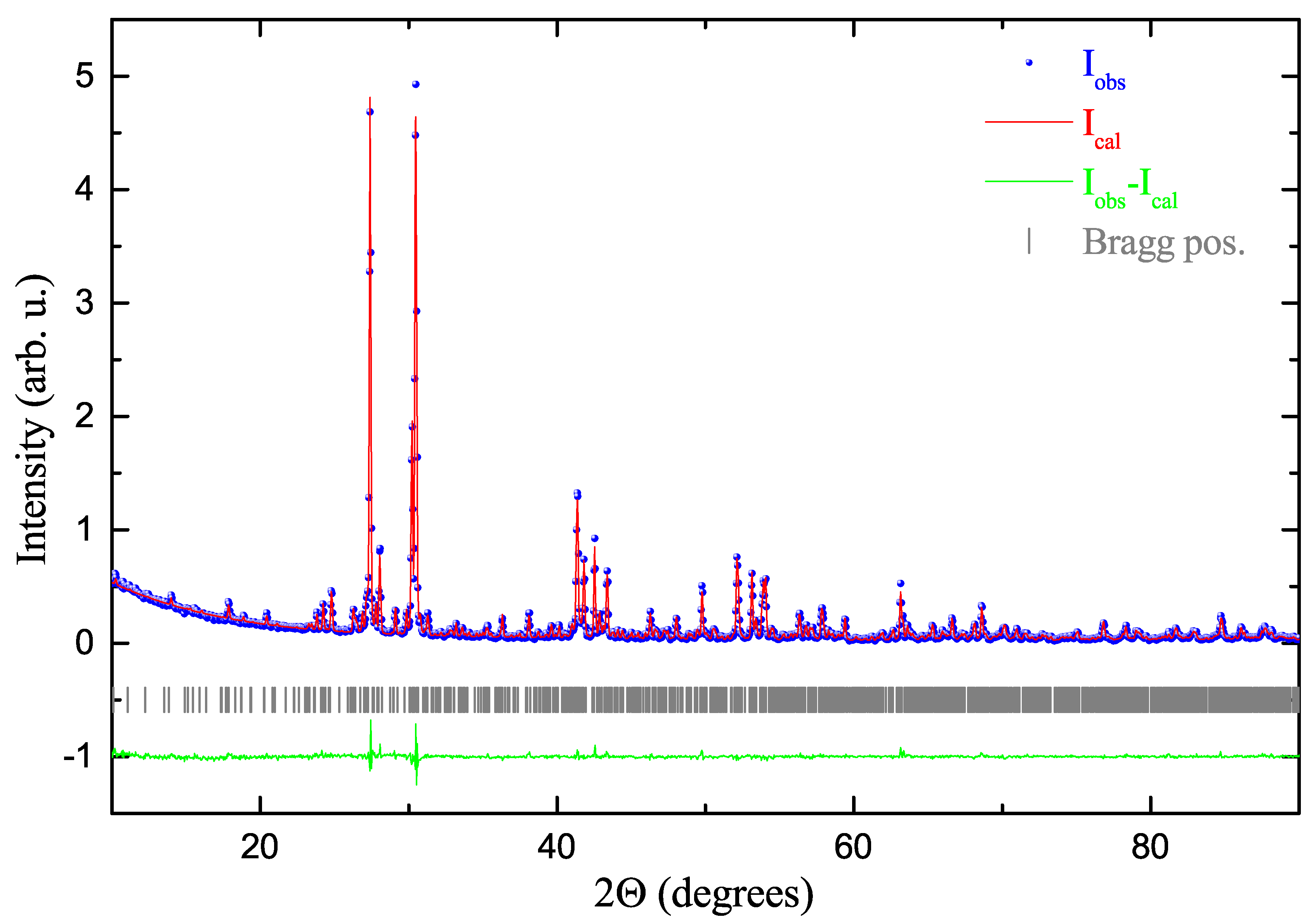
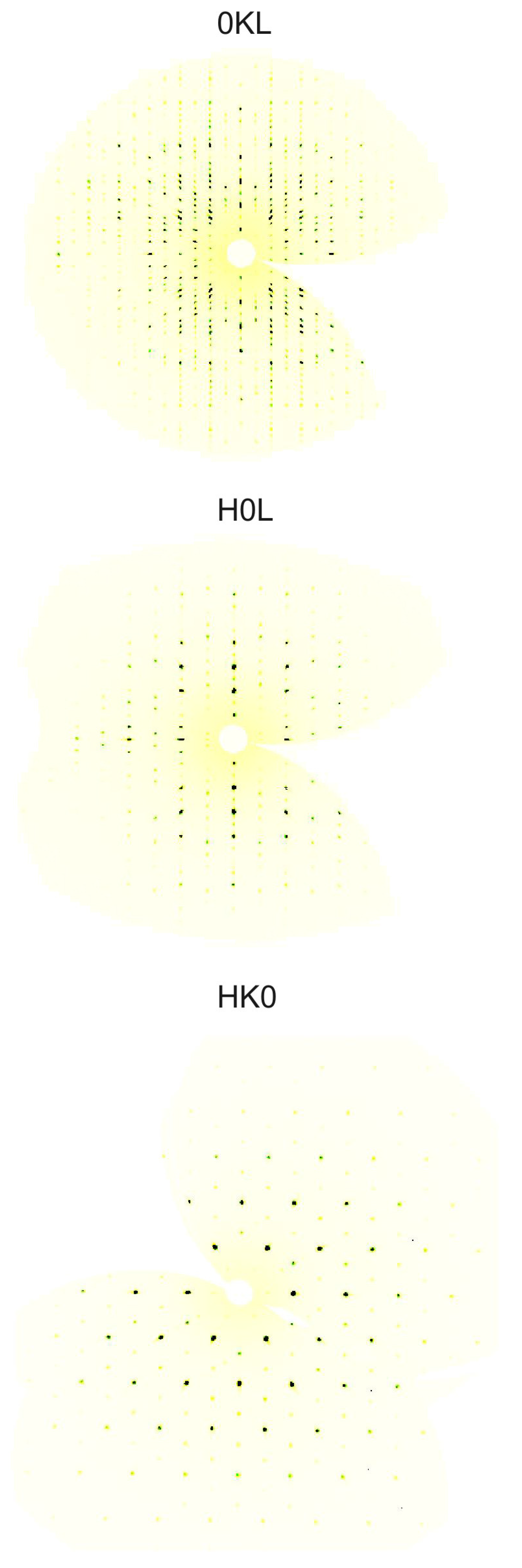
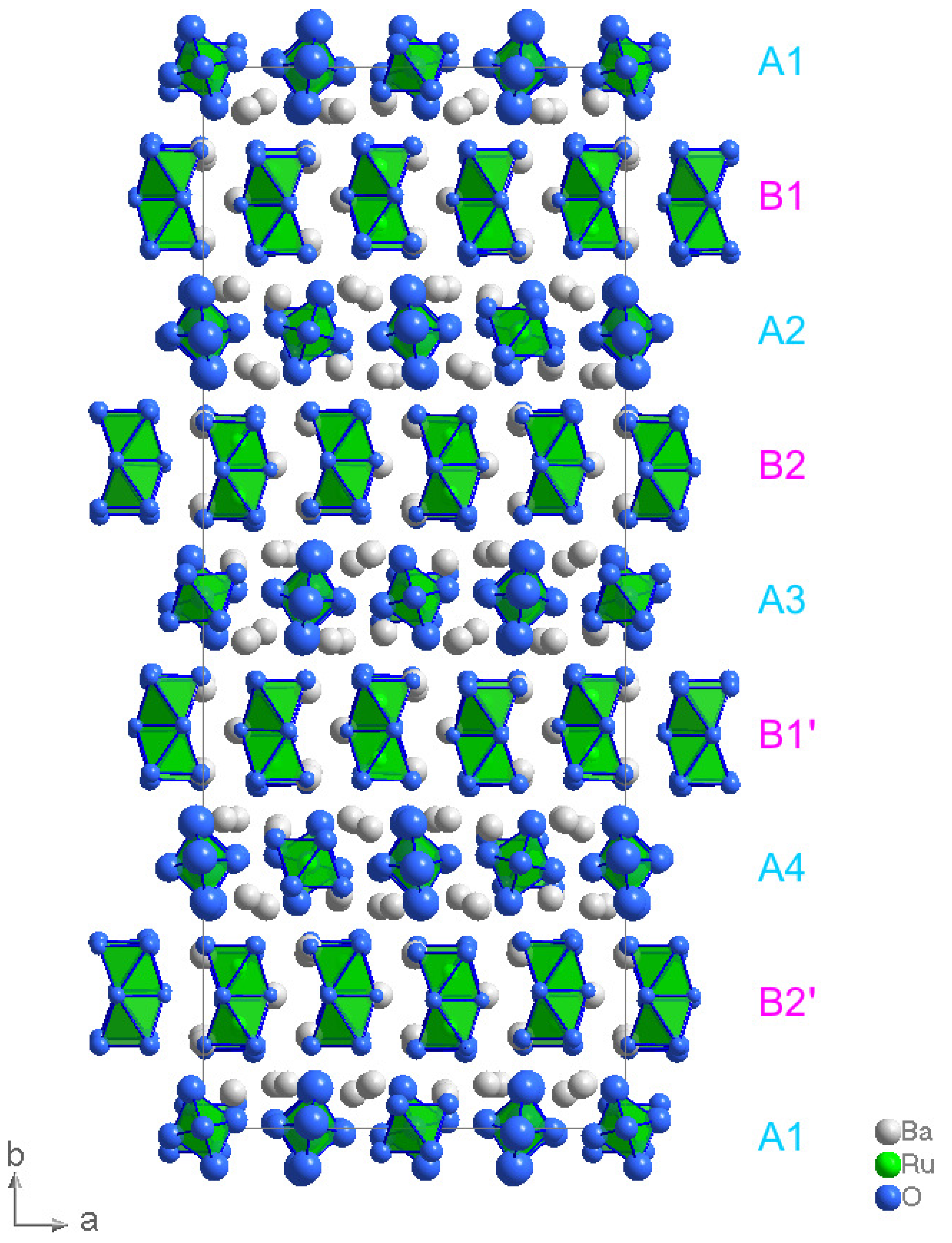
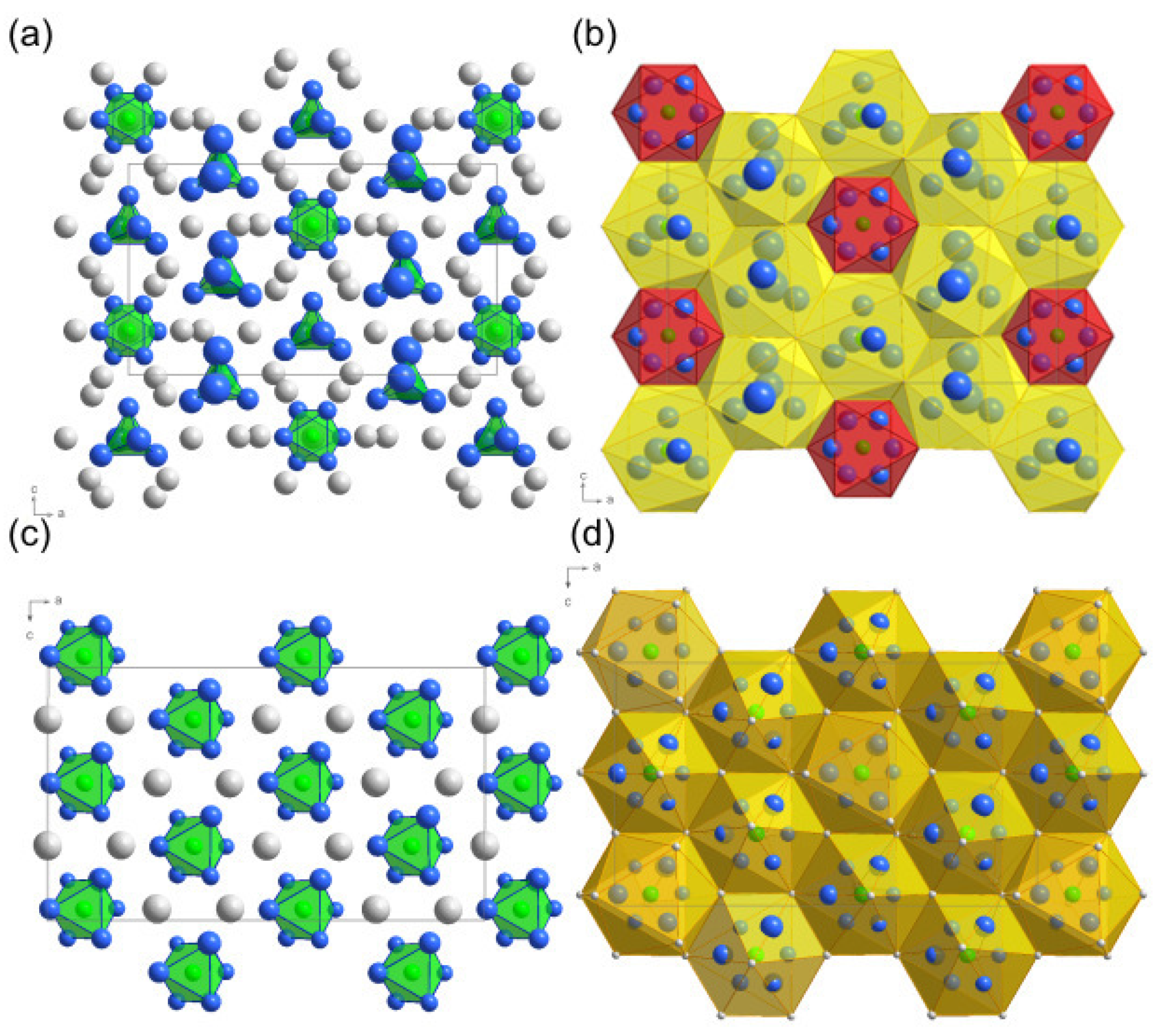
2. Results and Discussion
Structure
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Longo, J.M.; Raccah, P.M.; Goodenough, J.B. Magnetic Properties of SrRuO3 and CaRuO3. J. Appl. Phys. 1968, 39, 1327. [Google Scholar] [CrossRef]
- Grigera, S.A.; Perry, R.S.; Schofield, A.J.; Chiao, M.; Julian, S.R.; Lonzarich, G.G.; Ikeda, S.I.; Maeno, Y.; Millis, A.J.; Mackenzie, A.P. Magnetic field-tuned quantum criticality in the metallic ruthenate Sr3Ru2O7. Science 2001, 294, 329. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.S.; Galvin, L.M.; Grigera, S.A.; Capogna, L.; Schofield, A.J.; Mackenzie, A.P.; Chiao, M.; Julian, S.R.; Ikeda, S.I.; Nakatsuji, S.; et al. Metamagnetism and critical fluctuations in high quality single crystals of the bilayer ruthenate Sr3Ru2O7. Phys. Rev. Lett. 2001, 86, 2661. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Hashimoto, H.; Yoshida, K.; Nishizaki, S.; Fujita, T.; Bednorz, J.G.; Lichtenberg, F. Superconductivity in a layered perovskite without copper. Nature 1994, 372, 532. [Google Scholar] [CrossRef]
- Koster, G.; Klein, L.; Siemons, W.; Rijnders, G.; Dodge, J.S.; Eom, C.-B.; Blank, D.H.A.; Beasley, M.R. Structure, physical properties, and applications of SrRuO3 thin films. Rev. Mod. Phys. 2012, 84, 253. [Google Scholar] [CrossRef]
- Eremin, I.; Manske, D.; Ovchinnikov, S.G.; Annett, J.F. Unconventional superconductivity and magnetism in Sr2RuO4 and related materials. Ann. Phys. 2004, 13, 149–174. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Liu, C.-F.; Skoulatos, M.; Tjeng, L.; Komarek, A. Floating zone growth of Ba-substituted ruthenate Sr2-xBaxRuO4. J. Cryst. Growth 2015, 427, 94. [Google Scholar] [CrossRef]
- Li, Z.W.; Guo, H.; Liu, C.-F.; Bourdarot, F.; Schmidt, W.; Skoulatos, M.; Komarek, A.C. Spin fluctuations in Sr1.6Ba0.4RuO4: An inelastic neutron scattering study with polarization analysis. Phys. Rev. B 2017, 95, 045105. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Vijayaraghavan, R.; Varadaraju, U.V. Effects of oxygen non-stoichiometry and cationic substitutions on the properties of Sr2RuO4+x. Materials chemistry and physics. Mater. Chem. Phys. 1998, 56, 63–69. [Google Scholar] [CrossRef]
- Hong, S.-T.; Sleight, A.W.J. Scanning transmission electron microscopy (STEM) and X-ray absorption spectroscopy (XAS) investigations of catalytic systems. Solid State Chem. 1997, 128, 251–255. [Google Scholar] [CrossRef]
- Donohue, P.C.; Katz, L.; Ward, R. The crystal structure of barium ruthenium oxide and related compounds. Inorg. Chem. 1965, 4, 306–310. [Google Scholar] [CrossRef]
- Igarashi, T.; Nogami, Y.; Klein, Y.; Rousse, G.; Okazaki, R.; Taniguchi, H.; Yasui, Y.; Terasaki, I. X-ray Crystal Structure Analysis and Ru Valence of Ba4Ru3O10 Single Crystals. J. Phys. Soc. Jpn. 2013, 82, 104603. [Google Scholar] [CrossRef]
- Klein, Y.; Rousse, G.; Damay, F.; Porcher, F.; André, G.; Terasaki, I. Antiferromagnetic order and consequences on the transport properties of Ba4Ru3O10. Phys. Rev. B 2011, 84, 054439. [Google Scholar] [CrossRef]
- Dussarra, C.; Grasse, F.; Bontchev, R.; Darriet, J. Crystal structures and magnetic properties of Ba4Ru3O10 and Ba5Ru3O12. J. Alloys Compd. 1996, 233, 15–22. [Google Scholar] [CrossRef]
- Grasset, F.; Zakhour, M.; Darriet, J. Synthesis, crystal structure and magnetic properties of Ba5Ru2O9 (O2), Ba5Nb2O9 (O2) and Ba5Ru2O10 related to the perovskite-type structure, and structural relationships with corresponding sulfides. J. Alloys Compd. 1999, 287, 25–31. [Google Scholar] [CrossRef]
- Jia, Y.; Zurbuchen, M.A.; Wozniak, S.; Carim, A.H.; Schlom, D.G. Epitaxial growth of metastable Ba2RuO4 films with the K2NiF4 structure. Appl. Phys. Lett. 1999, 74, 3830. [Google Scholar] [CrossRef]
- Kafalas, J.A.; Longo, J.M. High pressure synthesis of (ABX3)(AX) n compounds. J. Solid State Chem. 1972, 4, 55. [Google Scholar] [CrossRef]
- Rodrguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physics B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345. [Google Scholar]
- Takeda, Y.; Kanamura, F.; Shimada, M.; Koizumi, M. The crystal structure of BaNiO3. Acta Cryst. B 1976, 32, 2464–2466. [Google Scholar] [CrossRef]
- Tillmanns, E.; Grosse, H.-P. Refinement of tribarium silicate. Acta Cryst. B 1978, 34, 649–651. [Google Scholar] [CrossRef]
- Bekker, T.B.; Rashchenko, S.V.; Seryotkin, Y.V.; Kokh, A.E.; Davydov, A.V.; Fedorov, P.P. BaO-B2O3 system and its mysterious member Ba3B2O6. J. Am. Ceram. Soc. 2018, 101, 450–457. [Google Scholar] [CrossRef]
- Kipka, R.; Mueller-Buschbaum, H. Uber Oxocuprate. XIX. Ein Oxohalogenocuprat (I): Ba2CuO2Cl. Anorg. Allg. Chem. 1977, 430, 250–254. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Fischer, D.; Hoppe, R.Z. Zur Konstitution von Alkaliruthenaten (VI).2. Uber den Aufbau von K2[RuO3(OH)2]. Anorg. Allg. Chem. 1991, 601, 41–46. [Google Scholar] [CrossRef]

| Empirical formula | BaRuO |
| Formula weight (g/mol) | 5696.1 |
| Temperature | room temperature |
| Wavelength | Mo K |
| Crystal system | orthorhombic |
| Space group | Fdd2 (43) |
| Unit cell dimensions | a = 20.4638(12) Å |
| b = 51.191(3) Å | |
| c = 11.7698(7) Å | |
| Volume | 12,329.5(13) Å |
| Z | 8 |
| Density (g/cm) | 6.1372 |
| Absorption coefficient | 19.234 |
| F(000) | 19,520 |
| Crystal size | ∼10 m |
| 2 | 64.12 |
| Index range | h: −30 → 30 |
| k: −76 → 76 | |
| l: −17 → 17 | |
| Reflections in total/independant | 340,765/21,135 |
| Observed reflections/independant | 256,056/19,031 |
| Internal R-value | 4.54% |
| Completeness up to 2 | 99.89% |
| Absorption correction | multi-scan |
| Max./min. transmission | 0.5463/0.7463 |
| Refinement method | least squares on |
| Reflections threshold | |
| Goodness of fit | 2.19 |
| R/R | 3.07%/7.80% |
| Largest minima in Fourier difference | −1.20 e Å |
| Largest maxima in Fourier difference | 1.94 e Å |
| Atom | x | y | z | U (Å) |
|---|---|---|---|---|
| Ba1 | 0.65079(2) | 0.208323(7) | 0.1563 | 0.01467(10) |
| Ba2 | 0.999319(17) | 0.085696(6) | 0.20177(7) | 0.00981(8) |
| Ba3 | 0.170861(19) | 0.041104(7) | 0.20207(6) | 0.01739(10) |
| Ba4 | 0.82290(2) | 0.219793(7) | 0.24056(5) | 0.01327(10) |
| Ba5 | 0.354973(17) | 0.032130(7) | 0.21110(6) | 0.01728(9) |
| Ba6 | 0.32153(2) | 0.216648(7) | 0.17447(5) | 0.01444(11) |
| Ba7 | 0.167785(18) | 0.124818(6) | 0.20302(8) | 0.01096(8) |
| Ba8 | 0.70055(2) | 0.041117(7) | 0.21087(6) | 0.02095(11) |
| Ba9 | 0.89407(3) | 0.040235(9) | 0.00885(5) | 0.02325(13) |
| Ba10 | 0.500566(18) | 0.080283(7) | 0.20496(7) | 0.01392(9) |
| Ba11 | 0.000746(16) | 0.164118(6) | 0.20603(7) | 0.00952(8) |
| Ba12 | 0.501251(17) | 0.162389(6) | 0.20568(7) | 0.00925(8) |
| Ba13 | 0.666246(17) | 0.124834(6) | 0.20821(8) | 0.01113(8) |
| Ru1 | 0.83331(2) | 0.103263(7) | 0.20564(8) | 0.00665(10) |
| Ru2 | 0.33370(2) | 0.151882(7) | 0.20507(8) | 0.00661(10) |
| Ru3 | 0.33503(2) | 0.097675(7) | 0.20691(8) | 0.00672(10) |
| Ru4 | 0.83275(2) | 0.156070(8) | 0.20657(8) | 0.00678(10) |
| Ru5 | 0.5 | 0 | 0.21206(10) | 0.00769(15) |
| Ru6 | 0.50515(3) | 0.249290(8) | 0.21046(10) | 0.01119(11) |
| Ru7 | 0 | 0 | 0.19733(10) | 0.01396(19) |
| O1 | 0.1229(2) | 0.57513(9) | 0.0740(4) | 0.0096(9) |
| O2 | 0.2552(2) | 0.58445(8) | 0.2062(5) | 0.0141(8) |
| O3 | 0.1258(3) | 0.32816(11) | 0.0876(5) | 0.0163(11) |
| O4 | 0.25532(19) | 0.17114(8) | 0.2035(5) | 0.0108(7) |
| O5 | 0.2429(2) | 0.42234(8) | 0.2063(5) | 0.0147(8) |
| O6 | 0.1296(3) | 0.82271(11) | 0.0898(5) | 0.0194(12) |
| O7 | 0.2545(2) | 0.67576(8) | 0.2060(5) | 0.0153(8) |
| O8 | 0.2028(3) | 0.37512(8) | 0.0939(4) | 0.0090(10) |
| O9 | 0.1223(3) | 0.07778(10) | 0.0714(4) | 0.0102(9) |
| O10 | 0.1262(3) | 0.42173(10) | 0.0887(4) | 0.0155(10) |
| O11 | 0.1281(3) | 0.91493(9) | 0.0864(4) | 0.0119(9) |
| O12 | 0.2042(2) | 0.87099(9) | 0.0928(4) | 0.0074(9) |
| O13 | 0.09182(19) | 0.87039(7) | 0.2025(5) | 0.0098(7) |
| O14 | 0.09121(19) | 0.37525(7) | 0.2059(5) | 0.0091(7) |
| O15 | 0.1229(2) | 0.17197(10) | 0.0751(4) | 0.0107(9) |
| O16 | 0.1229(3) | 0.66528(9) | 0.0729(4) | 0.0142(10) |
| O17 | 0.0472(3) | 0.12503(9) | 0.0677(4) | 0.0112(10) |
| O18 | 0.0421(2) | 0.47838(10) | 0.0913(4) | 0.0166(9) |
| O19 | 0.0279(3) | 0.96359(10) | 0.1966(6) | 0.0312(12) |
| O20 | 0.0786(2) | 0.52270(8) | 0.2076(5) | 0.0168(8) |
| O21 | 0.0150(4) | 0.71228(13) | 0.1873(7) | 0.0492(19) |
| O22 | 0.0078(3) | 0.21343(12) | 0.2369(6) | 0.0392(16) |
| O23 | 0.0756(3) | 0.00744(11) | 0.1199(5) | 0.0265(12) |
| O24 | 0.0452(3) | 0.62015(10) | 0.0702(4) | 0.0106(9) |
| O25 | 0.25 | 0.75 | 0.0983(6) | 0.0206(14) |
| O26 | 0.0736(3) | 0.25183(10) | 0.1366(5) | 0.0223(11) |
| O27 | 0.0785(3) | 0.75492(12) | 0.1292(5) | 0.0287(12) |
| O28 | 0.2101(3) | 0.22822(10) | 0.0825(4) | 0.0173(10) |
| O29 | 0.2418(4) | 0.49459(14) | 0.1135(6) | 0.0428(16) |
| Atom | U (Å) | U (Å) | U (Å) |
|---|---|---|---|
| Ba1 | 0.01598(19) | 0.01027(16) | 0.01775(19) |
| Ba2 | 0.01068(15) | 0.00795(12) | 0.01081(16) |
| Ba3 | 0.01431(16) | 0.01217(14) | 0.02569(19) |
| Ba4 | 0.01802(19) | 0.00930(15) | 0.01250(17) |
| Ba5 | 0.01249(15) | 0.01051(14) | 0.02885(19) |
| Ba6 | 0.0205(2) | 0.00795(15) | 0.0148(2) |
| Ba7 | 0.00906(14) | 0.01393(13) | 0.00988(16) |
| Ba8 | 0.02028(18) | 0.01712(17) | 0.0254(2) |
| Ba9 | 0.0248(2) | 0.0231(2) | 0.0218(2) |
| Ba10 | 0.01128(16) | 0.01918(15) | 0.01129(16) |
| Ba11 | 0.00932(14) | 0.00804(12) | 0.01119(15) |
| Ba12 | 0.00949(14) | 0.00785(12) | 0.01041(15) |
| Ba13 | 0.00979(15) | 0.01347(13) | 0.01013(16) |
| Ru1 | 0.00610(17) | 0.00638(16) | 0.00747(17) |
| Ru2 | 0.00665(18) | 0.00582(16) | 0.00736(18) |
| Ru3 | 0.00650(17) | 0.00651(16) | 0.00716(17) |
| Ru4 | 0.00673(18) | 0.00627(15) | 0.00733(18) |
| Ru5 | 0.0082(3) | 0.0076(2) | 0.0073(3) |
| Ru6 | 0.01110(19) | 0.00691(16) | 0.0156(2) |
| Ru7 | 0.0232(3) | 0.0082(2) | 0.0105(4) |
| Ba1 | −0.00120(14) | −0.00363(15) | −0.00249(13) |
| Ba2 | −0.00129(11) | −0.00031(16) | 0.00017(15) |
| Ba3 | −0.00332(13) | 0.00150(19) | −0.00277(16) |
| Ba4 | 0.00047(13) | 0.00177(13) | 0.00054(12) |
| Ba5 | 0.00103(12) | 0.00066(19) | 0.00177(16) |
| Ba6 | 0.00056(13) | −0.00395(14) | −0.00033(12) |
| Ba7 | 0.00103(12) | −0.0013(2) | −0.00129(15) |
| Ba8 | −0.00772(14) | 0.0032(2) | 0.00001(18) |
| Ba9 | 0.01344(18) | −0.00860(18) | −0.01232(16) |
| Ba10 | −0.00026(13) | 0.00042(17) | 0.0016(2) |
| Ba11 | −0.00102(11) | 0.00087(18) | −0.00106(16) |
| Ba12 | −0.00057(11) | 0.00028(18) | 0.00026(17) |
| Ba13 | 0.00243(11) | −0.0001(2) | 0.00155(15) |
| Ru1 | 0.00016(14) | −0.00077(19) | −0.0001(2) |
| Ru2 | −0.00025(14) | 0.0000(2) | 0.0002(2) |
| Ru3 | −0.00054(14) | −0.00053(19) | −0.0008(2) |
| Ru4 | 0.00022(14) | 0.0000(2) | −0.0001(2) |
| Ru5 | 0.0001(2) | 0 | 0 |
| Ru6 | −0.00109(14) | −0.0059(2) | 0.00244(17) |
| Ru7 | −0.0059(3) | 0 | 0 |
| Atoms | Distance | Coordination |
|---|---|---|
| Ru1-O2 | 1.867(4) | octahedral |
| Ru1-O11 | 1.861(5) | |
| Ru1-O12 | 2.022(5) | |
| Ru1-O13 | 2.042(4) | |
| Ru1-O16 | 1.861(5) | |
| Ru1-O24 | 2.068(5) | |
| Ru2-O3 | 1.909(6) | octahedral |
| Ru2-O4 | 1.883(4) | |
| Ru2-O8 | 2.044(5) | |
| Ru2-O9 | 1.893(5) | |
| Ru2-O14 | 2.071(4) | |
| Ru2-O17 | 2.053(5) | |
| Ru3-O5 | 1.895(4) | octahedral |
| Ru3-O8 | 2.075(5) | |
| Ru3-O10 | 1.884(5) | |
| Ru3-O14 | 2.049(4) | |
| Ru3-O15 | 1.884(5) | |
| Ru3-O17 | 2.062(5) | |
| Ru4-O1 | 1.874(5) | octahedral |
| Ru4-O6 | 1.914(6) | |
| Ru4-O7 | 1.892(4) | |
| Ru4-O12 | 2.069(5) | |
| Ru4-O13 | 2.054(4) | |
| Ru4-O24 | 2.044(5) | |
| Ru5-O18 | 1.997(5) | octahedral |
| Ru5-O18 | 1.997(5) | |
| Ru5-O20 | 1.986(4) | |
| Ru5-O20 | 1.986(4) | |
| Ru5-O28 | 1.980(5) | |
| Ru5-O28 | 1.980(5) | |
| Ru6-O21 | 1.924(7) | trigonal bipyr. |
| Ru6-O22 | 1.952(6) | |
| Ru6-O26 | 1.832(5) | |
| Ru6-O27 | 1.803(6) | |
| Ru6-O29 | 1.818(7) | |
| Ru7-O19 | 1.949(5) | trigonal bipyr. |
| Ru7-O19 | 1.949(5) | |
| Ru7-O23 | 1.835(6) | |
| Ru7-O23 | 1.835(6) | |
| Ru7-O25 | 1.777(7) |
| Atom | BVS | av. O-Distance | O-Coordination |
|---|---|---|---|
| Ba1 | 2.25(1) | 2.79(16) | 8-fold (2-5-1) |
| Ba2 | 2.25(1) | 2.88(17) | 10-fold (3-6-1) |
| Ba3 | 2.23(1) | 2.67(12) | octahedral (3-0-3) |
| Ba4 | 2.41(1) | 2.76(14) | 8-fold (3-4-1) |
| Ba5 | 2.25(1) | 2.80(20) | 8-fold (3-4-1) |
| Ba6 | 2.40(1) | 2.77(19) | 8-fold (3-4-1) |
| Ba7 | 1.95(1) | 2.98(11) | 12-fold (3-6-3) |
| Ba8 | 2.17(1) | 2.81(16) | 8-fold (3-3-2) |
| Ba9 | 2.16(1) | 2.82(18) | 8-fold (3-3-2) |
| Ba10 | 1.77(1) | 3.05(22) | 12-fold (3-6-3) |
| Ba11 | 2.36(1) | 2.87(18) | 10-fold (3-6-1) |
| Ba12 | 2.30(1) | 2.87(16) | 10-fold (3-6-1) |
| Ba13 | 2.06(1) | 2.96(12) | 12-fold (3-6-3) |
| average | 2.2(2) | ||
| Ru1 | 4.48(2) | 1.95(10) | octahedral (3-0-3) |
| Ru2 | 4.19(2) | 1.98(9) | octahedral (3-0-3) |
| Ru3 | 4.21(2) | 1.97(10) | octahedral (3-0-3) |
| Ru4 | 4.21(2) | 1.97(9) | octahedral (3-0-3) |
| Ru5 | 3.96(2) | 1.99(1) | octahedral (3-0-3) |
| Ru6 | 4.65(4) | 1.87(7) | trigonal bipyr. (1-3-1) |
| Ru7 | 4.63(4) | 1.87(8) | trigonal bipyr. (1-3-1) |
| average | 4.3(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Burkhardt, U.; Komarek, A.C. Synthesis of a New Ruthenate Ba26Ru12O57. Crystals 2020, 10, 355. https://doi.org/10.3390/cryst10050355
Lee J-E, Burkhardt U, Komarek AC. Synthesis of a New Ruthenate Ba26Ru12O57. Crystals. 2020; 10(5):355. https://doi.org/10.3390/cryst10050355
Chicago/Turabian StyleLee, Jeong-Eun, Ulrich Burkhardt, and Alexander Christoph Komarek. 2020. "Synthesis of a New Ruthenate Ba26Ru12O57" Crystals 10, no. 5: 355. https://doi.org/10.3390/cryst10050355
APA StyleLee, J.-E., Burkhardt, U., & Komarek, A. C. (2020). Synthesis of a New Ruthenate Ba26Ru12O57. Crystals, 10(5), 355. https://doi.org/10.3390/cryst10050355




