Structure of a Luminescent MOF-2 Derivative with a Core of Zn(II)-Terephthalate-Isoquinoline and Its Application in Sensing of Xylenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Measurements
2.2. Synthesis of {[Zn2(μ2-BDC)2(iQ)2]}∞, 1
2.3. Crystallographic Investigations
3. Results and Discussion
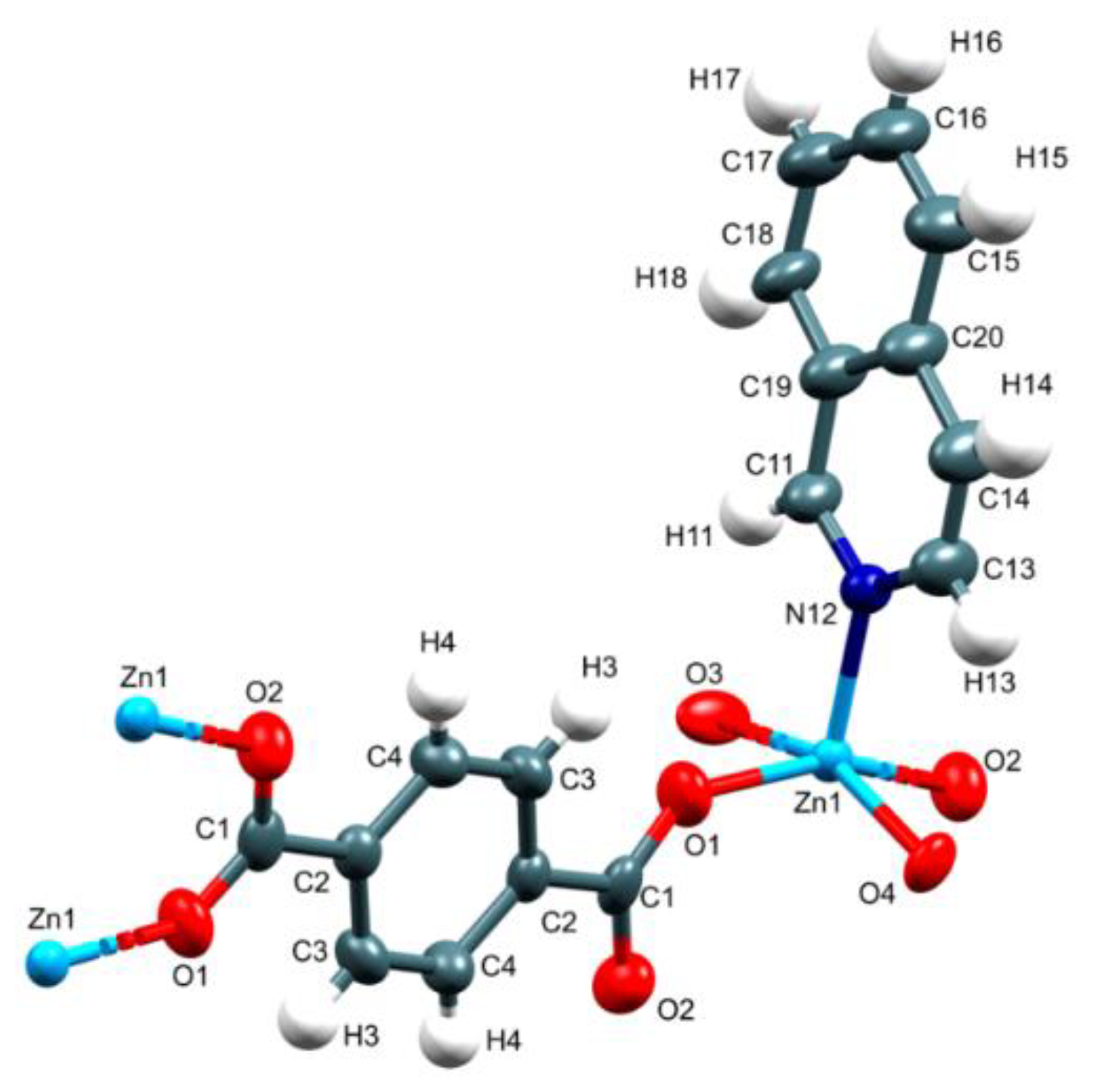
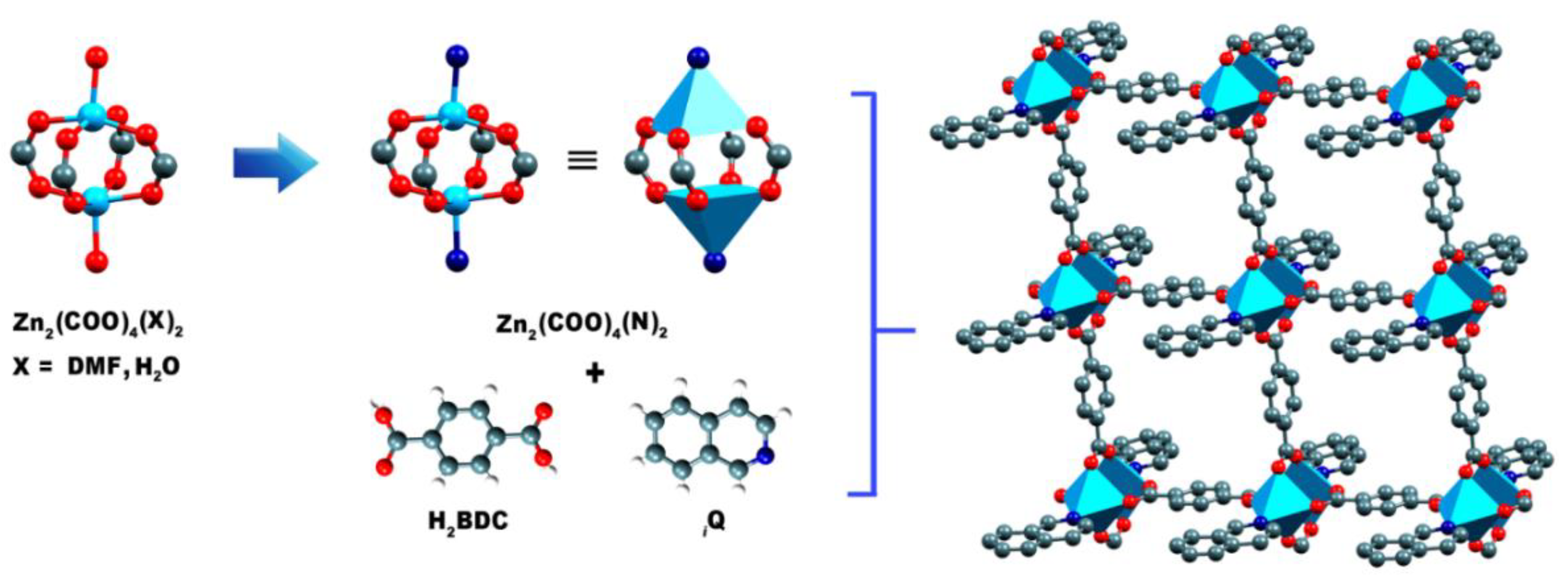
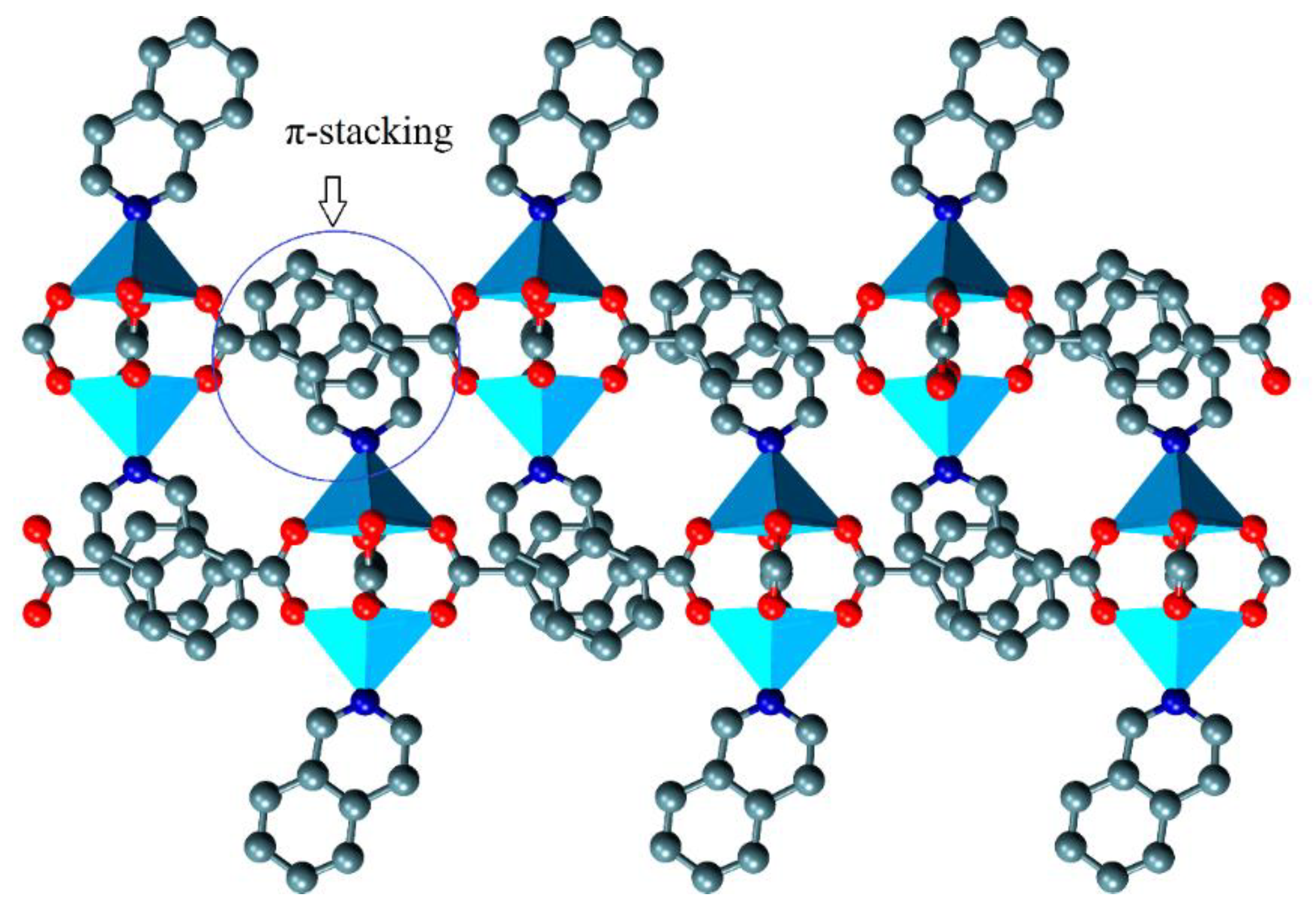
3.1. Crystal Structure of {[Zn2(μ2-BDC)2(iQ)2]}∞, 1
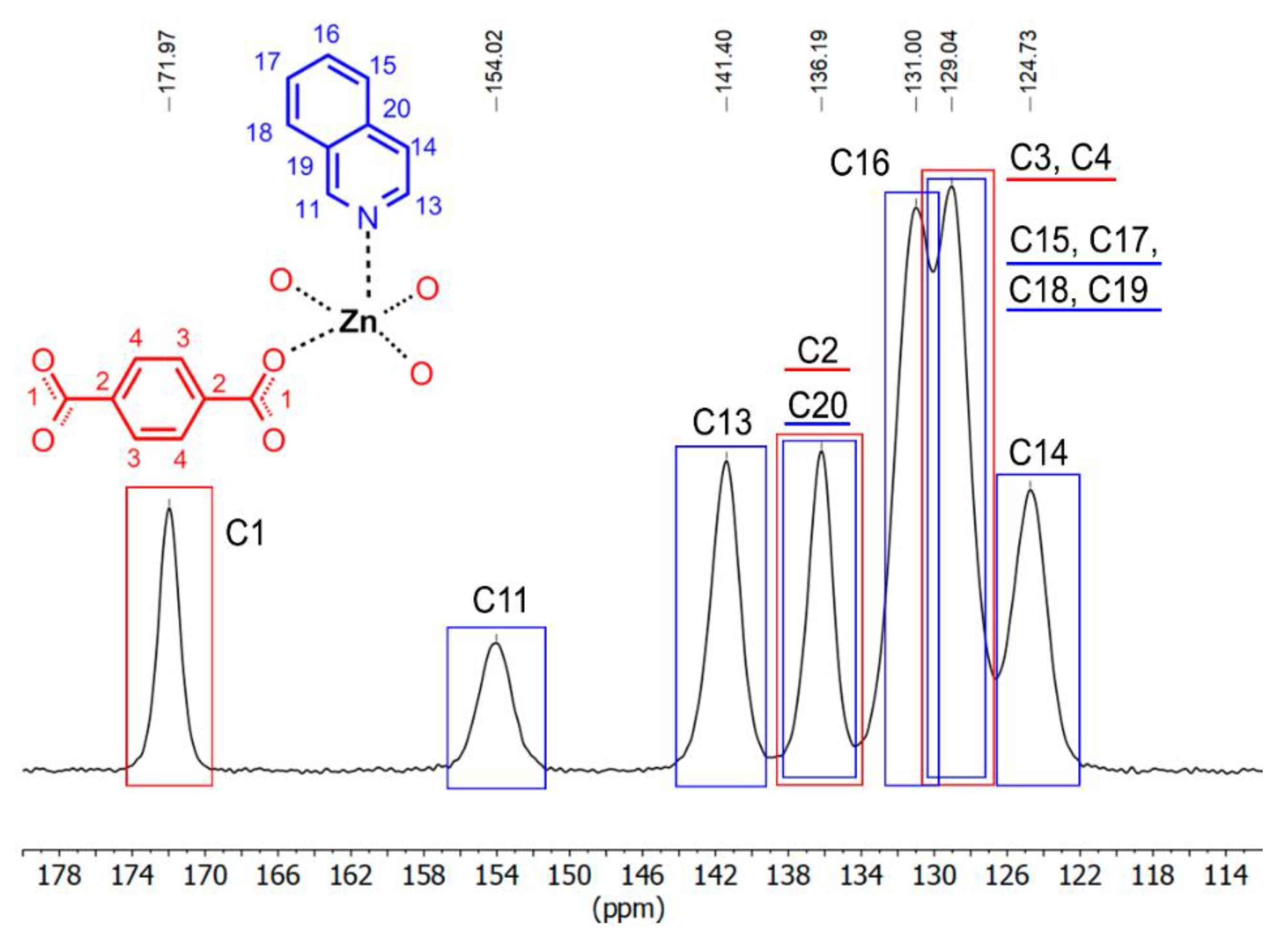
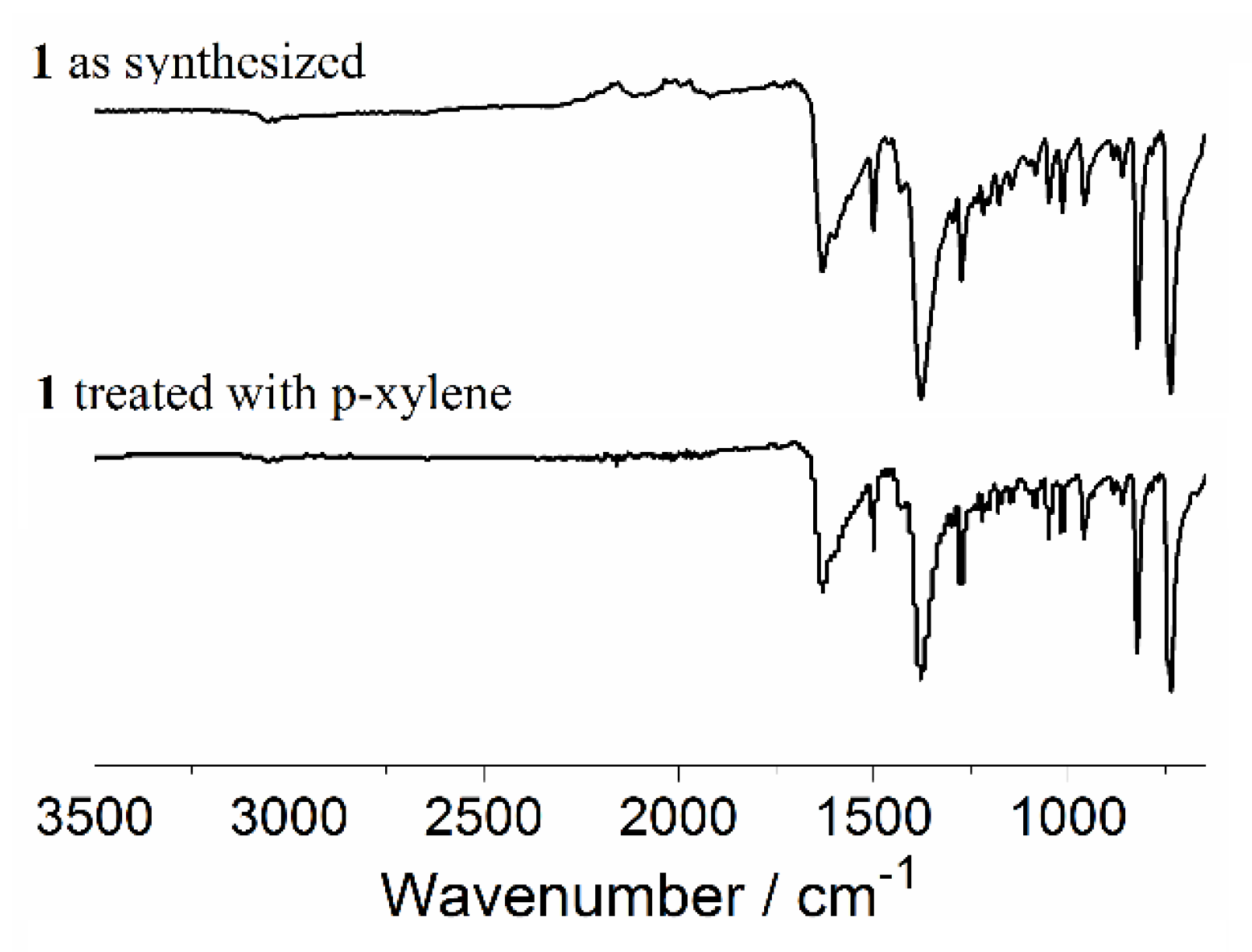
3.2. PXRD, Thermal Stability, IR-Correlation, and Solid-State Structure-CPMAS 13C NMR Correlation
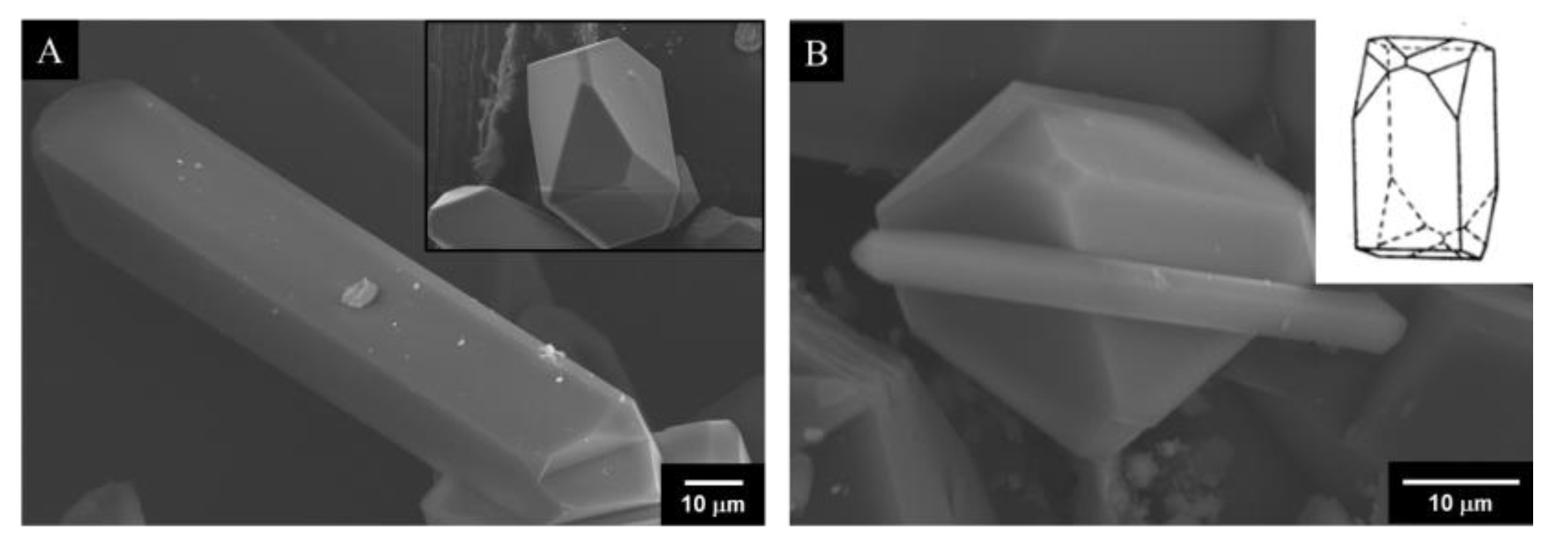
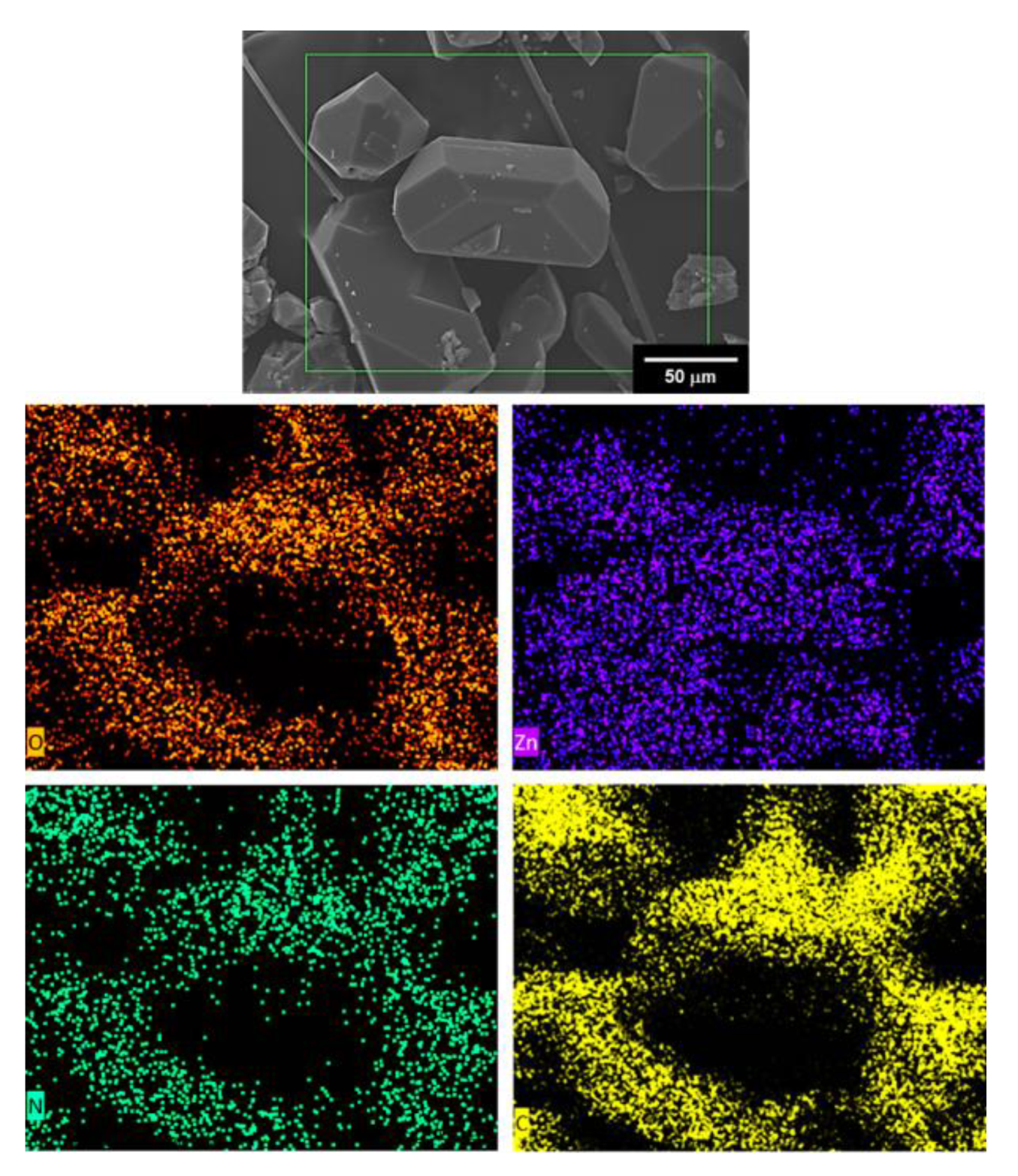
3.3. SEM-EDS Analysis
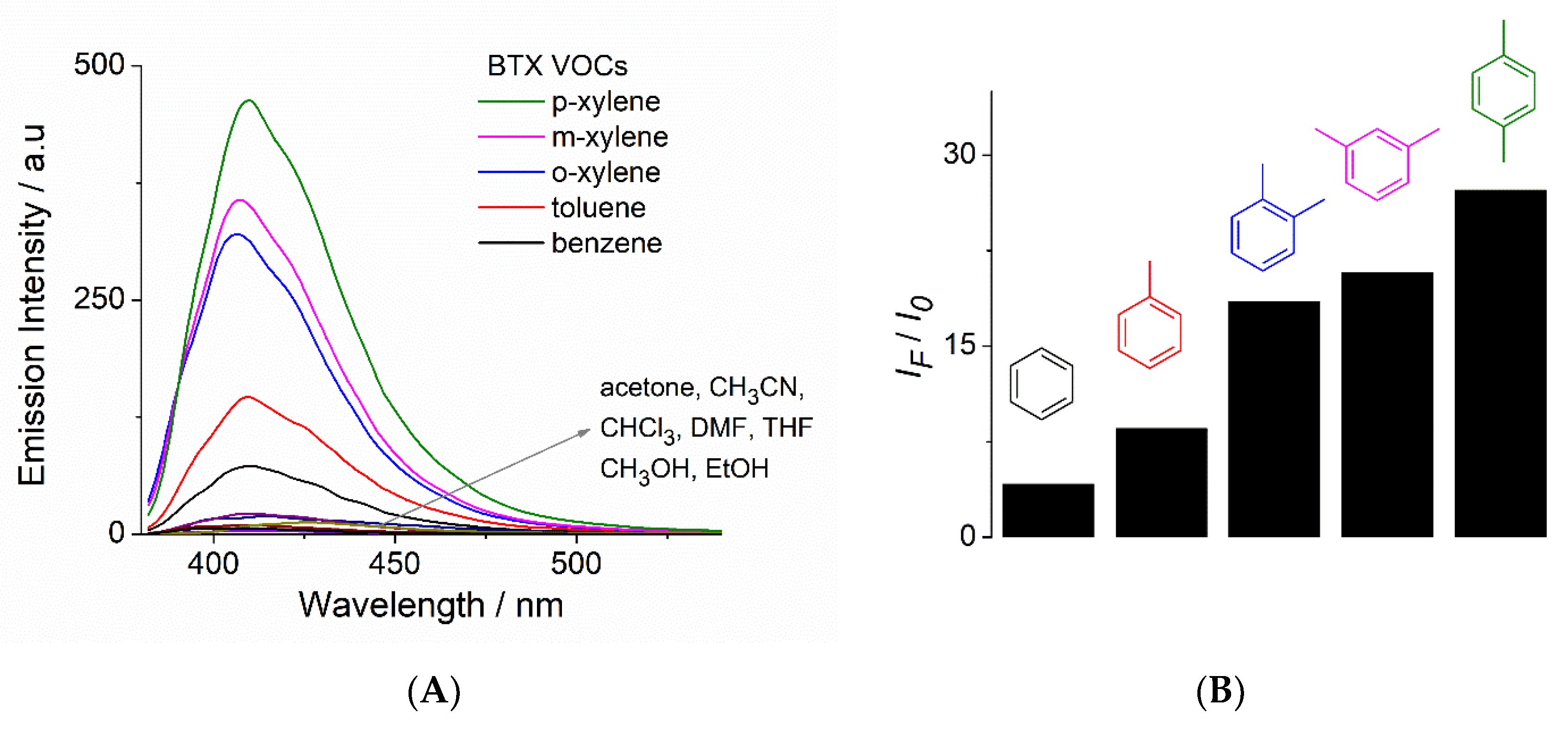
3.4. Photoluminescent Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lei, J.; Qian, R.; Ling, P.; Cui, L.; Ju, H. Design and sensing applications of metal-organic framework composites. Trends. Anal. Chem. 2014, 58, 71–78. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.D.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Xia, T.; Yu, E.; Cui, Y. Luminescent metal–organic framework thin films: From preparation to biomedical sensing applications. Crystals. 2018, 8, 338. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Zhang, N.; Guan, Q.L.; Liu, C.H.; Sun, Y.; Li, B.; Xing, Y.H.; Bai, F.Y. A rht-Type Luminescent Zn (II)-MOF Constructed by Triazine Hexacarboxylate Ligand: Tunable Luminescent Performance and White-light Emission Regulation through doping Eu3+/Tb3+. Appl. Organometal Chem. 2020, 34, 1–10. [Google Scholar] [CrossRef]
- Müller-Buschbaum, K.; Beuerle, F.; Feldmann, C. MOF based luminescence tuning and chemical/physical sensing. Microporous Mesoporous Mater. 2014, 216, 171–199. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.; Brown, R.J.C. Progress in Polymer Science Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar] [CrossRef]
- Rosales-Vázquez, L.D.; Valdes-García, J.; Bazany-Rodríguez, I.J.; Germán-Acacio, J.M.; Martínez-Otero, D.; Vilchis-Néstor, A.R.; Morales-Luckie, R.; Sánchez-Mendieta, V.; Dorazco-González, A. A sensitive photoluminescent chemosensor for cyanide in water based on a zinc coordination polymer bearing ditert-butyl-bipyridine. Dalt. Trans. 2019, 48, 12407–12420. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid Detection of the Biomarkers for Carcinoid Tumors by a Water Stable Luminescent Lanthanide Metal–Organic Framework Sensor. Adv. Funct. Mater. 2018, 28, 1–10. [Google Scholar] [CrossRef]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New Microporous Metal–Organic Framework Demonstrating Unique. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, Z.; Xin, X.; Sun, D. Metal–Organic frameworks based luminescent materials for nitroaromatics sensing. CrystEngComm 2015, 18, 193–206. [Google Scholar] [CrossRef]
- Rosales-Vázquez, L.D.; Sánchez-Mendieta, V.; Dorazco-González, A.; Martínez-Otero, D.; García-Orozco, I.; Morales-Luckie, R. Cadmium-1,4-cyclohexanedicarboxylato coordination polymers bearing different di-alkyl-2,2’-bipyridines: Syntheses, crystal structures and photoluminescence studies. Dalt. Trans. 2017, 46, 12516–12526. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Rananaware, A.; Rix, C.; Bhosale, S.V.; Latham, K. Highly Fluorescent Metal-Organic Framework for the Sensing of Volatile Organic Compounds. Cryst. Growth Des. 2016, 16, 3067–3071. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trends. Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Zhou, J.M.; Shi, W.; Xu, N.; Cheng, P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg. Chem. 2013, 52, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, C.; Wang, E.; Li, Y.; Hao, N.; Hu, C.; Xu, L. Syntheses, structures, and photoluminescence of a novel class of d10 metal complexes constructed from pyridine-3,4-dicarboxylic acid with different coordination architectures. Inorg. Chem. 2004, 43, 1850–1856. [Google Scholar] [CrossRef]
- Liu, G.X.; Zhu, K.; Chen, H.; Huang, R.Y.; Xu, H.; Ren, X.M. Two novel three-dimensional coordination polymers based on metal clusters: Hydrothermal syntheses, crystal structures and luminescence. Inorg. Chim. Acta 2009, 362, 1605–1610. [Google Scholar] [CrossRef]
- Rendón-Balboa, J.C.; Villanueva-Sánchez, L.; Rosales-Vázquez, L.D.; Valdes-García, J.; Vilchis-Nestor, A.R.; Martínez-Otero, D.; Martínez-Vargas, S.; Dorazco-González, A. Structure of a luminescent 3D coordination polymer constructed with a trinuclear core of cadmium-trimesate and isoquinoline. Inorg. Chim. Acta 2018, 483, 235–240. [Google Scholar] [CrossRef]
- Ma, B.Q.; Mulfort, K.L.; Hupp, J.T. Microporous pillared paddle-wheel frameworks based on mixed-ligand coordination of zinc ions. Inorg. Chem. 2005, 44, 4912–4914. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Topological analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem. Rev. 2014, 114, 1343–1370. [Google Scholar] [CrossRef]
- Munn, A.S.; Amabilino, S.; Stevens, T.W.; Daniels, L.M.; Clarkson, G.J.; Millange, F.; Lennox, M.J.; Düren, T.; Bourelly, S.; Llewellyn, P.L.; et al. Metal-organic frameworks from divalent metals and 1,4-benzenedicarboxylate with bidentate pyridine-N-oxide co-ligands. Cryst. Growth Des. 2015, 15, 891–899. [Google Scholar] [CrossRef]
- Hawxwell, S.M.; Brammer, L. Two-dimensional metal-organic frameworks containing linear dicarboxylates. Acta Cryst. Sect. B Struct. Sci. 2006, 3, 808–814. [Google Scholar] [CrossRef]
- Luisi, B.S.; Ma, E.Z.; Moulton, E.B. Tri-metal Secondary Building Units: Toward the Design of Thermally Robust Crystalline Coordination Polymers. J. Chem. Crystallogr. 2007, 37, 743–747. [Google Scholar] [CrossRef]
- Hailian, L.; Eddaoudi, M.; Groy, T.L.; Yaghi, O.M. Establishing Microporosity in Open Metal–Organic Frameworks: Gas Sorption Isotherms for Zn (BDC). J. Am Chem Soc. 1998, 120, 8571–8572. [Google Scholar]
- Tao, J.; Tong, M.-L.; Shi, J.-X.; Chen, X.-M.; Ng, S.W. Blue photoluminescent Zinc coordination polymers with supertetranuclear cores. Chem. Commun. 2000, 20, 2043–2044. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C. 2018, 6, 4342. [Google Scholar] [CrossRef]
- Martins, V.D.; Granato, M.A.; Rodrigues, A.E. Isobaric Vapor-Liquid Equilibrium for Binary Systems 2,2,4-Trimethylpentane with o-Xylene, m-Xylene, p-Xylene, and Ethylbenzene at 250 kPa. J. Chem. Eng. Data 2014, 59, 1499. [Google Scholar] [CrossRef]
- Rushi, A.D.; Datta, K.P.; Ghosh, P.S.; Mulchandani, A.; Shirsat, M.D. Selective Discrimination among Benzene, Toluene, and Xylene: Probing Metalloporphyrin-Functionalized Single-Walled Carbon Nanotube-Based Field Effect Transistors. J. Phys. Chem. C. 2014, 118, 24034. [Google Scholar] [CrossRef]
- Liu, F.F.; Escher, B.I.; Were, S.; Duffy, L.; Ng, J.C. Mixture effects of benzene, toluene, ethylbenzene, and xylenes (BTEX) on lung carcinoma cells via a hanging drop air exposure system. Chem. Res. Toxicol. 2014, 27, 952–959. [Google Scholar] [CrossRef]
- WHO (1993) Benzene, in Environmental Health Criteria 150, World Health Organization, International. Program on Chemical Safety. Available online: http://www.inchem.org/documents/ehc/ehc/ehc150.htm (accessed on 6 April 2020).
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects. 2019, 19, 100364. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Chen, J.; Deng, C.; Xu, N.; Shi, W.; Chen, P. Highly selective luminescent sensing of xylene isomers by a water stable Zn-organic framework. Inorg. Chem. Commun. 2016, 69, 1–3. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Sukhikh, T.S.; Filatov, E.Y.; Anosova, G.A.; Ryadun, A.A.; Kovalenko, K.A.; Potapov, A.S. Synthesis, crystal structure, and luminescent properties of novel zinc metal-organic frameworks based on 1,3-Bis(1,2,4-triazol-1-yl)propane. Cryst. Growth Des. 2017, 17, 5559–5567. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Long, S.; Peh, B.; Dong, J.; Wang, Y.; Karmakar, A.; Yuan, Y.D.; Cheng, Y.; Zhao, D. Luminescent Metal-Organic Frameworks for the Detection and Discrimination of o-Xylene from Xylene Isomers. Inorg. Chem. 2018, 57, 13631–13639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, J.; Chen, D.; Zhou, J.; Zhang, S.; Shia, W.; Cheng, P. Microporous heterometal-organic framework as a sensor for BTEX with high selectivity. J. Mater. Chem. A. 2014, 2, 20450–20453. [Google Scholar] [CrossRef]
- APEX 2 Software Suite; Bruker AXS Inc.: Madison, WI, USA, 2010.
- Sheldrick, M.G. SHELXT−Integrated space-group and crystal-structure determination . Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3. [Google Scholar] [CrossRef]
- Hübschle, B.C.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton. Trans. 1984, 1, 1349–1356. [Google Scholar] [CrossRef]
- Chisca, D.; Croitor, L.; Petuhov, O.; Kulikova, O.V.; Galina, F.; Volodina, G.F.; Coropceanu, E.B.; Masunov, A.E.; Fonari, M.S. Tuning structures and emissive properties in a series of Zn(II) and Cd(II) coordination polymers containing dicarboxylic acids and nicotinamide pillars. CrystEngComm 2018, 20, 432–447. [Google Scholar] [CrossRef]
- Cui, K.; Ma, J.; Huo, X.K.; Qu, Z.; Zhang, J.X. A new 2D Zinc(II)-organic framework with dinuclear units based on iodinated terephthalate: Synthesis, crystal structure and luminescence behavior. Z. Fur Nat. Sect. B. J. Chem. Sci. 2014, 69, 859–863. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, Z.; Jia, H.; Chen, S.; Qin, L.; Zheng, H. Zn(II)/Cd(II) terephthalate coordination polymers incorporating Bi-, Tri-, and tetratopic phenylamine derivatives: Crystal structures and photoluminescent properties. Cryst. Growth Des. 2016, 16, 2747–2755. [Google Scholar] [CrossRef]
- Wang, F.K.; Yang, S.Y.; Huang, R.B.; Zheng, L.S.; Batten, S.R. Control of the topologies and packing modes of three 2D coordination polymers through variation of the solvent ratio of a binary solvent mixture. CrystEngComm 2008, 10, 1211–1215. [Google Scholar] [CrossRef]
- Schoedel, A.; Yaghi, O.M. Porosity in Metal–Organic Compounds. In Macrocyclic and Supramolecular Chemistry How Izatt–Christensen Award Winners Shaped the Field; Reed, M.I., Ed.; John Wiley & Sons Inc: Chichester, West Sussex, UK, 2016; Volume 1, pp. 210–212. [Google Scholar]
- Dorazco-González, A.; Martinez-Vargas, S.; Hernández-Ortega, S.; Valdés-Martínez, J. Directed self-assembly of mono and dinuclear copper(II) isophthalates into 1D polymeric structures. Design and an unusual cocrystallization. CrystEngComm 2013, 15, 5961–5968. [Google Scholar]
- Bennett, T.D.; Todorova, T.K.; Baxter, E.F.; Reid, D.G.; Gervais, C.; Bueken, B.; Van de Voorde, B.; De Vos, D.; Keenf, D.A.; Mellot-Draznieks, C. Connecting defects and amorphization in UiO-66 and MIL-140 metal-organic frameworks: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2016, 18, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.A.; Hoffmann, A.; Höppe, H.A.; Janiak, C. Crystal structures and solid-state CPMAS 13C NMR correlations in luminescent zinc(II) and cadmium(II) mixed-ligand coordination polymers constructed from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzenedicarboxylate. Dalt. Trans. 2009, 10, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.J. Single-crystal structure validation with the program. PLATON J. Appl. Crystallogr. 2003, 36, 7. [Google Scholar] [CrossRef]



| Empirical formula | C34 H24 N2 O8 Zn2 |
| Formula weight | 719.29 |
| Temperature | 296(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P 2/n |
| Unit cell dimensions | a = 10.9454(4) Å, α = 90°. |
| b = 10.9146(4) Å, β = 112.457(2)°. | |
| c = 13.5283(5) Å, γ = 90°. | |
| Volume | 1493.59(10) Å3 |
| Z | 2 |
| Density (calculated) | 1.599 mg/m3 |
| Absorption coefficient | 1.664 mm−1 |
| F(000) | 732 |
| Crystal size | 0.207 x 0.170 x 0.156 mm3 |
| Theta range for data collection | 2.477° to 25.359°. |
| Index ranges | –13 <= h <= 12, –13 <= k <= 13, –14 <= l <= 16 |
| Reflections collected | 12,174 |
| Independent reflections | 2730 [R(int) = 0.0264] |
| Completeness to theta = 25.242° | 99.4% |
| Absorption correction | Analytical |
| Max. and min. transmission | 0.8479 and 0.7915 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 2730 / 216 / 268 |
| Goodness-of-fit on F2 | 1.173 |
| Final R indices [I > 2 sigma(I)] | R1 = 0.0529, wR2 = 0.1239 |
| R indices (all data) | R1 = 0.0554, wR2 = 0.1251 |
| Largest diff. peak and hole | 1.239 and −1.152 e.Å−3 |
| Zn(1)-O(4) | 2.003(3) | O(4)-Zn(1)-N(12) | 102.83(17) |
| Zn(1)-N(12) | 2.033(4) | O(4)-Zn(1)-O(2)#1 | 88.55(17) |
| Zn(1)-O(2)#1 | 2.042(4) | N(12)-Zn(1)-O(2)#1 | 103.56(17) |
| Zn(1)-O(3)#2 | 2.059(4) | O(4)-Zn(1)-O(3)#2 | 158.30(15) |
| Zn(1)-O(1) | 2.065(4) | O(4)-Zn(1)-O(1) | 87.57(17) |
| Zn(1)-Zn(1)#1 | 2.9740(11) | N(12)-Zn(1)-O(1) | 97.43(17) |
| N(12)-Zn(1)-O(3)#2 | 98.87(17) | ||
| O(2)#1-Zn(1)-O(3)#2 | 86.9(2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-Vázquez, L.D.; Bazany Rodríguez, I.J.; Hernández-Ortega, S.; Sánchez-Mendieta, V.; Vilchis-Nestor, A.R.; Cázares-Marinero, J.d.J.; Dorazco-González, A. Structure of a Luminescent MOF-2 Derivative with a Core of Zn(II)-Terephthalate-Isoquinoline and Its Application in Sensing of Xylenes. Crystals 2020, 10, 344. https://doi.org/10.3390/cryst10050344
Rosales-Vázquez LD, Bazany Rodríguez IJ, Hernández-Ortega S, Sánchez-Mendieta V, Vilchis-Nestor AR, Cázares-Marinero JdJ, Dorazco-González A. Structure of a Luminescent MOF-2 Derivative with a Core of Zn(II)-Terephthalate-Isoquinoline and Its Application in Sensing of Xylenes. Crystals. 2020; 10(5):344. https://doi.org/10.3390/cryst10050344
Chicago/Turabian StyleRosales-Vázquez, Luis D., Iván J. Bazany Rodríguez, Simón Hernández-Ortega, Víctor Sánchez-Mendieta, Alfredo R. Vilchis-Nestor, José de Jesús Cázares-Marinero, and Alejandro Dorazco-González. 2020. "Structure of a Luminescent MOF-2 Derivative with a Core of Zn(II)-Terephthalate-Isoquinoline and Its Application in Sensing of Xylenes" Crystals 10, no. 5: 344. https://doi.org/10.3390/cryst10050344
APA StyleRosales-Vázquez, L. D., Bazany Rodríguez, I. J., Hernández-Ortega, S., Sánchez-Mendieta, V., Vilchis-Nestor, A. R., Cázares-Marinero, J. d. J., & Dorazco-González, A. (2020). Structure of a Luminescent MOF-2 Derivative with a Core of Zn(II)-Terephthalate-Isoquinoline and Its Application in Sensing of Xylenes. Crystals, 10(5), 344. https://doi.org/10.3390/cryst10050344